Abstract
SGI-1776 is a small molecule Pim kinase inhibitor that primarily targets c-Myc-driven transcription and cap-dependent translation in mantle cell lymphoma (MCL) cells. Bendamustine is an alkylating chemotherapeutic agent approved for use in B-cell lymphoma that is known to induce DNA damage and to initiate response to repair. We hypothesized that while each drug leads to the effects as stated above, combination of these drugs will enhance SGI-1776-induced inhibition of global transcription and translation processes, while promoting bendamustine-triggered decrease of DNA synthesis and DNA damage response in B-cell lymphoma. Both SGI-1776 and bendamustine as single agents effectively induced apoptosis and when used in combination, additive effect in cell killing was observed in MCL cell lines, JeKo-1 and Mino, as well as MCL and splenic marginal zone lymphoma (a type of B-cell lymphoma) primary cells. As expected, SGI-1776 was effective in inducing decrease of global RNA and protein synthesis, while bendamustine significantly inhibited DNA synthesis and generated DNA damage response. When used in combination, effects were intensified in DNA, RNA and protein syntheses compared to single agent treatments. Together, these data provided foundation and suggested feasibility of using Pim kinase inhibitor in combination with chemotherapeutic agents such as bendamustine in B-cell lymphoma.
Keywords: Pim kinase, mantle cell lymphoma, SGI-1776, Bendamustine, combination strategy
Introduction
Proviral integration site for moloney murine leukemia virus (Pim) kinases are proto-oncogenes. There are three Pim kinase family members (Pim−1, −2 and −3) that are all Ser/Thr/Tyr kinases with known function in regulating transcription, translation, survival and cell cycle processes.1,2 For instance, c-Myc, a transcription regulator is a known Pim kinase substrate,3-5 as well as its co-activator, Histone H3.6 Translation regulator 4E-BP1 phosphorylation by Pim kinases is associated with increased cap-dependent protein synthesis.7 Meanwhile, phosphorylation of pro-apoptotic protein Bad is also mediated by Pim kinases.8 In addition, phosphorylation of cell cycle-regulating phosphatases cdc25A/C and kinase inhibitors p21 and p27 by Pim kinases regulate cell cycle processes.9-11
Several studies have established an increased expression of Pim kinases in hematological malignancies.2,12,13 At transcript level, Pim kinases are known to be overexpressed in B-cell malignancies including mantle cell lymphoma (MCL) and chronic lymphocytic leukemia (CLL).12,14,15 Pim-1 expression level is known to be associated with poor MCL patient outcome.14 Immunoblot analyses in MCL cell lines and primary lymphoma cells and immunohistochemistry assays in paraffin-embedded tissues demonstrated expression of all three Pim kinases proteins.16
Efforts have been devoted into developing Pim kinase inhibitors for clinical use, and SGI-1776, a potent and selective Pim kinase inhibitor, was the first one to enter the clinic.12 Pre-clinical studies with SGI-1776 have shown cytotoxicity in myeloid leukemia and lymphoid B-cell diseases, including MCL and CLL.12,13,16 In MCL, it has been demonstrated that besides inhibition of global RNA synthesis, and c-Myc-driven transcription, cap-dependent translation is impacted by SGI-1776.16 Decrease of short-lived transcripts and proteins such as anti-apoptotic Mcl-1 was also measured, as a potential mechanism of cell killing.16 However, single agent SGI-1776 therapy may have limited efficacy in these B-cell diseases, especially given that redundant pathways of Pim kinase are activated in B-cell lymphoma, and heterogeneous response was measured among cell lines and primary samples in MCL.16,17 Therefore, it may be advantageous to use SGI-1776 in combination with a broad-spectrum chemotherapeutic agent, to eliminate the effects by redundant pathways, while reducing the chance of developing chemoresistance in the clinic.
Bendamustine is an alkylating agent, with nitrogen mustard as an active component.18,19 Bendamustine functions primarily as an alkylating agent, inducing intra- and inter-strand DNA crosslinks, which lead to impaired DNA replication, repair and transcription processes in both quiescent and dividing cells.18,20 Meanwhile, traditional apoptosis pathways such as p53, Bad family protein (NOXA, Bax) and caspases are activated, causing mitotic catastrophe and cell death.21 Importantly, bendamustine does not show cross-resistance with other conventional chemotherapeutic agents, making it desirable for combination use.21
Bendamustine was first synthesized in the 1960s and was extensively used in Europe.22 In the US, it was approved by the FDA in 2008 for use in treating CLL and non-Hodgkin’s lymphoma, many of which are B-cell diseases.20 Currently, clinical trials are ongoing using bendamustine in combination with rituximab–an FDA approved anti-CD20 for B-cell malignancies, to treat non-Hodgkin’s lymphoma.23 In B-cell lymphomas, such as MCL, high relapse rate was observed following conventional chemotherapeutic regimens such as R-CHOP.24 So far, clinical trials using bendamustine in combination with rituximab have shown impressive results, and a phase III trial in follicular, indolent lymphoma and MCL showed a >90% overall survival rate, and >40% complete remission, indicating that bendamustine is an effective therapeutic agent in B-cell lymphoma. Importantly, this regimen was also well-tolerated by patients.25
With the existing knowledge on both drugs, we hypothesize that combination of transcription and translation targeting SGI-1776 with DNA-damaging bendamustine will additively or synergistically disrupt oncogenic processes by introducing DNA damage, disrupting DNA synthesis and repair, while blocking transcription and translation processes which ultimately lead to cell death in B-cell lymphoma. We used established MCL cell lines and fresh B-cell lymphoma primary cells to examine the cellular response to such combination therapy approach, with respect to cell death, reduction of global DNA, RNA and protein synthesis levels as well as DNA damage. Our study provides the foundation and establishes the basic evaluation of the therapeutic approach using Pim kinase inhibitor in combination with bendamustine in B-cell lymphoma, which can lead to more in-depth study in the future.
Material and Methods
Cell lines
MCL cell lines JeKo-1 and Mino were obtained from Dr. Hesham Amin at MD Anderson Cancer Center. The cell lines have been characterized for molecular marker expression.26 These cell lines are maintained in 10% and 20% fetal bovine serum in RPMI1640 media, respectively, as described previously.16 The cells were authenticated and were routinely tested for Mycoplasm contamination, as described before.16
Primary B-cell lymphoma samples
Patient samples were obtained from Lymphoma tissue bank at MD Anderson Cancer Center, collected from patients who consented in the Declaration of Helsinki through the institution review board-approval protocols. Peripheral blood mononuclear cells (PBMCs) were isolated from leukemic phase blood as previously described, and cells maintained at 107 cells/mL concentration.16 The PBMCs from MCL patient (age 57, male) contained 69% MCL cells, while percent malignant cells in PBMCs from SMZL patient (age 75, male) was unknown. The MCL sample contained 23.5 white blood count per μL blood, and 71% lymphocyte, 24% neutrophils and 2% monocytes while in the SMZL patient sample, these numbers were 63.4, 87%, 10% and 2%, respectively. These samples were not treated with growth factors.
Drugs
SGI-1776 was provided by SuperGen (now Tolero Pharmaceuticals, Inc., Salt Lake City, UT) in powder form and liquid stock was made and stored as previously described.16 Bendamustine hydrochloride was purchased from Selleckchem, USA (Houston, TX) as powder, and was dissolved in DMSO to make a 30mM stock solution and stored in −80°C. Upon use, lower concentration aliquots were prepared (5 and 10mM) and stored at −20°C.
Apoptosis assay
MCL cells were treated with DMSO, SGI-1776, bendamustine or simultaneous combination of the two drugs, and Annexin V/PI positivity was measured as described before to measure apoptosis.16
Macromolecule synthesis assay
Cells were incubated with [methyl- 3H]-thymidine, [5,6-3H]-uridine or [4,5-3H]-L-leucine (1.0mCi/mL stock, Moravek Biochemicals, Brea, CA) for 45mins of each experiment and then the incorporated radioactivity was measured as previously described to quantitate DNA, RNA and protein synthesis, respectively.12
Immunostaining of γ-H2AX
Post-treatment MCL cells were washed and incubated for 2hr at room temperature with primary phospho-Histone γ-H2AX (Ser139) antibody (EMD Millipore, Billerica, MA) followed by 1hr incubation with Alexa Fluor mouse IgG secondary antibody (488 goat, Invitrogen Corporation, Carlsbad, CA). Cells then were washed and co-incubated with 10μg/mL propidium iodine solution and 2.5μg/mL of DNAse-free RNAase (Roche Diagnostics, Basel, Switzerland). The cells then were analyzed for florescence signal shift to detect the extent of DNA damage, using a fluorescence-activated cell sorting (FACS) instrument.
Data analysis
All data plots were prepared and analyzed using GraphPad software (GraphPad). Cell line data were performed in triplicates and were shown as mean value ± SEM. DMSO was used as a vehicle control. Fractional analysis was used to determine if the drug combination caused less than, equal to or more than additive effect on inducing of apoptosis (Table 1). Data points fell into expected results ±20% were categorized into one of these three outcomes. Similar calculation was performed in determining SGI-1776 and bendamustine combination effect on inhibition of DNA, RNA and protein synthesis, as well as increased γ-H2AX phosphorylation in MCL cell lines and B-cell lymphoma primary samples.
Table 1.
Factional analysis of combination therapy of SGI-1776 with bendamustine in B-cell lymphoma.
| Expected cell death | Observed cell death | |||
|---|---|---|---|---|
| Cells | 5μM Bendamustine +5μM SGI-1776 |
10μM Bendamustine +5μM SGI-1776 |
5μM Bendamustine +5μM SGI-1776 |
10μM Bendamustine +5μM SGI-1776 |
| JeKo-1 | 10% | 18% | 18% | 28% |
| Mino | 9% | 26% | 13% | 31% |
| MCL | 23% | 24% | 18% | 21% |
| SMZL | 34% | 38% | 35% | 37% |
Formula for fractional analysis: %survival drug A X %survival drug B=Expected survival 1-%Expected survival rate=%Expected death
Expected death > Observed deaths → Less than additive to antagonistic
Expected death=Observed deaths → Additive
Expected death<Observed deaths More → than additive to synergistic
Results
Additive cell killing effect observed in B-cell lymphoma cells using SGI-1776 and bendamustine combination treatment
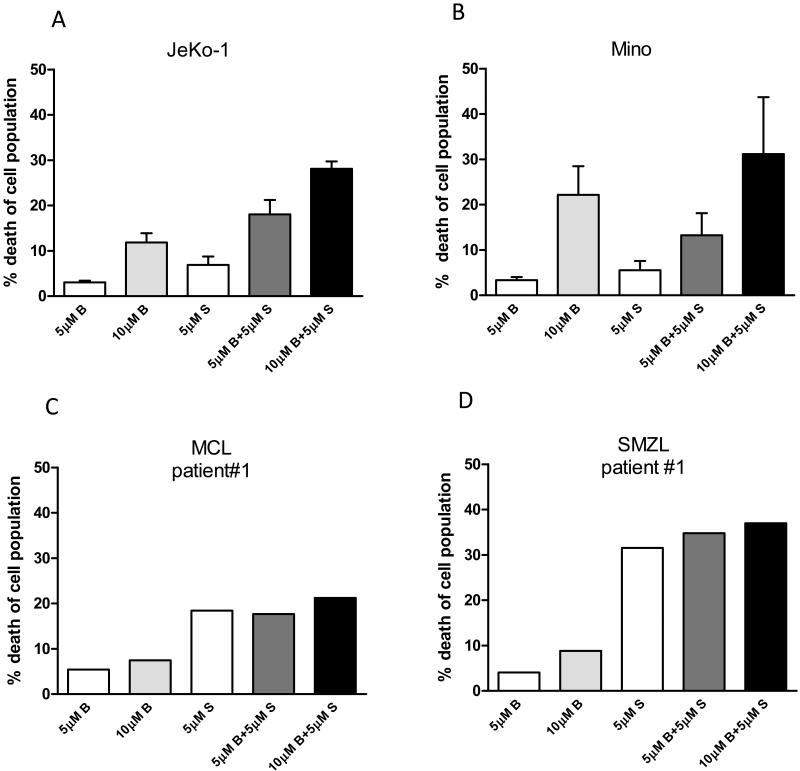
MCL cell lines JeKo-1 and Mino and primary B-lymphoma samples were treated with 5μM, 10μM bendamustine, 5μM SGI-1776, or combination of 5μM or 10μM bendamustine with 5μM SGI-1776 for 24hr, and then drug-induced apoptosis level was measured by Annexin V/PI positivity using flow cytometry (Figure 1). Data were represented as drug-induced cell death, where spontaneous cell death from DMSO treatment was subtracted from each drug treatment. In JeKo-1 cells, limited levels (<12%) of apoptosis were detected following 5 or 10μM bendamustine, or 5μM SGI-1776 single agent treatments. However, greater cell death was detected at both 5 and 10μM bendamustine in combination with 5μM SGI-1776 treatments, and there was 18% and 28% cell death measured, respectively. Combinations of SGI-1776 and bendamustine at these concentrations resulted in greater than additive killing (Table 1). In Mino cells, about 3-5% cell death was detected with 5μM of bendamustine and SGI-1776 single agent treatments for 24hr, whereas 10μM bendamustine resulted in 22% cell death. In combination studies, 13% and 31% of cell death was observed in cells treated by 5 or 10μM bendamustine with 5μM SGI-1776, respectively, which indicates that combination of the two drugs had additive killing effect in Mino (Table 1).
Figure 1. SGI-1776 and bendamustine combination treatment induced additive cell killing effect in B-cell lymphoma cells.
MCL cell lines (A) JeKo-1 and (B) Mino, or (C) MCL primary sample and (D) SMZL primary cells were treated with DMSO alone, or 5μM, 10μM bendamustine, 5μM SGI-1776, or 5μM, 10μM bendamustine combined with 5μM SGI-1776 for 24hr, then cells were collected and stained with Annexin V/PI and analyzed by flow cytometry to detect the changes in Annexin V/PI positivity. Annexin V/PI positive cells were presented as percent cell death, and endogenous cell death from DMSO treatment was subtracted from results of each drug treatment in this figure. Experiments in MCL cell lines were performed in triplicate and presented as mean ± SEM.
In primary B-lymphoma samples, heterogeneous results in cell death were observed. In the primary MCL cells, less than 10% cell death was detected when cells were treated with 5 or 10μM of bendamustine alone, while 18% cell death was observed with 5μM of SGI-1776 treatment. Combination of both drugs resulted in 21% cell death (Table 1). Similar to MCL sample, there was less than 10% cell death observed with bendamustine single agent treatment in the primary splenic marginal zone lymphoma (SMZL) sample, but extensive cell death occurred after treating with 5μM SGI-1776 (>30%). Additive cell killing was observed with combination of bendamustine and SGI-1776 (Table 1).
Inhibition of DNA synthesis with SGI-1776 and bendamustine as single agents and in combination
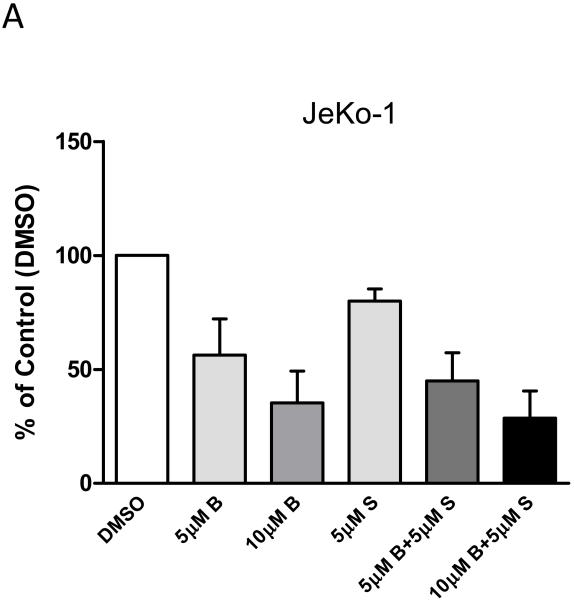
In order to determine effect of bendamustine and SGI-1776 combination treatment on DNA synthesis, radioactive thymidine incorporation assays were performed in JeKo-1 cell line to measure active DNA synthesis levels (Figure 2). With 5μM SGI-1776 alone there was 20% reduction of DNA synthesis level compared to DMSO treated control cells, while 40%-65% reduction of DNA synthesis was induced by 5 and 10μM of bendamustine single treatment in 24hr. When used in combination, 5 or 10μM of bendamustine with 5μM SGI-1776 reduced 55% and 71% of DNA synthesis, respectively. These data suggest that bendamustine and SGI-1776 lead to an additive effect in reducing DNA synthesis in JeKo-1 cells.
Figure 2. Effect of inhibition of DNA synthesis by SGI-1776 and bendamustine and their combination in JeKo-1 cells.
(A) JeKo-1 cells were treated with DMSO, or 5μM, 10μM bendamustine, 5μM SGI-1776, or 5μM, 10μM bendamustine combined with 5μM SGI-1776 for 24hr, then cells were incubated with [methyl-3H]-thymidine at 0.8μCi/mL concentration for 45min. Radioactive incorporation was then measured by scintillation count and values were recorded as disintegration per minute (DPM)/cell and normalized to DMSO control. Experiments were performed in triplicate and presented as mean ± SEM.
Inhibition of RNA synthesis with SGI-1776 and bendamustine as single agents and in combination
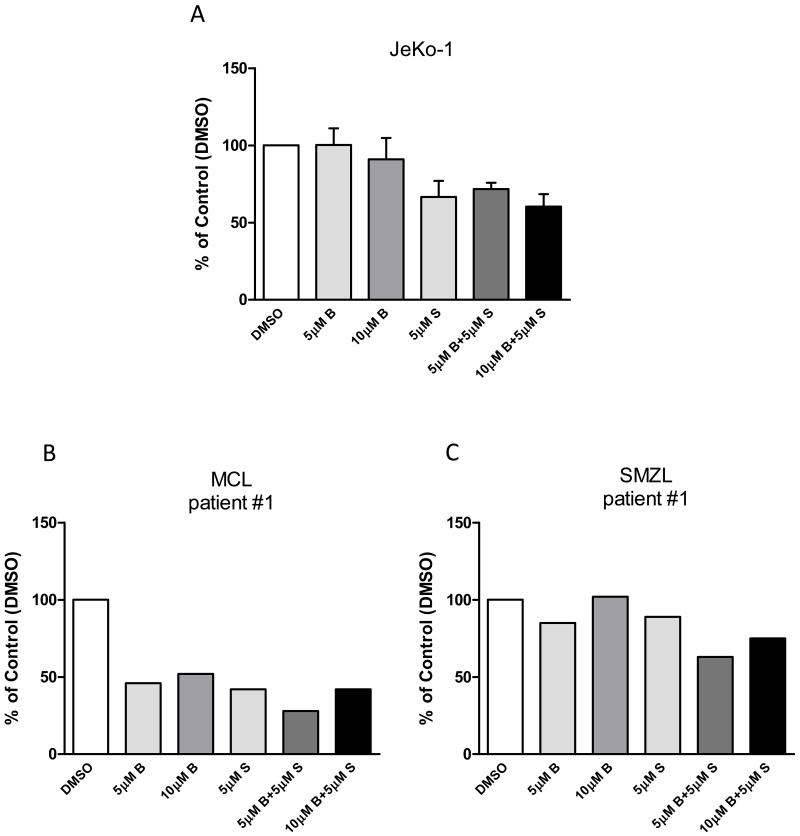
We performed radioactive uridine incorporation assays to measure the changes in global RNA synthesis levels (Figure 3). In JeKo-1 cells, 24hr of 5 or 10μM bendamustine treatment alone had no or minor changes in cellular RNA synthetic capacity. However, 5μM of SGI-1776 treatment alone resulted in 27% of RNA synthesis reduction. RNA synthesis inhibition elicited by SGI-1776 was maintained when combined with bendamustine.
Figure 3. Effect of inhibition of RNA synthesis by SGI-1776 and bendamustine and their combination in B-cell lymphoma cells.
Cells from (A) JeKo-1 cell line, or primary (B) MCL and (C) SMZL samples were treated with DMSO alone, or 5μM, 10μM bendamustine, 5μM SGI-1776, or 5μM, 10μM bendamustine combined with 5μM SGI-1776 for 24hr, then cells were incubated with [5,6-3H]- uridine at 0.8μCi/mL concentration for 45min. Radioactive incorporation was then measured by scintillation count and values were recorded as DPM/cell and normalized to DMSO control. Experiments in JeKo-1 were performed in triplicate and presented as mean ± SEM.
In primary MCL and SMZL samples, we observed somewhat different effects caused by combination treatment of bendamustine and SGI-1776 in reducing RNA synthesis levels. In MCL cells, 24hr treatment of bendamustine alone did not reduce RNA synthesis levels, while SGI-1776 single treatment lead to 7% decrease in RNA synthesis level compared to control. However, when used in combination, 5 or 10μM bendamustine with 5μM of SGI-1776 lowered RNA synthesis levels by 24% and 40% of control, respectively. On the other hand, in SMZL cells, while 5μM of bendamustine reduced global transcription by less than 10% after 24hr, 10μM bendamustine resulted in a 63% reduction of control. In contrast, SGI-1776 treatment alone did not decrease RNA synthesis. Combination of the two drugs showed additive effect in reducing global transcription levels, causing 4% and 79% less RNA synthesis compared to DMSO treatment. These data showed that combination treatment of SGI-1776 and bendamustine is effective in reducing cellular RNA synthetic capacity in quiescent primary samples and a replicating MCL cell line, JeKo-1, and more prominent effect was observed in primary lymphoma cells.
Effect of SGI-1776 and bendamustine as single agents and in combination on protein synthesis
SGI-1776 is known to inhibit protein synthesis in MCL cell lines and primary cells whereas bendamustine is known to cause DNA damage that leads to disruption of DNA synthesis/repair and transcription processes.16,19,20 We hypothesize that SGI-1776 will maintain translation-inhibition even when combined with bendamustine. To test this hypothesis, we conducted radioactive leucine incorporation assays to analyze the inhibitory effect of bendamustine and SGI-1776 both as single agents and in combination in MCL cell line (JeKo-1) and primary B-cell lymphoma cells (Figure 4).
Figure 4. Effect of protein synthesis inhibition by SGI-1776 and bendamustine and their combination in B-cell lymphoma cells.
Cells from (A) JeKo-1 cell line, or primary (B) MCL and (C) SMZL samples were treated with DMSO alone, or 5μM, 10μM bendamustine, 5μM SGI-1776, or 5μM, 10μM bendamustine combined with 5μM SGI-1776 for 24hr, then cells were incubated with [4,5-3H]-L-leucine at 0.8μCi/mL concentration for 45min. Radioactive incorporation was then measured by scintillation count and values were recorded as DPM/cell and normalized to DMSO control. Experiments in JeKo-1 were performed in triplicate and presented as mean ± SEM.
In JeKo-1 cells, there was 10% or less reduction of protein synthesis following 5 or 10μM bendamustine for 24hr treatment, while 33% reduction was observed in 5μM SGI-1776 treatment. In combination treatment, 28% and 40% less protein synthesis was observed when cells were treated with 5 or 10μM bendamustine combined with 5μM of SGI-1776 for 24hr.
In primary MCL cells, both single agent bendamustine and SGI-1776 lead to extensive inhibition of global protein synthesis. With both 5 or 10μM bendamustine alone or 5μM of SGI-1776 alone, there was 54%, 48% and 58% reduction of global translation compared to control, respectively. In combination treatments, 5 or 10μM bendamustine with 5μM of SGI-1776 lead to 72% and 58% protein synthesis reduction compared to DMSO treated cells. In primary SMZL cells, 5μM of bendamustine or SGI-1776 single agent induced 15% and 11% reduction in protein synthesis levels compared to control, while 10μM bendamustine did not cause such reduction. However, 5 or 10μM bendamustine with 5μM of SGI-1776 combination resulted in 37% and 25% reduction of protein synthesis compared to control, respectively. These data suggest that bendamustine and SGI-1776 alone and in combination were effective in reducing global translation processes in MCL cell line and B-cell lymphoma PBMCs.
Effect of SGI-1776 on bendamustine-induced γ-H2AX formation
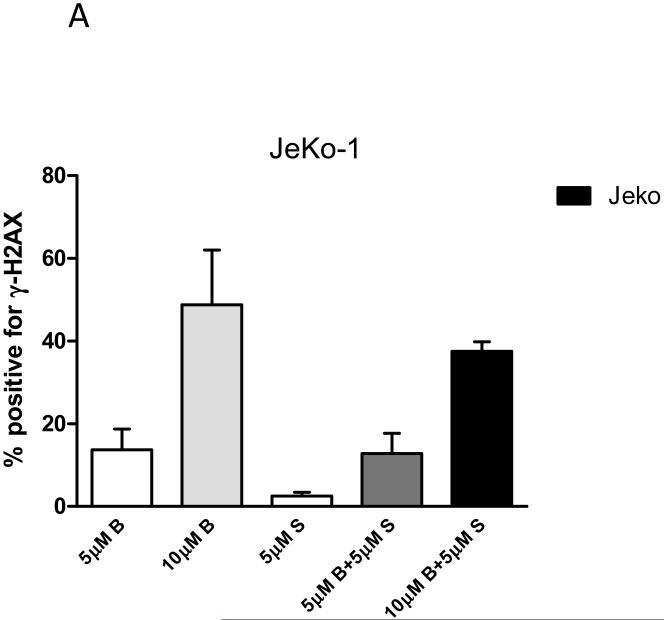
To determine whether bendamustine-induced DNA damage response was impacted by addition of SGI-1776, single agent and combination of the two drugs were evaluated in B-cell lymphoma cells. We performed immunostaining on JeKo-1 cells to analyze the change of a DNA damage marker, Histone 2A variant X (H2AX) at Ser139, also known as γ-H2AX. JeKo-1 cells were treated with bendamustine or SGI-1776 as single agents and also in combination for 24hr and γ-H2AX positivity was detected using flow cytometry (Figure 5). Untreated (DMSO only) cells showed low levels of endogenous H2AX phosphorylation (<1%), which was subtracted from the values of drug-treated cells. 5μM of SGI-1776 induced limited level of DNA damage, only about 3%. As expected from its mechanism, bendamustine as single agent was very effective in increasing γ-H2AX level, where 14% and 49% positivity for γ-H2AX was detected when cells were treated with 5 and 10μM of bendamustine, respectively. When used in combination, 5 or 10μM bendamustine with 5μM SGI-1776 lead to 13% and 38% γ-H2AX positivity, respectively. These results showed that SGI-1776 did not significantly impact bendamustine-induced γ-H2AX formation.
Figure 5. Effect of SGI-1776 on bendamustine-induced γ-H2AX formation.
(A) MCL cell line JeKo-1 were treated with DMSO, or 5μM, 10μM bendamustine, 5μM SGI-1776, or 5μM, 10μM bendamustine combined with 5μM SGI-1776 for 24hr, then cells were collected and incubated with 0.5% goat serum for 1hr, and then probed with primary phospho-Histone γ-H2AX (Ser139) antibody (1:500) following by 1hr incubation with Alexa Fluor mouse IgG secondary antibody (1:200). Then cells were washed and incubated with 10μg/mL propidium iodine solution and 2.5μg/mL of DNAse-free RNAase. Then the cells were analyzed for shift of florescence signal to detect the degree of DNA damage, using flow cytometry. Fluorescent positive cells were presented as percent positive for γ-H2AX, and endogenous H2AX phosphorylation from DMSO treatment was subtracted from results of each drug treatment in this figure. Experiments were performed in triplicate and presented as mean ± SEM.
Discussion
Bendamustine is an approved agent for B-cell malignancies including MCL.20 Mechanistically, bendamustine is known to cause intra- and inter-strand DNA crosslinks that initiates a DNA damage response.21,27 Repair of this damage leads to recovery and survival of cells, and hence a strategy to overcome this may be to combine bendamustine with agents that damage the cellular repair capacities.19 SGI-1776 is an experimental therapeutic agent that inhibits all three Pim kinase family proteins.12 Pim kinases have several substrates and modulate multiple pathways,1,2 however, in MCL, transcription and translation appear to be primary axes affected by SGI-1776.16
While molecular mechanisms were not tested, in this short study, we evaluated cellular mechanisms based on actions of both drugs as described above. It has been established that SGI-1776 downregulates transcription processes, by inhibiting c-Myc-driven transcription machinery while bendamustine disrupts DNA replication and repair.16,18 In MCL cell line and primary cells, SGI-1776 alone resulted in significant decrease in global RNA synthesis, while bendamustine showed little or no effect (Figure 3). The differential outcomes with patient samples may be due to patient heterogeneity, but the results from both cell line and primary cells indicated that combination of SGI-1776 and bendamustine was effective in reducing global RNA synthesis. The effect in decreasing transcription was more pronounced in primary cells compared to MCL cell line, JeKo-1 (Figure 3). This may be due to the difference that primary cells from patients are non-dividing versus JeKo-1 cell line that is highly proliferative. Additionally, molecular signatures of MCL, such as p53 and ATM status that affect Pim kinase inhibition or DNA damage response may be responsible for this variations.28 This needs to be explored in larger number of primary samples and cell lines.
Our previous study demonstrated that SGI-1776 inhibit cap-dependent translation process mediated by decreasing of 4E-BP1 phosphorylation at Thr37/46 in MCL cells.16 Consistent with this finding, we observed that 5μM of SGI-1776 treatment for 24hr effectively reduced the protein synthesis (Figure 4). This effect was also observed in MCL primary sample (60% decrease), and to a lesser extent in SMZL primary sample (10% reduction). These results suggest that SGI-1776 is effective in decreasing translation processes in B-cell lymphoma. With regards to effects on protein translation, bendamustine on the other hand, showed differential responses in these B-cell lymphoma models with 10%, 50% and 15% decrease in JeKo-1 cell line, MCL and SMZL primary cells, respectively (Figure 4). Bendamustine is not known to inhibit protein translation directly, and hence the observed decline may be a secondary effect following disruption of global RNA synthesis or DNA damage response (Figures 2, 3 and 5). This observation is intriguing and it is worth further investigation and could be used in biomarker studies. Combination treatment with SGI-1776 and bendamustine also showed differential responses in inhibition of global protein synthesis (Figure 4). Heterogeneity among patient samples is a likely reason for such variable results, however, in all of these B-cell lymphoma models, especially in primary cells, combination of SGI-1776 with bendamustine leads to greater inhibition of the global protein synthesis compared to single agent treatments.
Bendamustine is known to cause intra- and inter-strand DNA crosslinks, and γ-H2AX phosphorylation, a known marker for DNA double-stranded breaks, is associated with inter-strand crosslinks.18,20 γ-H2AX is important for recruiting and gathering DNA repair proteins along with cell cycle checkpoint proteins to the DNA double-stranded break sites, and can be detected using immunostaining and analyzed by flow cytometry.29,30 Our investigation demonstrated that bendamustine was indeed effective in decreasing total DNA synthesis (Figure 2) while increasing γ-H2AX levels in JeKo-1 cells when treated with the drug for 24hr (Figure 5). Compared to bendamustine, SGI-1776 had limited or no effect on DNA synthesis and γ-H2AX phosphorylation induction (Figures 2, 5). These results were expected, as Pim kinase substrates are mainly in transcription and translation regulation pathways, which occur downstream of DNA synthesis/repair.1,16 Interestingly, combination of SGI-1776 and bendamustine showed additive effect in blocking global DNA synthesis in JeKo-1 (Figure 2) without increasing bendamustine induced-γ-H2AX phosphorylation (Figure 5). Molecular markers related to DNA damage and repair, such as ATM, p53, aurora kinases, along with cell cycle checkpoint proteins may be relevant clinical markers to study Pim kinase inhibitor combination with bendamustine.21,27
Our study has demonstrated that Pim kinase inhibitor, SGI-1776, and bendamustine are effective in B-cell lymphoma both as single agent treatments and as combination therapy. SGI-1776 as a single agent was effective in inhibiting global RNA and protein synthesis in MCL cell line and B-cell lymphoma primary samples, which is consistent to our previous findings.16 Bendamustine as a single agent was more effective in reducing global DNA synthesis and inducing γ-H2AX phosphorylation in MCL cell line, JeKo-1 (Figures 2, 5). These results of single agent treatments of the two drugs are consistent with other published reports, that SGI-1776 primarily targets transcription and translation pathways, whereas bendamustine directly acts on DNA and disrupt DNA replication, repair and transcription processes.16,18,20 Comparable results in cell killing were observed in both MCL cell lines and B-cell lymphoma primary cells when treated with combination of bendamustine and SGI-1776, and in both models, this combination showed additive effects in apoptosis induction (Figure 1).
Our investigation with SGI-1776 and bendamustine serves as a proof-of-concept study since SGI-1776 will not be pursued further in the clinic due to toxicity concerns.31 However, such combinations of Pim kinase inhibitor with bendamustine is feasible since quite a few Pim kinase inhibitors are currently studied by both academic labs and pharmaceutical industries in preclinical and clinical settings, including SMI 4a (University of South Carolina), AZD-1208 (AstraZeneca plc), and GNE-652(Genentech, Inc.) and more.32-36
Conclusion
Our study was the first to use Pim kinase inhibitor, SGI-1776, and bendamustine both as single agents and in combination in B-cell lymphoma cells, and showed effective cellular response in respect of inducing apoptosis, inhibiting DNA, RNA and protein synthesis while promoting DNA damage response. As a single agent, SG-1776 was effective in inhibiting global RNA and protein synthesis while bendamustine effectively reduced global DNA synthesis and induced γ-H2AX expression; both were consistent and in complement with published reports. When used in combination, additive effect in cell killing was observed in JeKo-1 and Mino MCL cell lines, and in MCL, SMZL primary cells, while augmented effects were observed in total DNA, RNA and protein synthesis inhibitions compared to single agent treatments in these models.
Results from our investigation with SGI-1776 and bendamustine function as a proof-of-concept study and suggest feasibility of applying Pim kinase inhibitors with traditional DNA-damaging chemotherapeutic agents. New second-generation Pim kinase inhibitors are currently being developed and tested, and further investigation in combination with chemotherapeutic agents used in the clinic may yield optimum treatment strategies for hard-to-treat lymphomas. In addition, our study provides the foundation for future biomarker studies that can be evaluated in larger sample sets to validate the molecular pathways involved in similar treatment methods.
Acknowledgement
The authors would like to thank Dr. Rakesh Sharma, for obtaining and processing patient samples.
This work is supported by a Lymphoma SPORE (CA136411) to V.G., S.S.N., and Leukemia and Lymphoma Society Translational Research Award to V.G. The primary tumor samples were provided by The University of Texas M D Anderson Cancer Center Lymphoma SPORE Biospecimens Core and Tissue Bank, which are supported by the Cancer Center Support Grant CA16672.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All authors have no conflict of interest.
References
- 1.Chen LS, Balakrishnan K, Gandhi V. Inflammation and survival pathways: chronic lymphocytic leukemia as a model system. Biochem Pharmacol. 2010;80(12):1936–1945. doi: 10.1016/j.bcp.2010.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nawijn MC, Alendar A, Berns A. For better or for worse: the role of Pim oncogenes in tumorigenesis. Nat Rev Cancer. 2011;11(1):23–34. doi: 10.1038/nrc2986. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Wang Z, Li X, Magnuson NS. Pim kinase-dependent inhibition of c-Myc degradation. Oncogene. 2008;27(35):4809–4819. doi: 10.1038/onc.2008.123. [DOI] [PubMed] [Google Scholar]
- 4.Allen JD, Verhoeven E, Domen J, van der Valk M, Berns A. Pim-2 transgene induces lymphoid tumors, exhibiting potent synergy with c-myc. Oncogene. 1997;15(10):1133–1141. doi: 10.1038/sj.onc.1201288. [DOI] [PubMed] [Google Scholar]
- 5.Forshell LP, Li Y, Forshell TZ, et al. The direct Myc target Pim3 cooperates with other Pim kinases in supporting viability of Myc-induced B-cell lymphomas. Oncotarget. 2011;2(6):448–460. doi: 10.18632/oncotarget.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zippo A, De Robertis A, Serafini R, Oliviero S. PIM1-dependent phosphorylation of histone H3 at serine 10 is required for MYC-dependent transcriptional activation and oncogenic transformation. Nat Cell Biol. 2007;9(8):932–944. doi: 10.1038/ncb1618. [DOI] [PubMed] [Google Scholar]
- 7.Gingras AC, Raught B, Gygi SP, et al. Hierarchical phosphorylation of the translation inhibitor 4 E-BP1. Genes Dev. 2001;15(21):2852–2864. doi: 10.1101/gad.912401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan B, Zemskova M, Holder S, et al. The PIM-2 kinase phosphorylates BAD on serine 112 and reverses BAD-induced cell death. J Biol Chem. 2003;278(46):45358–45367. doi: 10.1074/jbc.M307933200. [DOI] [PubMed] [Google Scholar]
- 9.Bachmann M, Kosan C, Xing PX, Montenarh M, Hoffmann I, Moroy T. The oncogenic serine/threonine kinase Pim-1 directly phosphorylates and activates the G2/M specific phosphatase Cdc25C. Int J Biochem Cell Biol. 2006;38(3):430–443. doi: 10.1016/j.biocel.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Lwin T, Hazlehurst LA, Dessureault S, et al. Cell adhesion induces p27Kip1-associated cell-cycle arrest through down-regulation of the SCFSkp2 ubiquitin ligase pathway in mantle-cell and other non- Hodgkin B-cell lymphomas. Blood. 2007;110(5):1631–1638. doi: 10.1182/blood-2006-11-060350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Bhattacharya N, Mixter PF, Wei W, Sedivy J, Magnuson NS. Phosphorylation of the cell cycle inhibitor p21Cip1/WAF1 by Pim-1 kinase. Biochim Biophys Acta. 2002;1593(1):45–55. doi: 10.1016/s0167-4889(02)00347-6. [DOI] [PubMed] [Google Scholar]
- 12.Chen LSRS, Bearss D, Wierda WG, Gandhi V. Pim kinase inhibitor, SGI-1776, induces apoptosis in chronic lymphocytic leukemia cells. Blood. 2009;114:4150–4156. doi: 10.1182/blood-2009-03-212852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen LS, Redkar S, Taverna P, Cortes JE, Gandhi V. Mechanisms of cytotoxicity to Pim kinase inhibitor, SGI-1776, in acute myeloid leukemia. Blood. 2011;118(3):693–702. doi: 10.1182/blood-2010-12-323022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsi ED, Jung SH, Lai R, et al. Ki67 and PIM1 expression predict outcome in mantle cell lymphoma treated with high dose therapy, stem cell transplantation and rituximab: a Cancer and Leukemia Group B 59909 correlative science study. Leuk Lymphoma. 2008;49(11):2081–2090. doi: 10.1080/10428190802419640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez-Abad C, Pisonero H, Blanco-Aparicio C, et al. PIM2 inhibition as a rational therapeutic approach in B-cell lymphoma. Blood. 2011;118(20):5517–5527. doi: 10.1182/blood-2011-03-344374. [DOI] [PubMed] [Google Scholar]
- 16.Yang Q, Chen LS, Neelapu SS, Miranda RN, Medeiros LJ, Gandhi V. Transcription and translation are primary targets of Pim kinase inhibitor SGI-1776 in mantle cell lymphoma. Blood. 2012;(17):120, 3491–3500. doi: 10.1182/blood-2012-02-412643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammerman PS, Fox CJ, Birnbaum MJ, Thompson CB. Pim and Akt oncogenes are independent regulators of hematopoietic cell growth and survival. Blood. 2005;105(11):4477–4483. doi: 10.1182/blood-2004-09-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leoni LM, Bailey B, Reifert J, et al. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res. 2008;14(1):309–317. doi: 10.1158/1078-0432.CCR-07-1061. [DOI] [PubMed] [Google Scholar]
- 19.Rummel MJ, Gregory SA. Bendamustine’s emerging role in the management of lymphoid malignancies. Semin Hematol. 2011;48(Suppl 1):S24–36. doi: 10.1053/j.seminhematol.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Gandhi V, Burger JA. Bendamustine in B-Cell Malignancies: The New 46-Year-Old Kid on the Block. Clin Cancer Res. 2009;15(24):7456–7461. doi: 10.1158/1078-0432.CCR-08-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leoni LM, Hartley JA. Mechanism of action: the unique pattern of bendamustine-induced cytotoxicity. Semin Hematol. 2011;48(Suppl 1):S12–23. doi: 10.1053/j.seminhematol.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Forero-Torres A, Saleh MN. Bendamustine in non-Hodgkin lymphoma: the double-agent that came from the Cold War. Clin Lymphoma Myeloma. 2007;8(Suppl 1):S13–17. doi: 10.3816/clm.2007.s.028. [DOI] [PubMed] [Google Scholar]
- 23.Horn J, Kleber M, Hieke S, Schmitt-Graff A, Wasch R, Engelhardt M. Treatment option of bendamustine in combination with rituximab in elderly and frail patients with aggressive B-non-Hodgkin lymphoma: rational, efficacy, and tolerance. Ann Hematol. 2012;91(10):1579–1586. doi: 10.1007/s00277-012-1503-5. [DOI] [PubMed] [Google Scholar]
- 24.Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG) J Clin Oncol. 2005;23(9):1984–1992. doi: 10.1200/JCO.2005.08.133. [DOI] [PubMed] [Google Scholar]
- 25.Goy A, Kahl B. Mantle cell lymphoma: the promise of new treatment options. Crit Rev Oncol Hematol. 2011;80(1):69–86. doi: 10.1016/j.critrevonc.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Amin HM, McDonnell TJ, Medeiros LJ, et al. Characterization of 4 mantle cell lymphoma cell lines. Arch Pathol Lab Med. 2003;127(4):424–431. doi: 10.5858/2003-127-0424-COMCLC. [DOI] [PubMed] [Google Scholar]
- 27.Gaul L, Mandl-Weber S, Baumann P, Emmerich B, Schmidmaier R. Bendamustine induces G2 cell cycle arrest and apoptosis in myeloma cells: the role of ATM-Chk2-Cdc25A and ATM-p53-p21- pathways. J Cancer Res Clin Oncol. 2008;134(2):245–253. doi: 10.1007/s00432-007-0278-x. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Galan P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011;117(1):26–38. doi: 10.1182/blood-2010-04-189977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fillingham J, Keogh MC, Krogan NJ. GammaH2AX and its role in DNA double-strand break repair. Biochem Cell Biol. 2006;84(4):568–577. doi: 10.1139/o06-072. [DOI] [PubMed] [Google Scholar]
- 30.Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10(15):886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- 31.Schatz JH, Wendel HG. Targeted cancer therapy: what if the driver is just a messenger? Cell Cycle. 2011;10(22):3830–3833. doi: 10.4161/cc.10.22.18288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beharry Z, Mahajan S, Zemskova M, et al. The Pim protein kinases regulate energy metabolism and cell growth. Proc Natl Acad Sci U S A. 2011;108(2):528–533. doi: 10.1073/pnas.1013214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keeton EMK, Alimzhanov M, et al. Efficacy and biomarker modulation by AZD1208, a novel, potent and selective pan-Pim kinase inhibitor, in models of acute myeloid leukemia; Proceedings: AACR 103rd Annual Meeting 2012; 2012. p. 8. [Google Scholar]
- 34.Keeton EPS, Alimzhanov M, et al. AZD1208, a Novel, Potent and Selective Pan PIM Kinase Inhibitor, Demonstrates Efficacy in Models of Acute Myeloid Leukemia; 53 rd ASH Annual Meeting: 2011; 2011; Abstract 1540. [Google Scholar]
- 35.Munugalavadla VBBL, Chen Y-H, et al. A selective PIM kinase inhibitor is highly active in multiple myeloma: mechanism of action and signal transduction studies; 53 rd ASH annual meeting: 2011; 2011; Abstract 4084. [Google Scholar]
- 36.Blanco-Aparicio C, Carnero A. Pim kinases in cancer: Diagnostic, prognostic and treatment opportunities. Biochem Pharmacol. 2012 doi: 10.1016/j.bcp.2012.09.018. [DOI] [PubMed] [Google Scholar]