Abstract
Grain amaranth is a pseudocereal with unique agricultural, nutritional, and functional properties. This study was undertaken to determine the effect of different heat-processing methods on physicochemical and nutraceutical properties in two main grain amaranth species, of Amaranthus hypochondriacus L. and Amaranthus cruentus L. Grains were prepared by roasting and popping, milled and analyzed for changes in in vitro protein digestibility, gruel viscosity, pasting characteristics, antioxidant activity, flavonoids, and total phenolics. In vitro protein digestibility was determined using the pepsin-pancreatin enzyme system. Viscosity and pasting characteristics of samples were determined using a Brookfield Viscometer and a Rapid Visco Analyzer, respectively. The grain methanol extracts were analysed for phenolics using spectrophotometry while antioxidant activity was determined using the DPPH (2,2-diphenyl-1-picrylhydrazyl) method. Heat treatment led to a reduction in protein digestibility, the effect being higher in popped than in roasted samples. Viscosities for roasted grain amaranth gruels were significantly higher than those obtained from raw and popped grain amaranth gruels. The results for pasting properties were consistent with the results for viscosity. In both A. hypochondriacus L. and A. cruentus L., the order of the viscosity values was roasted>raw>popped. The viscosities were also generally lower for A. cruentus L. compared to A. hypochondriacus L. Raw samples for both A. hypochondriacus L. and A. cruentus L. did not significantly differ in total phenolic content (TPC), total flavonoid content (TFC), and total antioxidant activity values. Thermal processing led to an increase in TFC and antioxidant activity. However, TPC of heat-processed samples remained unchanged. From the results, it can be concluded that heat treatment enhances antioxidant activity of grain amaranth and causes rheological changes dependent on the nature of heat treatment.
Keywords: Antioxidant activity, flavonoids, grain amaranth, pasting properties, phenolics, protein digestibility
Introduction
Grain amaranth has the potential to contribute to improvement in nutrition of populations, especially in developing countries, because of its unique agricultural, nutritional, and functional properties. It is fast-growing, high-yielding, stress-resistant, nutritious, and has nutraceutical properties. Grain amaranth is rich in proteins, lipids, energy, and fiber (Muyonga et al. 2008). Grain amaranth protein is of superior amino acid profile compared to proteins found in most other plant foods. Amaranth grains contain twice the level of calcium in milk, five times the level of iron in wheat, higher sodium, potassium, and vitamins A, E, C, and folic acid than cereal grains (Becker et al. 1981).
Grain amaranth has been shown to exhibit antioxidant activity and this has been attributed to its content of polyphenols, anthocyanins, flavonoids, and tocopherols (Klimczak et al. 2002; Escudero et al. 2011). Phenolic content of grain amaranth varies between species and may be affected by environmental conditions (Escudero et al. 2011). The antioxidant activity of phenolics is associated with inhibition of lipid peroxidation (Charanjit et al. 2009). Animal models have shown protective effects of grain amaranth against serum and liver intoxication (López et al. 2011). Amaranth oil has been shown, in animal studies, to lower total serum triglycerides and levels of low-density lipoproteins (Berger et al. 2003; Escudero et al. 2006; Martirosyan et al. 2007). Consumption of grain amaranth has been associated with health benefits in humans, including recovery of severely malnourished children and increase in the body mass index of people formerly wasted by HIV/AIDS (Tagwira et al. 2006).
A variety of heat-processing methods are applied to grain amaranth, in preparation for consumption. Heat processing affects the level of phytochemicals (Xu et al. 2007), antioxidant activity (Xu et al. 2007; Queiroz et al. 2009), functional properties (Muyonga et al. 2001), and nutritional value (Rehman and Shah 2005) of foods. Rheological properties of grain amaranth have also been shown to vary among species (Kong et al. 2009). The aim of this study was to investigate the effect of different heat-processing methods commonly applied to grain amaranth on the protein digestibility, rheology, phenolic content, and antioxidant activity of two grain amaranth species.
Material and Methods
Grain amaranth
Amaranth (A. hypochondriacus L. and A. cruentus L.) grains were procured from farmers in Kamuli district, Uganda, who had previously been supplied with seeds for the two species by Makerere University School of Agricultural Sciences. Grains were color sorted to ensure sample purity.
Grain processing
Grains of the two types were separately processed by roasting and popping. Roasting was performed in a Gallenkamp oven 282A (Fistreem International, Leicestershire, U.K.). About 1 kg of dry amaranth grain was spread uniformly on a baking tray of ∼0.3 × 0.6 m in size. The amaranth was roasted at 200°C for 8 min after which it was cooled and later milled into flour.
Amaranth was popped by heating on an aluminum pan using an Ariston K3G2/G gas cooker (Ariston Appliances, Boston, MA) set to maximum heat. A handful of dry grain amaranth (∼100 g) of grain was placed on the pan at a time and heated for about 1–2 min, while stirring using a wooden ladle. The grains began to pop after heating for ∼30 sec. Heating was continued until almost all the grain turned whitish. The total heating time per batch was ∼90 sec. On completion of popping, the popped grains were passed over a 1-mm mesh to separate the popped from those that had not popped grains. The grains that had not popped passed through the screen while the popped grains did not.
Raw grains of the two types as well as the grains processed by the different heat treatment methods were ground using a Waring Blender HGB55E (Waring Blender Co., McConnellsburg, PA) and passed through a Retsch 500-μm sieve (Haan, Germany). The resulting flour was stored (for ≤30 days) in airtight glass jar at room temperature (∼25°C) until analysis was undertaken.
Determination of physicochemical properties
Protein digestibility
In vitro protein digestibility was determined using the pepsin–pancreatin enzyme system (Saunders et al. 1973; Chavan et al. 2001) with minor modifications. About 1 g of sample was suspended in 60 mL of 0.1 mol\L HCl containing 6 mg of pepsin, followed by gentle shaking for 15 min at 37°C. The resulting solution was then neutralized with 0.5 mol\L NaOH and treated with 16 mg of pancreatin from porcine pancreas (activity equivalent to 4× US pharmacopeia) in 30 mL of phosphate buffer (0.1 mol\L, pH 8.0). The mixture was then shaken for 24 h at 37°C in a water bath shaker. The undigested solid was separated by filtration using glass wool (of known weight) under suction from a vacuum pump and washed twice with 10 mL distilled water. The protein content in the undigested solid and initial protein content of both cooked and raw samples was determined using the Kjeldahl method. Digestibility was calculated using the formula:
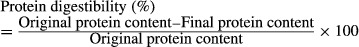
Viscosity
Viscosity was determined using a Brookfield DV-II+Pro Viscometer LVDV-11+P (Brookfield Engineering Laboratories, Inc., Middleboro, MA). For all the flour samples, the same mass to volume ratio (1:10) was used to prepare porridge. Typically, 50 ± 10 g of flour and 500 ± 100 mL of water were used. The mixture of flour in water was boiled for 20 before pre-boiled hot water was added to attain the total water volume required to make 6% or 9% solid content. Boiling was continued for an additional 5 min. The beakers and their contents were then placed in a water bath maintained at 60°C until the gruels cooled to a temperature of 60–62°C. Upon recording the temperature of 60–62°C, the gruel were analysed for viscosity using spindle #4 and at spindle speed of 30 revolutions per min. Viscosity readings were taken 60 sec after turning off the rotor.
Pasting properties
Pasting characteristics of the flours were determined using a Rapid Visco Analyzer RVA-4 (Newport Scientific Pty. Ltd., Warriewood, Australia). The flour suspensions (6.72 g in 25.28 mL H2O) corrected to 14% humidity base were exposed to the following time/temperature sequence: 50°C for 1 min, heating from 50°C to 95°C at 12.16°C/min, maintained at 95°C for 2.5 min, and cooled from 95°C to 50°C at 11.84°C/min rate. The apparent viscosity was expressed in rapid visco units. Peak viscosity, trough, breakdown, final viscosity, set back, peak time, and pasting temperature were recorded.
Determination of bioactive compounds and antioxidant activity
Extraction of flavonoids and phenolic compounds
Methanol extracts were obtained from all samples (Makkar 2000). Briefly, ∼0.1 g of flour sample was extracted for 30 min with 5 mL of methanol:water (50:50 v/v) mixture at room temperature, while intermittently shaking. The extract was cooled by keeping the extract tube in a freezer for 10 min and then centrifuged at 3000 g for 10 min using a FisherScientific centrifuge 225 (Fisher Scientific, Leicestershire, U.K.). The supernatant was recovered and the pellet re-extracted for 45 min under the same conditions. Finally, the two supernatants were pooled and used for total antioxidant activity, total phenolic content (TPC), and total flavonoid content (TFC).
Antioxidant activity
The antioxidant activity of the methanol extracts was estimated using the DPPH (1,1-diphenyl-2-pycrylhydrazyl) free radical-scavenging assay (Kim et al. 2002). To 2.95 mL of freshly prepared absolute methanol solution of 100 μmol\L of DPPH, 50 μL of the sample extract or control (50% [v/v] methanol) was added. The mixture was shaken and allowed to stand at room temperature in the dark for 30 min. The absorbance of the resulting solution was measured at 517 nm against a blank (absolute methanol). The free radical-scavenging activity was calculated as follows:
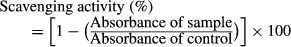
A standard of ascorbic acid was run using several concentrations ranging from 0.002 to 0.1 mg/mL. A standard curve was constructed by plotting the percentage of free radical-scavenging activity of ascorbic acid versus its concentration (R2 = 0.992). The final result was expressed as mg vitamin C equivalent per 1 g dry weight (mg VCE/g dw).
Determination of total phenolic content
Total phenolic content was determined using spectrophotometry (Makkar 2000). To a sample of 100 μL, distilled water was added to make the quantity 0.5 mL. This was followed by the addition of 0.25 mL of Folin-Ciocalteu reagent (1 N) and 1.25 mL of sodium carbonate (20%). After 40 min at room temperature, the absorbance at 725 nm was read on a GENESYS spectrophotometer 10 ultraviolet (Thermo Electron Corporation, Marietta, OH) against a blank that contained methanol instead of a sample. The calibration curve was constructed within the concentration range 0.025–0.225 mg/mL (R2 = 0.999). The TPC values were expressed in milligrams of gallic acid equivalents/gram dry weight (mg GAE/g dw) of plant material using equation:
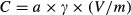
where C is the total amount of phenolic compounds (mg GAE/g dw sample), a is the dilution number, γ is the concentration obtained from the calibration curve (mg/mL), V is the volume of aqueous methanol used for extraction (mL), and m is the weight of dry plant material (g).
Determination of total flavonoid content
Total flavonoid content was measured using a colorimetric assay (Muanda et al. 2011). A quantity of 250 μL standard solution of catechin at different concentrations or appropriately diluted samples was added to a 10 mL volumetric flask containing 1 mL of double distilled waters (ddH2O). At time 0 min, 75 μL of NaNO2 (5%) was added to the flask. After 5 min, 75 μL of AlCl3 (10%) was added. At 6 min, 500 μL of NaOH (1N) was added to the mixture. The solution was then diluted by adding 2.5 mL double-distilled H2O and mixed thoroughly. The absorbance of the pink mixture was determined at 510 nm against a blank that contained distilled water instead of a sample. The calibration curve was constructed within the concentration range 0.025–0.225 mg/mL (R2 = 0.999). The TFC values for samples were expressed as milligrams of catechin equivalents/gram dry weight (mg CE/g dw) of plant material using the equation:
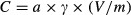
where C is the total amount of phenolic compounds (mg GAE/g dw sample), a is the dilution number, γ is the concentration obtained from the calibration curve (mg/mL), V is the volume of aqueous methanol used for extraction (mL), and m is the weight of dry plant material (g).
Statistical analysis
Data for all parameters corresponding to different treatments were subjected to analysis of variance (ANOVA) at α = 0.05 using the SPSS (version 16, SPSS Inc., Chicago, IL). Means were separated using least significance difference (LSD). All analytical measurements were performed in triplicate.
Results and Discussion
Protein digestibility
The protein content was found to be 12.37 ± 0.71% and 13.04 ± 0.98%, respectively, for the A. cruentus L. and A. hypochondriacus L. variety. Protein digestibility for A. cruentus L. (73.85%) grain amaranth and A. hypochondriacus L. (71.93%) were not significantly different. In vitro digestibility of 61–76% has previously been reported for raw grain amaranth proteins (Correa et al. 1986). Heat treatments led to a reduction in protein digestibility (Table 1). This is in agreement with an earlier work (Písaŕíkova et al. 2005) that reported a reduction in in vitro protein digestibility from 68.1 to 50.6 as a result of popping. The reduction in protein digestibility in this study was higher in popped than in roasted samples. The reduction in protein digestibility resulting from heat processing of grain amaranth might be attributed to amino acid degradation, formation of intramolecular disulfide bonds and Maillard reaction, changes associated with dry heat processing (Hurrell et al. 1976; Hsu et al. 1977; Nestares et al. 1993). The lower digestibility for popped seeds as compared to roasted ones point to more pronounced protein changes popping. This is not surprising as popping temperatures tend to be higher than those registered during roasting. The in vitro protein digestibility values recorded from grain amaranth were higher than reported digestibility values for whole raw maize (66.6%) and sorghum (55.8–59.1%) (Duodu et al. 2002). Grain amaranth proteins therefore seem to exhibit higher digestibility than these cereals. Heat treatment has been reported to cause reduction in protein digestibility for sorghum and increased digestibility for maize (Duodu et al. 2002). The nature of the change in protein digestibility resulting from heat treatment seems to relate partly to the extent of formation of complexes between proteins and other grain components and the level of matrix disintegration, which impacts the access of proteolytic enzymes to protein bodies.
Table 1.
Protein digestibility (%) of raw, roasted, and popped grain amaranth
| Treatment | Protein digestibility (%) |
|---|---|
| A. cruentus L. raw | 73.85 ± 2.11a |
| A. cruentus L. roasted | 63.34 ± 1.23b |
| A. cruentus L. popped | 52.81 ± 1.34c |
| A. hypochondriacus L. raw | 71.93 ± 3.03a |
| A. hypochondriacus L. roasted | 60.60 ± 2.23b |
| A. hypochondriacus L. popped | 50.51 ± 1.44c |
Data are expressed as means ± SE for triplicate experiments. Means with same superscript are not significantly different.
Viscosity
Samples of A. hypochondriacus L. consistently exhibited higher viscosity compared with those for A. cruentus L. (Table 2). Viscosity differences among grain amaranth cultivars have been shown to correlate positively with amylose content (Kong et al. 2009). Studies on starch from different sources showed that amylose content and amylopectin branch chain length distribution predominantly affect starch pasting properties (Jane et al. 1999). The difference in pasting properties of the two grain amaranth species in this study may therefore reflect differences in amylose content and/or nature of amylopectin in their starches. Popped samples exhibited much lower viscosity than raw samples (Table 2). This can be attributed to pregelatinization of starch due to heat treatment. On the contrary, roasted samples showed significantly higher viscosity than raw and popped samples. During heating, starch granules may disintegrate, becoming more susceptible to hydration which is associated with high viscosity (Lai 2001). Leached amylose may also associate resulting in limited rehydration potential and lower peak viscosity. Heat pretreatment may therefore increase or reduce paste viscosity. In this case, the observed high viscosity for roasted grain amaranth may be attributed to disintegration of starch granules, increasing their susceptibility to hydration while the low viscosity of flour from popped grain seems to arise from the association of amylose, possibly because of the extreme dehydration impact of popping. The viscosity values show that the drinking consistency (∼3000 cP) is achieved at a flour rate of between 6% and 9% for raw grain amaranth. Much higher rates are required in the case of popped amaranth while much lower rates would be required for roasted grain.
Table 2.
Viscosity of 6% and 9% gruels of Amaranthus hypochondriacus L. and A. cruentus L
| Amaranth variety | A. hypochondriacus L. | A. cruentus L. | |
|---|---|---|---|
| Treatment | % flour | Viscosity, cP at 40°C | Viscosity, cP at 40°C |
| Raw | 6 | 2240 ± 34.2d,1 | 1999 ± 30.1d,2 |
| 9 | 8798 ± 54.6c,1 | 6299 ± 81.1c,2 | |
| Roasted | 6 | 31643 ± 712.1b,1 | 17496 ± 621.1b,2 |
| 9 | 37268 ± 862.5a,1 | 31593 ± 822.8a,2 | |
| Popped | 6 | 322.9 ± 11.7f,1 | 213.0 ± 16.1f,2 |
| 9 | 834 ± 19.0e,1 | 625.9 ± 41.1e,2 | |
Data are expressed as means ± SE for triplicate experiments. Means within a column with the same superscript are not significantly different. Means within the same row with the same numeral are not significantly different.
Pasting properties
The results for pasting properties were consistent with the results for viscosity. In both A. hypochondriacus L. and A. cruentus L., the order of viscosity values was roasted>raw>popped (Fig. 1). The viscosities were also generally lower for A. cruentus L. compared to A. hypochondriacus L. Amylose content has been established as a key determinant of pasting viscosity for grain amaranth cultivars (Kong et al. 2009). The observed differences in pasting temperature between species seem to suggest that the two differ in their amylose content. A study of 15 grain amaranth cultivars grown in China (11 belong to A. cruentus L. and 2 to A. hypochondriacus L.) revealed amylose content of 5.4–5.8% for A. hypochondriacus L. and 4.7–12.5% for A. cruentus L. (Kong et al. 2009). The pasting temperatures for raw A. hypochondriacus L. and A. cruentus L. in this study were found to be 75.9°C and 77.6°C, respectively (Table 3). Pasting temperatures of 63.4–74°C have been reported for grain amaranth from previous studies (Lai 2001; Kong et al. 2009). The effect of roasting and popping on pasting viscosity was generally similar to that reported above for viscosity. High pasting viscosity was associated with high breakdown viscosity, high final viscosity, high setback, low pasting time, and temperature. A similar trend was reported when comparing pasting properties of different grain amaranth cultivars (Kong et al. 2009). This trend can be explained by the fact that all these attributes are dependent on the pace and level of starch granule disintegration. Samples which register more extensive granule disintegration seem also likely to exhibit a high extent of retrogradation reflected in the values for setback.
Figure 1.
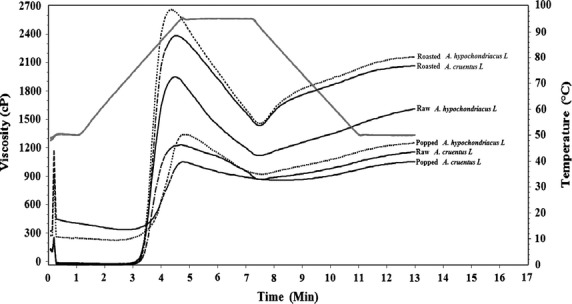
The RVA profiles of raw, roasted, and popped Amaranthus hypochondriacus L. and A. cruentus L.
Table 3.
Pasting properties of raw, roasted, and popped Amaranthus hypochondriacus and A. cruentus L. amaranth
| Amaranth variety | Pasting viscosity | Breakdown viscosity | Final viscosity | Setback | Pasting time (min) | Pasting temperature (°C) |
|---|---|---|---|---|---|---|
| Raw A. cruentus L. | 1222.5 ± 12.0e | 258 ± 7.07e | 1159.5 ± 7.8e | 207 ± 4.2e | 4.68 ± 0.01c | 77.63 ± 0.1c |
| Roasted A. cruentus L. | 2405.5 ± 33.2b | 963.5 ± 26.16b | 2078.5 ± 14.9b | 636.5 ± 7.8b | 4.57 ± 0.05d | 76.73 ± 0.1d |
| Popped A. cruentus L. | 1006.0 ± 66.5f | 176.5 ± 28.99f | 1026 ± 41.0f | 196.5 ± 3.5f | 4.84 ± 0.05a | 81.23 ± 0.5a |
| Raw A. hypochondriacus L. | 1963.5 ± 21.9c | 847 ± 24.04c | 1625 ± 21.2c | 508.5 ± 23.3e | 4.47 ± 0.00e | 75.93 ± 0.1e |
| Roasted A. hypochondriacus L. | 2711.5 ± 75.7a | 1213 ± 15.55a | 2204 ± 63.6a | 705.5 ± 3.5a | 4.33 ± 0.00f | 75.95 ± 0.1f |
| Popped A. hypochondriacus L. | 1359.5 ± 26.2d | 417.5 ± 3.54d | 1255.5 ± 7.8d | 313.5 ± 21.9d | 4.77 ± 0.05b | 80.03 ± 0.1b |
Data are expressed as means ± SE for triplicate experiments. Means within a column with the same superscript are not significantly different.
Total phenolic content
No significant difference in TPC was observed between raw and roasted or popped grain for both A. cruentus L. and A. hypochondriacus L. (Table 4). Earlier studies have reported conflicting results with respect to the effect of heat on total phenolics. Heat has been reported to cause reduction in TPC of roasted and popped grain amaranth (Yanez et al. 1986). This reduction may be attributed to thermal degradation due to the processing conditions used in the study (Griffith and Castell-Perez 1998). Roasting of sesame seeds at 200°C for 20 min, on the other hand, was found to significantly increase TPC (Devi et al. 2011). Increased phenolic content has been reported for tomatoes treated at 88°C for 30 min (Choi et al. 2006). The increased phenolic content of thermally processed foods can be attributed to the heat-induced release of more bound phenolics (Dewanto et al. 2002; Jannat et al. 2010). Therefore, the results of this study may be attributed to the heat-associated increase in phenolic compounds offsetting their degradation by heat.
Table 4.
Total phenolic content, total flavonoid content, and DPPH for Amaranthus hypochondriacus L. and A. cruentus L
| Material tested | Total phenolic content (mg GAE/g dw) | Total flavonoid content (mg CE/g dw) | Total antioxidant activity (mg VCE/g dw) |
|---|---|---|---|
| Raw A. cruentus L. | 3.63 ± 0.08ab | 0.54 ± 0.13b | 0.24 ± 0.12bc |
| Roasted A. cruentus L. | 3.93 ± 0.22a | 1.06 ± 0.18a | 0.56 ± 0.07a |
| Popped A. cruentus L. | 3.41 ± 0.32ab | 0.93 ± 0.16a | 0.31 ± 0.13bc |
| Raw A. hypochondriacus L. | 3.34 ± 0.22b | 0.47 ± 0.09b | 0.09 ± 0.01c |
| Roasted A. hypochondriacus L. | 3.70 ± 0.33ab | 0.54 ± 0.07b | 0.33 ± 0.08b |
| Popped A. hypochondriacus L. | 2.99 ± 0.29b | 0.78 ± 0.11a | 0.13 ± 0.07c |
Data are expressed as means ± SE for triplicate experiments. Means within a column with the same superscript are not significantly different.
Flavonoid content
Heat treatment generally led to an increase in the flavonoid content in grain amaranth. An increase in flavonoid content has also been reported following heating of Shiitake (Lentinus edodes) mushroom (Choi et al. 2006). This was attributed to enhanced extractability of bound flavonoid compounds resulting from heat-induced disruption of the plant cell wall. Heat-induced increase in flavonoid content has also been associated with deactivation of endogenous oxidative enzymes, thereby preventing enzymatic oxidation which causes loss of the antioxidant compounds in the raw plant materials (Jeong et al. 2004; Jannat et al. 2010).
Antioxidant activity
Roasting resulted in a significant increase in antioxidant activity of both A. hypochondriacus L. and A. cruentus L. Roasting has been previously reported to increase the antioxidant activity of sesame seeds (Devi et al. 2011). Much of the antioxidant activity of plant materials is attributable to flavonoids and other phenolics (Kahkonen et al. 1999; Nicoli et al. 1999). Therefore, the increase in antioxidant activity might be due to the observed increase in total flavonoids (Table 4). Popping on the other hand did not cause a significant change in antioxidant activity of both A. hypochondriacus L. and A. cruentus L. This trend may be attributed to the negative and positive effect of heat on different phenolic compounds.
Conclusion
The two varieties of grain amaranth (A. hypochondriacus L. and A. cruentus L.) studied differ in physicochemical properties, with A. cruentus L. exhibiting higher protein content and higher antioxidant activity than A. hypochondriacus L. and A. hypochondriacus L. exhibiting higher viscosity. When exposed to dry heat processes typically used to prepare grain amaranth, both protein digestibility and antioxidant activity are affected. Popping has a higher negative impact on protein digestibility while roasting is more damaging to the antioxidant activity. Heat processing also leads to change in viscosity and pasting behavior of grain amaranth. The results show that roasting would be preferred in the production of flour to be used as a thickening agent or for low-calorie gruels. On the other hand, popping is suitable for the production of flour for high nutrient density gruels.
Acknowledgments
This study was funded by the McKnight Foundation under the Collaborative Crop Research Program.
Funding Information
This study was funded by the McKnight Foundation under the Collaborative Crop Research Program.
Conflict of Interest
None declared.
References
- Becker R, Wheeler EL, Lorenz K, Stafford AE, Grosjean OK, Betschart AA, et al. A compositional study of amaranth grain. J. Food Sci. 1981;46:1175. [Google Scholar]
- Berger A, Gremaud G, Baumgartner M, Rein D, Monnard I, Kratky E, et al. Cholesterol-lowering properties of amaranth grain and oil in hamsters. Int. J. Vitam. Nutr. Res. 2003;73:39–47. doi: 10.1024/0300-9831.73.1.39. [DOI] [PubMed] [Google Scholar]
- Charanjit K, Joshi S, Kapoor HC. Antioxidants in onions (Allium cepa L.) cultivars grown in India. J. Food Chem. 2009;33:184–200. [Google Scholar]
- Chavan UD, Mckenzie DB, Shahidi F. Functional properties of protein isolates from beach pea Lathyrus maritius L. Food Chem. 2001;74:177–187. [Google Scholar]
- Choi Y, Lee AM, Chun J, Lee HB, Lee J. Influence of heat treatment on the antioxidant activities and polyphenolic compounds of Shiitake (Lentinus edodes) mushroom. Food Chem. 2006;99:381–387. [Google Scholar]
- Correa AD, Jokl L, Carlsson R. Chemical constituents, in vitro digestibility, and presence of antinutritional substances in amaranth grains. Arch. Latinoam. Nutr. 1986;36:319–326. [PubMed] [Google Scholar]
- Devi PB, Vijayabharathi R, Sathyabama S, Malleshi NG, Priyadarisini VB. Health benefits of finger millet (Eleusine coracana L.) polyphenols and dietary fiber: a review. J. Food Sci. Technol. 2011:1–20. doi: 10.1007/s13197-011-0584-9. doi: 10.1007/s13197-011-0584-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewanto V, Wu X, Kafui K, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002;50:3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- Duodu KG, Nunes A, Delgadillo I, Parker ML, Mills ENC, Belton PS, et al. Effect of grain structure and cooking on sorghum and maize in vitro protein digestibility. J. Cereal Sci. 2002;35:161–174. [Google Scholar]
- Escudero NL, Zirulnik F, Gomez NN, Mucciarelli SI, Ginènez MS. Influence of a protein concentrate from Amaranthus cruentus seeds on lipid metabolism. Exp. Biol. Med. 2006;231:50–59. doi: 10.1177/153537020623100106. [DOI] [PubMed] [Google Scholar]
- Escudero NL, Alabarracín GJ, López L, Giménez MS. Antioxidant activity and phenolic content of flour and protein concentrate of Amaranthus cruentus seeds. J. Food Biochem. 2011;35:1327–1341. [Google Scholar]
- Griffith LD, Castell-Perez ME. Effects of roasting and malting on physicochemical properties of select cereals and legumes. Cereal Chem. 1998;75:780–784. [Google Scholar]
- Hsu HW, Vavak DL, Satterlee LD, Miller GA. A multi-enzyme technique for estimating protein digestibility. J. Food Sci. 1977;42:1269–1273. [Google Scholar]
- Hurrell RF, Carpenter KJ, Sinclair WJ, Otterburn MS, Asquith RS. Mechanisms of heat damage in proteins. 7. Significance of lysine-containing isopeptides and of lanthionine in heated proteins. Br. J. Nutr. 1976;35:383–395. doi: 10.1079/bjn19760044. [DOI] [PubMed] [Google Scholar]
- Jane J, Chen YY, Lee LF, McPherson AE, Wong KS, Radosavljevic M, et al. Effects of amylopectin branch chain length and amylose content on the gelatinization and pasting properties of starch. Cereal Chem. 1999;76:629–637. [Google Scholar]
- Jannat B, Oyeisi MR, Sadeghi N, Hajimahmoodi M, Behzad M, Choopankari E, et al. Effects of roasting temperature and time on healthy nutraceuticals of antioxidants and total phenolic content in Iranian sesame seeds (Sesamum indicum L.) Iran. J. Environ. Health Sci. Eng. 2010;7:97–102. [Google Scholar]
- Jeong SM, Kim SY, Kim DR, Nam KC, Ahn DU, Lee SC. Effect of seed roasting conditions on the antioxidant activity of defatted sesame meal extract. J. Food Sci. 2004;69:377–381. [Google Scholar]
- Kahkonen MP, Hopia AI, Vuorela HJ, Rauha J, Pihlaja K, Kajula TS, et al. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999;47:3954–3962. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- Kim DO, Lee KW, Lee HJ, Lee CY. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agric. Food Chem. 2002;50:3713–3717. doi: 10.1021/jf020071c. [DOI] [PubMed] [Google Scholar]
- Klimczak I, Małecka M, Pachołek B. Antioxidant activity of ethanolic extracts of amaranth seeds. Nahrung. 2002;46:184–1866. doi: 10.1002/1521-3803(20020501)46:3<184::AID-FOOD184>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Kong X, Bao J, Corke H. Physical properties of Amaranthus starch. Food Chem. 2009;113:371–376. [Google Scholar]
- Lai H. Effects of hydrothermal treatment on the physicochemical properties of pregelatinized rice flour. Food Chem. 2001;72:455–463. [Google Scholar]
- López VR, Razzeto GS, Gimenéz MS, Escudero NL. Antioxidant properties of Amaranthus hypochondriacus seeds and their effect on the liver of alcohol-treated rats. Plant Foods Hum. Nutr. 2011;66:157–162. doi: 10.1007/s11130-011-0218-4. [DOI] [PubMed] [Google Scholar]
- Makkar HPS. Quantification of tannins in tree foliage: a laboratory manual for the FAO/IAEA co-ordinate research project on use of nuclear and related techniques to develop simple tannin assay for predicting and improving the safety and efficiency of feeding ruminants on the tanniniferous tree foliage. Vienna, Austria: Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture; 2000. [Google Scholar]
- Martirosyan DM, Miroshnichenko LA, Kulakova SN, Pogojeva AV, Zoloedov VI. Amaranth oil application for coronary heart disease and hypertension. Lipids Health Dis. 2007;6:1–12. doi: 10.1186/1476-511X-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muanda NF, Kon′e D, Dicko A, Soulimani R, Younos C. Phytochemical composition and antioxidant capacity of three malian medicinal plant parts. Evid. Based Complement. Alternat. Med. 2011;2011:1–9. doi: 10.1093/ecam/nep109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyonga JH, Ramteke RS, Eipeson WE. Predehydration steaming changes physicochemical properties of unripe banana flour. J. Food Process. Preserv. 2001;25:35–47. [Google Scholar]
- Muyonga JH, Nabakabya D, Nakimbugwe D, Masinde D. Efforts to promote amaranth production and consumption in Uganda to fight malnutrition. In: Robertson GL, Lupien JR, editors. Using food science and technology to improve nutrition and promote national development. International Union of Food Science & Technology; 2008. Available at http://www.iufost.org/publications/books/documents/chapter8.pdf (accessed October 2013) [Google Scholar]
- Nestares T, Lopez-Jurado M, Sanz A, Lopez-Frias M. Nutritional assessment of two vegetable protein concentrates in growing rats. J. Agric. Food Chem. 1993;41:1282–1286. [Google Scholar]
- Nicoli MC, Anese M, Parpinel MT, Franceschi S. Influence of processing on the antioxidant properties of fruits and vegetables. Trends Food Sci. Technol. 1999;10:94–100. [Google Scholar]
- Písaŕíkova B, Zralý Z, Kráčmar S, Trčková M, Herzig I. Nutritional value of amaranth (genus Amaranthus L.) grain in diets for broiler chicken. Czech J. Anim. Sci. 2005;50:568–573. [Google Scholar]
- Queiroz YS, Manólio Soares RA, Capriles VD, Torres EA, Areas JA. Effect of processing on the antioxidant activity of amaranth grain. Arch. Latinoam. Nutr. 2009;59:419–424. [PubMed] [Google Scholar]
- Rehman Z, Shah WH. Thermal heat processing effects on antinutrients, protein and starch digestibility of food legumes. Food Chem. 2005;91:327–331. [Google Scholar]
- Saunders RM, Connor MA, Booth AN, Bickoff EN, Kohier CO. Measurement of digestibility of alfalfa protein concentrate by in vitro and in vivo methods. J. Nutr. 1973;103:530–535. doi: 10.1093/jn/103.4.530. [DOI] [PubMed] [Google Scholar]
- Tagwira M, Tagwira F, Dugger R, Okum B. Using grain amaranth to fight malnutrition. RUFORUM Working Doc. 2006;1:201–206. [Google Scholar]
- Xu G, Ye X, Chen J, Liu D. Effect of heat treatment on the phenolic compounds and antioxidant capacity of citrus peel extract. J. Agric. Food Chem. 2007;55:330–335. doi: 10.1021/jf062517l. [DOI] [PubMed] [Google Scholar]
- Yanez GA, Messinger JK, Walker CE, Raphow JH. Amaranthus hypodriacus: starch isolation and partial characterization. Cereal Chem. 1986;63:273–276. [Google Scholar]


