Abstract
Inflammation is a key mediator in the development of malignant mesothelioma (MM) which has a dismal prognosis and poor therapeutic strategies. Curcumin, a naturally occurring polyphenol in turmeric, has been shown to possess anti-carcinogenic properties through its anti-inflammatory effects. Inflammasomes, a component of inflammation, control the activation of caspase-1 leading to pyroptosis and processing of pro-inflammatory cytokines, interleukin (IL)-1β and IL-18. In the present study, we investigate the role of curcumin in pyroptotic cell death of MM cells. Using in vitro models with mouse and human MM cells, curcumin is shown to induce pyroptosis through activation of caspase-1 and increased release of High Mobility Group Box1 (HMGB1) without processing of IL-1β and IL-18. Absence of IL-1β processing in response to curcumin-mediated caspase-1 activation is attributed to blockade of pro-IL-1β priming through inhibition of the Nuclear Factor kappaB (NF-κB) pathway. Furthermore, curcumin’s cytotoxicity in MM cells is demonstrated to be dependent on pyroptosis as inhibition of caspase-1 resulted in protection against curcumin-induced cell death. We also demonstrate that curcumin-mediated caspase-1 activation is oxidant dependent by using N-acetyl-L-cysteine (NAC) to inhibit pyroptosis. PCR Array analysis using the human inflammasome template revealed that curcumin significantly downregulated levels of inflammasome-related gene expression involved in inflammation, e.g., NF-κB, toll-like receptors (TLR) and IL-1β. Our data indicates that curcumin has a double effect on MM cells through induction of pyroptosis while subsequently protecting against inflammation.
Keywords: malignant mesothelioma, curcumin, pyroptosis, caspase-1, inflammasome
Introduction
Malignant mesothelioma (MM) is an asbestos-related malignancy with a dismal prognosis and poor therapeutic strategies. MM arises from the mesothelial cells that line the pleural cavity, pericardium, and peritoneum. Therapeutic interventions for MM include chemotherapy, surgery, radiation, immunotherapy, targeted molecular therapy and gene therapy, however the median survival remains poor at 9–12 months from time of diagnosis (1). Curcumin is a naturally occurring polyphenol in the spice turmeric, which comes from the rhizomes of the herb Curcuma Longa. Prior research has identified a broad range of anti-carcinogenic potential of curcumin in various cancer types through its anti-inflammation, anti-proliferative, anti-angiogenic, pro-apoptotic and enhancing chemoradiation properties (2–7). However, little research has explored the therapeutic role of curcumin in MM. If proven to be effective in MM treatment, curcumin would serve as an excellent addition to available therapeutic options as this compound has a relatively low cost and low cytotoxic profile compared to its chemotherapy counterparts (8). Furthermore, effective compounds will be urgently needed due to the incidence and mortality of MM in less developed countries with economically disadvantaged populations (9).
Inflammation plays an important role in development of multiple cancers including MM (10, 11). Caspase-1 mediates the activation of interleukin (IL)-1β and IL-18 and an inflammatory cell death process termed pyroptosis that is distinct from necrosis and apoptosis (12). Initially synthesized as an inactive protein (pro-caspase-1), caspase-1 becomes biologically functional through autoprocessing into active caspase-1 which consists of two small (p10) and two large subunits (p20) (13). Autoprocessing of caspase-1 typically occurs through classical inflammasomes composed of nod-like receptors (NLR) and an adaptor protein, apoptosis-associated speck like protein containing a caspase-1 recruitment domain known as PYCARD or ASC (14). Additional modes of caspase-1 activation may occur through inflammasomes with direct caspase-1 recruitment domains (ASC independent) such as NLRC4 and NLRP1 (15) or through an assembly of ASC dimers known as the pyroptosome (16). One of the best characterized inflammasome is that consisting of NLRP3, ASC and caspase-1 which has previously been shown to be activated by asbestos in macrophages (17) and mesothelial cells (unpublished data). Here we are the first to report the ability of curcumin to enhance MM cell killing via induction of pyroptosis without activation of classical inflammasome-related cytokines, IL-1β and IL-18, due to inhibition of toll like receptor (TLR) and NF-κB pathways. Additionally, we demonstrate that curcumin-initiated pyroptosis is dependent on reactive oxygen species (ROS) production and independent of the NLRP3 inflammasome.
Materials and Methods
Reagents
Curcumin (77.5% curcumin, 18.27% demethoxycurcumin, 4.21% bisdemethoxycurcumin; also called C3 complex) was kindly supplied by Dr. Bharat Aggarwal (MD Anderson, Houston, TX) and curcumin (≥94% curcuminoid content, ≥80% Curcumin) was purchased from Sigma-Aldrich (Saint Louis, MO). A 10 mM solution of curcumin was prepared in dimethyl sulfoxide DMSO) and stored in 100 uL aliquots at −20°C and diluted as needed in cell culture medium. Crocidolite asbestos was obtained from NIEHS and physical and chemical characterization has been previously reported (18). N-acetyl-L-cysteine (NAC) was purchased from Sigma-Aldrich. DMSO was used as a control treatment in all in vitro experiments.
Cell culture and exposure to reagents
Mouse MM cells (#40) were obtained from Dr. Agnes Kane (Brown University, Providence RI) and maintained in High Glucose DMEM containing 10% FBS and supplemented with penicillin (50 units/mL) and streptomycin (100 µg/mL). Human mesothelial LP9/TERT-1 (LP9), cell line phenotypically and functionally resembling normal human mesothelial cells (19), were obtained from Dr. James Rheinwald (Bringham and Women's Hospital, Boston, MA). HMESO cells have been previously characterized by Reale et al. (20). H2595 and H2461 were contributed by Dr. Harvey Pass (New York University, New York, NY) (21). Cell lines were validated by STR DNA fingerprinting using the Promega CELL ID System (Promega, Madison, WI). All cells were maintained in appropriate cell culture medium as described before (22). Crocidolite asbestos fibers were prepared and added to cell culture medium as previously described (23). For NAC treatments, HMESO cells were grown to 80–90% confluency and treated with NAC (Sigma, Saint Louis, MO) 10 mM for 18 h after pH adjustment (24) prior to curcumin treatments. In experiments involving Actinomycin D (Sigma, Saint Louis, MO), cells were treated with 10 µg/mL of Actinomycin D for 30 min prior to curcumin treatments.
MTS Assay
MM cells were treated with different concentrations of curcumin (0–50 µM) for 24–72 h, and cell viability was measured using the colorimetric MTS Assay, CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega) as per the manufacturer’s recommendations (22).
Quantitative real-time PCR (qRT-PCR)
Total RNA (1 µg) from different cell types was reverse-transcribed as described previously (23) with random primers using the Promega AMV Reverse Transcription System (Promega, Madison, WI). In LP9 cells with various exposure times to asbestos and curcumin, NLRP3 and pro-IL-1β genes were evaluated using the ΔΔCt method. Hypoxanthine phosphoribosyl transferase (hprt) and 18s ribsosomal RNA controls were used as normalization controls.
PCR Array
HMESO cells were grown to 90% confluency and treated with curcumin (40 µM for 48 h). RNA was isolated and purified using a Qiagen RNeasy plus kit (Valencia, CA) as previously described (22). After quality assessment, 500 ng of RNA was employed for cDNA synthesis using the RT2 First Strand Kit (SABiosciences, Frederick, MD). Quantitative Real-Time PCR (qRT-PCR) was performed by the Vermont Cancer Center DNA Analysis Facility using RT2 Real-Time™ SYBR Green PCR Master Mix and Human Inflammasomes RT2 Profiler PCR Arrays (Qiagen) (7900HT Sequence Detection System, Applied Biosystems). Data were analyzed using an on-line spreadsheet-based data analysis template (SABiosciences). qRT-PCR (TaqMan) was used to validate selected genes using Assay on Demand (AOD) primers and probes from Applied Biosystems.
Cell viability determination
Viability of HMESO cells after caspase-1 inhibition and curcumin treatment was studied by growing HMESO cells to 90% confluency per well in a 12 well plate (n=3). At 90% confluency, HMESO cells were maintained in low serum containing medium (0.5% FBS) for 24 h before treatment with caspase-1 inhibitor (EMD Biosciences, Darmstadt, Germany, 40 µM for 1 h) followed by curcumin (40 µM for 48 h). Cells were trypsinized and counted using a hemocytometer.
Caspase-1 activity assay
Caspase-1 activity was measured using the Caspase-1 Colorimetric Assay (R&D Systems, Minneapolis, MN) according to the manufacturer’s directions and normalized to total protein.
Detection of HMGB1, IL-1β and caspase-1 (p20) in medium
The medium from treated cells were collected prior to cell lysis and 500–1000 µL was concentrated in Amcion® ultra centrifugal filters with a 10K membrane (Millipore, Billerica, MA) by spinning at 14,000g for 30 min. Western blot analyses were performed on concentrated supernatants. A rabbit polyclonal HMGB1 antibody (Abcam, Cambridge, MA) was used at a dilution of 1:500. A rabbit polyclonal caspase-1 (p20) antibody (Cell Signaling Technology, Danvers, MA) was used at a dilution of 1:500. A rabbit polyclonal IL-1β antibody (Cell Signaling Technology, Danvers, MA) was used at a dilution of 1:500.
Western blot analysis
Cells were exposed to agents as described above, and lysates were prepared as previously described (23). The amount of protein was determined using the RC DC protein assay (Bio-Rad, Hercules, CA). Western blots were performed as described previously (23), using monoclonal rabbit NLRP3 antibodies (Novus Biologicals, Littleton, CO) at a dilution of 1:500 and a mouse β-Actin antibody (Abcam, Cambridge, MA) at a dilution of 1:2000. QuantityOne was used to quantify band density. Blots are representative of at least two or more different experiments.
ELISA for IL-1β and IL-18
All source material for IL-1β and IL-18 ELISAs came from cell culture medium that was concentrated as described above. Human and mouse IL-1β kits (Biolegend, San Diego, CA) and human and mouse IL-18 ELISA kits (Medical & Biological Laboratories, Nagoya, Japan) were used. All ELISA assays were performed according to the manufacturer's instructions. The entire concentrate from 500–1000 µL of cell supernatant was used from each dish.
MM cell transfection with siNLRP3
On-Target plus Non-targeting siRNA #1 (scrambled controL) or On-Target plus SMART pool mouse NLRP3 and ASC siRNA (5 nM; Dharmacon, Lafayette, CO) were transfected into mouse MM cells at 80% confluency using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) as previously described (25). The efficiency of NLRP3 or ASC knockdown was determined by qRT-PCR after 30 h. After 30 h post transfection, mouse MM cells were treated with 40 µM of curcumin for 24 h and caspase-1 activity was measured as described above.
Nitro Blue Tetrazolium (NBT) assay for ROS detection
HMESO cells were grown to 90% confluency in 12 well plates and treated with curcumin (40 µM for 6 h). Cells were washed with HBSS without phenol red and incubated with NBT purchased from Sigma-Aldrich (Saint Louis, MO) for 45 min. One set of dishes were fixed in 100% methanol for 5 min and the other were trypsinized and counted using a hemocytometer. Fixed cells were then washed twice with HBSS without phenol red. NBT was solubilized in 560 µL of 2 M KOH and 480 µL DMSO and 100 µL was transferred to a 96 well plate and read at wavelength 630.
In vivo MM tumor model
For allograft model, mouse MM cells #40 (2×106 cells in 50 µL 0.9% NaCl, pH 7.4) were injected into the lower left quadrant of the peritoneal cavity of 8 week-old male C57/BL6 mice. For xenograft model, HMESO cells (2×106 cells in 50 µl 0.9% NaCl, pH 7.4) were injected into the lower left quadrant of the peritoneal cavity of 6–8 week-old male Fox Chase SCID mice. Each treatment group ranged from 4 to 8 mice. Curcumin treatments were initiated 24 hours to one week post MM cell injections. Mice were treated daily with oral curcumin via gavage or 3× per week IP injections of curcumin with a vehicle of corn oil or DMSO. Cisplatin 2 mg/kg IP injections were performed at week 1 and week 2 post-MM inoculations alone and in combination with curcumin. At 4 weeks post-MM cell injection, mice were euthanized by IP injection of sodium pentobarbital. Following euthanization, MM tumors were collected, weighed and measured using calipers. All experiments using mice were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Vermont College of Medicine (Burlington, VT).
Statistical analyses
Statistical significance was determined using a one way ANOVA followed by a Newman-Keuls multiple comparisons test or a Student’s t-test. Comparisons yielding p values below 0.05 were determined to be statistically significant from each other.
Results
Curcumin induced NLRP3 inflammasome priming and caspase-1 activation, but not cytokine maturation in mouse MM cells
Cytotoxic doses of curcumin in mouse MM cells were established using MTS assays by treating cells with escalating doses of curcumin (0–50 µM) for 24–72 h. As shown in Fig. 1A, all curcumin doses of 40 and 50 µM for 48 and 72 h significantly inhibited mouse MM cell growth compared to control. For this reason, the concentration of curcumin 40 µM was selected for subsequent experiments.
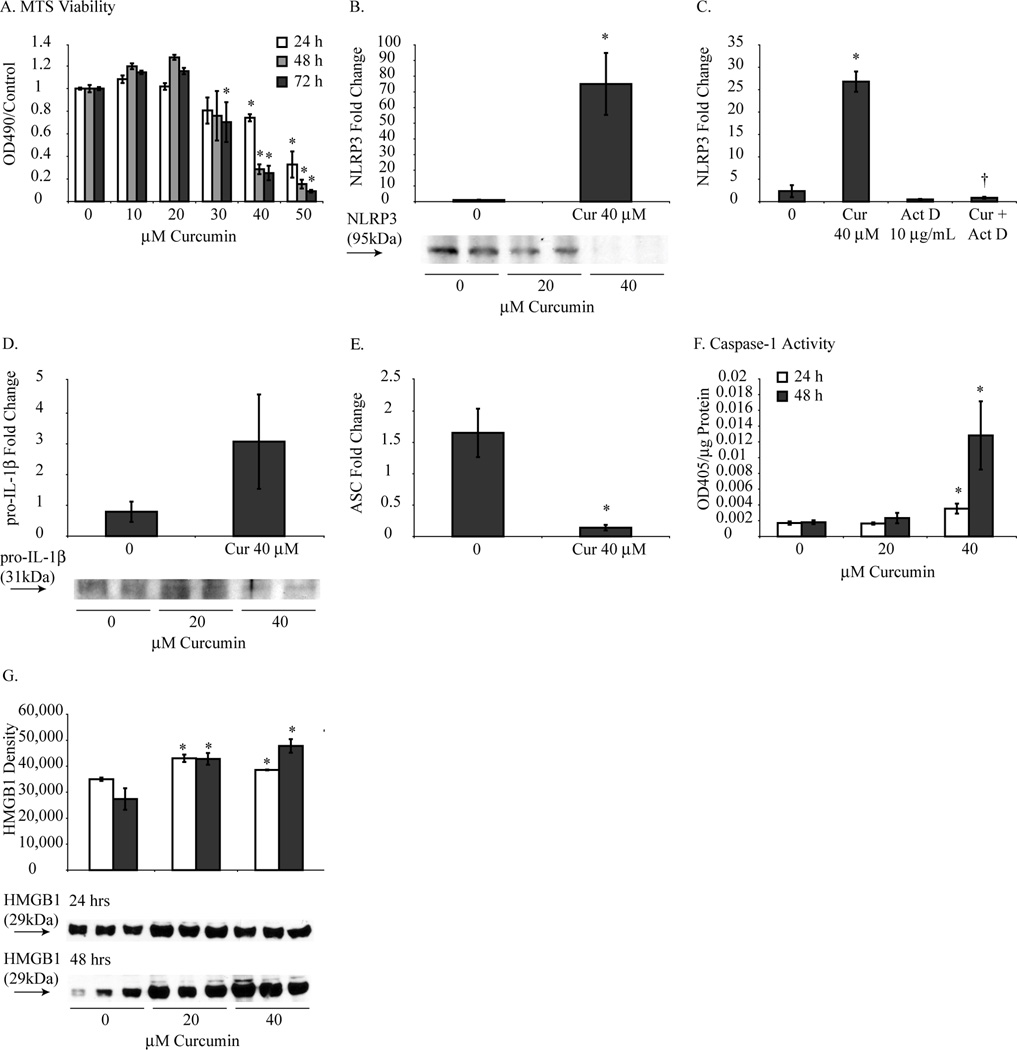
Figure 1.
Curcumin induced NLRP3 priming and caspase-1 activation in mouse MM cells without pro-inflammatory cytokine processing. (A) MTS assays revealed that curcumin inhibited mouse MM cell growth in a manner that was dose and time dependent. Mouse MM cells treated with curcumin (40 µM for 48 h) resulted in increased mRNA levels of NLRP3 (B) compared to control MM cells which was inhibited by Actinomycin D pretreatment for 1 hour (C). Pro-IL-1β mRNA levels were not significantly altered by curcumin (D) while ASC mRNA levels (E) were significantly decreased. Caspase-1 activation (F) and HMGB-1 release (G) significantly increased in mouse MM cells in response to curcumin (20 and 40 µM for 48 h). *p value ≤ 0.05 when compared to control. †p value ≤ 0.05 when compared to curcumin only treatment group.
Inflammasome activation is a two-step process that requires priming followed by activation (26). To investigate the priming step of NLRP3 activation, mRNA levels of NLRP3 were analyzed following curcumin treatment. Treatment with curcumin 40 µM for 48 h in mouse MM cells resulted in significantly increased NLRP3 mRNA levels (Fig. 1B) which was a result of increased transcription as demonstrated through amelioration of this effect by Actinomycin D (Act D) pretreatment (Fig. 1C). Control (DMSO) and curcumin treated mouse MM cells did not show a significant difference in pro-IL-1β mRNA levels (Fig. 1D). In addition, curcumin downregulated mRNA levels of ASC (Fig. 1E).In support of pyroptosis, caspase-1 activation was significantly increased in response to curcumin treatment (Fig. 1F). HMGB1 is marker of cell death (27) and was analyzed as a parameter to support the occurrence of pyroptosis. Curcumin treatment resulted in significantly increased extracellular (secreted) levels of HMGB1 in the medium of mouse MM cells (Fig. 1G).
As previously described, active caspase-1 is responsible for activation of the pro-inflammatory cytokines, IL-1β and IL-18. Processing of these cytokines was investigated by ELISA which demonstrated undetectable levels of IL-1β and IL-18 in control and curcumin treated mouse MM cells (data not shown). Additionally, IL-1β Western blot analysis was performed and also revealed no detectable levels of IL-1β in curcumin treated mouse MM cells (data not shown), thus confirming that IL-1β was not processed as a result of caspase-1 activation by curcumin.
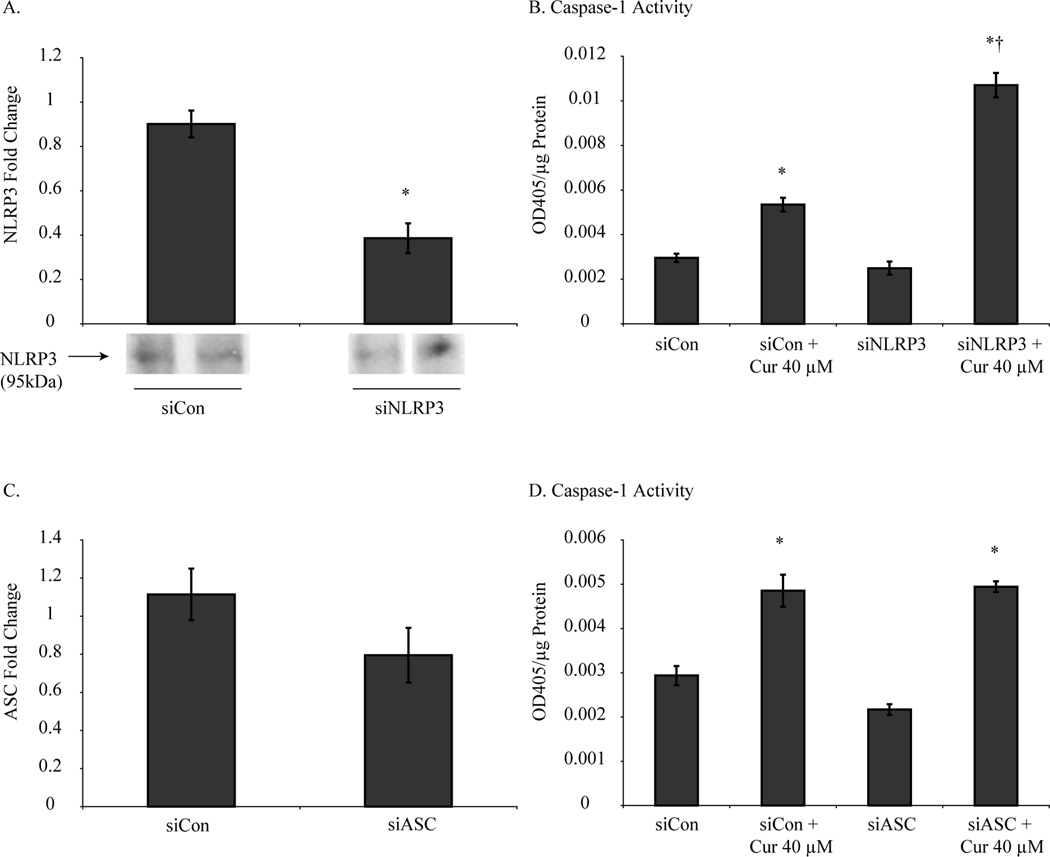
Silencing of NLRP3 or ASC by siRNA did not inhibit curcumin-induced caspase-1 activation in mouse MM cells
As an attempt to link our findings of NLRP3 priming with caspase-1 activation in curcumin treated mouse MM cells, we evaluated if curcumin-induced caspase-1 activation was NLRP3 dependent. NLRP3 mRNA was significantly reduced by siRNA in mouse MM cells (Fig. 2A), however, caspase-1 activation by curcumin was not affected (Fig. 2B). Next, we knocked down expression of ASC in mouse MM cell by siRNA (Fig. 2C) which again had no effect on curcumin’s ability to activate caspase-1 (Fig. 2D).
Figure 2.
NLRP3 and ASC inhibition did not attenuate curcumin-induced caspase-1 activation. Mouse MM cells transfected with siNLRP3 or siASC suppressed NLRP3 (A) and ASC (C) mRNA levels, however did not inhibit caspase-1 activity (B and D) when treated with curcumin (40 µM for 24 h). *p value ≤0.05 when compared to control. †p value ≤ 0.05 when compared to siCon + curcumin.
Curcumin treatment in human MM cells resulted in NLRP3 priming and caspase-1 activation without pro-inflammatory cytokine secretion
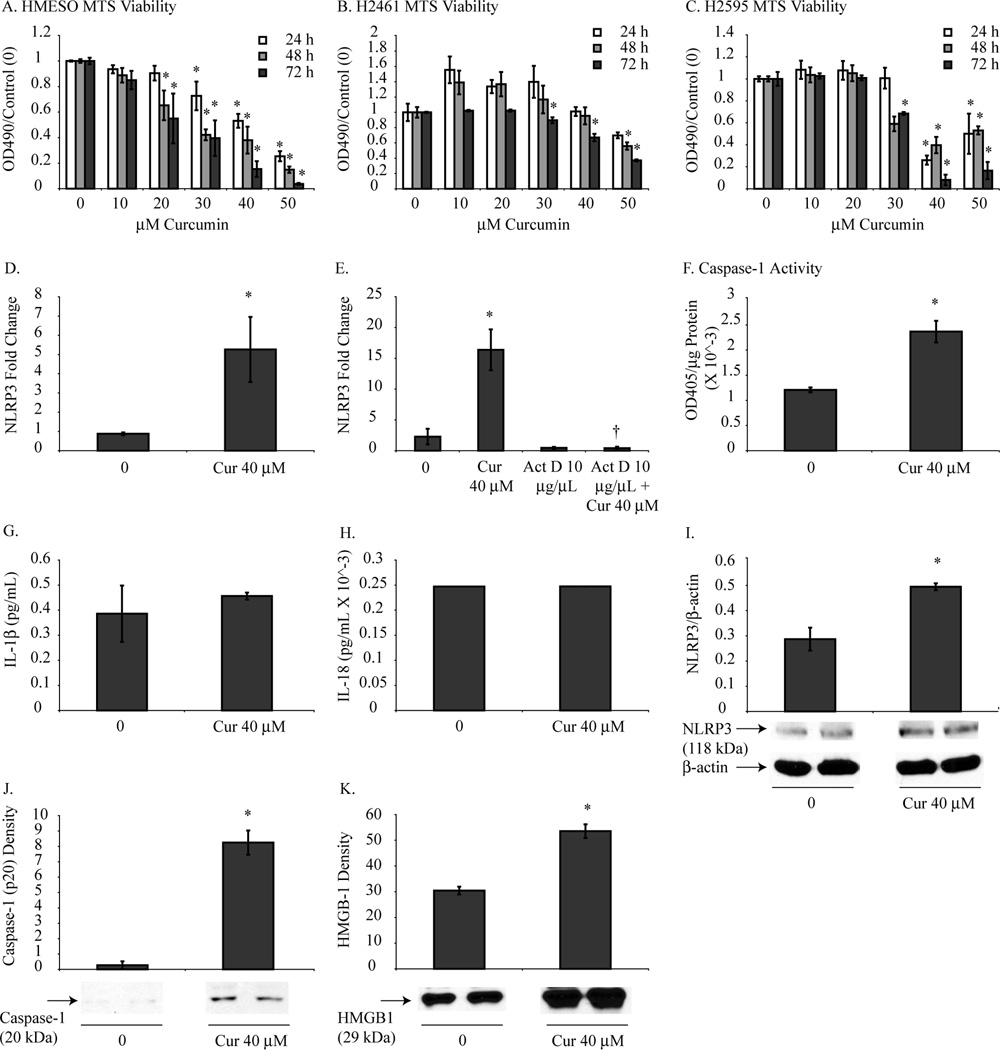
MTS assays were performed to confirm cytotoxic curcumin doses in HMESO, H2595 and H2461 cells. Fig. 3A, 3B and 3C reveals that curcumin had a dose and time dependent inhibitory effect on cell growth of human MM cells. Similar to mouse MM cells, curcumin doses of 30–50 µM at all-time points resulted in significant cytotoxicity of human MM cells. Curcumin-induced priming of NLRP3 in HMESO cells was demonstrated by significantly increased mRNA (Fig. 3D) levels of NLRP3 compared to control cells. Actinomycin D pretreatment diminished the curcumin-induced increase in NLRP3 mRNA (Fig. 3E) thus indicating this effect occurred by transcriptional upregulation of NLRP3 and not stabilization of mRNA. Caspase-1 activity significantly increased in HMESO cells in response to curcumin treatment as shown by increased caspase-1 activity measured by caspase-1 assay (Fig. 3F). Consistent with the cytokine analysis in mouse MM cells, curcumin did not induce significant changes in processed IL-1β and IL-18 levels in HMESO cells (Fig. 3G and H). NLRP3 protein (Fig. 3I) and caspase-1 p20 protein was also increased by curcumin (Fig. 3J). Additionally, HMBG1 was increased in the medium of HMESO cells treated with curcumin (Fig. 3K).
Figure 3.
Curcumin mediates NLRP3 priming and caspase-1 activation without pro-inflammatory cytokine secretion in human MM cells. MTS assays revealed that curcumin inhibited HMESO (A), H2461 (B) and H2595 (C) cell growth in a manner that was dose and time dependent. HMESO cells treated with curcumin (40 µM for 48 h) resulted in increased mRNA levels (D) and protein levels (I) of NLRP3 compared to control MM cells. Pretreatment with Actinomycin D 1 h prior to curcumin treatment inhibited curcumin-induced increased mRNA levels of NLRP3 (E). Caspase-1 activation was significantly increased in response to curcumin (40 µM for 48 h) when measured by caspase-1 activity assay (F) and western blot analysis of p20 (J) without significant change in secretion of IL-1β (G) and IL-18 (H). Curcumin treatment (40 µM for 48 h) resulted in increased HMBG1 release in HMESO cells compared to control cells (K). *p value ≤ 0.05 when compared to control. †p value < 0.05 when compared to curcumin only treatment group.
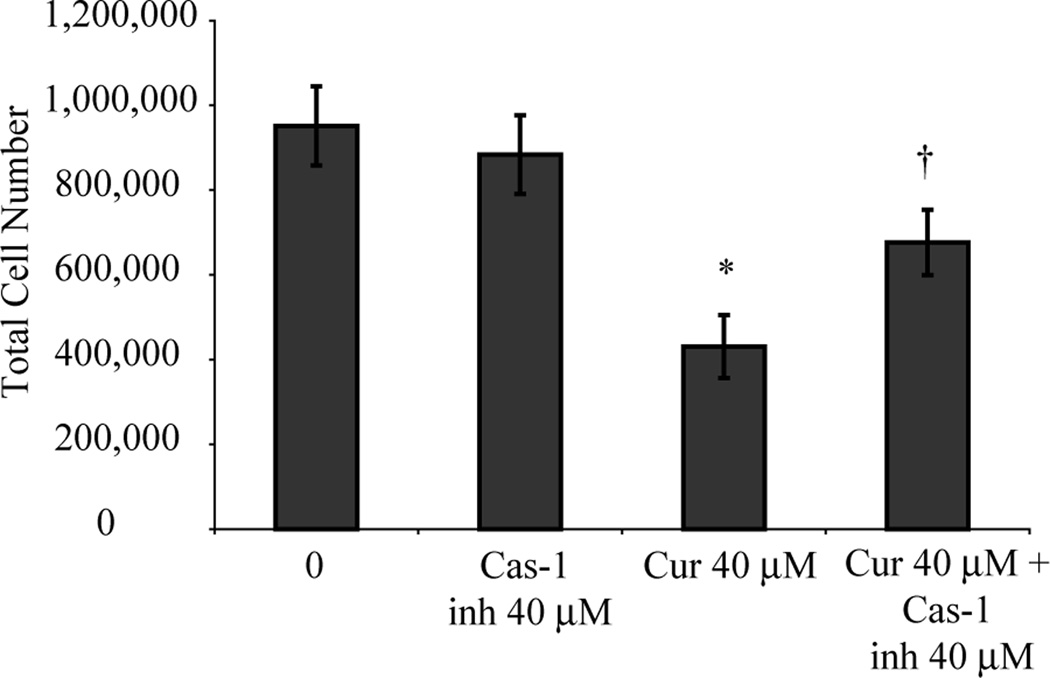
Curcumin kills HMESO cells through pyroptosis
Pyroptotic cell death requires active caspase-1. To prove that curcumin induces cell death through pyroptosis, HMESO cells were pretreated with a caspase-1 inhibitor and then treated with curcumin (40 µM for 48 h). Fig. 4 demonstrates that HMESO cells were significantly less susceptible to curcumin-induced cytotoxicity in the presence of caspase-1 inhibition.
Figure 4.
Curcumin kills human MM cells through pyroptosis. Curcumin (40 µM for 48 h) significantly decreased HMESO cells compared to control. Pretreatment with caspase-1 inhibitor for 1 h significantly reduced the inhibitory effect of curcumin. *p value ≤ 0.05 when compared to control; †p value ≤ 0.05 when compared to curcumin only treatment group.
Curcumin-induced priming of NLRP3 and pyroptosis in HMESO cells is ROS dependent
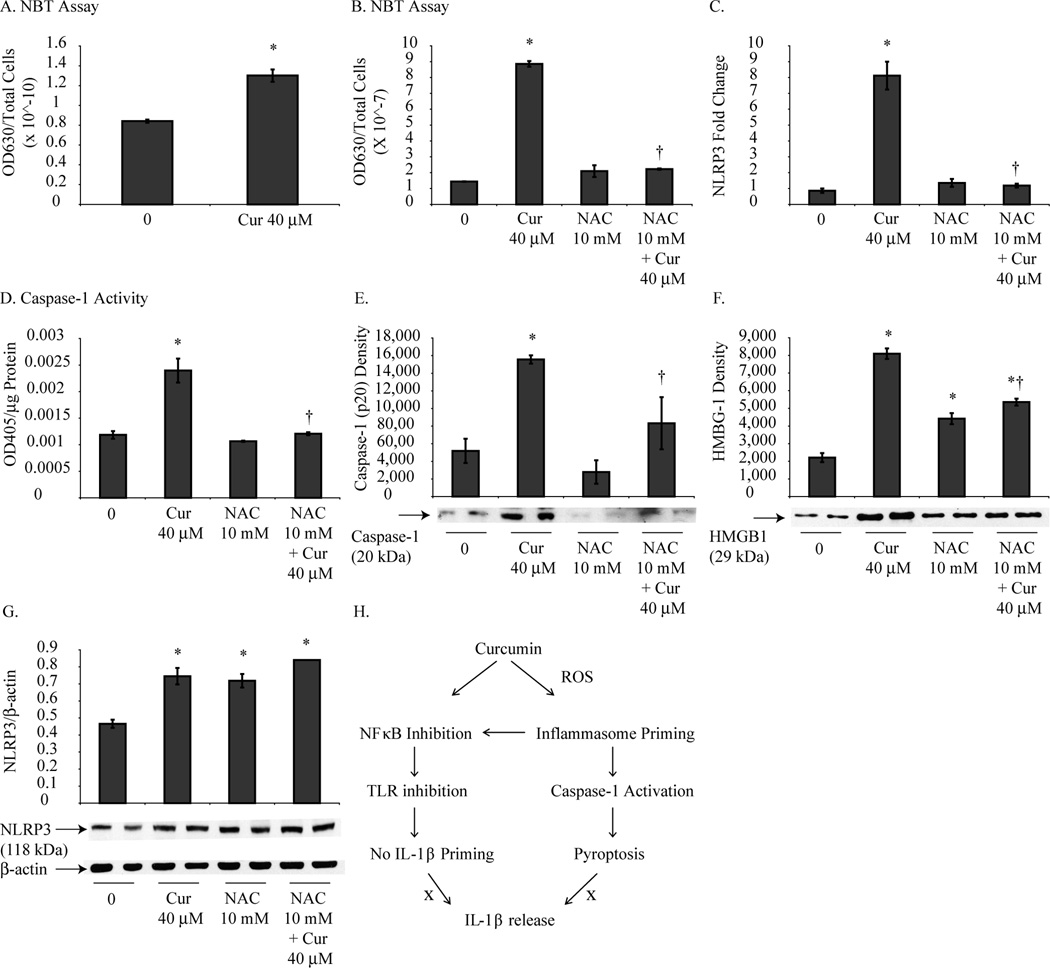
ROS is a known activator of the NLRP3 inflammasome in macrophages (17). Curcumin has been shown to have both anti-oxidant and pro-oxidant effects in different cell types and experimental settings (28–30) although no data exist regarding curcumin regulated ROS in MMs. For this reason, NBT assays were performed to determine if curcumin alters ROS production in HMESO cells. As shown in Fig. 5A, curcumin treatment (40 µM for 6 h) of HMESO cells increased ROS production compared to control cells which was inhibited by NAC pretreatment (Fig. 5B). Additionally, pretreatment with NAC decreased curcumin-induced increases in NLRP3 mRNA (Fig. 5C), caspase-1 activity (Fig. 5D), cleavage of p20 (Fig. 5E) and HMGB-1 release (Fig. 5F) in HMESO cells, however, no significant effect of NAC was observed on NLRP3 protein expression (Fig. 5G).
Figure 5.
Curcumin-induced pyroptosis is ROS dependent. Curcumin treatment (40 µM for 6 h) resulted in significantly increased ROS in HMESO cells (A) compared to control cells which was attenuated by NAC pretreatment (40 µM for 18 h) (B). Pretreatment with NAC (40 µM for 18 h) blocked increased NLRP3 mRNA levels (C), caspase-1 activity (D), cleavage of p20 (E), and HMGB1 release (F) by curcumin (40 µM for 48 h) in HMESO cells. Expression of NLPR3 protein (G) was not altered with NAC pretreatment. (H) Schema showing regulation of pyroptosis by curcumin. *p value ≤ 0.05 when compared to control; †p value < 0.05 when compared to curcumin only treatment group.
Curcumin alters inflammasome-related gene expression in HMESO cells
A PCR Array using a ‘Human Inflammasomes’ template on HMESO cells treated with curcumin (40 µM for 48 h) compared to control cells showed that curcumin treatment resulted in significantly (p ≤ 0.05) reduced levels of inflammasome-related gene expression involved in inflammation, NF-κB, toll like receptors (TLR) and IL-1 pathways (Table 1). Additionally, MYD88, NLRC4 and TXNIP were downregulated by curcumin. Genes that were significantly upregulated by curcumin in HMESO cells included heat shock protein 90 kDa alpha class A member 1 (HSP90AA1), IL-12, IL-6 and Mediterranean fever (MEFV).
Table 1.
PCR Array analysis showing significant altered expression of important tumorigenesis related genes by curcumin in HMESO cells as compared to control
| Gene Name | Increase/Decrease (fold) | Function | Validation (QRT-PCR) |
|---|---|---|---|
| Caspase 8 (CASP8) | −1.76 | apoptosis | |
| CASP8 and FADD-like apoptosis regulator (CFLAR) | −1.66 | apoptosis | |
| Inhibitor of kappa light polypeptide gene enhancer in B cells, kinase beta (IKBKB) | −1.89 | NF-κB pathway promoter | Yes |
| Interleukin 1-beta (IL-1β) | −3.11 | inflammation, drug resistance | Yes |
| Interleukin-1 receptor associated kinase (IRAK1) | −2.28 | IL-1 pathway promoter | |
| Myeloid differentiation primary response gene 88 (MYD88) | −2.3 | IL-1 and TLR signal transducer | Yes |
| Nuclear factor of kappa light polypeptide gene enhancer in B cells 1 (NFKB1) | −3.13 | inflammation, proliferation | |
| NLR family, CARD domain containing 4 (NLRC4) | −1.76 | inflammasome | |
| PYD and CARD containing (PYCARD) | −2.4 | caspase-1 adaptor protein | Yes |
| TGF-β activated kinase 1/MAP3K7 binding protein 1 (TAB1) | −2.66 | signal transduction TGF-β, IL-1 | |
| TGF-β activated kinase 1/MAP3K7 binding protein 2 (TAB2) | −1.97 | signal transduction IL-1, NF-κB | |
| Toll-interleukin 1 receptor domain containing adaptor protein (TIRAP) | −2.09 | TLR adapter protein, NF-κB activation | |
| Thioredoxin interacting protein (TXNIP) | −4.89 | thioredoxin inhibitor | Yes |
| Heat shock protein 90kDa alpha, class A member 1 (HSP90AA1) | 10.15 | ROS production | |
| Interleukin-12 A (IL-12A) | 18.79 | Th1 response | Yes |
| Interleukin-6 (IL-6) | 3.73 | inflammation, maturation of B cells | |
| Mediterranean fever (MEFV) | 1.57 | inflammasome adapter protein |
Curcumin in combination with asbestos resulted in increased NLRP3 and pro-IL-1β priming in mesothelial cells
In order to determine curcumin’s effect on asbestos-initiated inflammasome processes in human mesothelial cells, LP9 cells were pretreated with curcumin followed by asbestos exposure. The combination of curcumin pre-treatment followed by asbestos exposure resulted in increased steady state mRNA levels of NLRP3 (Supplementary Fig. S1A) and pro-IL-1β (Supplementary Fig. S1B) compared to control and asbestos only treated LP9 cells. No accumulative effects of curcumin pretreatment in combination with asbestos were present in caspase-1 activation (Supplementary Fig. S1C) as asbestos only and curcumin pretreatment with asbestos treatment groups were not significantly different. Curcumin treatment alone had no effect on LP9 cells when analyzed for the above parameters.
Curcumin treatment in vivo did not reduce MM tumor burden
Various doses of curcumin administered through different routes in murine allograft and xenograft models of MM did not reduce tumor burden (Supplementary Fig. 2A–C). Additionally, curcumin did not have synergistic effects with cisplatin treatment in the allograft model (Supplementary Fig. 2B).
Discussion
Prior studies have shown that curcumin causes cell death by apoptosis (31) and autophagy (32), however we are the first to demonstrate that curcumin induces cell death by pyroptosis in mouse and human MM cells. Pyroptosis is a caspase-1 mediated, programmed, pro-inflammatory cell death that results in loss of plasma membrane integrity and release of cytoplasmic contents (12). Impaired pyroptosis has been reported to enhance inflammation-induced colon cancer (33). Consistent with the above report, induction of caspase-1 has been shown to be beneficial in other cancers such as prostate and renal cancer (34, 35). Here we use two different techniques (caspase-1 activity assays and Western blot analysis of secreted p20) to clearly demonstrate that curcumin mediates caspase-1 activation in MM cells. Furthermore, we show that caspase-1 was necessary to exert the cytotoxic effects of curcumin as caspase-1 inhibition prior to curcumin treatment resulted in protection against curcumin-induced cell death. Even more intriguing is the finding that curcumin can induce pyroptosis while preventing processing of pro-inflammatory cytokines, IL-1β and IL-18. This finding is consistent with research performed in dendritic cells that reports inflammasome independent regulation of pyroptosis and cytokine processing in response to Listeria monocytogenes (Lm) p60 protein (36). Additionally, research performed in pathogen-infected macrophages suggests that two distinct inflammasome complexes may form which differentially mediate cytokine secretion and/or pyroptosis (37).
The ability of curcumin to provide protection against processing of classical inflammasome cytokines is an important finding because of the detrimental effects these cytokine may convey. IL-1β has been linked to inflammasome-dependent carcinogenic inflammation through its role in tumor progression and chemoresistance in multiple cancer types (14). In addition, IL-1β has been shown to promote mesothelial cell proliferation and transformation leading to MM development (38). IL-18 has also been linked to tumor progression as this cytokine was shown to be immunosuppressive and support metastatic function in melanoma and colon carcinoma (39). In our in vitro models, curcumin may be preventing cytokine processing by blocking priming of cytokine precursors, pro-IL-1β and pro-IL-18, which is a necessary step required prior to cytokine activation (40). We show that pro-IL-1β was not primed by curcumin in mouse MM cells and found to be significantly downregulated in human MM cells. Cytokine priming typically involves TLR stimulation with downstream involvement of NF-κB (41, 42). In our studies, we found that curcumin treatment of MM cells resulted in downregulation of inflammasome-related genes belonging to the TLR, IL-1 and NF-κB pathways thus providing a link to curcumin-induced inhibition of pro-cytokine expression and subsequent cytokine processing (maturation) (Fig. 5H).
Another parameter to support the occurrence of pyroptosis is HMGB1 which is passively released into the extracellular space upon loss of membrane integrity (43). HMGB1 is a damage associated molecular pattern (DAMP), and has been studied as a positive prognostic biomarker for response to chemotherapy in breast cancer patients (27). This study concluded that HMGB1 was increased in the plasma of patients who had a positive response to chemotherapy (epirubicin/docetaxeL) compared to patients who were non-responders. Other recent studies suggest that HMGB1 requires cytokines, such as IL-1β, to induce strong inflammatory responses which involves signaling through TLRs and RAGE receptors (44). Our data showing increased HMGB1 release by curcumin suggest that HMGB1 is not inducing pro-inflammatory effects in this setting as curcumin blocks IL-1β processing and promotes downregulation of TLR signaling pathway genes (MYD88, IRAK1, TIRAP).
The molecular pathway by which curcumin activates caspase-1 leading to pyroptosis remains unclear. Here we show for the first time that curcumin upregulates NLRP3 mRNA as well as protein levels in human MM cells. Despite these findings, our data indicate that activation of the NLRP3 inflammasome is not required for curcumin-induced pyroptosis as reduction in NLRP3 or ASC mRNA levels via siRNA did not attenuate caspase-1 activation by curcumin. In support of this finding, we demonstrated that curcumin downregulates the gene expression of TXNIP in MM cells which is a direct ligand for activation of the NLRP3 inflammasome (45). Another possibility could be that reduction in NLRP3 expression leads to a compensatory upregulation of other inflammasomes that are capable of caspase-1 activation. Additionally, our data indicate that casapse-1 is not activated by curcumin through a pyroptosome or other inflammasomes that require ASC because siASC failed to suppress curcumin-induced caspase-1 activation. Furthermore, curcumin treatment in mouse and human MM cells significantly downregulated ASC. It is possible that curcumin could activate caspase-1 through ASC-independent inflammasomes although our PCR array data indicates that curcumin downregulates NLRC4 gene expression. In addition, we performed analysis of NLRP1 via PCR array and qRT-PCR in MM cells treated with and without curcumin (data not shown). PCR array data initially revealed an upregulation of NLRP1 gene expression by curcumin. However, the values generated from the analysis were very close to the threshold cutoff values. For this reason, we attempted validation with qRT-PCR using Assay on Demand (AOD) primers and probes for NLRP1 which repeatedly showed undetectable levels of mRNA expression of NLRP1 in control and curcumin treated MM cells. These results suggest that curcumin is not signaling through NLRC4 or NLRP1 inflammasomes either, however, confirming these findings would require siNLRC4 and siNLRP1 experiments.
Regardless of the exact mechanisms involved in curcumin-induced caspase-1 activation in MM cells, our findings implicate ROS in this process. Prior studies have demonstrated that curcumin exerts its biological effects through pro-oxidant mechanisms (28, 29). Here we show that curcumin increases ROS production in MM cells and that ROS production is required, in part, for NLRP3 priming, HMBG1 release and caspase-1 activation by curcumin. In contrast, NLRP3 protein expression was not altered by reduction in curcumin-induced ROS which further suggest NLRP3 is not mediating pyroptosis. Taken together, our data implies that curcumin generates ROS which may then activate inflammasomes leading to caspase-1 activation or may directly activate caspase-1 resulting in pyroptosis (Fig. 5H). The source of ROS generated by curcumin remains unclear. One possible link may be HSP90 which was upregulated in MM cells by curcumin. HSP90 has been shown to regulate nicotinamide adenine dinucleotide phosphate oxidases (Nox) and increase ROS production (46, 47).
Although curcumin upregulated NLRP3 may not be necessary for pyroptosis, this protein may serve as a mediator for regulation of NF-κB pathway by curcumin in MM cells. Numerous reports have highlighted curcumin as an inhibitor of NF-κB in multiple cancers (48). The NF-κB pathway is implicated in mesothelial cell transformation and MM tumor progression (49). Our PCR array data revealed that curcumin downregulated gene expression of NFKB1 in addition to other genes involved in promotion of the NF-κB pathway (IKBKB, TIRAP, TAB2) in MM cells. Interestingly, it has been reported that increased levels of NLRP3 alone (42) and ASC alone block NF-κB activity through inhibition of p65 nuclear translocation. However, co-expression of NLRP3 and ASC resulted in NF-κB activation (50). These reports indicate that the balance of NLRP3 and ASC expression influence regulation of NF-κB signaling and provide a context to interpret our findings that curcumin upregulates NLRP3 mRNA and protein levels and downregulates gene and mRNA expression of ASC in MM cells. Hence, curcumin may be downregulating NF-κB by upregulation of NLRP3 levels with subsequent downregulation of ASC.
We also investigated the role of curcumin in asbestos-initiated inflammasome and caspase-1 processes in mesothelial cells. To our surprise, we found that curcumin significantly enhances asbestos-induced increases of NLRP3 and pro-IL-1β mRNA levels in mesothelial cells. As described previously, increased levels of NLRP3 may have biological signaling importance, however it remains unclear how curcumin modulates the detrimental effects of asbestos in mesothelial cells.
While our in vitro data strongly showed the curcumin is cytotoxic to MM cells through induction of pyroptosis, our in vivo experiments failed to show corresponding results. These findings are likely a consequence of the low solubility and poor bioavailability of curcumin which has limited its role as a cancer therapy in clinical trials (8). Our future studies involve use of synthetic curcumin analog to increase the cellular uptake.
In conclusion, we demonstrate that curcumin induces cytotoxic effects on MM cells through pyroptosis in a process involving ROS production that is independent of the NLRP3 inflammasome. Additionally, curcumin has anti-inflammatory effects by blocking cytokine processing of IL-1β and IL-18 and genes involved in the NF-κB pathway. These results provide evidence that curcumin warrants further investigation as a therapeutic agent in MM although future studies must include improved curcumin analogs or enhanced modes of delivery to overcome curcumin’s most challenging feature which is limited bioavailability.
Supplementary Material
Acknowledgements
We thank Jennifer L. Díaz for assistance in manuscript preparation. We acknowledge the Vermont Cancer Center (VCC) DNA Analysis Facility for generation of qRT-PCR and PCR Array data.
Grant Support
This work was supported by a grant from the Mesothelioma Applied Research Foundation (MARF) and NIEHS grants R01 ES021110 and T32 ES07122.
Footnotes
The Authors have no conflicts to disclose.
References
- 1.Robinson BW, Lake RA. Advances in malignant mesothelioma. The New England journal of medicine. 2005;353:1591–1603. doi: 10.1056/NEJMra050152. [DOI] [PubMed] [Google Scholar]
- 2.Schaffer M, Schaffer PM, Zidan J, Bar Sela G. Curcuma as a functional food in the control of cancer and inflammation. Current opinion in clinical nutrition and metabolic care. 2011;14:588–597. doi: 10.1097/MCO.0b013e32834bfe94. [DOI] [PubMed] [Google Scholar]
- 3.Goel A, Aggarwal BB. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutrition and cancer. 2010;62:919–930. doi: 10.1080/01635581.2010.509835. [DOI] [PubMed] [Google Scholar]
- 4.Kanai M, Yoshimura K, Asada M, Imaizumi A, Suzuki C, Matsumoto S, et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer chemotherapy and pharmacology. 2011;68:157–164. doi: 10.1007/s00280-010-1470-2. [DOI] [PubMed] [Google Scholar]
- 5.Prakobwong S, Khoontawad J, Yongvanit P, Pairojkul C, Hiraku Y, Sithithaworn P, et al. Curcumin decreases cholangiocarcinogenesis in hamsters by suppressing inflammation-mediated molecular events related to multistep carcinogenesis. International journal of cancer Journal international du cancer. 2011;129:88–100. doi: 10.1002/ijc.25656. [DOI] [PubMed] [Google Scholar]
- 6.Tharakan ST, Inamoto T, Sung B, Aggarwal BB, Kamat AM. Curcumin potentiates the antitumor effects of gemcitabine in an orthotopic model of human bladder cancer through suppression of proliferative and angiogenic biomarkers. Biochemical pharmacology. 2010;79:218–228. doi: 10.1016/j.bcp.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Zhang C, Li B, Zhang X, Hazarika P, Aggarwal BB, Duvic M. Curcumin selectively induces apoptosis in cutaneous T-cell lymphoma cell lines and patients' PBMCs: potential role for STAT-3 and NF-kappaB signaling. The Journal of investigative dermatology. 2010;130:2110–2119. doi: 10.1038/jid.2010.86. [DOI] [PubMed] [Google Scholar]
- 8.Basnet P, Skalko-Basnet N. Curcumin: an anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011;16:4567–4598. doi: 10.3390/molecules16064567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishikawa KTK, Karjalainen A, Wen CP, Furuya S, Hoshuyama T, Todoroki M, Kiyomoto Y, Wilson D, Higashi T, Ohtaki M, Pan G, Wagner G. Recent mortality from pleural mesothelioma, historical patterns of asbestos use adoption of bans: a global assessment. Environ Health perspect. 2008;116:1675–1680. doi: 10.1289/ehp.11272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller J, Shukla A. The role of inflammation in development and therapy of malignant mesothelioma. Am Med J. 2012;3:240–248. [Google Scholar]
- 11.Hillegass JM, Shukla A, Lathrop SA, MacPherson MB, Beuschel SL, Butnor KJ, et al. Inflammation precedes the development of human malignant mesotheliomas in a SCID mouse xenograft model. Annals of the New York Academy of Sciences. 2010;1203:7–14. doi: 10.1111/j.1749-6632.2010.05554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Couillin I, Petrilli V, Martinelli M. The Inflammasomes. Springer: Basel AG; 2011. [Google Scholar]
- 13.Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nature immunology. 2012;13 doi: 10.1038/ni.2237. 333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zitvogel L, Kepp O, Galluzzi L, Kroemer G. Inflammasomes in carcinogenesis and anticancer immune responses. Nature immunology. 2012;13:343–351. doi: 10.1038/ni.2224. [DOI] [PubMed] [Google Scholar]
- 15.Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunological reviews. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandes-Alnemri T, Alnemri ES. Assembly, purification, and assay of the activity of the ASC pyroptosome. Methods in enzymology. 2008;442:251–270. doi: 10.1016/S0076-6879(08)01413-4. [DOI] [PubMed] [Google Scholar]
- 17.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell WJ, Huggins CW, Wylie AG. Chemical and physical characterization of amosite, chrysotile, crocidolite and nonfibrous tremolite for oral ingestion studies in US Bureau of Mines Reports of Investigations. Washington DC: NIEHS; 1980. [Google Scholar]
- 19.Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, et al. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Molecular and cellular biology. 2000;20:1436–1447. doi: 10.1128/mcb.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reale FR, Griffin TW, Compton JM, Graham S, Townes PL, Bogden A. Characterization of a human malignant mesothelioma cell line (H-MESO 1): a biphasic solid and ascitic tumor model. Cancer research. 1987;47:3199–3205. [PubMed] [Google Scholar]
- 21.Pass HI, Stevens EJ, Oie H, Tsokos MG, Abati AD, Fetsch PA, et al. Characteristics of nine newly derived mesothelioma cell lines. The Annals of thoracic surgery. 1995;59:835–844. doi: 10.1016/0003-4975(95)00045-m. [DOI] [PubMed] [Google Scholar]
- 22.Shukla A, Hillegass JM, MacPherson MB, Beuschel SL, Vacek PM, Pass HI, et al. Blocking of ERK1 and ERK2 sensitizes human mesothelioma cells to doxorubicin. Molecular cancer. 2010;9:314. doi: 10.1186/1476-4598-9-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shukla A, Barrett TF, Nakayama KI, Nakayama K, Mossman BT, Lounsbury KM. Transcriptional up-regulation of MMP12 and MMP13 by asbestos occurs via a PKCdelta-dependent pathway in murine lung. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2006;20:997–999. doi: 10.1096/fj.05-4554fje. [DOI] [PubMed] [Google Scholar]
- 24.Shukla A, Flanders T, Lounsbury KM, Mossman BT. The gamma-glutamylcysteine synthetase and glutathione regulate asbestos-induced expression of activator protein-1 family members and activity. Cancer research. 2004;64:7780–7786. doi: 10.1158/0008-5472.CAN-04-1365. [DOI] [PubMed] [Google Scholar]
- 25.Shukla A, Hillegass JM, MacPherson MB, Beuschel SL, Vacek PM, Butnor KJ, et al. ERK2 is essential for the growth of human epithelioid malignant mesotheliomas. International journal of cancer Journal international du cancer. 2011;129:1075–1086. doi: 10.1002/ijc.25763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hornung V, Latz E. Critical functions of priming and lysosomal damage for NLRP3 activation. European journal of immunology. 2010;40:620–623. doi: 10.1002/eji.200940185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnold T, Michlmayr A, Baumann S, Burghuber C, Pluschnig U, Bartsch R, et al. Plasma HMGB-1 after the initial dose of epirubicin/docetaxel in cancer. European journal of clinical investigation. 2013;43:286–291. doi: 10.1111/eci.12043. [DOI] [PubMed] [Google Scholar]
- 28.Aggeli IK, Koustas E, Gaitanaki C, Beis I. Curcumin Acts as a Pro-Oxidant Inducing Apoptosis Via JNKs in the Isolated Perfused Rana ridibunda Heart. Journal of experimental zoology Part A, Ecological genetics and physiology. 2013;319:328–339. doi: 10.1002/jez.1797. [DOI] [PubMed] [Google Scholar]
- 29.Reuss DE, Mucha J, Hagenlocher C, Ehemann V, Kluwe L, Mautner V, et al. Sensitivity of malignant peripheral nerve sheath tumor cells to TRAIL is augmented by loss of NF1 through modulation of MYC/MAD and is potentiated by curcumin through induction of ROS. PloS one. 2013;8:e57152. doi: 10.1371/journal.pone.0057152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhullar KS, Jha A, Youssef D, Rupasinghe HP. Curcumin and its carbocyclic analogs: structure-activity in relation to antioxidant and selected biological properties. Molecules. 2013;18:5389–5404. doi: 10.3390/molecules18055389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Rishi AK, Wu W, Polin L, Sharma S, Levi E, et al. Curcumin suppresses growth of mesothelioma cells in vitro and in vivo, in part, by stimulating apoptosis. Molecular and cellular biochemistry. 2011;357:83–94. doi: 10.1007/s11010-011-0878-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamauchi Y, Izumi Y, Asakura K, Hayashi Y, Nomori H. Curcumin induces autophagy in ACC-MESO-1 cells. Phytotherapy research: PTR. 2012;26:1779–1783. doi: 10.1002/ptr.4645. [DOI] [PubMed] [Google Scholar]
- 33.Hu B, Elinav E, Huber S, Booth CJ, Strowig T, Jin C, et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21635–21640. doi: 10.1073/pnas.1016814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winter RN, Rhee JG, Kyprianou N. Caspase-1 enhances the apoptotic response of prostate cancer cells to ionizing radiation. Anticancer research. 2004;24:1377–1386. [PubMed] [Google Scholar]
- 35.Ueki T, Takeuchi T, Nishimatsu H, Kajiwara T, Moriyama N, Narita Y, et al. Silencing of the caspase-1 gene occurs in murine and human renal cancer cells and causes solid tumor growth in vivo. International journal of cancer Journal international du cancer. 2001;91:673–679. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1113>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt RL, Lenz LL. Distinct licensing of IL-18 and IL-1beta secretion in response to NLRP3 inflammasome activation. PloS one. 2012;7:e45186. doi: 10.1371/journal.pone.0045186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Broz P, von Moltke J, Jones JW, Vance RE, Monack DM. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell host & microbe. 2010;8:471–483. doi: 10.1016/j.chom.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Faux SP, Hallden G, Kirn DH, Houghton CE, Lemoine NR, et al. Interleukin-1beta and tumour necrosis factor-alpha promote the transformation of human immortalised mesothelial cells by erionite. International journal of oncology. 2004;25:173–178. [PubMed] [Google Scholar]
- 39.Terme M, Ullrich E, Aymeric L, Meinhardt K, Desbois M, Delahaye N, et al. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer research. 2011;71:5393–5399. doi: 10.1158/0008-5472.CAN-11-0993. [DOI] [PubMed] [Google Scholar]
- 40.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nature immunology. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barker BR, Taxman DJ, Ting JP. Cross-regulation between the IL-1beta/IL-8 processing inflammasome and other inflammatory cytokines. Current opinion in immunology. 2011;23:591–597. doi: 10.1016/j.coi.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Connor W, Jr, Harton JA, Zhu X, Linhoff MW, Ting JP. Cutting edge: CIAS1/cryopyrin/PYPAF1/NALP3/CATERPILLER 1.1 is an inducible inflammatory mediator with NF-kappa B suppressive properties. J Immunol. 2003;171:6329–6333. doi: 10.4049/jimmunol.171.12.6329. [DOI] [PubMed] [Google Scholar]
- 43.Willingham SB, Allen IC, Bergstralh DT, Brickey WJ, Huang MT, Taxman DJ, et al. NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and -independent pathways. J Immunol. 2009;183:2008–2015. doi: 10.4049/jimmunol.0900138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sha Y, Zmijewski J, Xu Z, Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol. 2008;180:2531–2537. doi: 10.4049/jimmunol.180.4.2531. [DOI] [PubMed] [Google Scholar]
- 45.Dowling JK, O'Neill LA. Biochemical regulation of the inflammasome. Critical reviews in biochemistry and molecular biology. 2012;47:424–443. doi: 10.3109/10409238.2012.694844. [DOI] [PubMed] [Google Scholar]
- 46.Chen F, Yu Y, Qian J, Wang Y, Cheng B, Dimitropoulou C, et al. Opposing actions of heat shock protein 90 and 70 regulate nicotinamide adenine dinucleotide phosphate oxidase stability and reactive oxygen species production. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:2989–2999. doi: 10.1161/ATVBAHA.112.300361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen F, Pandey D, Chadli A, Catravas JD, Chen T, Fulton DJ. Hsp90 regulates NADPH oxidase activity and is necessary for superoxide but not hydrogen peroxide production. Antioxidants & redox signaling. 2011;14:2107–2119. doi: 10.1089/ars.2010.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh S, Khar A. Biological effects of curcumin and its role in cancer chemoprevention and therapy. Anti-cancer agents in medicinal chemistry. 2006;6:259–270. doi: 10.2174/187152006776930918. [DOI] [PubMed] [Google Scholar]
- 49.Carbone M, Yang H. Molecular pathways: targeting mechanisms of asbestos and erionite carcinogenesis in mesothelioma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18:598–604. doi: 10.1158/1078-0432.CCR-11-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stehlik C, Fiorentino L, Dorfleutner A, Bruey JM, Ariza EM, Sagara J, et al. The PAAD/PYRIN-family protein ASC is a dual regulator of a conserved step in nuclear factor kappaB activation pathways. The Journal of experimental medicine. 2002;196:1605–1615. doi: 10.1084/jem.20021552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.