Abstract
Objective
This pilot study assesses the degree of hemolysis induced by cardiopulmonary bypass (CPB) and determines its association with acute kidney injury (AKI) in pediatric patients. Further, it evaluates the degree to which the use of urinary biomarkers neutrophil gelatinase-associated lipocalin (NGAL) and cystatin C correlate with the presence of AKI and hemolysis following CPB.
Design
Prospective observational study
Setting
A 13-bed pediatric cardiac intensive care unit in a university hospital
Patients
Children undergoing cardiac surgery with the use of CPB
Interventions
None
Measurements and Main Results
Blood and urine samples were obtained at multiple time points before and after CPB. Hemolysis was assessed by measuring levels of plasma hemoglobin and haptoglobin. AKI was defined as a doubling in serum creatinine from preoperative baseline and by using the pediatric-modified RIFLE criteria. Urinary NGAL and Cystatin C levels were measured. A total of 40 patients (range: 3 days to 4.8 years) were enrolled. Plasma hemoglobin levels increased markedly on separation from CPB with a concurrent decrease in haptoglobin. This was associated with an increase in protein oxidation in the plasma. Hemolysis was more evident in younger patients with a longer duration of bypass and in those requiring a blood-primed circuit. 40% of patients had a doubling in serum creatinine and 88% of patients developed acute kidney injury when defined by the pediatric-modified RIFLE criteria. Controlling for CPB time, persistently elevated levels of plasma hemoglobin were associated with a 5 fold increase in AKI. Further, urinary NGAL measured 2 hours after separation from CPB was associated with AKI and with elevations in plasma hemoglobin.
Conclusions
CPB in pediatric patients results in significant hemolysis, which is associated with the development of AKI. The biomarker NGAL correlates with both AKI and hemolysis in this population.
Keywords: cardiopulmonary bypass, hemolysis, neutrophil gelatinase-associated lipocalin, cystatin C, acute kidney injury, pediatrics
Introduction
Cardiopulmonary bypass (CPB) is required to facilitate surgical correction or palliation of congenital heart defects. However, it can lead to significant inflammation and oxidant stress that contribute to multi-organ dysfunction. In adults, the presence of acute kidney injury (AKI) following CPB greatly elevates the risk of mortality [1]. Similarly, the 30 to 40% of children that develop AKI following CPB will demonstrate significantly prolonged mechanical ventilation and of length of intensive care and hospital stay [2, 3].
The pathophysiology of AKI following congenital heart surgery is complex and multifactorial. Contributing intraoperative factors may include low perfusion pressure with non-pulsatile blood flow [3–5] and reperfusion injury upon separation from bypass [5]. The risk of developing postoperative AKI may be further increased by the presence of hemolysis, a known but often overlooked sequela of CPB that has the potential to generate oxidant stress and cause renal tubular injury.
Cardiopulmonary bypass-induced hemolysis, caused in part by exposure of blood to a non-endothelialized circuit and to mechanical shear stress, traditionally has been thought to be minimal and inconsequential in adults. However, increasing evidence suggests that its impact on oxidant generation and microcirculatory blood flow may exacerbate kidney injury following cardiac surgery [6]. The degree of hemolysis and its effects are likely to be further magnified in the infant population secondary to the greater disparity between an infant’s circulating blood volume and the volume of the CPB circuit that requires that the CPB circuit be primed with stored blood. Stored blood is more prone to hemolysis, particularly with increased storage age [4, 7–10].
Clinical diagnosis of AKI traditionally relies on elevation of serum creatinine. However, serum creatinine is a relatively insensitive, nonspecific, and delayed marker of acute injury. To address these issues, several biomarkers recently have been shown to predict the development of AKI within hours of injury [11]. Among these are neutrophil gelatinase-associated lipocalin (NGAL) and cystatin C, both of which can be detected in the urine after inflammation and ischemia. These markers have shown utility in identifying adults and children who go on to develop AKI following CPB [12–14].
Our primary objectives were to assess the degree of hemolysis induced by CPB and to determine the association of CPB-induced hemolysis and AKI in pediatric patients with congenital heart disease following surgical repair or palliation. We hypothesized that patients that are younger, require a blood-primed CPB circuit, and have a longer CPB time would experience more hemolysis and that this would be associated with a greater incidence of AKI. Our secondary objectives were to assess the degree to which urinary NGAL and cystatin C provide earlier diagnosis of AKI following CPB and to determine whether changes in these biomarkers correlate with hemolysis.
Methods
Patients and Study Design
The study protocol was approved by the Institutional Review Board at Duke University Medical Center. Children weighing < 20 kg and scheduled for cardiac surgery with CPB were eligible for enrollment. This weight limit was chosen because patients < 20 kg are managed using a roller head arterial pump while those > 20 kg are typically maintained on a centrifugal pump. Patients were excluded only if consent could not be obtained. Subjects were prospectively enrolled from January through November 2011. Some eligible patients undergoing surgery during this time point were not approached due to logistical reasons (e.g. study personnel not available to obtain consent or for sample collection). Written informed consent from a parent or legal guardian was obtained prior to surgery.
All patients were intubated for surgery and had arterial lines, central venous lines, and Foley catheters placed. The type of oxygenator used was determined by patient weight according to institutional practice (Sorin D100 or Maquet Neo-I for patients weighing <5kg, Sorin D101 or Maquet Neo-I for patients weighing 5–10 kg, and Sorin D101 or Maquet Pedi-I for patients weighing 10–20 kg). The CPB pump was cycled at an indexed output of approximately 3.0 LPM. All CPB circuits were primed with sodium bicarbonate, heparin, and methylprednisolone. Packed red blood cells (pRBCs, approximately 300 mL) and fresh frozen plasma (FFP, approximately 250–300 mL) were included in the prime for all patients weighing ≤ 10 kg and for those patients who were anticipated to have complex repairs with a prolonged duration of CPB. Additional pRBCs, platelets, FFP and/or cryoprecipitate were given at the discretion of the surgeon and anesthesiologist. All patients were managed during CPB with unfractionated heparin adjusted according to activated clotting time. Heparin was reversed with protamine at the end of CPB after approximately 15 minutes of modified ultrafiltration.
Demographic information collected on each patient included congenital heart lesion, presence of cyanosis, presence of genetic and other anomalies, and complexity of surgery as categorized according to Aristotle Basic Score [15, 16]. Additional surgical and clinical data including surgical repair, duration of CPB, aortic cross-clamping, presence and duration of hypothermia the use of circulatory arrest, and the age of PRBCs used to prime the CPB pump (when applicable) were recorded during surgical hand-off in the Pediatric Cardiac Intensive Care Unit and verified against the anesthesia and perfusion records from each case. Blood and urine samples were collected from each patient from indwelling arterial lines and Foley catheters or urine collection bag at a maximum of 7 time points: pre-cardiopulmonary bypass, on immediate separation from CPB after modified ultrafiltration, at 2, 6, 24, 48, and 72 hours post separation from CPB as described below. Blood samples (2mL per time point) were collected in a syringe containing dry lithium heparin and centrifuged at 1000 × g for 10 minutes. Plasma was immediately aliquoted, frozen on dry ice, and stored at −80°C until time of analysis. Urine samples were aliquoted and frozen (−80°C) immediately after collection. Blood and urine collection for study purposes automatically ended when the patient’s arterial line was removed. Laboratory data measured routinely pre- and post-operatively, including serum BUN and creatinine, were recorded at time points most closely coinciding with sample collection. Samples were collected from all patients from baseline through 6 hours (n=40). Additional samples were collected at 24 hours (n=39), 48 hours (n=27), and 72 hours (n=18) depending on the duration of arterial access. Serum creatinine was measured in 100% of patients through 24 hours, in 95% at 48 hours and in 85% at 72 hours.
Hemolysis
Hemoglobin was measured in plasma samples using the Quantichrom Hemoglobin Assay kit (BioAssay Systems, Hayward, CA). Based on the Triton/NaOH method, a calibrator was added to the samples and the resulting color was determined by spectrophotometric plate reader (BioTek Instruments, Winooski, VT).
Human Haptoglobin in plasma was determined by a quantitative, competitive enzyme immunoassay ELISA (Assaypro, St. Charles, MO) according to the manufacturer’s instructions. Color development was measured by spectrophotometer.
Protein Oxidation
Protein oxidation in the plasma was determined by Oxyblot detection kit (EMD-Millipore, Billerica, MA). Protein samples were derivatized with DNP-hydrazone and transferred to PVDF membrane by slot blot. Membranes were incubated with a primary antibody specific to DNP, followed by incubation with a secondary goat anti-rabbit IgG HRP-conjugate and detection by ECL chemiluminescent reagent (GE Healthcare, Pittsburgh, PA). Protein concentration was determined by DC™ colorimetric assay (BioRad, Hercules, CA) using a bovine serum albumin standard in a microplate format. Results were standardized to protein concentration and displayed as fold change from baseline for each patient.
Acute Kidney Injury
AKI was defined as a doubling of the preoperative serum creatinine at any point during the study period. This degree of increase was chosen as a more clinically meaningful demonstration of AKI given the low baseline level of serum creatinine in infants and young children. AKI was also defined and classified using the pediatric-modified RIFLE criteria (pRIFLE), which stratify AKI into 3 categories (“Risk,” “Injury,” and “Failure”) according to severity based on decreases in estimated creatinine clearance (eCCl) from preoperative baseline [17]. “Risk” corresponds to an eCCl decrease of 25 – 49%, “Injury” to a decrease of 50 – 74%, and “Failure” to a decrease of ≥ 75% or an absolute eCCl <35mL/min/1.73m2. The Schwartz formula was used to calculate eCCl [18, 19]. The majority of neonatal patients had a baseline or subsequent eCCl close to or below 35mL/min/1.73 m2. However, as these values were within normal limits for age, neonates were given a diagnosis of “Failure” only if eCCl decreased by at least 75% from preoperative baseline and not based on absolute eCCl.
Human NGAL (Lipocalin-2) in urine samples was quantitatively determined by ELISA sandwich enzyme immunoassay (Abnova, Taipei City, Taiwan). The assay was performed per the manufacturer’s protocol for NGAL standards, quality controls, and diluted patient samples. Product absorption was measured by spectrophotometry. Patient samples were assayed in triplicate. All patient samples were initially assayed at a 10x dilution. Samples that did not fit the standard curve were repeated at higher or lower dilutions as needed.
Human Cystatin C in urine was determined by ELISA immunoassay (R&D Technologies, North Kingston, RI). The assay was performed per the manufacturer’s protocol with cystatin C standards, controls, and diluted patient samples incubated in microtiter wells pre-coated with monoclonal cystatin C antibody. The resulting signal was determined by spectrophotometry. Patient samples were assayed in triplicate. All patient samples were initially assayed at a 5x dilution. Samples that did not fit the standard curve were repeated at higher or lower dilutions as needed.
Statistical Analysis
Descriptive statistics for continuous variables are presented as means with standard deviations, while categorical variables are expressed as frequency and proportion. To assess change over time, we used one-way analysis of variance (ANOVAs). Two-way analysis of variance was used to compare continuous variables over time between age groups and between groups with and without AKI. Paired t-tests were performed if the ANOVA was found to be significant to assess the change from baseline at each time point. No correction was made for multiple comparisons. AKI was defined by a doubling in serum creatinine from preoperative baseline or a pRIFLE of “Injury” or greater, which indicates a decrease in eCCl by at least 50%. We used univariable regression to assess predictors of hemolysis as defined by changes in plasma hemoglobin levels. Potential predictors included duration of CPB, complexity of repair, age, presence of a cyanotic heart lesion, presence and duration of aortic cross clamping, and a blood primed CPB pump. Univariable and multivariable logistic regression were used to assess predictors of renal injury following CPB as measured by a doubling in serum creatinine or a pRIFLE classification of “Injury” or greater. Triplicate values for the NGAL and cystatin C ELISA analysis were used to calculated a mean value for each sample and the concentration determined by a four-parameter linear curve-fit to standard concentrations and corresponding mean at 450nm absorbance with final correction by appropriate dilution factor. Differences were considered significant at p<0.05. We were powered at 80% to detect a difference in plasma hemoglobin of 31 mg/dL between subjects with and without AKI as measured by a doubling in serum creatinine given an estimated SD = 35 in plasma hemoglobin levels with α= 0.05.
Results
Of the 40 patients enrolled, 10 were neonates (≤ 28 days, 6.7 +/−6.5 days), 15 were infants (1 – 12 months, 4.1 +/− 1.4 months), and 15 were greater than one year of age (37.1 +/− 12.8 months) (Table 1). All neonates were > 36 weeks gestation and had no structural renal abnormalities detected on routine preoperative ultrasound screening. The average complexity score of the surgical procedures according to the Aristotle Basic Score was 7.7 +/− 2.6. The mean duration of CPB was 151 +/− 89.2 minutes. The average CPB duration for neonates was significantly higher than that for non-neonates (235.5 +/−96.5 versus 122.4 +/−67.0, p<0.01). Seventy-five percent of patients received a blood primed pump to maintain a hematocrit of approximately 25 – 30% while on CPB. Based on the patients’ weight and an estimated blood volume of 85 mL/kg, this amounted to a volume of approximately 86 ± 35.9% of the patients’ blood volume (112.2 +/− 16.6% for neonates and 69.3 +/− 34.5% for non-neonates, p<0.01). The average duration of storage for PRBCs used to prime the CPB pump was 13.0 +/− 4.7 days. The duration of storage for neonates was not statistically different from that of non-neonates (12.44 +/− 3.84 days versus 13.28 +/− 5.19 days). None of the patients enrolled had a known pre-operative diagnosis of chronic renal failure or hemolytic disease. In-hospital mortality was 2.5% (n=1).
Table 1. Demographic and clinical characteristics.
Continuous variables are reported as mean (±SD). Categorical variables are reported as n (%). “Other” surgical repairs consists of right ventricle to pulmonary artery conduit replacement, partial anomalous pulmonary vein repair, and pulmonary artery reconstruction, (n=1 for each).
| n=40 | |
|---|---|
| Age (months) | 15.5 (±18.7) |
| Male gender (%) | 17 (43%) |
| Weight (kg) | 7.5 (±4.8) |
| Cyanotic (%) | 19 (48%) |
| Blood prime (%) | 30 (75%) |
| Surgical procedures: | |
| Atrial/ventricular septal defect repair | 8 (20%) |
| Atrioventricular septal defect repair | 4 (10%) |
| Arterial switch | 4 (10%) |
| Norwood | 2 (5%) |
| Bidirectional Glenn/Fontan | 11 (27.5%) |
| Tetralogy of Fallot repair | 4 (10%) |
| Left ventricular outflow/aortic repair | 4 (10%) |
| Other | 3 (7.5%) |
| Cardiopulmonary bypass time (minutes) | 150.7 (±89.2) |
| CPB blood flow indexed | 2.63 (±0.36) |
| Aortic cross clamp (%) | 31 (78%) |
| Aortic cross clamp time (minutes) | 81.2 (±47.5) |
| Duration of modified ultrafiltration (minutes) | 14.6 (±4.85) |
| Aristotle basic score | 7.7 (±2.6) |
| Duration of mechanical ventilation (hours) | 108 (±188) |
| Length of ICU stay (days) | 13.13 (±17.21) |
| Length of hospital stay (days) | 21.25 (±22.89) |
Hemolysis occurs following CPB
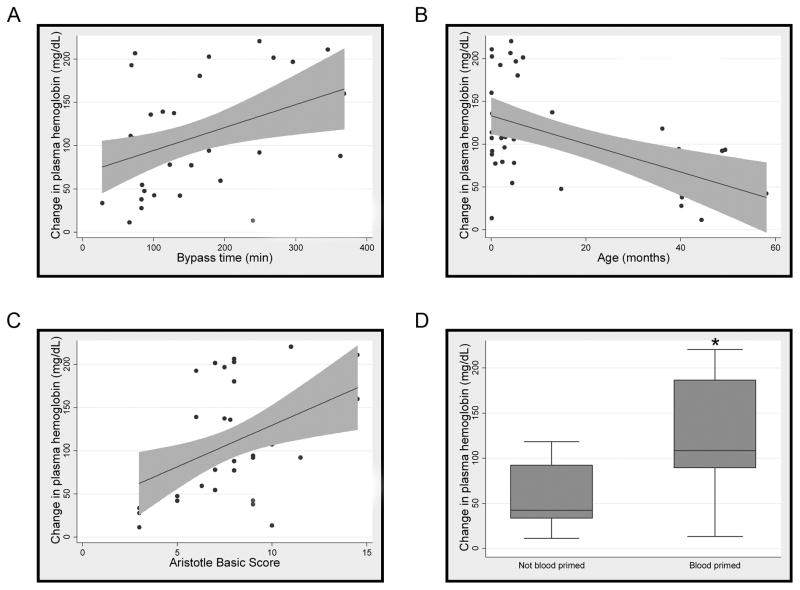
Plasma hemoglobin levels increased markedly from a baseline of 51.0 mg/dL (+/− 56.86) to a peak of 146.2 mg/dL (+/− 81.38) immediately after separation from CPB with a return to baseline over 24 hours (Figure 1A). Concurrent with the increase in hemoglobin, there was significant consumption of the hemoglobin binding protein, haptoglobin, with a maximal decrease to < 50% of its baseline 6 hours following separation from CPB with a return to baseline after 24 to 48 hours (Figure 1B). To determine the relationship between hemolysis and clinical characteristics, we performed correlation analysis and found four distinct risk factors associated with hemolysis. Longer duration of CPB, higher complexity of surgical repair, a blood-primed CPB circuit, and younger age at time of surgery all correlated with greater peak changes in plasma hemoglobin (Figure 2). There was no correlation between hemoglobin levels and either the presence of cyanosis or the use or duration of aortic cross-clamp. In addition, there was no increase in the amount of plasma hemoglobin in those patients whose CPB pump was primed with PRBCs stored for less than 14 days when compare to those stored 14 days or longer (data not shown).
Figure 1. Cardiopulmonary bypass is associated with hemolysis.
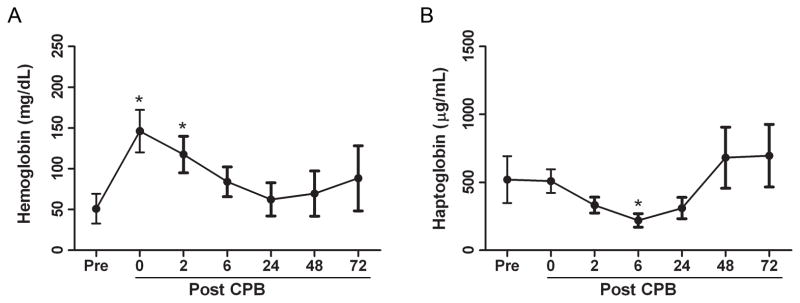
Plasma obtained from study patients immediately pre- and 0, 2, 6, 24, 48, and 72 hours post-cardiopulmonary bypass was analyzed for plasma hemoglobin (A) and haptoglobin (B). Groups were compared by one-way ANOVA. Paired t-tests were performed if the ANOVA was found to be significant to assess the change from baseline at each time point. Data are expressed as means with 95% confidence intervals, *p<0.001versus pre-cardiopulmonary bypass values.
Figure 2. Hemolysis is associated with duration of cardiopulmonary bypass time, patient age, complexity of repair, and a blood-primed circuit.
Association between peak change in plasma free hemoglobin following cardiopulmonary bypass and cardiopulmonary bypass time (minutes) (R2 = 0.16) (A), age (months) (R2 = 0.27) (B), surgical complexity as defined by Aristotle basic score (R2 = 0.18) (C), and blood-primed cardiopulmonary bypass pump (D) were assessed by linear regression, p≤0.01 for all variables.
Protein oxidation occurs following CPB
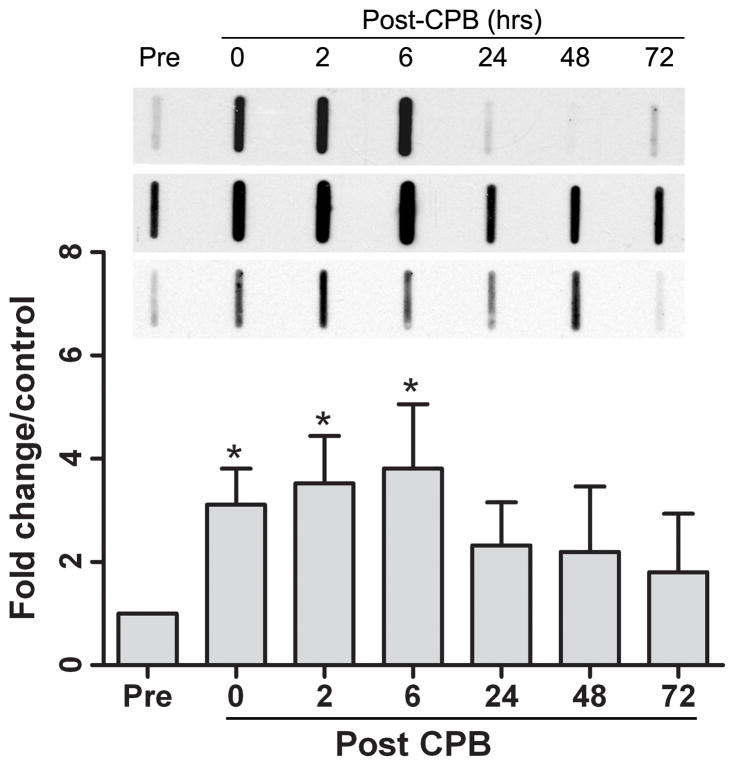
Acute elevation in plasma free hemoglobin levels can rapidly overwhelm antioxidant defenses and induce oxidant injury [20]. Therefore, given the significant increase in plasma hemoglobin following CPB, we next assessed protein oxidation, as manifested by increased carbonyl formation in plasma following CPB. We found a significant increase in protein oxidation on separation from bypass with a continued increase through 6 hours post-CPB (Figure 3). This timing coincided with both peak plasma hemoglobin levels and maximum consumption of haptoglobin.
Figure 3. Protein oxidation increases immediately following cardiopulmonary bypass.
Carbonyl content in plasma was measured immediately pre- and at 0, 2, 6, 24, 48, and 72 hours post-cardiopulmonary bypass to assess protein oxidation. Data are expressed as means with 95% confidence interval. Three representative blots are shown.
CPB-induced hemolysis is associated with increased AKI
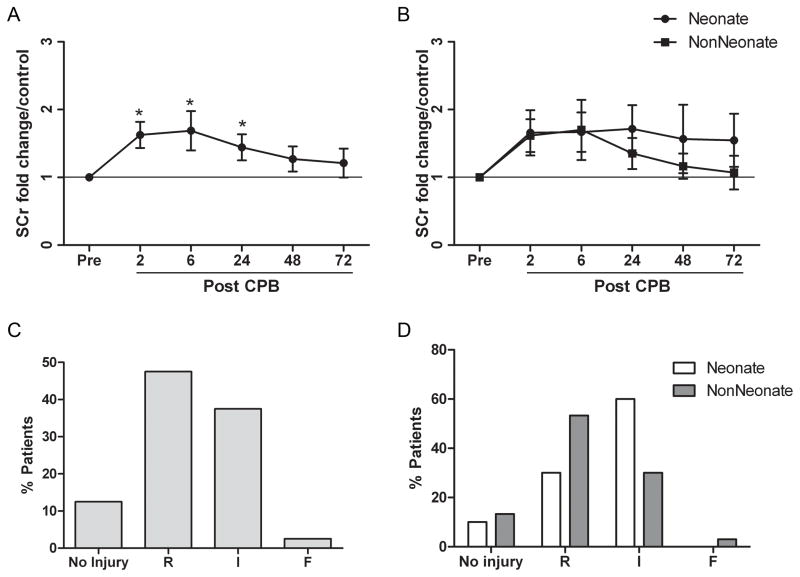
We found that 40% of our patients developed AKI following CPB as defined by a doubling in baseline serum creatinine and that this injury was evident as early as 2 hours after separation from CPB (Figure 4A). Using the pRIFLE criteria, 87.5% demonstrated some evidence of AKI with 47.5% of patients in the “Risk” category, 37.5% in the “Injury” category, and 2.5% in the “Failure” category (Figure 4B). ECCl did not fall below 35 ml/min/1.73m2 in the any of the non-neonatal patients. Changes in serum creatinine in infants and children returned to baseline over 24 to 72 hours following CPB while neonates had more variability in the duration of AKI (Figure 4C). Injury as measured by the pRIFLE criteria showed a tendency toward greater severity in the neonates, with 60% of neonates demonstrating a decrease in eCCl of 50% or greater while only 33% of infants and children showed a similar level of injury (Figure 4D).
Figure 4. Acute kidney injury is associated with the use of cardiopulmonary bypass.
Acute kidney injury, measured as fold change in baseline serum creatinine is shown for all patients (A) and for patients stratified by age (B). Data are expressed as means with 95% confidence interval, *p<0.05. Percentage of patients after cardiopulmonary bypass in each category of pRIFLE score at maximal level of injury during the study period is shown for all patients (C) and for patients stratified by age (D).
To determine the relative impact of a number of clinical and CPB factors on the subsequent development of AKI, we performed additional correlation analysis and found that both change in plasma hemoglobin and duration of CPB were significantly associated with AKI (p<0.05). Aortic cross clamp time and the presence of a blood primed circuit showed a trend toward an effect with p<0.1. Age, complexity of surgery score, use and degree of hypothermia, and duration of modified ultrafiltration showed no significant effect.
To determine the association between hemolysis and the development of AKI following CPB, we performed multivariable analyses that controlled for the duration of CPB time alone. Those patients with an elevated hemoglobin level ≥ 100 mg/dL that persisted for 2 hours after CPB demonstrated a greater than 5-fold increased risk of doubling serum creatinine (p = 0.05) or a pRIFLE criteria of “Injury” or greater independent of CPB time (p=0.03) (Table 2).
Table 2. Hemolysis is associated with an increased risk of acute kidney injury after controlling for duration of cardiopulmonary bypass.
Multivariate logistic regression was used to assess the degree to which plasma hemoglobin ≥ 100 mg/dL 2 hours after separation from CPB correlated with AKI as defined by a doubling in serum creatinine or a pRIFLE of “Injury” or greater when corrected for duration of CPB.
| Odds Ratio Adjusted for CPB time | 95% Confidence Interval | p-value | |
|---|---|---|---|
| Plasma free hemoglobin ≥ 100 mg/dl 2 hours after separation from CPB | |||
| Doubling of SCr - | 5.05 | 1.0 – 25.7 | 0.05 |
| pRIFLE “Injury” or greater | 5.95 | 1.2 – 30.2 | 0.03 |
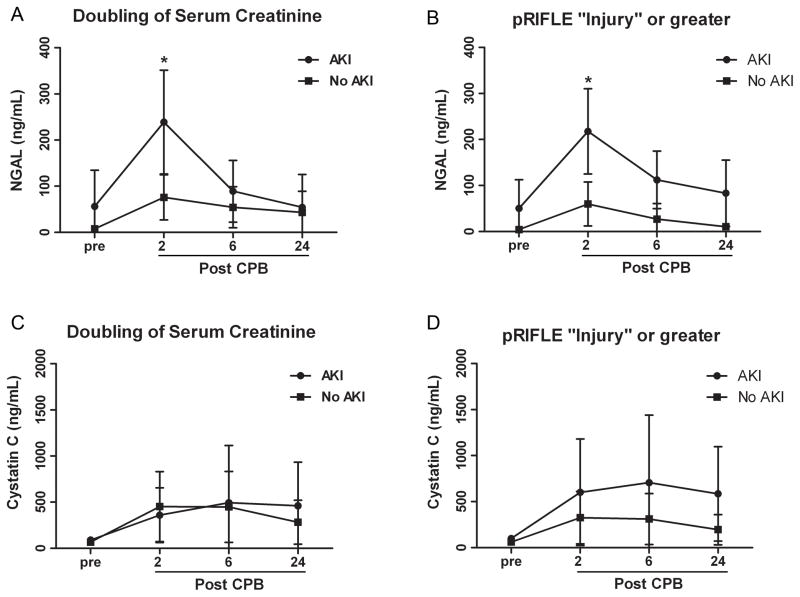
To determine the utility of urinary biomarkers to assess for CPB-induced AKI in the presence of elevated levels of plasma hemoglobin, we next measured urinary NGAL at multiple time points over the first 24 hours after CPB. NGAL levels peaked 2 hours after separation from CPB and were significantly correlated with the development of AKI as measured by either a doubling of serum creatinine or by pRIFLE category of “Injury” or greater (Figure 5A, B). Further, we found that after controlling for CPB time, the maximal change in hemoglobin predicted an increase in NGAL measured 2 hours after separation from CPB with an increase of 1.03 ng/mL for each mg/dL of plasma hemoglobin (95% CI of 0.08, 1.98; p=0.03).
Figure 5. Urinary neutrophil gelatinase-associated lipocalin is associated with cardiopulmonary bypass-induced acute kidney injury.
Urinary neutrophil gelatinase-associated lipocalin (A, B) and cystatin C (C, D) levels were measured pre- and at 2, 6, and 24 hours post-cardiopulmonary bypass and stratified by the presence or absence of acute kidney injury as diagnosed by doubling in serum creatinine and pRIFLE criteria of “Injury” or greater. Data are expressed as means with 95% confidence interval, *p<0.001.
NGAL was low in all patients prior to CPB (27.23 ± 84.86 ng/mL) with the exception of two outliers in the group that subsequently developed AKI. These two infants each had a significant illness in the weeks prior to surgery; however, they had normal baseline serum creatinine prior to CPB. Both infants subsequently developed a doubling in serum creatinine. Cystatin C was also measured. We found that cystatin C increased somewhat though not significantly after separation from CPB. However, there was considerable variability at each of the time points studied (Fig. 5C, D).
Discussion
This study demonstrates that CPB in pediatric patients with congenital heart disease is associated with significant hemolysis and the development of AKI. The presence of CPB-induced hemolysis is supported by markedly increased plasma hemoglobin levels and significantly decreased haptoglobin levels on separation from bypass. We also demonstrate that neonates and infants are likely to be at more substantial risk from CPB-induced hemolysis given its greater severity in younger patients who had a longer duration of CPB, required a blood-primed circuit, and had a more complex surgical repair. This study also establishes an association between the high incidence of CPB-induced AKI and the presence of hemolysis, with five times greater odds of developing renal injury with plasma hemoglobin levels ≥ 100 mg/dl as was seen in close to half of our patients. Finally, the association between elevated levels NGAL and AKI as well as the correlation between NGAL levels 2 hours after separation from CPB and plasma hemoglobin levels, suggest that this urinary biomarker may provide a useful measure of renal injury in the presence of CPB-associated hemolysis.
The incidence and severity of organ dysfunction following CPB in children is associated with increased inflammation [21–23]. While the mechanisms behind this exaggerated response to CPB are not fully understood, the development of CPB-associated systemic inflammatory response in children is known to be preceded by oxidant generation [24]. One potential cause of CPB-induced oxidant generation is the liberation of free hemoglobin associated with hemolysis, which can rapidly saturate the protective effects of the endogenous hemoglobin ligand haptoglobin [20]. Liberation of hemoglobin in our study was associated with both haptoglobin consumption and protein oxidation. Unbound plasma hemoglobin and its breakdown products, heme and iron, can readily participate in the Fenton reaction to amplify oxidant-mediated cytotoxicity, activate pro-inflammatory pathways, and initiate apoptosis [25–28]. Reactive oxygen species generated by excess heme may activate the oxidant-mediated transcription factor, NFκB and induce production of proinflammatory cytokines. In addition, unbound hemoglobin can act as a nitric oxide scavenger to decrease the bioavailability of nitric oxide and impair tissue perfusion [25, 27, 29].
Serum creatinine, while the traditional measure of AKI, is a fairly late and insensitive marker, rising to an abnormal level only after the glomerular filtration rate has declined by at least 50% and has reached a steady state following injury [5, 11]. A somewhat surprising finding in our study was the time course of elevation in serum creatinine. Typically, peak serum creatinine levels in children following CPB are not evident until 24–48 hours following surgery [12, 13, 30]. Our patients experienced a significant increase in serum creatinine as early as 2 hours following completion of CPB, with peak serum creatinine levels occurring at 6 hours after CPB. This discrepancy between our results and those of earlier studies may be due to a variety of factors including a greater number of neonates in our study.
A number of recent studies support the use of biomarkers for a more sensitive measure of AKI in children undergoing CPB as a means to provide more timely protection and intervention [31, 32]. Consistent with these studies, elevated urinary NGAL 2 hours after separation from CPB in our study strongly correlated to the development of AKI measured either by a doubling in serum creatinine or by pRIFLE criteria. In addition to the expected correlation with other measures of renal injury, urinary NGAL significantly correlated with elevated levels of plasma hemoglobin, independent of CPB time. Further investigation is necessary to determine if this association is a direct effect of unbound plasma hemoglobin or its metabolites or whether it represents a specific renal insult. We were unable to show an association between cystatin C and AKI in infants and children following CPB, likely due to the considerable variability we found in a small and heterogeneous population. More work needs to be done to firmly establish the meaning and utility of the numerous individual biomarkers identified to date [32].
None of our patients required renal replacement therapy and the majority had recovery of renal function during the study period. This is consistent with a previous study demonstrating that CPB associated AKI frequently resolves within 5 days following surgery [3]. However, despite this resolution, numerous studies in both adults and children have shown that small changes in serum creatinine following cardiothoracic surgery are predictive of prolonged length of ventilation, length of stay, and increased 30-day mortality, independent of the underlying severity of cardiac disease and duration of CPB [3, 33, 34]. Further, there is a growing body of evidence to suggest that even mild episodes of AKI in patients without preexisting renal dysfunction increase a patient’s risk for eventual progression to chronic kidney disease (CKD) and that these patients may be at greater risk of injury with subsequent insults [35]. Very little is truly known at this time about the transition from AKI to CKD in pediatrics, particularly in the face of multiple or additive insults. The use of NGAL may offer more sensitive and timely identification of injury to provide a means to minimize additive insults and investigate potential therapies [36]. This would be particularly useful in those patients expected to endure additional renal insults, such as those CHD patients who require staged repairs with multiple episodes of CPB.
A surprising finding of our study is that we were unable to detect a difference in the degree of hemolysis that occurred in patients whose CPB pumps were primed with blood that was < 14 days versus those primed with blood stored for 14 days or more. Previous data support that stored blood lyses and releases free hemoglobin and iron over time [8, 37]. It is likely that our results differ due to our small sample size as well as the variability in our patient population. Additionally, we may have seen a greater effect if we focused on blood that had been stored for less than 7 days, However, there were too few patients in our group (n=2) to assess this.
There were several limitations to our study. This study was not designed to show a direct link between CPB-induced hemolysis and AKI. Further, our patient population was fairly small and heterogeneous. While this did not allow us to elucidate which aspects of CPB were most responsible for hemolysis in our patients (e.g. cardiotomy suction use, infusion of cell saver products, etc.) or the impact of transfusion in the post-operative period, we were able to demonstrate consistent hemolysis at a range of ages and cardiac lesions. Such pilot data provide a compelling rationale to further investigate the mechanisms by which hemolysis may exacerbate AKI following CPB.
Conclusion
Significant hemolysis occurs during CPB and is associated with the development of postoperative AKI in neonates, infants, and children undergoing congenital heart surgery. Decreasing CPB-induced hemolysis or attenuating the effects of CPB-induced hemolysis by augmenting endogenous mechanisms that exist to scavenge and remove free hemoglobin may provide a means to more rapidly clear excess plasma free hemoglobin, decrease oxidant injury, and minimize the toxic effects of acute hemolysis. The effects of these therapies may be best assessed by biomarkers as opposed to serum creatinine.
Acknowledgments
This research was funded by the Children’s Miracle Network (LSM). Dr. Smith received salary support for research from the NIH and the U.S. Department of Health and Human Services (NICHD 1K23HD060040-01 and DHHS-1R18AE000028-01).
We wish to thank Christine Hiller PNP, Bronwyn Bartle PNP, and Coleen Miller PNP for their assistance with patient enrollment and consent acquisition, as well as the pediatric cardiac anesthesiologists and the intensive care nurses for their assistance with sample collection. We also thank the CPB perfusionists and Drs. Rasheed Gbadegesin, Andrew Ghio, and George Ofori-Amanfo for their helpful contributions to the completion of this study.
Footnotes
For reprints or information regarding this article: jennifer.turi@duke.edu
No reprints will be ordered.
References
- 1.Thakar CV, Worley S, Arrigain S, Yared JP, Paganini EP. Influence of renal dysfunction on mortality after cardiac surgery: modifying effect of preoperative renal function. Kidney Int. 2005;67(3):1112–1119. doi: 10.1111/j.1523-1755.2005.00177.x. [DOI] [PubMed] [Google Scholar]
- 2.Li S, Krawczeski CD, Zappitelli M, Devarajan P, Thiessen-Philbrook H, Coca SG, Kim RW, Parikh CR. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Crit Care Med. 2011;39(6):1493–1499. doi: 10.1097/CCM.0b013e31821201d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zappitelli M, Bernier PL, Saczkowski RS, Tchervenkov CI, Gottesman R, Dancea A, Hyder A, Alkandari O. A small post-operative rise in serum creatinine predicts acute kidney injury in children undergoing cardiac surgery. Kidney Int. 2009;76(8):885–892. doi: 10.1038/ki.2009.270. [DOI] [PubMed] [Google Scholar]
- 4.Kozik DJ, Tweddell JS. Characterizing the inflammatory response to cardiopulmonary bypass in children. Ann Thorac Surg. 2006;81(6):S2347–2354. doi: 10.1016/j.athoracsur.2006.02.073. [DOI] [PubMed] [Google Scholar]
- 5.Cruz DN, Ronco C, Katz N. Neutrophil gelatinase-associated lipocalin: a promising biomarker for detecting cardiac surgery-associated acute kidney injury. J Thorac Cardiovasc Surg. 2010;139(5):1101–1106. doi: 10.1016/j.jtcvs.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Vermeulen Windsant IC, Hanssen SJ, Buurman WA, Jacobs MJ. Cardiovascular surgery and organ damage: time to reconsider the role of hemolysis. J Thorac Cardiovasc Surg. 2011;142(1):1–11. doi: 10.1016/j.jtcvs.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Jones TJ, Elliott MJ. Paediatric CPB: bypass in a high risk group. Perfusion. 2006;21(4):229–233. doi: 10.1191/0267659106pf873oa. [DOI] [PubMed] [Google Scholar]
- 8.Marwah SS, Blann A, Harrison P, Lumley MA, Wright J, McDowell J, Phillips JD, Rea C, Bareford D. Increased non-transferrin bound iron in plasma-depleted SAG-M red blood cell units. Vox Sang. 2002;82(3):122–126. doi: 10.1046/j.1423-0410.2002.00153.x. [DOI] [PubMed] [Google Scholar]
- 9.Mumby S, Chaturvedi RR, Brierley J, Lincoln C, Petros A, Redington AN, Gutteridge JM. Iron overload in paediatrics undergoing cardiopulmonary bypass. Biochim Biophys Acta. 2000;1500(3):342–348. doi: 10.1016/s0925-4439(00)00003-x. [DOI] [PubMed] [Google Scholar]
- 10.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM, et al. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci U S A. 2007;104(43):17063–17068. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore E, Bellomo R, Nichol A. Biomarkers of acute kidney injury in anesthesia, intensive care and major surgery: from the bench to clinical research to clinical practice. Minerva Anestesiol. 2010;76(6):425–440. [PubMed] [Google Scholar]
- 12.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 13.Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, Syed H, Ali S, Barasch J, Devarajan P. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3(3):665–673. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villa P, Jimenez M, Soriano MC, Manzanares J, Casasnovas P. Serum cystatin C concentration as a marker of acute renal dysfunction in critically ill patients. Crit Care. 2005;9(2):R139–143. doi: 10.1186/cc3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacour-Gayet F, Clarke D, Jacobs J, Gaynor W, Hamilton L, Jacobs M, Maruszewski B, Pozzi M, Spray T, Tchervenkov C, et al. The Aristotle score for congenital heart surgery. Seminars in thoracic and cardiovascular surgery Pediatric cardiac surgery annual. 2004;7:185–191. doi: 10.1053/j.pcsu.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien SM, Clarke DR, Jacobs JP, Jacobs ML, Lacour-Gayet FG, Pizarro C, Welke KF, Maruszewski B, Tobota Z, Miller WJ, et al. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg. 2009;138(5):1139–1153. doi: 10.1016/j.jtcvs.2009.03.071. [DOI] [PubMed] [Google Scholar]
- 17.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71(10):1028–1035. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58(2):259–263. [PubMed] [Google Scholar]
- 19.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34(3):571–590. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol Lett. 2005;157(3):175–188. doi: 10.1016/j.toxlet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Carmona F, Manso PH, Vicente WV, Castro M, Carlotti AP. Risk stratification in neonates and infants submitted to cardiac surgery with cardiopulmonary bypass: a multimarker approach combining inflammatory mediators, N-terminal pro-B-type natriuretic peptide and troponin I. Cytokine. 2008;42(3):317–324. doi: 10.1016/j.cyto.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Liu KD, Altmann C, Smits G, Krawczeski CD, Edelstein CL, Devarajan P, Faubel S. Serum Interleukin-6 and interleukin-8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: a case-control study. Crit Care. 2009;13(4):R104. doi: 10.1186/cc7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hovels-Gurich HH, Schumacher K, Vazquez-Jimenez JF, Qing M, Huffmeier U, Buding B, Messmer BJ, von Bernuth G, Seghaye MC. Cytokine balance in infants undergoing cardiac operation. Ann Thorac Surg. 2002;73(2):601–608. doi: 10.1016/s0003-4975(01)03391-4. discussion 608–609. [DOI] [PubMed] [Google Scholar]
- 24.Christen S, Finckh B, Lykkesfeldt J, Gessler P, Frese-Schaper M, Nielsen P, Schmid ER, Schmitt B. Oxidative stress precedes peak systemic inflammatory response in pediatric patients undergoing cardiopulmonary bypass operation. Free Radic Biol Med. 2005;38(10):1323–1332. doi: 10.1016/j.freeradbiomed.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Aft RL, Mueller GC. Hemin-mediated oxidative degradation of proteins. J Biol Chem. 1984;259(1):301–305. [PubMed] [Google Scholar]
- 26.Balla G, Vercellotti GM, Muller-Eberhard U, Eaton J, Jacob HS. Exposure of endothelial cells to free heme potentiates damage mediated by granulocytes and toxic oxygen species. Lab Invest. 1991;64(5):648–655. [PubMed] [Google Scholar]
- 27.Balla J, Vercellotti GM, Jeney V, Yachie A, Varga Z, Jacob HS, Eaton JW, Balla G. Heme, heme oxygenase, and ferritin: how the vascular endothelium survives (and dies) in an iron-rich environment. Antioxid Redox Signal. 2007;9(12):2119–2137. doi: 10.1089/ars.2007.1787. [DOI] [PubMed] [Google Scholar]
- 28.Jeney V, Balla J, Yachie A, Varga Z, Vercellotti GM, Eaton JW, Balla G. Pro-oxidant and cytotoxic effects of circulating heme. Blood. 2002;100(3):879–887. doi: 10.1182/blood.v100.3.879. [DOI] [PubMed] [Google Scholar]
- 29.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, 3rd, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8(12):1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 30.Krawczeski CD, Woo JG, Wang Y, Bennett MR, Ma Q, Devarajan P. Neutrophil gelatinase-associated lipocalin concentrations predict development of acute kidney injury in neonates and children after cardiopulmonary bypass. J Pediatr. 2011;158(6):1009–1015. e1001. doi: 10.1016/j.jpeds.2010.12.057. [DOI] [PubMed] [Google Scholar]
- 31.Dent CL, Ma Q, Dastrala S, Bennett M, Mitsnefes MM, Barasch J, Devarajan P. Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: a prospective uncontrolled cohort study. Crit Care. 2007;11(6):R127. doi: 10.1186/cc6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng J, Xiao Y, Yao Y, Xu G, Li C, Zhang Q, Li H, Han L. Comparison of Urinary Biomarkers for Early Detection of Acute Kidney Injury After Cardiopulmonary Bypass Surgery in Infants and Young Children. Pediatr Cardiol. 2012 doi: 10.1007/s00246-012-0563-6. [DOI] [PubMed] [Google Scholar]
- 33.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15(6):1597–1605. doi: 10.1097/01.asn.0000130340.93930.dd. [DOI] [PubMed] [Google Scholar]
- 34.Lassnigg A, Schmid ER, Hiesmayr M, Falk C, Druml W, Bauer P, Schmidlin D. Impact of minimal increases in serum creatinine on outcome in patients after cardiothoracic surgery: do we have to revise current definitions of acute renal failure? Critical care medicine. 2008;36(4):1129–1137. doi: 10.1097/CCM.0b013e318169181a. [DOI] [PubMed] [Google Scholar]
- 35.Mammen C, Al Abbas A, Skippen P, Nadel H, Levine D, Collet JP, Matsell DG. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis. 2012;59(4):523–530. doi: 10.1053/j.ajkd.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 36.Ricci Z, Luciano R, Favia I, Garisto C, Muraca M, Morelli S, Di Chiara L, Cogo P, Picardo S. High-dose fenoldopam reduces postoperative neutrophil gelatinase-associated lipocaline and cystatin C levels in pediatric cardiac surgery. Crit Care. 2011;15(3):R160. doi: 10.1186/cc10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ozment CP, Mamo LB, Campbell ML, Lokhnygina Y, Ghio AJ, Turi JL. Transfusion-related biologic effects and free hemoglobin, heme, and iron. Transfusion. 2013;53(4):732–740. doi: 10.1111/j.1537-2995.2012.03837.x. [DOI] [PubMed] [Google Scholar]