Abstract
Protein Disulfide Isomerase (PDI), an important endoplasmic reticulum-resident oxidoreductase chaperone can bind to estrogens as well as intact with its receptor proteins (i.e., estrogen receptors (ER) α and β). It has been postulated that PDI also acts as an intracellular 17β-estradiol (E2)-binding protein that transports and accumulates E2 in live cells. Drop in E2 level promotes dissociation of E2 from PDI and released in cytosol; the released E2 can augment estrogen receptor-mediated transcriptional activity and mitogenic action in cultured cells by modulating the ERβ/ERα ratio. In this study, we observed rotenone-induced damage to PDI leads to significant increase in ERβ/ERα ratio by down-regulating ERα and up-regulating ERβ. We demonstrated that nitrosative stress induced disruption of the cellular estrogenic status can be prevented through diphenyl difluoroketone (EF24, curcumin analog) intervention by protecting PDI from reactive oxygen species (ROS)-induced damage. Together, our study suggests that both PDI and EF24 can play a vital role in maintaining cellular estrogenic homeostasis.
Keywords: protein disulfide isomerase (PDI), nitrosative stress, EF24, ERα, ERβ
Introduction
Breast cancer is the second leading cause of cancer related deaths in women worldwide and approximately 1.5 million women are diagnosed with breast cancer annually [Friedenreich et al., 2011]. Estrogen hormone plays important physiological roles in the progression of breast cancer in women. Biological effects of estrogen are mediated by estrogen receptors (ER) [Jensen et al., 2005] ER super family is consisted by two homologous nuclear receptors; estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ) [Nilsson et al., 2001]. ERα and ERβ exhibit differential transcriptional activities and functions in breast cancer [Duong et al., 2006]. ERα is expressed in approximately 70% of human breast cancer patients [Ali et al., 2000]. The ratio between ERα and ERβ is critical for maintaining the cellular homeotsasis. Overexpression of ERα and attenuation of ERβ expression may lead to breast carcinogenesis [Paruthiyil et al., 2004]. Binding of estrogen with ERα activates several oncogenes which are associated with angiogenesis, cell proliferation and invasiveness during breast cancer [Pegueux et al., 2012].
Mitochondrial respiratory chain is responsible to produce free radicals, principally reactive oxygen species (ROS), along with ATP production. Rotenone-induced inhibition of mitochondrial respiratory chain is greatly attributed to the production of excess nitrogen species, which are categorized as ROS [Chou et al., 2010]. The mitochondrial derived ROS are vital not only because mitochondrial respiratory chain components are present in almost all eukaryotic cells, but also because the ROS produced in mitochondria can readily influence mitochondrial function without having to cope with long diffusion times from the cytosol [Li et al., 2003]. If not effectively dissipated, ROS can predominantly accumulate and damage resident proteins, lipids, and DNA [Ghaffari et al. 2008]. In absence of redox regulatory and DNA repair enzymes accumulated ROS and/or unrepaired DNA damage can lead to initiation and progression of cancer [Ghaffari et al. 2008]. Recent studies have shown that excess nitrogen species-mediated damage to PDI influences estrogenic status in MCF7 breast cancer cells by altering the ERα and ERβ ratio in cell [Roy et al., 2007].
PDI, a 60-KDa intracellular protein, is primarily localized in the endoplasmic reticulum [Turano et al., 2002], although it is also present in cytosolic and nuclear compartments [Turano et al., 2002; Coppari et al., 2002]. PDI has four domains that are homologous to thioredoxin (TRX) (termed a, b, b′, and a′). PDI acts as a catalase of thiol-disulfide exchange reactions through two active sites (CGHC), one each in the amino (a) and carboxy (a′) termini, that catalyze disulfide bond formation, reduction, and isomerization [Edman et al., 1985]. Earlier it has been studied that PDI facilitates oxidative protein folding by catalizing thiol-disulfide exchange [Pal et al., 2010]. In addition, it was suggested that PDI can interact directly with ERα with its ER-interacting property very similar to Hsp90 and Hsp70, two well-known chaperone proteins that can alter ER functions [Xiong et al., 2012]. PDI, with its possible ERα chaperoning activity, acts as a modulator of estrogen’s hormonal activity in different target cells. The intracellular PDI-bound estrogens can be released from PDI to instigate the ER-mediated transcriptional activity as well as mitogenic actions [Xiong et al., 2012]. Studies suggest that PDI plays a critical role in estrogen responsiveness by functioning as a molecular chaperone and PDI alone is capable of interacting with ERα and influencing its activity [Updike et al., 2007; Schultz et al., 2007; Fu et al., 2011]. It is well-known that nitrosative stress, originating from elevated levels of nitric oxide (NO), results in the S-nitrosylation of PDI cysteines (i.e. the covalent modification of PDI active site cysteines by NO) and blocks the chaperone activity of PDI [Pal et al., 2011].
The human estrogen receptors are predominantly expressed in breast cancer MCF7 cells and are greatly attributed to the tumorigenic processes [Chaudhri et al., 2012]. To better understand the changes in receptor proteins level, we have studied PDI-ERα interaction in MCF-7 cell by using a variety of molecular and biochemical approaches.
In our earlier study, we have shown polyphenol, EF24, scavenges free radicals and prevent PDI from getting S-nitrosylated [Pal et al., 2011]. Current studies on EF24 are going on as an anticancer drug. It is also found that EF24 is not only more efficacious in anticancer screens but also considerably less toxic, a commonly used chemotherapeutic agent. In this study, we chose to assay PDI’s ER-binding property to maintain ERα and ERβ levels in MCF7 cell under homeostasis and nitrosative stress conditions. EF24 was then introduced to determine whether they can rescue PDI function and furthermore, prevent the change in receptor proteins levels. Our results indicate that EF24 prevents nitrosative stress-induced alteration of ERα and ERβ levels and promotes the homeostasis of estrogen in target cell.
Materials and methods
Materials
PDI was expressed and purified as previously described [Wang et al., 2008]. Reduced and oxidized dithiothreitol (DTTred and DTTox) were purchased from Sigma Chemical Co and used without further purification. EF24 was synthesized in our lab as reported by Adams et al. [Adams et al., 2004]. It was further purified by reversed-phase HPLC and characterized by mass spectrometry. HPLC analysis of re-purified EF24 showed purity ≥ 99%. Tetra-nitromethane (TNM, obtained from Sigma-Aldrich, St. Louis, MO, USA) was used as a model nitrogen species (NOx) donor. Other reagents were purchased as follows: Mouse monoclonal PDI antibody (Abcam, Cambridge, MA, USA), rabbit polyclonal ERα antibody (Santa cruz, Santa cruz, CA, USA), ERβ antibody (Invitrogen, Carlsbad, CA, USA) and GAPDH (Glyceraldehyde 3-phosphate dehydrogenase, Cell Signalling, Danvers, MA, USA), horseradish peroxidase (HRP)-conjugated goat anti-mouse and/or goat anti-rabbit IgG (KPL Biomedical) and MCF7 (Obtained from ATCC, Manassas, VA).
Cell Culture
HEK 293 cells and HELA cells were cultured in DMEM with 10% fetal bovine serum (Atlanta Biologicals) and 100 U/ml penicillin-streptomycin (Sigma). MCF7 cells were cultured in RPMI-1640 media (Hyclone Laboratory) supplemented with 10% fetal bovine serum (Atlanta Biologicals) and 100 U/ml penicillin-streptomycin (Sigma). Cells were incubated in 5% CO2 and 78% relative humidity at 37 °C. All cell lines were either cultured (0.5×106 cells/well) onto 6-well plates or 4-well chambered slides in growth medium at 37 °C in 5% CO2 incubator.
Cell treatment
Cells were treated with various concentration of rotenone for 2 hrs after 3 hrs of preincubation with various concentration of EF24. Total cellular protein extracts then were prepared by washing the cells with PBS, collected by centrifugation (3000g, 5 min at 4° C), and extracted by sonicating in buffer containing 10 mM Tris–HCl (pH 7.4), 10 mM EDTA, 2% (w/v) SDS and protease inhibitors (Sigma). Total protein concentrations were measured using a bicinchoninic acid kit (Pierce) and subjected to immunoblot analyses.
Cytotoxicity assays of Rotenone and EF24
The effect of EF24 and rotenone were tested utilizing MCF-7 cell lines. Cells in 96-well plate cultured in DMEM medium supplemented with 10% fetal bovine serum, 1% penicillin–streptomycin. The cells were grown at 37°C with 5% carbon dioxide. Cells were incubated for 24 hrs and images were captured in live mode. Several controls were included in each experiment. As a control for non-specific effects, the diluents of rotenone and EF24, DMSO, as contained in the experimental samples, was included at final concentration of 2.5 v/v. H2O2 and PBS were used as positive and negative control respectively.
In this study, it was used automated microscopic imaging to ascertain cell death via the use of a Differential Nuclear Staining (DNS) that was recently validated as high throughput screening (HTS) assay. For each experimental and control well was added a mixture of two fluorescent dyes, Propidium iodide (MP Biomedicals, Solon, OH) and Hoechst 33342 (Invitrogen, Eugene, OR) at a final concentration of 1 μg/ml, 1h prior to image recording. Both dyes are nucleic acids intercalators. Hoechst has the property to easily cross cell membranes of healthy and dead cells and stains nucleic DNA, while PI is only capable to stain dead cells with compromised plasma membranes. The fluorescence signal from each individual fluorophore emitted by stained cells was captured in two separate channels, in fulfillment with the dye emission requirements. Images were acquired directly form a tissue culture multi-well plate utilizing a BD Pathway 855 Bioimager system (BD Biosciences Rockville, MD). To acquire adequate numbers of cells for statistical analysis purpose, montages (3×3) from nine adjacent image fields were captured per well utilizing a 20× objective. Captured images and data analysis determining the percentage of death cells from each individual well was performed by using BD AttoVision™ v1.6.2 software (BD Biosciences Rockville, MD), designed to assist the Bioimager system. Every experimental point, as well as all controls, was assessed in quintuplicate.
Immunoblot analyses
Blots were incubated in blocking buffer (5%, w/v, dried skimmed milk in Tris-buffered saline, pH 7.4, and 0.1% Tween 20) then developed using anti-PDI monoclonal antibody (1:500 abcam) or anti-ERα antibody(1:100 dilution) or anti-ER-β antibody (1:100 dilution) or anti-GAPDH (Glyceraldehyde 3-phosphate dehydrogenase, 1:1000 dilution) diluted in blocking buffer and applied for 1 h at room temperature and incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse or anti-rabbit (KPL Biomedical) in 1% BSA in Tris buffered saline with Tween 20 (TBST) for 30 min. Chemiluminescence (ECL-plus or SuperSignal West Pico Chemiluminescent Substrate) was used according to the manufacturer’s (Amersham or Pierce Biotechnology Inc.) instructions for detection of protein. Antibody binding was revealed using peroxidase-conjugated secondary antibodies and enhanced Chemiluminescence (ECL-plus or SuperSignal West Pico Chemiluminescent Substrate) according to facturer’s (Amersham or Pierce Biotechnology Inc.) protocols.
Confocal microscopy and immunocytochemistry
For confocal microscopy, cells were grown in 4-well chambered slides. Treated or untreated cells were fixed with methanol (100%) and incubated at −20 °C for 5 min before blocking with 5% normal goat serum (NGS). Followed by blocking, cells were incubated with respective primary antibodies overnight at 4°C i.e., anti-PDI (monoclonal, 1:500), anti-ERα or anti-ER-β (1:100) in 1% NGS. The slides were washed and reacted with Fluorescein isothiocyanate (FITC) conjugated goat anti-rabbit IgG (for ERα or ERβ), and Rhodamine conjugated goat anti-mouse IgG (for PDI) in 1:100 dilution. The cells were counter-stained with 4′-6-Diamidino-2-phenylindole (DAPI), mounted with DAKO medium (DAKO Corporation, CA) and visualized by confocal microscopy (Zeiss LSM 700 confocal)
Statistical analyses
Each data point was executed at least three times. To note experimental changeability, data are presented as mean with its corresponding standard deviation. Statistical analysis was performed in Origin Pro (OriginLAB Corporation, Northamton, MA)Two-tailed paired Student’s t-tests were calculated to denote the statistical significance of variances between two groups of samples. To identify if there is a significant difference between two groups, a value of P < 0.05 was considered significant.
Results
Impact of reactive oxygen species on PDI and attenuation by polyphenol EF24 in MCF7 cells
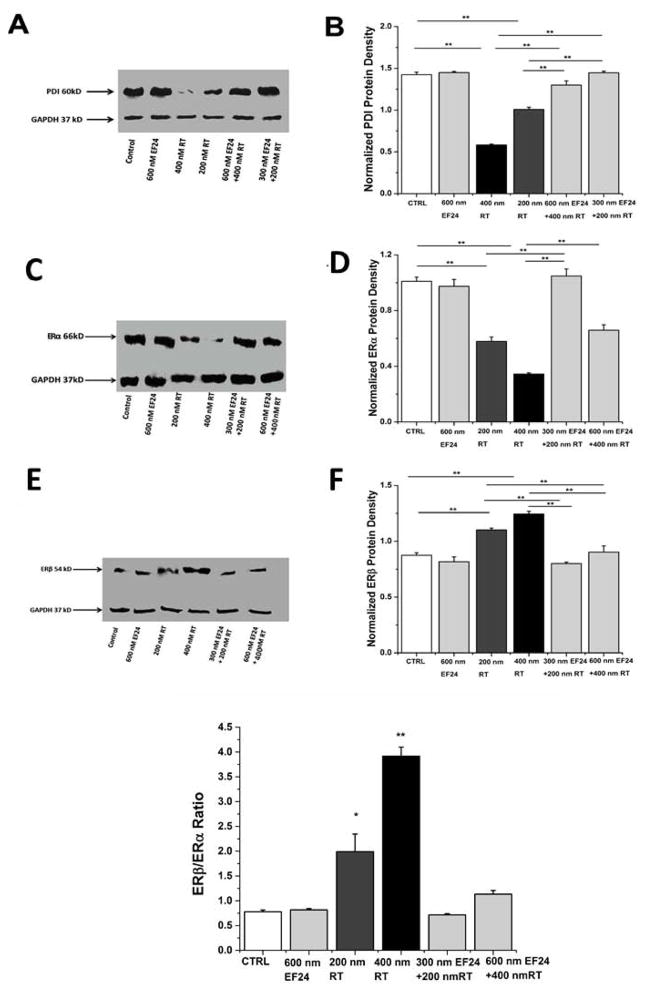
Assay shows the level of expression of PDI as a function of increasing concentrations of rotenone (Fig. 2A). A dose-dependent decrease in oxidoreductase expression with increasing concentration of rotenone was evidenced by immunoblot analysis. This attenuation in PDI-expression may couple with the modification of the oxidoreductase active site and loss of oxidoreductase activity. Remarkably, cells treated with EF24 prior to rotenone infusion show that the polyphenol is able to rescue PDI expression. Densitometric analysis (Fig. 2B) of this immunoblot assay, using GAPDH as the control, confirmed that intervention of EF24 restores PDI levels to those observed in the absence of rotenone-dependent ROS infusion. The concentrations of rotenone and EF24 used for this study were chosen after does-response assays repetitively performed in MCF7 cell line (Fig. 1).
Figure 2. Effect of EF24 and rotenone on PDI, ERα and ERβ expression in MCF7 cells.
Cells were treated with rotenone and EF24 at different concentrations and cell lysates were analyzed by immunoblot using PDI antibody. Cells treated with rotenone for 2 hrs after pre-incubation with EF24 for 3 hrs. GAPDH was used as a loading control. (A) Immunoblot shows PDI expression level (B) Densitometric analysis of immunoblot assay of PDI protein level (C) Immunoblot shows ERα expression level (D) Densitometric analysis of immunoblot assay of ERα protein (E) Immunoblot shows ERβ expression level (F) Densitometric analysis of immunoblot assay of ERβ protein level. Representative images (A, C, E) are shown from at least three replicates (G) Bar diagram shows ERβ/ERα ratio. Bar diagram is made by using OriginPro8.6 software. Error bars represent s.e. from the mean ** p <0.01 (Tukey statistical analysis for p value).
Figure 1. Cytotoxicity assay with rotenone and EF24 in MCF7 cell.
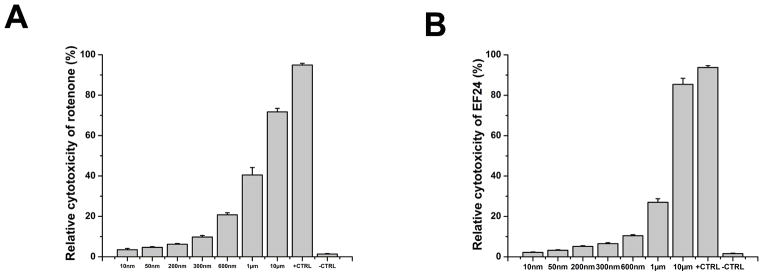
(A) Rotenone cytotoxicity. (B) EF24 cytotoxicity.
Rotenone-induced nitrosative stress on PDI in MCF7 cells alters ERα and ERβ expression level
Immunoblot assay was done with ERα and ERβ antibodies to show the effect of rotenone and EF24 on expression level of ERα and ERβ in MCF7 cells (Fig. 2C and E). Immunoblotting and densitometric analysis of these immunoblot assays using GAPDH as a control confirmed that PDI-dependent ERα level was significantly decreased as a function of rotenone concentration (Fig. 2C&D). In contrast, and in agreement with previous studies, ERβ level was elevated as a function of rotenone concentration and PDI compromise (Fig. 2E&F). Regulation of these two ER-proteins in two opposite directions leads significant increase in ERβ/ERα ratio (Fig. 2G).
Our evidences show cells treated with EF24 prior to rotenone addition alters both ERα and ERβ levels and rescued to a significant extent. The observed ERβ/ERα ratio was also found to be in agreement with the results obtained in the absence of rotenone (untreated cell).
PDI is a chaperone for ERα. Earlier study has shown redox sensitive cysteine residues at the active sites of PDI can be blocked by SNO-PDI formation in presence of reactive nitrogen species [Xiong et al., 2012]. Formation of SNO-PDI interferes with the chaperone activity of PDI and diminishes the interaction with ERα in MCF7 cells [Xiong et al., 2012], which can alter ERβ/ERα ratio. It is well-established that rotenone stress causes S-nitrosylation of cysteines in MCF7 cell [Wang et al., 2005]. Our data clearly indicates MCF7 cells with nitrosative stress on PDI would have significantly increased ERβ to ERα ratio, an important parameter that determines the responses of target tissues or cells to estrogens. While, ERβ to ERα ratio can be rescued by EF24 intervention.
Effect of rotenone and EF24 on ERα expression level in MCF7 cells
Confocal microscopy and immunocytochemistry were performed to analyze the effect of rotenone and EF24 on MCF7 cells (Fig. 3). Findings show ERα expression level goes down significantly when cells were stressed with 400 nm rotenone for 2 hrs (Fig. 3F). Again, cells pretreated with 600 nm EF24 for 3 hrs and followed by 400 nm rotenone insult for 2 hrs show ERα expression level very similar to controls (Fig. 3G). As control for non-specific effect, untreated (Fig. 3D) and 600 nM EF24 treated (Fig. 3E) cells were included. These results show that EF24 has prophylactic effect against nitrosative stress and plays vital role in maintaining ERα expression level in MCF7 cells.
Figure 3. Confocal images showing expression and localization of PDI and ERα in MCF7 cells, and effect of EF24 and rotenone on ERα distribution in MCF7 cells.
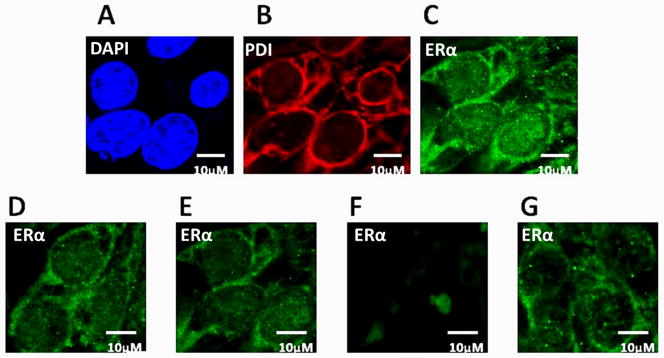
(A) Cells stained with DAPI showing nucleus in blue color (B) Cells stained with rhodamine showing PDI in red color, (C) Cells stained with FITC showing ERα in green color, (D) Expression of ERα in untreated cells, (E) ERα expression level when cells were treated with 600 nM EF24 alone, (F) ERα expression level when cells were exposed to 400 nm rotenone for 2 hrs, and (G) Cells pretreated with 600 nM EF24 for 3 hrs followed by 400 nM rotenone treatment for 2 hrs. All the cover-glasses were counterstained with DAPI, to delimitate the nucleolus (blue color). Images are taken by Zeiss LSM 700 confocal microscopy and analyzed in imajeJ. Representative images are shown from at least 8–10 replicates. The scale bar represents 10 μM.
Effect of rotenone and EF24 on ERα and PDI localization in MCF7 cells
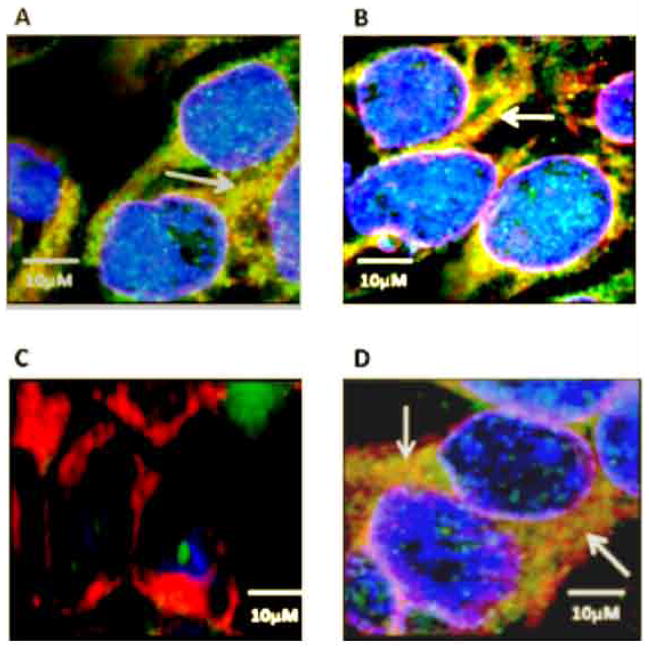
As it has been earlier studied that PDI binds with ERα and helps to maintain ERα level in MCF7 cells, we were interested to see the colocalization of PDI and ERα under homeostasis and nitrosative stress condition. We hypothesized that under nitrosative stress condition, active sites of PDI will be chemically modified, leads to PDI dysfunction and interference in PDI and ERα colocalization. We were further interested to know if, pretreatment with EF24 is able to rescue PDI from getting chemically modified under nitrosative stress condition and keep its active sites functional. Confocal images show ERα and PDI colocalization goes down significantly when cells were stressed 2 hrs with 400 nm rotenone (Fig. 4C). While, cells pretreated with 600 nm EF24 for 3 hrs and followed by 400 nm rotenone insult for 2 hrs, show colocalization very similar to controls (Fig. 4D). As control for non-specific effects, untreated (Fig. 4A) and 600 nM EF24 treated (Fig. 4B) cells were included. These results demonstrate that EF24 has prophylactic effect against nitrosative stress and plays vital role in maintaining homeostasis of ERα in MCF7 cells.
Figure 4. Fluorescence images of MCF7 cells reveal the colocalization (yellow zone indicated by White arrow) of PDI and ERα in cells.
MCF7 cells were untreated or stressed with rotenone and processed for immunofluorescence as detailed in the Materials and methods section. (A) Colocalization in untreated cell, (B) Colocalization after cells were treated with 600 nM EF24 treatment, (C) Colocalization in cells after 400 nM rotenone insult for 2 hrs, and (D) Colocalization was compared to control when cells were pretreated with 600 nm EF24 for 3 hrs followed by 400 nM rotenone treatment for 2 hrs. All the cover-glasses were counterstained with DAPI, rhodamine, and FITC to delimitate the nucleolus (in blue color), PDI (in red color), and ERα (in green color) respectively. Images are taken by Zeiss LSM 700 confocal microscopy and analyzed in imajeJ. Representative images are shown from at least 8–10 replicates. The scale bar represents 10 μM.
Effect of rotenone on ERα and PDI localization in HELA and HEK 293 cells
Effect of rotenone on ERα and PDI localization was further investigated by using non-malignant cell (HEK 293) (Fig. 5) and another malignant cell (HELA) (Fig. 6) lines. Both cell lines show ERα and PDI highly colocalized during homeostasis, whereas marked decrease in ERα and PDI colocalization was observed in the cells treated with 400 nm rotenone for 2 hrs. These results indicate that effect of rotenone on ERα and PDI colocalization is not specific to cell types; rotenone is capable of inhibiting the interaction between ERα and PDI by chemical modification of active sites of PDI in various cell types. Like MCF7 cells, Rotenone significantly decreases ERα and PDI expression level in HELA cells (Fig. 6B). PDI expression level was decreased with rotenone treatment compared to untreated cells in non-malignant HEK 293 cells (Fig. 5B); however, no significant change was observed in ERα expression level with rotenone treated HEK 293 cells (Fig. 5B).
Figure 5. Fluorescence images of HEK 293 cells show the colocalization of PDI and ERα in cells.
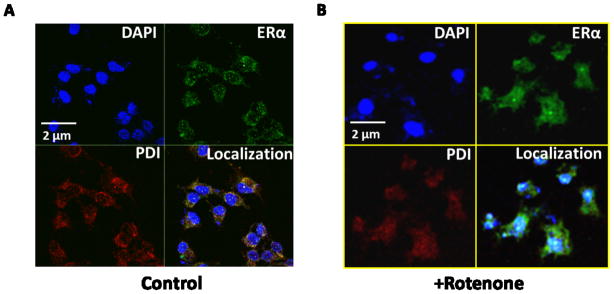
HEK 293 cells were untreated or stressed with rotenone and processed for immunofluorescence as detailed in the Materials and methods section. (A) Colocalization in untreated cell, (B) Colocalization in cells after 400 nM rotenone treatment for 2 hrs. All the cover-glasses were counterstained with DAPI, rhodamine, and FITC to delimitate the nucleolus (in blue color), PDI (in red color), and ERα (in green color) respectively. Images are taken by Zeiss LSM 700 confocal microscopy and analyzed in imajeJ. Representative images are shown from at least 8–10 replicates. The scale bar represents 2 μM.
Figure 6. Fluorescence images of HELA cells show the colocalization of PDI and ERα in cells.
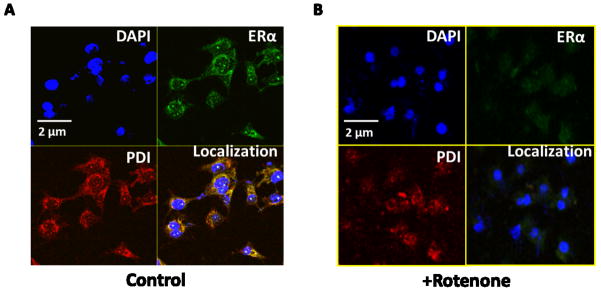
HELA cells were untreated or treated with rotenone and processed for immunofluorescence as detailed in the Materials and methods section. (A) Colocalization in untreated cell, (B) Colocalization in cells after 400 nM rotenone treatment for 2 hrs. All the cover-glasses were counterstained with DAPI, rhodamine, and FITC to delimitate the nucleolus (in blue color), PDI (in red color), and ERα (in green color) respectively. Images are taken by Zeiss LSM 700 confocal microscopy and analyzed in imajeJ. Representative images are shown from at least 8–10 replicates. The scale bar represents 2 μM.
Discussion
The results of our present studies show that PDI, a well-known protein folding catalyst [Kemmink et al., 1997] that is highly expressed in many tissues and also cancer cell line [Xu et al., 2012], is a high-capacity intracellular estrogen-binding protein and can modulate the estrogen receptor proteins level and magnitude. The estrogen receptor protein-interacting ability of PDI may offer an explanation for the intriguing property of estrogen status maintenance in target tissues (such as breast cancer tissue). Inherent interaction property of PDI with estrogen receptor protein is naturally endowed with great potentials as an important modulator of estrogen’s hormonal activity [Davis et al., 2002]. However, the exact biological roles of PDI in this respect have not been characterized. Our results provide definitive evidence demonstrating that PDI can significantly alter the ratio of ERβ to ERα by altering the levels of these two proteins in opposite directions under nitrosative stress condition. This unique function of PDI offers new mechanistic insights into the modulation of estrogenic status in target cells. Nitrosative stress represents a big threat to the estrogen receptor protein level-maintenance activity of PDI and has been shown to be associated with onset of defective reproductive organs development.
Our findings demonstrate that bisphenolic phytochemical; EF24 can rescue PDI from becoming chemically modified under nitrosative stress condition and maintains its activity. In the presence of model NOx donor PDI loses its activity due to the modification of PDI active sites by NOx. In the presence of EF24, PDI remains chemically unmodified even NOx donor is added in the experimental condition. Although the mechanism of action and location of NOx on EF24 is not investigated here, our study demonstrated that PDI function can remain unaffected under conditions of nitrosative stress as long as scavenging bisphenol is present.
To further testify the specificity in effect of rotenone, we have investigated the effect of rotenone in both malignant and non-malignant cell types. We found effect of rotenone on PDI expression level and function is very similar in both types of cells. However, similarity in effect of rotenone on estrogen receptor α in two different malignant cell lines and dissimilarity in effect between malignant and non-malignant cell types suggesting that effect of rotenone on estrogen receptor may be specific to cancer patients.
It is likely that the differential regulation of ERα and ERβ by PDI is biologically important. It is been well-known that relative expression levels of these two ER subtypes are crucial determinants of cellular responses to the proliferative-antiproliferative actions of certain estrogens and PDI is an important regulator of ERβ/ERα ratio. Therefore, in our study, we were very keen to ameliorate our knowledge on changes in levels of ER receptor proteins putting the crucial ratio-regulator (PDI) under nitrosative stress. We also extended our curiosity further by observing EF24’s prophylactic effect in prevention of nitrosative damage on PDI due to chemical modification by NOx donor and changes in levels of ER receptor protein. Our study provides a confirmatory statement on EF24’s prophylactic effect to rescue PDI from chemical modification by scavenging NOx under nitrosative stress condition and plays a crucial role to maintain ERβ/ERα ratio in target tissues.
Conclusions
Taken together, our study has established that polyphenolic phytochemical, EF24, is able to protect PDI function under conditions of nitrosative stress by scavenging free radical (NOx) and forming stable adducts which result in homeostasis of ERβ/ERα ratio. This finding offers new mechanistic insights into the regulation of estrogenic status in target cells. The prophylactic mechanism of polyphenol might permit the development of new therapeutic approaches for cancer and other disorders associated with excessive ROS production related PDI dysfunction.
Acknowledgments
We wish to acknowledge Dr. M. Narayan (UTEP) and Dr. Siddhartha Das (UTEP) for providing funding from start-up/seed money from UTEP and funding from ADRF. We would like to thank Jeremiah for the synthesis of EF24. We thank the Biomolecule Analysis Core Analysis at the Border Biomedical Research Center/Biology/UTEP (NIH grant 5G12RR008124).
Contributor Information
Dr. Debarshi Roy, Email: roy.debarshi@mayo.edu, Department of Experimental Pathology, Mayo Clinic College of Medicine, Rochester, MN 55905, USA. Ph: (507) 284-4516 Fax: (507) 284-1678
Parijat Kabiraj, Email: pkabiraj@miners.utep.edu, Parijat Kabiraj, Department of Chemistry, University of Texas at El Paso. 500 W. Univ. Ave., El Paso, TX 79968; Ph: (915) 747-8717.
Dr. Rituraj Pal, Email: rpal@bcm.edu, Department of Molecular Physiology and Biophysics, Baylor College of Medicine. One Baylor Plaza, Houston, Texas 77030, USA. Ph: (915) 747-6614; Fax: (713) 798-3475
References
- Adams BK, Ferstl EM, Davis MC, Herold M, Kurtkaya S, Camalier RF, et al. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorg Med Chem. 2004;12:3871–3883. doi: 10.1016/j.bmc.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Ali S, Coombes RC. Estrogen receptor alpha in human breast cancer: occurrence and significance. J Mammary Gland Biol Neoplasia. 2000;5:271–281. doi: 10.1023/a:1009594727358. [DOI] [PubMed] [Google Scholar]
- Chaudhri RA, Olivares-Navarrete R, Cuenca N, Hadadi A, Boyan BD, Schwartz Z. Membrane estrogen signaling enhances tumorigenesis and metastatic potential of breast cancer cells via estrogen receptor-α36 (ERα36) J Biol Chem. 2012;287(10):7169–81. doi: 10.1074/jbc.M111.292946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppari S, Altieri F, Ferraro A, Chichiarelli S, Eufemi M, Turano C. Nuclear localization and DNA interaction of protein disulfide isomerase ERp57 in mammalian cells. J Cell Biochem. 2002;185:325–333. doi: 10.1002/jcb.10137. [DOI] [PubMed] [Google Scholar]
- Chou AP, Li S, Fitzmaurice AG, Bronstein JM. Mechanisms of rotenone-induced proteasome inhibition. Neurotoxicology. 2010;31(4):367–72. doi: 10.1016/j.neuro.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CH, Raulston JE, Wyrick PB. Protein Disulfide Isomerase, a Component of the Estrogen Receptor Complex, Is Associated with Chlamydia trachomatis Serovar E Attached to Human Endometrial Epithelial Cells. Infect Immun. 2002;70(7):3413–8. doi: 10.1128/IAI.70.7.3413-3418.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong V, Licznar A, Margueron R, Boulle N, Busson M, Lacroix M, Katzenellenbogen BS, Cavaillès V, Lazennec G. ER alpha and ER beta expression and transcriptional activity are differentially regulated by HDAC inhibitors. Oncogene. 2006;25(12):1799–806. doi: 10.1038/sj.onc.1209102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman JC, Ellis L, Blacher RW, Roth RA, Rutter WJ. Sequence of protein disulfide isomerase and implications of its relationship to thioredoxin. Nature. 1985;317 (6034):267–270. doi: 10.1038/317267a0. [DOI] [PubMed] [Google Scholar]
- Friedenreich CM. Physical activity and breast cancer: review of the epidemiologic evidence and biological mechanisms. Recent Results Cancer Res. 2011;188:125–139. doi: 10.1007/978-3-642-10858-7_11. [DOI] [PubMed] [Google Scholar]
- Fu XM, Wang P, Zhu BT. Characterization of the Estradiol-Binding Site Structure of Human Protein Disulfide Isomerase (PDI) PLoS One. 2011;6(11):e27185. doi: 10.1371/journal.pone.0027185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffari S. Oxidative Stress in the Regulation of Normal and Neoplastic Hematopoiesis. Antioxid Redox Signal. 2008;10:1923–1940. doi: 10.1089/ars.2008.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen EV, Jacobson HI. Fate of steroid estrogens in target tissues. In: Pincus G, Vollmer EP, editors. Biological Activities of Steroids in Relation to Cancer. Academic Press; New York: 1960. pp. 61–174. [Google Scholar]
- Kemmink J, Darby NJ, Dijkstra K, Nilges M, Creighton TE. ) The folding catalyst protein disulfide isomerase is constructed of active and inactive thioredoxin modules. Curr Biol. 1997;7(4):239–45. doi: 10.1016/s0960-9822(06)00119-9. [DOI] [PubMed] [Google Scholar]
- Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, Robinson JP. Mitochondrial Complex I Inhibitor Rotenone Induces Apoptosis through Enhancing Mitochondrial Reactive Oxygen Species Production. J Biol Chem. 2003;278 (10):8516–8525. doi: 10.1074/jbc.M210432200. [DOI] [PubMed] [Google Scholar]
- Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, et al. Mechanisms of Estrogen Action. Physiol Rev. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- Pal R, Cristan EA, Schnittker K, Narayan M. Rescue of ER oxidoreductase function through polyphenolic phytochemical intervention: Implications for subcellular traffic and neurodegenerative disorders. Biochem Biophys Res Commun. 2010;392:567–571. doi: 10.1016/j.bbrc.2010.01.071. [DOI] [PubMed] [Google Scholar]
- Pal R, Miranda M, Narayan M. Nitrosative stress-induced Parkinsonian Lewy-like aggregates prevented through polyphenolic phytochemical analog intervention. Biochem Biophys Res Commun. 2011;404 (1 ):324–329. doi: 10.1016/j.bbrc.2010.11.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC. Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. 2004;1;64(1):423–8. doi: 10.1158/0008-5472.can-03-2446. [DOI] [PubMed] [Google Scholar]
- Péqueux C, Raymond-Letron I, Blacher S, Adlanmerini M, Fouque MJ, Rochaix P. Stromal estrogen receptor-α promotes tumor growth by normalizing an increased angiogenesis. Cancer Res. 2012;72(12):3010–9. doi: 10.1158/0008-5472.CAN-11-3768. [DOI] [PubMed] [Google Scholar]
- Roy D, Cai Q, Felty Q, Narayan S. Estrogen-induced generation of reactive oxygen and nitrogen species, gene damage, and estrogen-dependent cancers. J Toxicol Environ Health B Crit Rev. 2007;10(4):235–57. doi: 10.1080/15287390600974924. [DOI] [PubMed] [Google Scholar]
- Schultz-Norton JR, Gabisi VA, Ziegler YS, McLeod IX, Yates JR, Nardulli AM. Interaction of estrogen receptor α with proliferating cell nuclear antigen. Nucleic Acids Res. 2007;35 (15):5028–5038. doi: 10.1093/nar/gkm533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turano C, Coppari S, Altieri F, Ferraro Proteins of the PDI family: unpredicted non-ER locations and functions. J Cell Physiol. 2002;193(2):154–163. doi: 10.1002/jcp.10172. [DOI] [PubMed] [Google Scholar]
- Updike MS, Sawdy JC, Wang LS, Liu S, Huang YW, Ye W, et al. Primary Cultured Human Breast Epithelial Cells Up-regulate Protein Disulfide Isomerase in Response to Zeranol. Anticancer Res. 2007;27 (1A):407–410. [PubMed] [Google Scholar]
- Xiong Y, Manevich Y, Tew KD, Townsend D. S-Glutathionylation of Protein Disulfide Isomerase Regulates Estrogen Receptor α Stability and Function. Int J Cell Biol. 2012;2012:273549. doi: 10.1155/2012/273549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Narayan M. Assessing the Magnitude of Folding Forces along the Oxidative Folding Pathway of Multi-Disulfide-Containing Proteins. The Protein Journal. 2008;27:181–185. doi: 10.1021/ja0305398. [DOI] [PubMed] [Google Scholar]
- Wang T, Tamae D, LeBon T, Shively J, Yen Y, Li J. The role of peroxiredoxin II in radiation-resistant MCF-7 breast cancer cells. Cancer Research. 2005;65(22):10338–10346. doi: 10.1158/0008-5472.CAN-04-4614. [DOI] [PubMed] [Google Scholar]
- Xu S, Butkevich AN, Yamada R, Zhou Y, Debnath B, Duncan R, et al. Discovery of an orally active small-molecule irreversible inhibitor of protein disulfide isomerase for ovarian cancer treatment. Proc Natl Acad Sci U S A. 2012;109(40):16348–53. doi: 10.1073/pnas.1205226109. [DOI] [PMC free article] [PubMed] [Google Scholar]