Abstract
Prions are self-perpetuating protein isoforms that cause fatal and incurable neurodegenerative disease in mammals. Recent evidence indicates that a majority of human proteins involved in amyloid and neural inclusion disorders possess at least some prion properties. In lower eukaryotes, such as yeast, prions act as epigenetic elements, which increase phenotypic diversity by altering a range of cellular processes. While some yeast prions are clearly pathogenic, it is also postulated that prion formation could be beneficial in variable environmental conditions. Yeast and mammalian prions have similar molecular properties. Crucial cellular factors and conditions influencing prion formation and propagation were uncovered in the yeast models. Stress-related chaperones, protein quality control deposits, degradation pathways and cytoskeletal networks control prion formation and propagation in yeast. Environmental stresses trigger prion formation and loss, supposedly acting via influencing intracellular concentrations of the prion-inducing proteins, and/or by localizing prionogenic proteins to the prion induction sites via heterologous ancillary helpers. Physiological and environmental modulation of yeast prions points to new opportunities for pharmacological intervention and/or prophylactic measures targeting general cellular systems rather than the properties of individual amyloids and prions.
Keywords: amyloid, chaperone, cytoskeleton, heat shock, quality control, ubiquitin
Introduction: The prion concept
Prions are self-perpetuating protein conformations that are transmitted via extracellular infection in mammals or inherited via the cytoplasm in lower eukaryotes such as yeast. Originally prions were identified as causative agents of mammalian neurodegenerative diseases such as human Creutzfeld-Jakob disease, “mad cow” disease and sheep scrapie (Aguzzi & O'Connor, 2010). Mammalian prion (PrPSc), an altered conformational isoform of a normal cellular protein (PrPc), is the only established prion that is infectious to humans. PrPSc generates fibrous β-rich ordered aggregates (called amyloids) in the brains of infected animals and humans. PrPSc is an infectious protein because it recruits and converts its normal isoform (PrPC) into its pathogenic conformation (Prusiner, 1998, Colby & Prusiner, 2011). A broad range of neurodegenerative disorders (including Alzheimer's, Parkinson's and Huntington's diseases) is characterized by the formation of amyloid or amyloid-like fibrils of various proteins, specific to each disease but distinct from PrP (Prusiner, 2012). Like prions, most of these pathogenic proteins are transmissible at the cellular level, and in experimental conditions, some of them can be transmissible at organismal level (Aguzzi & Rajendran, 2009). Thus, a current trend is to broaden the term “prion” to include all self-perpetuating conformational diseases. Many amyloid diseases are fatal and incurable. Genetic amyloid diseases are caused by mutations, such as expansion of a polyglutamine (poly-Q) stretch in the huntingtin protein in case of Huntington's disease. Others, such as most cases of Alzheimer's or Parkinson's diseases are idiopathic, occurring sporadically. Alzheimer's disease is already 6th most frequent cause of death and increasing in frequency. Age-dependence of Alzheimer's disease and other amyloidoses forecasts a further increase in health care costs as the human population ages.
Yeast prions
Lower eukaryotes, such as yeast Saccharomyces cerevisiae and other fungi also contain self-perpetuating transmissible amyloids that posses prion properties (for the recent review, see (Liebman & Chernoff, 2012). Due to convenient genetic and phenotypic assays and relatively cheap cultivation techniques, yeast prions provide a useful model system for studying mechanisms of amyloid formation and propagation that are applicable to mammalian and human diseases.
A number of phylogenetically unrelated prions, some of them with the potential to impact a wide range of cellular processes have now been described in S. cerevisiae (Table 1). This list is definitely not complete, as many protein domains from yeast that can confer prion properties when fused to a reporter construct have not yet been studied for their ability to maintain a prion state of their native proteins (Alberti, et al., 2009).
Table 1.
Yeast prions
| Protein | Prion | Protein function | Prion phenotype | Reference |
|---|---|---|---|---|
| Ure2 | [URE3] | Regulatory protein in the nitrogen metabolism pathway | Use of poor nitrogen source | (Lacroute, 1971, Wickner, 1994) |
| Sup35 | [PSI+] | Translation termination factor | Increased nonsense suppression | (Cox, 1965, Wickner, 1994) |
| Rnq1 | [PIN+]/[RNQ+] | Unknown | Increase de novo formation of other prions | (Derkatch, et al., 1997, Derkatch, et al., 2001, Sondheimer & Lindquist, 2000) |
| Swi1 | [SWI+] | Subunit of chromatin remodeling complex | Altered carbon source utilization | (Du, et al., 2008) |
| Cyc8 | [OCT+] | Transcriptional co-repressor | Altered carbon source utilization, flocculation | (Patel, et al., 2009) |
| Mot3 | [MOT+] | Transcriptional co-repressor | Change in cell wall composition | (Alberti, et al., 2009) |
| Sfp1 | [ISP+] | Transcriptional activator | Antisuppression | (Rogoza, et al., 2010) |
| Mod5 | [MOD+] | tRNA modification enzyme | Increased level of ergosterol and resistance to antifungal drugs | (Suzuki, et al., 2012) |
| Nup100 | [NUP100+] | FG-nucleoporin | Increase in the rate of nuclear import | (Halfmann, et al., 2012) |
All yeast prions share several common features. First of all, soluble and amyloid states of the same protein cause distinct phenotypes. Furthermore, most prions can adopt multiple amyloid conformations with different fragmentation and elongation rates. These create prion “strains” or “variants” that have different ratios of the soluble and amyloid isoforms, and thus with different phenotypes and propagation abilities (Tanaka , et al., 2006). “Weak” prion strains typically show weaker phenotype, larger aggregate size, and less efficient mitotic transmission, compared to “strong” prion strains.
If the function of the normal cellular protein is compromised to some extent when it forms a prion aggregate, then the prion phenotype will reflect this loss of function. Indeed, the phenotypes associated with at least 4 known yeast prions ([URE3], [PSI+], [SWI+], [OCT+]) resemble those caused by partial “loss-of-function” mutations in the respective genes. As an example, Figure 1 illustrates the phenotypic differences between yeast cells with the non-prion vs. prion isoforms of the translational termination factor Sup35. In the presence of the prion, the translational termination activity of Sup35 is compromised (loss of function) so cells terminate translation less efficiently at nonsense codons (Liebman & Chernoff, 2012). However, sometimes phenotypic effects of prions are masked; for example, phenotypic detection of [NUP100+] prion is hard due to redundancy of FG nucleoporins (Halfmann, et al., 2012). In other cases, the phenotypes associated with a prion are more difficult to reconcile with the partial loss of function. Some prion phenotypes appear to reflect a gain of function for the prion protein. The presence of [PIN+] facilitates the de novo appearance of other yeast prions, including [PSI+] and [URE3] (Liebman & Chernoff, 2012). The prion formed by the Mot3 transcription factor, [MOT3+], regulates the acquisition of multicellular forms in budding yeast. The traits governed by [MOT3+] involve both gains and losses of Mot3 regulatory activity. Both effects are required for the full spectrum of [MOT3+] phenotypes (Holmes, et al., 2013).
Figure 1. Detection of prion [PSI+] by the nonsense-suppression assay.
In a [psi−] ade1-14 reporter strain, stop codon UGA, introduced in the middle of ADE1 gene, is normally recognized by the translation termination complex including release factor Sup35. This results in termination of translation, synthesis of truncated Ade1 protein, inability of cells to grow on the medium lacking adenine (–Ade) and accumulation of red color on complete YPD medium. In the [PSI+] strain, Sup35 is present in the partially inactive prion form and termination is inefficient, resulting in the synthesis of complete Ade1 protein, growth on –Ade medium and white color on YPD medium. Other phenotypes associated with [PSI+] presence include: aggregation of Sup35-GFP, distribution of Sup35 into the pellet fraction after centrifugation of cell lysates, presence of SDS-resistant polymers (see Liebman & Chernoff, 2012).
Genetically, yeast prions manifest themselves as non-Mendelian elements because they are based not on a mutation in DNA inherited with a chromosome, but on a self-propagating protein conformation inherited extrachromosomally. Since prion aggregates capture and convert non-prion protein into the prion conformation, prion traits are typically dominant. In meiosis, prions either are inherited by all meiotic progeny or show irregular retention/loss, rather than 1:1 segregation as one might have expected if the phenotypes were based on two different alleles of a gene. Yeast prions are infectious via cytoplasmic exchange and are therefore efficiently transferred by a technique called cytoduction, the transient fusion of donor and recipient cells without nuclear fusion. In contrast to viruses and other cytoplasmic nucleic acid, loss of yeast prions is reversible in principle, as soluble protein present in the non-prion cell can reform the prion. Transient overproduction of the soluble protein induces de novo appearance of its prions, apparently due to increased misfolding into a prion form (for review, see Liebman & Chernoff, 2012).
While known yeast prion proteins are not homologous to each other, they share several common structural characteristics. All known yeast prion proteins contain specific regions, termed prion domains or PrDs, which are required and sufficient for prion formation and propagation. At least some PrDs appear to be dispensable for the normal cellular function of a prion protein. With the exception of [MOD+], all known yeast PrDs contain aggregation-prone glutamine/asparagine (QN)-rich stretches that are similar to the poly-Q stretch of mammalian huntingtin but absent in mammalian PrP or in the prion protein HET-s from the fungus Podospora (for review, see Liebman & Chernoff, 2012)). Mod5 has a PrD enriched in hydrophobic residues instead of Q/N (Suzuki, et al., 2012). Despite sequence differences, mechanisms of amyloid formation by yeast prions appear to be surprisingly similar to amyloid formation by mammalian proteins.
Prions and adaptation
Existing evidence clearly indicates that similar to many human and mammalian amyloids, at least some [PSI+] and [URE3] variants are harmful to yeast (McGlinchey, et al., 2009, Wickner, et al., 2011). Prion variants that do not cause toxicity on their own may become toxic in combination with other factors. For example, endogenous prions, such as [PIN+] and/or [PSI+] (Gokhale, et al., 2005, Gong, et al., 2012, Kochneva-Pervukhova, et al., 2012, Zhao, et al., 2012) promote aggregation and toxicity of expanded polyQ constructs in the yeast huntingtin model. Overproduction of Sup35 (Chernoff, et al., 1992, Derkatch, et al., 1997, Vishveshwara, et al., 2009) or Rnq1 (Douglas, et al., 2008, Stein & True, 2011, Treusch & Lindquist, 2012) is toxic to prion containing cells. At least in some cases this toxicity is associated with the sequestration of prion-interacting proteins.
However, with about 10 yeast prions proven by now (see above, Table 1), about 20 PrD-like regions capable of acquiring and maintaining the prion state when fused to other reporter proteins (Alberti, et al., 2009), and as many as 200 domains sharing sequence patterns with known PrDs (Michelitsch & Weissman, 2000, Harrison & Gerstein, 2003), it has become obvious that prions are widespread in yeast, and possibly in other organisms. Although [PSI+] and [URE3] prions are apparently extremely rare in natural or industrial isolates of yeast, the [PIN+] prion is found in about 6-12% of strains (Chernoff, et al., 2000, Resende, et al., 2003, Nakayashiki, et al., 2005, Halfmann, et al., 2012). Moreover, about one-third of the natural and industrial Saccharomyces strains tested exhibit phenotypes that are curable by transient inactivation of Hsp104 (Halfmann, et al., 2012), a chaperone that is required for the propagation of most known yeast prions (see below). This indicates that yet-unidentified prions are widespread in wild yeast, and raises the possibility that yeast might actively utilize reversible prion conversion as a molecular switch for regulating certain cellular functions. Moreover, it is possible that different variants of the same prion protein could be either pathogenic or adaptive, or even one and the same variant could be detrimental or adaptive, depending on conditions. Considering prion formation as a “mutation” occurring at the protein level (Chernoff, 2001), one could expect different effects on fitness for different prion variants or in different conditions, in the same way as mutations with deleterious or beneficial effects may arise in a single gene, or even one and the same mutation could be deleterious or beneficial depending on conditions.
In organisms other than yeast, proven or postulated positive biological roles of amyloids include scaffolding of melanin polymerization (Fowler & Kelly, 2009), storage of peptide hormones (Maji, et al., 2009), protection from stress (Iconomidou & Hamodrakas, 2008), silk production (Romer & Scheibel, 2008), substrate attachment (Gebbink, et al., 2005), biofilm formation (Blanco, et al., 2012), and a connection to long term memory (Si, et al., 2003, Si, et al., 2010, Heinrich & Lindquist, 2011, Majumdar, et al., 2012). However in most cases, these are either reversible amyloids or amyloid-like formations whose prion capabilities are not yet entirely proven. [Het-s] from mycellial fungus Podospora was the first proven prion shown to provide a biological advantage to its host (Coustou, et al., 1997, Wickner, 1997). It controls vegetative incompatibility, an adaptive trait, by causing death of non-prion mycellium at the position of contact (Saupe, 2007, Saupe, 2011). It was proposed that defect of translation caused by the prion state of yeast translation termination factor Sup35, [PSI+], may uncover hidden genetic variation and produce new heritable phenotypes due to synthesis of expanded proteins (True & Lindquist, 2000, True, et al., 2004). Indeed, some [PSI+] strains show resistance to some stressors and increased growth in certain conditions (Eaglestone, et al., 1999, True & Lindquist, 2000, True, et al., 2004, Halfmann, et al., 2012), although these effects are genotype-specific, and no consistent effect of [PSI+] on adaptation to novel environments has been reported (Joseph & Kirkpatrick, 2008). [PSI+] also modulates programmed frameshifting in the antizyme gene, responsible for feedback regulation of polyamine biosynthesis (Namy, et al., 2008), although adaptive role of this effect remains unclear (see Chernoff, 2008).
Presence of the [MOD+] prion increases yeast resistance to azoles, a common class of antifungal agents, and to 5-fluorouracil (5FU) (Suzuki, et al., 2012). Therefore, [MOD+] prion is selected for in the presence of fluconazole. However, this prion causes slight disadvantage in normal conditions. Thus it is lost from population following the prolonged growth after removal of selective pressure.
Perhaps the most intriguing case of an environmentally regulated and potentially adaptive yeast prion is [MOT3+]. It is a prion form of transcriptional factor Mot3, which regulates genes involved in biosynthesis of cell wall and ergosterol biosynthesis in yeast. Mot3 is also involved in repression of anaerobic genes during aerobic growth (Grishin, et al., 1998), and reduction in Mot3 levels occurring in hypoxic cells results in the derepression of the target anaerobic genes (Sertil, et al., 2003). Mot3 protein shows a high frequency (10−4) of spontaneous conversion between prion and non-prion states (Alberti et al. 2009). The [MOT3+] prion is found in about 6% of yeast strains tested (Halfmann, et al., 2012).
Interestingly, the presence of [MOT3+] prion interferes with cell separation after division, enabling yeast differentiation into multicellular structures (Holmes, et al., 2013). [MOT3+] prion is not simply an “on/off switch” for Mot3. Instead, the prion phenotype results in both losses and gains of Mot3 function. Formation, elimination and phenotypic manifestation of [MOT3+] prion each respond to specific environmental conditions. Ethanol stress increases the frequency of [MOT3+] formation, while hypoxia eliminates [MOT3+], possibly due to a decrease of Mot3 protein levels. In natural conditions, yeast cultures frequently undergo transitions from high ethanol stress (caused by utilization of sugars via brewing) to hypoxia. Thus, formation and loss of Mot3 prion works as molecular switch that couples natural environmental changes, frequently encountered by yeast, to heritable changes in gene expression.
Rapid phenotypic changes are most beneficial when an organism faces environmental stresses causing cell death, while for the adaptation to a new environment that is not deleterious the organism may use random genomic mutations (Suzuki & Tanaka, 2013). Even in cases when prions per se are not adaptive, they may originate as by-products of the adaptive process. For example, aggregate formation during unfavorable conditions may protect proteins from degradation (see Chernoff, 2007). While such aggregates are normally disassembled by chaperones upon return to normal conditions, some of them may convert into self-perpetuating prions. Indeed, stress granules, assemblies that protect pre-initiation mRNA complexes during stress, are formed with participation of a protein containing a prion-like QN-rich domain (Gilks, et al., 2004). Toxicity of filamentous aggregates formed by overproduced Sup35 protein (see below) is ameliorated after their conversion into mature prions (Ganusova, et al., 2006). For higher organisms, it has been suggested that amyloid formation associated with neurodegenerative diseases may represent a positive environmental adaptation of aging cells (Suzuki & Tanaka, 2013). Neurons could respond to oxidative stress in the aging cells by converting toxic soluble monomers to lesser or non- toxic amyloids in order to gain survival advantages. It has been speculated that eukaryotic cells have evolved to deal with aging associated stresses by using protein conversion into prion-like amyloid form and this way avoiding the dependence on changes in the genome associated with the risk of damaging genetic mutations (Suzuki & Tanaka, 2013).
Role of heat shock proteins in formation and propagation of the yeast prions
Role of chaperones in prion propagation under normal and stress conditions
Cellular defense systems, aimed at protecting the cells from aggregation of stress-damaged proteins, also recognize amyloid aggregates and stress-related proteins and serve as major modulators of prion formation and propagation in yeast. Prion propagation depends on the chaperone machinery, and perturbing chaperone function often results in an increased rate of prion appearance or loss (or both) (Liebman & Chernoff, 2012, Winkler, et al., 2012). As first shown for [PSI+] (Chernoff, et al., 1995), propagation of yeast prions in vivo, with apparent exception of the nuclear prion [ISP+](Rogoza, et al., 2010), requires Hsp104, a chaperone that can promote breakage of amyloid fibers and generate new ends for immobilization of the newly synthesized protein molecules into amyloids (Kushnirov & Ter-Avanesyan, 1998, Reidy & Masison, 2011, Liebman & Chernoff, 2012, Winkler, et al., 2012). Inhibition of the ATPase activity of Hsp104 during growth in the presence of guanidine-HCl leads to a gradual prion loss in cell divisions (Ferreira, et al., 2001, Jung, et al., 2002, Ness, et al., 2002). Interestingly, effect of guanidine-HCl is different from the effect of Hsp104 depletion or mutational inactivation in both kinetics of prion loss and lack of increase in aggregate size, at least for [PSI+] (Wegrzyn, et al., 2001, Park, et al., 2012), suggesting that possibly some “trimming” rather than “fragmenting” activity of Hsp104 is possibly retained in the presence of guanidine (Park, et al., 2012). Overproduction of Hsp104 antagonizes yeast prions [PSI+] (Chernoff, et al., 1995) and [MOD+] (Suzuki, et al., 2012) but not the other known yeast prions. Possible molecular basis of the effect of overproduced Hsp104 on prions is discussed below.
Hsp104 and its bacterial ortholog, ClpB, are also implicated in disaggregation of stress-damaged proteins (Glover & Lum, 2009). Hsp104 works together with other chaperones, specifically with the members of the Hsp70 and Hsp40 families in both prion propagation and disaggregation of heat damaged proteins. Yeast contains two major cytosolic Hsp70 subfamilies, namely Ssa, encoded by 4 genes, SSA1-4, and working with the Hsp40 cochaperones Ydj1 and Sis1(Verghese, et al., 2012), and Ssb, encoded by two genes, SSB1 and SSB2, and working with Hsp40-Zuo1 and unusual Hsp70-related co-chaperone Ssz1 (Gautschi, et al., 2002, Hundley, et al., 2002). Ssa levels are increased during stress, and at least one SSA gene must be present for normal growth (Werner-Washburne, et al., 1987). Ssb is nonessential, not heat inducible, associated to the ribosome, and implicated in folding of nascent polypeptides (Kim, et al., 2013). Notably, Ssa and Ssb exhibit opposite effects on the [PSI+] prion. Overproduction of Ssa facilitates prion propagation in the presence of excess Hsp104 (Newnam, et al., 1999, Allen, et al., 2005), while deletion of SSA2, the major constitutively expressed member of the Ssa family, destabilizes weak strains of [PSI+] (Newnam, et al., 2011). In contrast, Ssb overproduction increases [PSI+] elimination by excess Hsp104 while double ssb1/2 deletion decreases it (Chernoff, et al., 1999). The peptide-binding domain of Hsp70 is responsible for the differences in the effects of Ssa and Ssb on [PSI+] (Allen, et al., 2005). Some ssa mutations antagonize [PSI+] and resemble effects of Hsp104 overproduction (for review, see Reidy & Masison, 2011). Ssa also influences the [URE3] prion, with Ssa1 and Ssa2 proteins having different effects: [URE3] is antagonized by overproduction of Ssa1 (but not Ssa2), and by mutation of Ssa2 (but not Ssa1) (Roberts, et al., 2004, Sharma & Masison, 2008) Single substitution at position 83 is responsible for these differential effects of Ssa1 and Ssa2 (Sharma & Masison, 2011). Numerous data also implicate the Hsp40 proteins Ydj1 and Sis1 in prion propagation (for detailed review, see Liebman & Chernoff, 2012).
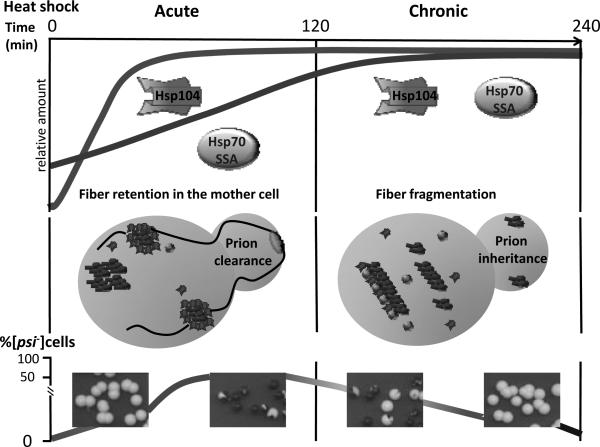
It appears that consequences of chaperone action on Sup35 aggregates depend on the balance between Hsp104 and Ssa, rather than on the amount of each of these proteins per se (Figure 2). Indeed, during short-term (30-60 min) mild (39°C) heat shock of exponential yeast cultures, Hsp104 levels increase faster then levels of other Hsps, including Ssa. If yeast cells containing a weak [PSI+] strain are shifted to normal temperature after the short-term heat shock, the prion is destabilized, leading to frequent [PSI+] loss during cell divisions following the heat shock treatment (Newnam, et al., 2011). Longer (several hours) incubation at high temperature before return to normal conditions, results in restoration of the Hsp104/Ssa balance and leads to restoration of [PSI+] stability. Deletions of SSA genes increase [PSI+] destabilization and counteract prion recovery. Notably, [PSI+] loss in cell divisions following heat shock is asymmetric and is preferentially confined to daughter cells (buds). This indicates that after stress, prion aggregates are preferentially trapped in the mother cells, resembling the distribution of aggregates of the oxidatively damaged proteins (Aguilaniu, et al., 2003, Liu, et al., 2011, Newnam, et al., 2011).
Figure 2. A model of prion destabilization by alteration of the chaperone balance during stress.
In the exponentially growing yeast cells, Hsp104 is present at a low level. It is rapidly accumulated during the first 30 min of heat shock and only moderately rises further during next 3.5 hrs at high temperature. In contrast, Hsp70-Ssa is present at the considerably higher background levels in exponential cells, compared to Hsp104. As a result, its levels rise slowly during heat shock, reaching maximum only by about 4 hrs of incubation at high temperature. Efficient fragmentation of Sup35 prion aggregates occurs when Hsp70-Ssa binds to aggregates and recruits Hsp104 to break prion polymers into prion fragments, propagons, inheritable by a daughter cell. When Hsp104 is present in an excess, it binds prion polymers on its own in the absence of Hsp70-Ssa but is not able to efficiently fragment them. We propose that the same occurs after short-term exposure to heat shock, as confirmed by increased size of Sup35 aggregates. As a result, large aggregates become trapped in the mother cell after a resumption of cell division, possibly in association with the quality control deposits of heat-damaged proteins. This apparently leads to prion clearance from a daughter cell, detected in the experiment. After longer incubation at high temperature, the balance between Hsp104 and Hsp70-Ssa is partly restored. This coincides with the prion recovery, suggesting that normal fragmentation and propagation of aggregates is resumed due to restoration of the chaperone balance. Images show colonies of a yeast strain bearing a weak prion variant, that are plated after various exposures to 39°C. Red color indicates prion loss. For details, see (Chernova, et al., 2011, Newnam, et al., 2011).
Recent data (Specht, et al., 2011, Seyffer, et al., 2012, Winkler, et al., 2012, Winkler, et al., 2012) show that efficient fragmentation of Sup35 prion aggregates occurs when Hsp70-Ssa (apparently in combination with one of Hsp40s, probably Sis1) binds to aggregates and then recruits Hsp104. The resulting complex breaks prion polymers into smaller fragments. When Hsp104 is present in excess and binds prion polymers in the absence of Hsp70-Ssa, it is not able to efficiently fragment them and instead, directs prion polymers to non-productive pathway eventually resulting in prion loss. At least during stress, this non-productive pathway is associated with asymmetric prion distribution in the cell division (see Figure 2 and Newnam, et al., 2011). Non-productive interaction between Sup35 polymers and Hsp104 apparently involves the portion of the Sup35M domain that immediately follows the prion domain, Sup35N (Helsen & Glover, 2012, Helsen & Glover, 2012).
As Hsp104, Hsp70 and Hsp40 represent the major complex involved in disaggregation and refolding of stress-damaged proteins in yeast (Glover & Lindquist, 1998, Glover & Lum, 2009), it is evident that the same chaperone machinery is employed in protection against environmental stresses and in modulation of amyloid propagation. An amyloid becomes a self-perpetuating transmissible entity (prion) in vivo as a result of its interactions with chaperones (Liebman & Chernoff, 2012).
One more player modulating the chaperone machinery of prion propagation is the Small Glutamine-rich Tetratricopeptide-containing cochaperone Sgt2. This protein was initially identified as a component of the Guided Entry of Tail-anchored (TA) proteins (GET) trafficking pathway (Stefanovic & Hegde, 2007, Favaloro, et al., 2008, Schuldiner, et al., 2008), and was thought to play a role in protection of the hydrophobic tails of TA proteins by promoting their association with the chaperones and other GET pathway components. Recent data show that Sgt2 also interacts with yeast prions, and modulates interactions of the Hsp104 and Ssa proteins with prion polymers, apparently via generating additional sites accessible for the Ssa/Hsp104/Hsp40 complex (Kiktev, et al., 2012). Sgt2, which is induced in response to stress, to alterations of the GET pathway, and/or to the presence of amyloids in the cell, provides an additional tool for physiological regulation of prion propagation.
Hsp70 and Hsp40 families are conserved in other organisms, including humans (Rikhvanov, et al., 2007) and likely interact with mammalian amyloids as well. Bacterial homologs of these proteins were recently found to be capable of replacing yeast chaperones in propagating yeast prions (Reidy, et al., 2012). Although Hsp104 orthologs are conserved in bacteria, plants and protists, they have not been found in multicellular animals. However, animal cells do exhibit a phenomenon of induced thermotolerance, attributed to Hsp104 in yeast. This suggests that the functional analog of Hsp104 is present in animal cells. Recent data suggest that unusual Hsp70-related chaperone Hsp110 can cooperate with Hsp70 and Hsp40 to achieve protein disaggregation (Shorter, 2011, Muralidharan, et al., 2012, Mattoo, et al., 2013). Although the mammalian protein disaggregation machinery was not able to remodel the polymers of yeast prion Sup35, it may have similar activity for mammalian prion-like proteins in native cellular context (Newby & Lindquist, 2013).
Role of chaperones in de novo prion formation
In addition to their crucial role in prion propagation, some chaperones also modulate de novo prion formation (for detailed review, see (Liebman & Chernoff, 2012)). Ssa overproduction or depletion of Ssb increases de novo [PSI+] induction by excess Sup35 (Chernoff, et al., 1999, Newnam, et al., 1999, Allen, et al., 2005). Ssb1/2Δ also increases spontaneous [PSI+] formation at normal levels of Sup35. Ssb is implicated in the folding of nascent polypeptides (James, et al. 1997) and antagonizing the accumulation of misfolded proteins (Willmund, et al., 2013), potential seeds for prion nucleation. Alteration of the heat shock factor (Hsf), regulating Hsp expression, drastically influences initial prion formation and changes the spectrum of the de novo induced [PSI+] variants (Park, et al., 2006). Excess Hsp104 increases de novo induction of the [URE3] prion (Kryndushkin, et al., 2011). Alterations in levels of Hsp40-Ydj1 (Kryndushkin, et al., 2011) or Sse1, yeast ortholog of Hsp100 (Fan, et al., 2007), also influence induction of [PSI+] and [URE3] prions. It should be noted that in most cases, effects of chaperones on de novo prion formation depend on the presence of nucleation factors, such as [PIN+] prion (see in more detail below). This indicates that in such cases, chaperones do not directly control the initial step of prion nucleation. For example, excess Hsp104 probably increases fragmentation of the [PIN+] prion and in this way, generates additional nuclei for cross-seeding of the [PSI+] prion.
Role of protein concentrations and heterologous aggregates in de novo prion formation
Although many proteins can generate amyloids in vitro (Chiti & Dobson, 2006), only a fraction of proteins possess prion-forming capabilities in vivo. The process of prion spread by aggregate shearing and nucleated polymerization, termed propagation, has been extensively studied in various model systems. However, the mechanisms leading to the formation of an initial prion nucleus, starting from the native conformation of the protein, remain a mystery. Yeast models offer a unique opportunity for studying prion formation due to the availability of large numbers and convenient phenotypic assays.
De novo formation of prion nuclei is facilitated when prion-forming protein is present at a high local concentration. This process is best understood in yeast, where a prion can be induced de novo by transient overproduction of a prion-forming protein. In the case of the [PSI+] prion, spontaneous de novo prion formation in yeast occurs at a low efficiency (less that 1 per million cells), while overproduction of the Sup35 protein or its PrD can increase the frequency of [PSI+] formation up to 10-50% of the cells (Chernoff, et al., 1993, Derkatch, et al., 1996). Prion induction by transient overproduction was also described for most of the other known yeast prions (Wickner, 1994, Derkatch, et al., 2001, Alberti, et al., 2009, Patel, et al., 2009, Rogoza, et al., 2010, Halfmann, et al., 2012, Suzuki, et al., 2012). One possible reason for overproduction to induce prion formation is that the increase in protein levels may also mean in an increase in the abundance of the misfolded protein molecules. Moreover, overproduction may make misfolding more likely to occur, for example because of an insufficient supply of chaperones. Accumulation of misfolded proteins may overwhelm proteolytic pathways and help misfolded protein molecules to escape degradation. Still, overproduction per se is not always sufficient for prion formation. Frequency of prion induction by transient overproduction is facilitated dramatically in the presence of other (heterologous) preexisting prions or prion-like aggregates (Figure 3). These observations in yeast have certain parallels in humans, e.g. the aggregation of amyloid β in Alzheimer's disease is typically accompanied by aggregation of another protein, tau, even though the causative relationships between these aggregates remain a matter of debate (Clavaguera, et al., 2009, Frost, et al., 2009). There is growing evidence that in a variety of neurodegenerative diseases protein aggregation is initiated by protein templating, or seeding, through interaction with a misfolded form of the protein by a mechanism resembling seeded crystallization (Walker & LeVine, 2012).
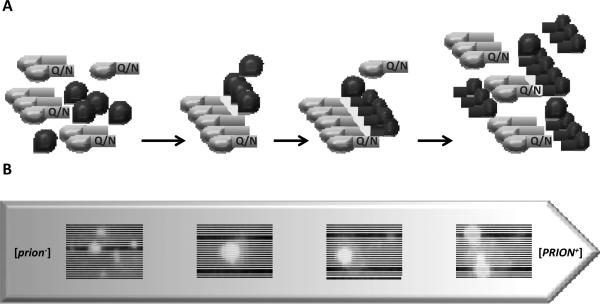
Figure 3. Preexisting Q/N-rich aggregates promote conversion of soluble heterological protein into a prion form.
(A) Cross-seeding model: Q/N rich aggregate (green) serves as a nucleation factor for initial aggregation of prion protein (red).
(B) Time-lapse confocal microscopy images of cells expressing Q/N rich protein Lsb2-GFP and prion protein Sup35NM-dsRED (Chernova, et al., 2011). Small aggregates of Sup35 fuse and grow into large aggregates in dynamic association with Lsb2. Aggregates of Lsb2 seed conversion of Sup35 into prion [PSI+].
The best studied example of prion cross-seeding in yeast is the dramatic enhancement of [PSI+] induction in the presence of [PIN+] (a prion form of the Rnq1 protein), other QN-rich prions, or overproduced aggregated QN-rich proteins (Derkatch, et al., 1997, Derkatch, et al., 2001, Osherovich & Weissman, 2001). This distinguishes initial prion nucleation from prion propagation, as [PIN+] is not required for the propagation of preexisting [PSI+] (Derkatch, et al., 2000). [PIN+] also enhances the de novo appearance of [URE3], although less dramatically (Bradley, et al., 2002), promotes aggregation of expanded polyglutamines (Osherovich & Weissman, 2001, Meriin, et al., 2002), and slightly (about twofold) increases the formation of the non-QN rich Podospora prion [Het-s] in yeast (Taneja, et al., 2007). This suggests that [PIN+] may function as a universal nucleating factor, not restricted only to QN-rich proteins.
Aggregates of other prionogenic proteins also promote nucleation of heterologous prions. Another prion, [URE3], facilitates de novo [PSI+] induction, and either [PIN+] or [PSI+] can facilitate formation of other prions when the other proteins are overexpressed (Derkatch & Liebman, 2007).
Furthermore, overexpression of a variety of proteins with QN-rich domains, forming aggregates in yeast efficiently induces [PSI+] formation in yeast cells overproducing the Sup35 and lacking any known pre-existing prions (Derkatch, et al., 2001, Derkatch, et al., 2004). In some cases, heterologous prion cross-seeding by overproduced nucleating protein may occur even without overproducing the “target” protein (Ross, et al., 2009, Vishveshwara & Liebman, 2009). Eleven heterologous [PSI+] inducers, including the cytoskeleton-associated protein Lsb2, or Pin3 (see below) were identified in a genetic screen for yeast proteins whose overproduction enables overproduced Sup35 to form [PSI+] in the absence of [PIN+] (that is, prion form of Rnq1), causing the so called prion inducing, or Pin+ phenotype (Derkatch, et al., 2001). Interestingly, three of the initially identified 11 proteins with the Pin+ effect after overproduction are also known to form prions on their own (Ure2, Swi1 and Cyc8), and at least two other proteins (New1, Lsm4) contain prion-like domains that propagate a prion state when fused to a reporter protein. However, the QN-rich domains of other prion-inducing proteins, such as Lsb2, Nup116 and Yck1, have not been shown to form a stable prion themselves, despite numerous tries (Alberti, et al., 2009, Chernova, et al., 2011). It therefore remains unclear whether a heterologous protein must form its own (even though unstable) prion in order to promote prion formation by another protein, or its aggregation and/or accumulation at a specific site (see below) is sufficient for nucleating another prion.
Two models were proposed to explain the heterologous prion cross-seeding: (1) titration of the cellular factors (e. g. chaperones) inhibiting prion formation by pre-existing aggregates, and/or (2) ability of a pre-existing aggregate to serve as the initial nucleus for de novo aggregation of the heterologous protein (Derkatch, et al., 2001, Osherovich & Weissman, 2001) (see Figure 3). While sequestered factors have not been identified thus far, despite intensive screens (e. g. (Osherovich & Weissman, 2002), there is significant evidence in support of the cross-seeding model. In vitro, Rnq1 fibers enhance the fibrillization of Sup35 PrD and vice versa (Derkatch, et al., 2004, Vitrenko, et al., 2007). Likewise, yeast Sup35 PrD overexpressed in bacteria formed amyloid fibers, but only if another QN-rich amyloid was already present (Garrity, et al., 2010).
It should be noted that in addition to enhancing each other's initial nucleation, heterologous prions may also interfere with each other's propagation. Certain variants of [PIN+] destabilize weak [PSI+] (Bradley & Liebman, 2003), [PSI+] and [URE3] prions destabilize each other (Bradley, et al., 2002, Schwimmer & Masison, 2002). Overexpression of some mutants of Rnq1, or fragments of Rnq1 PrD in the presence of [PIN+] inhibits propagation of [PSI+] and (in case of Rnq1 fragments) [URE3] (Kurahashi, et al., 2008, Kurahashi, et al., 2011). In some cases, prion destabilization is accompanied by increase in prion size and possible segregation defect (e. g. (Kurahashi, et al., 2011). Overexpression of some QN-rich proteins destabilizes pre-existing [PSI+] and [URE3] prions, apparently due to sequestering Hsp104 and Sis1 chaperones away from the diffuse cytoplasmic pool, and thus interfering with the ability of the prion aggregates to be sheared (Yang, et al., 2013).
Effects of the ubiquitin-proteasome system (UPS) on prions
Alterations in pathways of protein degradation have been linked to both heritable and sporadic aggregation-related neurodegenerative diseases (Ciechanover & Brundin, 2003). Protein aggregates can be disposed of by either the autophagy pathways (Kirkin, et al., 2009) or by the ubiquitin proteasome system (UPS). Protein ubiquitination is a reversible post-translational modification in which the 76 aa polypeptide called ubiquitin (Ub) is covalently linked, via its C-terminal glycine residue, to the ε-amino group of lysine residues in target proteins (Hershko & Ciechanover, 1998), via the sequential action of a Ub-activating enzyme, E1, Ub-conjugating enzymes, E2, and Ub-ligases, E3 (Kerscher, et al., 2006). Target protein can be either monoubiquitinated, or polyubiquitinated via subsequent rounds of ubiquitination, attaching additional Ub units to the chain initiated by the first Ub unit. As Ub contains several acceptor lysine residues, poly-Ub chains of various topologies can be generated (Pickart & Fushman, 2004, Ikeda & Dikic, 2008). K48-linked poly-Ub chain, of at least four Ub units or longer, serves as signals targeting proteins for degradation by the 26S proteasome (Welchman, et al., 2005). UPS failure leads to the accumulation and aggregation of misfolded proteins (Ciechanover & Brundin, 2003, Goldberg, 2003), which may result in enhanced nucleation of amyloids. On the other side, accumulation of protein aggregates can sequester Ub and other UPS components, inhibiting the proteasome and exerting pleiotropic effects on cellular metabolism (Lowe, et al., 1988, Mayer, et al., 1989, Andre & Tabrizi, 2012).
UPS defects have been linked to certain amyloid and neural inclusion diseases in mammals and humans (Dennissen, et al., 2012). Intracellular deposits of amyloid aggregates contain significant amounts of ubiquitin. Further, proteasome inhibitors affect the turnover of mammalian prion proteins, and proteasome activity is inhibited by disease-associated prion protein oligomers (Ma & Lindquist, 2001, Yedidia, et al., 2001, Apodaca, et al., 2006, Kristiansen, et al., 2007, Deriziotis & Tabrizi, 2008, Andre & Tabrizi, 2012).
In yeast, UPS alterations influence formation and propagation of the [PSI+] prion (Chernova, et al., 2003, Allen, et al., 2007). De novo [PSI+] induction by excess Sup35 is more efficient at increased Ub levels, and is reduced by a decrease in the levels of free Ub, for example in the strains lacking the deubiquitinating enzyme Ubp6 (Chernova et al. 2003). Mechanisms responsible for this phenomenon are not yet clear. One possibility is that increased targeting of Sup35 to UPS leads to generation of the partially degraded Sup35 fragments (including the QN-rich PrD region), which are more efficient in de novo prion nucleation, compared to the complete Sup35 protein (Derkatch, et al., 1996, Derkatch, et al., 1997, Kochneva-Pervukhova, et al., 1998). Indeed, it is known that degradation of polyglutamine sequences by the proteasome is inefficient (Venkatraman, et al., 2004).
Deletion of UBC4, which encodes one of the major yeast ubiquitin conjugating (E2) enzymes, increases both [PSI+] resistance to excess of chaperone Hsp104 and de novo [PSI+] formation (Allen et al. 2007). Notably, the increase of [PSI+] formation by ubc4Δ is detected even in the absence of any other prions, although it requires the presence of the Rnq1 protein. Genome-wide screens (Tyedmers, et al., 2008) identified additional genes, coding for UPS components, which influence de novo formation of [PSI+]. Respective UPS related proteins include: Rpn4, a regulator of the expression of proteasome genes; Ubp7, a Ub-specific protease; Pre9, the only nonessential protein of the 20S proteasome subunit; and Doa1, a Ub-binding protein that influences cellular Ub levels (Mullally, et al., 2006).
The simplest explanation for the effect of ubc4Δ (and possibly, other UPS-deficient deletions) on [PSI+] would be that a defect in ubiquitination prevents degradation of misfolded Sup35, thereby increasing its abundance and conversion into a prion. However, there is no evidence for direct ubiquitination of Sup35 (Allen et al. 2007). Another (not mutually exclusive) explanation could be that ubc4Δ acts via auxillary factors. Indeed, ubc4Δ increases the amount of the Hsp70-Ssa chaperone associated with Sup35 (Allen et al. 2007). Hsp70-Ssa is known to promote the formation and propagation of [PSI+] (see above), and is itself ubiquitinated (Peng, et al., 2003).
Protein quality control deposits and prions
In mammalian cells, inhibition of UPS by polyQ-containing proteins and other aggregates induces formation of the aggresome (Johnston, et al., 1998), a huge perinuclear structure that is assembled with the help of the microtubular cytoskeleton (Bennett, et al., 2005) and is probably degraded via autophagy (Iwata, et al., 2005). Aggresome formation is also detected in yeast, for example when the aggregation-prone fragment of the human huntingtin protein, containing the expanded polyQ region followed by P-rich region, is expressed in the yeast cell (Wang, et al., 2009).
Yeast aggresome colocalizes with the components of spindle body (a yeast counterpart of the centrosome), and its formation is inhibited by benomyl, an antagonist of the microtubular cytoskeleton (Wang, et al., 2009). PolyQ constructs assembled into an aggresome are present in amyloid-like detergent-resistant deposits (Gong, et al., 2012). The presence of the huntingtin's P-rich region greatly increases the efficiency of aggresome formation (Wang, et al., 2009). In the yeast cells containing the [PIN+] prion, expanded polyQ constructs lacking the P-rich region form multiple cytotoxic peripheral aggregates, inhibiting endocytosis due to sequestration of actin assembly proteins (Meriin, et al., 2002). In contrast, polyQP constructs are assembled into a single aggresome and are not cytotoxic (Wang, et al., 2009). However, aggresomes become cytotoxic in the presence of [PSI+] prion, due to sequestration of the release factor Sup45 (eRF1) mediated by interactions of the prion form of its partner, release factor Sup35 (eRF3) with an aggresome (Gong, et al., 2012). Thus, endogenous prions interact with quality control deposits and modulate their cytotoxicity.
Upon UPS impairment, misfolded yeast proteins are partitioned to two distinct compartments, the juxtanuclear quality control compartment, JUNQ and the insoluble protein deposit, IPOD (Kaganovich, et al., 2008). Protein deposits similar to JUNQ and IPOD have also been identified in mammalian cells (Weisberg, et al., 2012). In contrast to the aggresome, JUNQ typically contains soluble ubiquitinated proteins marked for proteasomal degradation. In cultured human cells, accumulation of insoluble aggregates in JUNQ inhibits the degradation of other misfolded proteins by sequestering the essential chaperone Hsp70 and thereby blocking the path of quality control substrates to the proteasome. Rerouting toxic aggregates from JUNQ to an insoluble polyQ inclusion restores protein degradation in JUNQ and rescues cell viability (Weisberg, et al., 2012).
IPOD contains insoluble aggregates, including amyloidogenic proteins. Targeting a soluble misfolded protein to IPOD instead of JUNQ can alter its solubility properties by inducing its conversion into an insoluble aggregate. Overproduction of Rnq1 protein in the cells containing the [PIN+] prion leads to formation of IPOD that sequesters spindle body components and causes cytotoxicity (Treusch & Lindquist, 2012). This suggests that formation of aggresome and IPOD may be based on similar or overlapping molecular mechanisms but result in different consequences: perinuclear aggresome does not interfere with the cell division apparatus, while IPOD may become toxic by relocating some spindle body components to the cell periphery.
Formation of protein quality control deposits in yeast is regulated by chaperones and by stress-inducible protein-sorting factors of Hook family. One of such proteins is Btn2, a yeast ortholog of the human protein Hook1 whose levels are elevated in case of Batten disease associated with the lysosomal disfunction (Weimer, et al., 2005). Yeast Btn2 interacts with the chaperones Hsp42 and Hsp40-Sis1 in directing misfolded protein to the peripheral (IPOD) or perinuclear (JUNQ) quality control deposits respectively (Malinovska, et al., 2012). Btn2 paralog, Cur1, mediates Btn2-dependent depletion of Sis1 from cytosol (Malinovska, et al., 2012). Overproduction of Btn2 or Cur1 causes elimination of the [URE3] prion (Kryndushkin, et al., 2008), suggesting that sequestration of prion aggregates and prion-controlling chaperones into quality control deposits may interfere with prion propagation.
Interactions of actin cytoskeleton with prions
The actin cytoskeleton plays an important role in trafficking proteins to specific intracellular locations, including deposition of damaged and aggregated proteins (Liu, et al., 2010). Yeast prion protein Sup35 interacts with various components of the actin cortical cytoskeleton (Bailleul, et al., 1999, Ganusova, et al., 2006), that are involved in endocytosis and are part of the regulatory network centered on the actin assembly factor Las17 (Toret & Drubin, 2006), a yeast homolog of the mammalian Wiskott-Aldrich syndrome protein (WASP). Prolonged disruption of the yeast actin cytoskeleton with latrunculin-A leads to destabilization and loss of the weak variant of [PSI+] (Bailleul-Winslett, et al., 2000). Mutation in actin or deletion of the genes coding for some of actin assembly proteins, such as Sla1, Sla2 or End3 (Bailleul, et al., 1999, Ganusova, et al., 2006), as well as Las17, Sac6 or Vps5 (Manogaran, et al., 2011), decrease both formation of aggregated structures and de novo [PSI+] induction by excess Sup35. Specifically, large filamentous structures are generated in the process of [PSI+] induction but are not present in cells containing mature [PSI+] prion (Ganusova, et al., 2006, Zhou, et al., 2001). These structures probably correspond to intermediates of the prion induction pathway and are affected by alterations of actin cytoskeleton. Peripheral filamentous structures overlap with peripheral actin patches and punctuated structures formed by actin assembly proteins such as Sla1 and Sla2. Internal filamentous structures surround the vacuole. Filamentous structures overlapped with clumps of preautophagosomal markers Atg8 and Atg14 (Tyedmers, et al., 2010). This suggests that misfolded Sup35 is assembled at the periphery of the cell and then transported to the vicinity of vacuole (Ganusova, et al., 2006, Mathur, et al., 2010). It appears that misfolded Sup35 (and possibly other misfolded amyloidogenic proteins) is (are) recruited to the actin cytoskeleton network, then targeted to and concentrated at quality control deposits with the purpose of detoxification and subsequent degradation. However, the increase of local protein concentration occurring as a result of this process stimulates de novo prion formation. Thus, quality control deposits may act as prion induction sites (Ganusova, et al., 2006, Tyedmers, et al., 2010). Initial filamentous agglomerates of Sup35 are toxic (Zhou, et al., 2001, Ganusova, et al., 2006, Manogaran, et al., 2011); thus, conversion of a misfolded protein into a prion may help to ameliorate toxicity when protein misfolding is increased and degradation machinery is overwhelmed. Deletions of genes coding for actin assembly proteins Arf1 and Bem1, the vesicle-trafficking protein Bug1, and the regulator of osmotic response and actin polymerization Hog1 reduced [PSI+] induction without affecting Sup35 filament formation, suggesting that these proteins probably act at later stages in the prion formation pathway (Manogaran, et al., 2011).
Some actin assembly proteins (e. g. Pan1, Sla2, Lsb2) contain QN-rich domains resembling PrDs. Las17-binding protein Lsb2 (Madania, et al., 1999), also called Pin3, is capable of aggregation and promotes [PSI+] induction by excess Sup35 in the absence of other prions, such as [PIN+], when present in high concentration (Derkatch, et al., 2001, Chernova, et al., 2011). Lsb2 association with actin patches and its polarized distribution in the budding cell suggest its role at the early steps of actin assembly regulated by actin nucleation factor Las17. Expression of the LSB2 gene is regulated by the stress-inducible transcription factor Hsf1 (Harbison, et al., 2004), and Lsb2 mRNA levels are induced 20-to 30-fold by various stresses (Gasch, et al., 2000). Accordingly, Lsb2 protein levels are dramatically increased by heat shock but return to pre-stress conditions after heat treatment is prolonged for more than 30 min (Chernova, et al., 2011). Notably, the levels of Lsb2, accumulated during heat stress, are similar to the levels promoting prion induction. Thus, Lsb2 accumulation in response to physiological stresses could be a trigger that induces prions (Figure 4). Indeed, a variety of environmental stresses have been shown to increase the frequency of de novo [PSI+] formation (see below). Association of Lsb2 with the actin cytoskeleton is required for its ability to aggregate and nucleate [PSI+] (Chernova, et al., 2011). Overproduced Lsb2 is transiently colocalized with overproduced Sup35, promoting its assembly into cytologically detectable aggregated structures. It appears that Lsb2 promotes assembly of misfolded proteins into prion-inducing sites (Chernova, et al., 2011). In the absence of Lsb2, [PSI+] destabilization by mild short-term heat shock (see above) is significantly increased, further confirming that Lsb2 mediates effects of stresses, the ubiquitin proteasome system and the actin cytoskeleton on a prion (Chernova, et al., 2011).
Figure 4. Model for the role of Lsb2 as a stress-dependent prion auxillary factor.
Under normal conditions, levels of the Q/N rich aggregation-prone cytoskeleton-associated protein Lsb2 in the cell are low and are tightly regulated by ubiquitinproteasome system. When level of Lsb2 is increased, protein become ubiquitinated (higher molecular weight bands) and quickly degraded. Short-term rise of the temperature to 39°C leads to strong increase in the Lsb2 protein levels, that may trigger the accumulation of misfolded Sup35 at the cytoskeleton-associated cortical locations. In the [PSI+] cells, this partially protects [PSI+] from uncontrolled agglomeration and elimination. In a small fraction of [psi−] cells, Lsb2 could seed conversion of accumulated Sup35 into the prion form [PSI+]. The proteolytically stable prion aggregates escape the quality control compartments, while Lsb2 aggregates are recycled and/or degraded.
Dramatic increase in levels of Lsb2, caused by heat shock and other stresses, may trigger assembly of misfolded Sup35 at the cytoskeleton associated cortical locations (Figure 4). This partly protects the preexisting [PSI+] prion from uncontrolled agglomeration and elimination during short-term heat shock. In [psi−] cells, the increased local concentration of Sup35 could facilitate prion formation (Chernova, et al., 2011). This effect probably becomes more pronounced in a small fraction of cells that have accumulated Lsb2 in a transiently aggregated state for longer periods of time. At the end, proteolytically stable prion aggregates could escape the quality control compartments while Lsb2 could be recycled or degraded. Remarkably, the increase in levels of Lsb2 caused 81% loss of [URE3] prion, suggesting that increased levels of Lsb2 induced by thermal stress could influence phenotypic variation by altering the balance of chaperones needed for prion propagation, in response to environmental stimuli (Yang, et al., 2013). Thus, Lsb2 can be considered as a multifunctional “stress-dependent prion modifier”, which can rearrange complex prion composition in the cell in response to environmental stress.
Lsb2 is not among those yeast proteins shown to form a prion in vivo (Alberti, et al., 2009, Chernova, et al., 2011). Since Lsb2 is a short-lived protein, it is possible that it forms a transient prion-like state (also supported by T. Chernova, D. Kiktev, A. Romanyuk, K. Wilkinson, Y. Chernoff, unpublished data). It is an attractive possibility that such a short-lived prion-like state of Lsb2 is needed to restrict its ability to target misfolded proteins to the quality control compartments or serve as prion induction sites after stress conditions are relieved. Notably, [PSI+] prions are frequently unstable when they first appear (Derkatch, et al., 1996, Derkatch, et al., 2000). Formation and quick loss of unstable prions could be an efficient way to control misfolded protein toxicity in changing environments.
Interestingly, the endocytosis-related components of the actin cytoskeleton also interact with the peripheral aggregates formed by huntingtin-derived constructs with expanded polyQ regions in yeast (Meriin, et al., 2003). In the presence of [PIN+] prion, polyQ toxicity is associated with a block in endocytosis (Meriin, et al., 2003). The [PIN+] prion apparently plays a role of nucleating factor for polyQ aggregates, and possibly mediates or facilitates their interactions with cortical cytoskeleton. A human homolog of the yeast actin assembly protein Sla2, HIP1, also interacts with huntingtin in mammalian cells (for review, see (Bhattacharyya, et al., 2008)).
Segregation of protein aggregates and prions in cell divisions
Efficient mitotic transmission of prions requires that prions distribute into both mother and daughter cells. It is not known if this distribution occurs in result of simple diffusion of multiple prion units, or “propagons”, see (Cox, et al., 2007, Byrne, et al., 2009), generated in result of prion fragmentation by the Hsp104/70/40 machinery, or involves the cell quality control partitioning apparatus.
In S. cerevisiae, the process of cell division (budding) is morphologically asymmetric, producing one mother cell and one daughter cell. Segregation of damaged (for example, carbonylated) and aggregated proteins is also asymmetric as they are preferentially accumulated in the mother cell. Although some arguments were made in favor of the model explaining mother-specific retention of protein aggregates by decreased diffusion through bud neck (Zhou, et al., 2011), solid experimental evidence suggests that this process is active, and involves Hsp104, actin cytoskeleton, sirtuin Sir2, and possibly a polarisome (Aguilaniu, et al., 2003, Erjavec, et al., 2007, Tessarz, et al., 2009, Liu, et al., 2010, Liu, et al., 2011). Mother cells can produce only a limited number of buds and then it dies. Asymmetric accumulation of the large aggregates of stress-damaged proteins in the older mother cells serves as a mechanism protecting “rejuvenated” daughter cells free from toxic protein aggregation (Aguilaniu, et al., 2003, Liu, et al., 2010). It is also likely that aggregate accumulation contributes to the process of aging of mother cells. It is possible that confinement of aggregates to the quality control deposits (see above) may serve as one of the general mechanisms for excluding aggregates from nascent daughter cells (Spokoini, et al., 2012).
As mentioned above, yeast prion [PSI+] exhibits asymmetric mother-specific distribution in the cell divisions after heat stress, leading to increased prion loss (Newnam, et al., 2011). Therefore, segregation of a yeast prion parallels the segregation of heat-damaged aggregated proteins (see above, Figure 2). An attractive hypothesis is that when the chaperone-based prion fragmentation machinery is inefficient due to chaperone imbalance, Sup35 prion aggregates are associated with the quality control deposits composed of heat-damaged proteins and therefore trapped in mother cells. Effects of chaperones and actin assembly protein Lsb2 on [PSI+] stability after heat shock (see above) do indeed point in this direction. Notably, mother specific retention and daughter-specific loss of [PSI+] was also detected in conditions when Hsp104-mediated polymer fragmentation is inhibited by guanidine-HCl and the number of propagons is diluted in cell divisions (Cox, et al., 2003, Cox, et al., 2007). Aging yeast cells accumulate larger Sup35 aggregates, consistent with their decreased transmission to daughters (Derdowski, et al., 2010). Asymmetric segregation of damaged aggregated proteins has also been detected in the yeast Schizosaccharomyces pombe, despite the lack of morphological asymmetry in cell division (Erjavec, et al., 2008). There is little doubt in that divisions of mammalian cells, for example in the process of cell differentiation, are functionally asymmetric (e. g., see Inaba & Yamashita, 2012). It is therefore possible that intracellular amyloid proteins are asymmetrically distributed and accumulated in certain cell types in mammals and humans as well.
Prion induction by stress
In mammals, few environmental factors have been shown to induce amyloids, although a prominent example is exposure to a pesticide linked to Parkinson disease (Song, et al., 2011). In yeast, formation of [URE3], [PSI+] and [PIN+] is facilitated by prolonged incubation at low temperature (M. Aigle, unpublished data quoted in (Chernoff, et al., 1995, Derkatch, et al., 2000, Chernoff, 2007). Formation of [PSI+] is also induced by a variety of other environmental stresses including heat stress, osmotic and oxidative stresses, and the unfolded protein response (Tyedmers, et al., 2008). In mutants defective in ribosome-associated peroxiredoxins, [PSI+] formation is induced by Sup35 oxidation (Sideri, et al., 2010, Sideri, et al., 2011). Ethanol stress induces formation of the [MOT3+] prion (see above, Holmes, et al., 2013). Mechanisms by which stressful conditions influence prion formation remain largely unknown. One possibility is that environmental stresses may act via influencing intracellular concentrations of the prionogenic and/or heterologous prion-inducing proteins, or by localizing (perhaps via heterologous ancillary proteins) prionogenic proteins to the prion induction sites. Heterologous proteins, whose levels are regulated via the Ub system, could serve as sensors connecting the process of prion/amyloid initiation to physiological and environmental signals (see an example of Lsb2 above). The action of such a regulated prion/amyloid induction pathway could be widespread, triggering the formation of many amyloids (both pathological and adaptive) in response to environmental signals.
Concluding remarks
In addition to their pathogenic effects, yeast prions manifest themselves as epigenetic regulators of phenotype, providing a robust but dynamic system of response to changing conditions. Prion formation and propagation are controlled by the yeast cell via multiple mechanisms, involving chaperones, cytoskeleton, quality control deposits and ubiquitinproteasome system. We propose that heterologous proteins, whose levels are regulated via the cellular quality control system, serve as sensors connecting the process of prion/amyloid initiation and propagation to physiological and environmental signals. This links physiological and environmental conditions to prions via alterations in protein homeostasis. Such a regulatory mechanism, if applicable beyond yeast, may present new opportunities for pharmacological interventions and the prophylactic measures aimed at amyloid disorders. The power of yeast genetics and availability of tractable prion-dependent phenotypes make yeast an excellent model for understanding the general mechanisms controlling physiological and environmental regulation of prions and amyloids.
Summary.
Cellular chaperones, degradation pathways and protein trafficking networks regulate prion formation and maintenance in a response to physiological and environmental changes.
Acknowledgments
This work was supported by the grant GM093294 (K.D.W, Y.O.C, T.A.C) from NIH, by the Ministry of Education and Science of Russian Federation, agreement 8874 (Y.O.C.), and by the research grant 1.50.2218.2013 from St. Petersburg State University (Y.O.C.). The content is solely the responsibility of the authors and does not necessary represent the official views of funding organizations.
References
- Aguilaniu H, Gustafsson L, Rigoulet M, Nystrom T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science. 2003;299:1751–1753. doi: 10.1126/science.1080418. [DOI] [PubMed] [Google Scholar]
- Aguzzi A, Rajendran L. The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron. 2009;64:783–790. doi: 10.1016/j.neuron.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Aguzzi A, O'Connor T. Protein aggregation diseases: pathogenicity and therapeutic perspectives. Nat Rev Drug Discov. 2010;9:237–248. doi: 10.1038/nrd3050. [DOI] [PubMed] [Google Scholar]
- Alberti S, Halfmann R, King O, Kapila A, Lindquist S. A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell. 2009;137:146–158. doi: 10.1016/j.cell.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen KD, Chernova TA, Tennant EP, Wilkinson KD, Chernoff YO. Effects of ubiquitin system alterations on the formation and loss of a yeast prion. J Biol Chem. 2007;282:3004–3013. doi: 10.1074/jbc.M609597200. [DOI] [PubMed] [Google Scholar]
- Allen KD, Wegrzyn RD, Chernova TA, et al. Hsp70 chaperones as modulators of prion life cycle: novel effects of Ssa and Ssb on the Saccharomyces cerevisiae prion [PSI+]. Genetics. 2005;169:1227–1242. doi: 10.1534/genetics.104.037168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre R, Tabrizi SJ. Misfolded PrP and a novel mechanism of proteasome inhibition. Prion. 2012;6:32–36. doi: 10.4161/pri.6.1.18272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apodaca J, Kim I, Rao H. Cellular tolerance of prion protein PrP in yeast involves proteolysis and the unfolded protein response. Biochem Biophys Res Commun. 2006;347:319–326. doi: 10.1016/j.bbrc.2006.06.078. [DOI] [PubMed] [Google Scholar]
- Bailleul PA, Newnam GP, Steenbergen JN, Chernoff YO. Genetic study of interactions between the cytoskeletal assembly protein sla1 and prion-forming domain of the release factor Sup35 (eRF3) in Saccharomyces cerevisiae. Genetics. 1999;153:81–94. doi: 10.1093/genetics/153.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailleul-Winslett PA, Newnam GP, Wegrzyn RD, Chernoff YO. An antiprion effect of the anticytoskeletal drug latrunculin A in yeast. Gene Expr. 2000;9:145–156. doi: 10.3727/000000001783992650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett EJ, Bence NF, Jayakumar R, Kopito RR. Global impairment of the ubiquitin-proteasome system by nuclear or cytoplasmic protein aggregates precedes inclusion body formation. Mol Cell. 2005;17:351–365. doi: 10.1016/j.molcel.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya NP, Banerjee M, Majumder P. Huntington's disease: roles of huntingtin-interacting protein 1 (HIP-1) and its molecular partner HIPPI in the regulation of apoptosis and transcription. FEBS J. 2008;275:4271–4279. doi: 10.1111/j.1742-4658.2008.06563.x. [DOI] [PubMed] [Google Scholar]
- Blanco LP, Evans ML, Smith DR, Badtke MP, Chapman MR. Diversity, biogenesis and function of microbial amyloids. Trends Microbiol. 2012;20:66–73. doi: 10.1016/j.tim.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley ME, Liebman SW. Destabilizing interactions among [PSI(+)] and [PIN(+)] yeast prion variants. Genetics. 2003;165:1675–1685. doi: 10.1093/genetics/165.4.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley ME, Edskes HK, Hong JY, Wickner RB, Liebman SW. Interactions among prions and prion “strains” in yeast. Proc Natl Acad Sci U S A 99 Suppl. 2002;4:16392–16399. doi: 10.1073/pnas.152330699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne LJ, Cole DJ, Cox BS, Ridout MS, Morgan BJ, Tuite MF. The number and transmission of [PSI] prion seeds (Propagons) in the yeast Saccharomyces cerevisiae. PLoS One. 2009;4:e4670. doi: 10.1371/journal.pone.0004670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoff YO. Mutation processes at the protein level: is Lamarck back? Mutat Res. 2001;488:39–64. doi: 10.1016/s1383-5742(00)00060-0. [DOI] [PubMed] [Google Scholar]
- Chernoff YO. Stress and prions: lessons from the yeast model. FEBS Lett. 2007;581:3695–3701. doi: 10.1016/j.febslet.2007.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoff YO. Identity determinants of infectious proteins. Proc Natl Acad Sci U S A. 2008;105:13191–13192. doi: 10.1073/pnas.0806234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoff YO, Derkach IL, Inge-Vechtomov SG. Multicopy SUP35 gene induces de-novo appearance of psi-like factors in the yeast Saccharomyces cerevisiae. Curr Genet. 1993;24:268–270. doi: 10.1007/BF00351802. [DOI] [PubMed] [Google Scholar]
- Chernoff YO, Lindquist SL, Ono B, Inge-Vechtomov SG, Liebman SW. Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [psi+]. Science. 1995;268:880–884. doi: 10.1126/science.7754373. [DOI] [PubMed] [Google Scholar]
- Chernoff YO, Newnam GP, Kumar J, Allen K, Zink AD. Evidence for a protein mutator in yeast: role of the Hsp70-related chaperone ssb in formation, stability, and toxicity of the [PSI] prion. Mol Cell Biol. 1999;19:8103–8112. doi: 10.1128/mcb.19.12.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoff YO, Galkin AP, Lewitin E, Chernova TA, Newnam GP, Belenkiy SM. Evolutionary conservation of prion-forming abilities of the yeast Sup35 protein. Mol Microbiol. 2000;35:865–876. doi: 10.1046/j.1365-2958.2000.01761.x. [DOI] [PubMed] [Google Scholar]
- Chernoff YO, Inge-Vechtomov SG, Derkach IL, Ptyushkina MV, Tarunina OV, Dagkesamanskaya AR, Ter-Avanesyan MD. Dosage-dependent translational suppression in yeast Saccharomyces cerevisiae. Yeast. 1992;8:489–499. doi: 10.1002/yea.320080702. [DOI] [PubMed] [Google Scholar]
- Chernova TA, Allen KD, Wesoloski LM, Shanks JR, Chernoff YO, Wilkinson KD. Pleiotropic effects of Ubp6 loss on drug sensitivities and yeast prion are due to depletion of the free ubiquitin pool. J Biol Chem. 2003;278:52102–52115. doi: 10.1074/jbc.M310283200. [DOI] [PubMed] [Google Scholar]
- Chernova TA, Romanyuk AV, Karpova TS, et al. Prion induction by the short-lived, stress-induced protein Lsb2 is regulated by ubiquitination and association with the actin cytoskeleton. Mol Cell. 2011;43:242–252. doi: 10.1016/j.molcel.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- Ciechanover A, Brundin P. The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron. 2003;40:427–446. doi: 10.1016/s0896-6273(03)00606-8. [DOI] [PubMed] [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby DW, Prusiner SB. Prions. Cold Spring Harb Perspect Biol. 2011;3:a006833. doi: 10.1101/cshperspect.a006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coustou V, Deleu C, Saupe S, Begueret J. The protein product of the het-s heterokaryon incompatibility gene of the fungus Podospora anserina behaves as a prion analog. Proc Natl Acad Sci U S A. 1997;94:9773–9778. doi: 10.1073/pnas.94.18.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox B, Ness F, Tuite M. Analysis of the generation and segregation of propagons: entities that propagate the [PSI+] prion in yeast. Genetics. 2003;165:23–33. doi: 10.1093/genetics/165.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BS. ψ, a cytoplasmic suppressor of super-suppressor in yeast. Heredity. 1965;20:505–521. [Google Scholar]
- Cox BS, Byrne LJ, Tuite MF. Prion stability. Prion. 2007;1:170–178. doi: 10.4161/pri.1.3.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennissen FJ, Kholod N, van Leeuwen FW. The ubiquitin proteasome system in neurodegenerative diseases: culprit, accomplice or victim? Prog Neurobiol. 2012;96:190–207. doi: 10.1016/j.pneurobio.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Derdowski A, Sindi SS, Klaips CL, DiSalvo S, Serio TR. A size threshold limits prion transmission and establishes phenotypic diversity. Science. 2010;330:680–683. doi: 10.1126/science.1197785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deriziotis P, Tabrizi SJ. Prions and the proteasome. Biochim Biophys Acta. 2008;1782:713–722. doi: 10.1016/j.bbadis.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Derkatch IL, Liebman SW. Prion-prion interactions. Prion. 2007;1:161–169. doi: 10.4161/pri.1.3.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkatch IL, Bradley ME, Hong JY, Liebman SW. Prions affect the appearance of other prions: the story of [PIN(+)]. Cell. 2001;106:171–182. doi: 10.1016/s0092-8674(01)00427-5. [DOI] [PubMed] [Google Scholar]
- Derkatch IL, Chernoff YO, Kushnirov VV, Inge-Vechtomov SG, Liebman SW. Genesis and variability of [PSI] prion factors in Saccharomyces cerevisiae. Genetics. 1996;144:1375–1386. doi: 10.1093/genetics/144.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkatch IL, Bradley ME, Zhou P, Chernoff YO, Liebman SW. Genetic and environmental factors affecting the de novo appearance of the [PSI+] prion in Saccharomyces cerevisiae. Genetics. 1997;147:507–519. doi: 10.1093/genetics/147.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkatch IL, Uptain SM, Outeiro TF, Krishnan R, Lindquist SL, Liebman SW. Effects of Q/N-rich, polyQ, and non-polyQ amyloids on the de novo formation of the [PSI+] prion in yeast and aggregation of Sup35 in vitro. Proc Natl Acad Sci U S A. 2004;101:12934–12939. doi: 10.1073/pnas.0404968101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkatch IL, Bradley ME, Masse SV, Zadorsky SP, Polozkov GV, Inge-Vechtomov SG, Liebman SW. Dependence and independence of [PSI(+)] and [PIN(+)]: a twoprion system in yeast? EMBO J. 2000;19:1942–1952. doi: 10.1093/emboj/19.9.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas PM, Treusch S, Ren HY, Halfmann R, Duennwald ML, Lindquist S, Cyr DM. Chaperone-dependent amyloid assembly protects cells from prion toxicity. Proc Natl Acad Sci U S A. 2008;105:7206–7211. doi: 10.1073/pnas.0802593105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z, Park KW, Yu H, Fan Q, Li L. Newly identified prion linked to the chromatin-remodeling factor Swi1 in Saccharomyces cerevisiae. Nat Genet. 2008;40:460–465. doi: 10.1038/ng.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaglestone SS, Cox BS, Tuite MF. Translation termination efficiency can be regulated in Saccharomyces cerevisiae by environmental stress through a prion-mediated mechanism. EMBO J. 1999;18:1974–1981. doi: 10.1093/emboj/18.7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erjavec N, Larsson L, Grantham J, Nystrom T. Accelerated aging and failure to segregate damaged proteins in Sir2 mutants can be suppressed by overproducing the protein aggregation-remodeling factor Hsp104p. Genes Dev. 2007;21:2410–2421. doi: 10.1101/gad.439307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erjavec N, Cvijovic M, Klipp E, Nystrom T. Selective benefits of damage partitioning in unicellular systems and its effects on aging. Proc Natl Acad Sci U S A. 2008;105:18764–18769. doi: 10.1073/pnas.0804550105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q, Park KW, Du Z, Morano KA, Li L. The role of Sse1 in the de novo formation and variant determination of the [PSI+] prion. Genetics. 2007;177:1583–1593. doi: 10.1534/genetics.107.077982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro V, Spasic M, Schwappach B, Dobberstein B. Distinct targeting pathways for the membrane insertion of tail-anchored (TA) proteins. J Cell Sci. 2008;121:1832–1840. doi: 10.1242/jcs.020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira PC, Ness F, Edwards SR, Cox BS, Tuite MF. The elimination of the yeast [PSI+] prion by guanidine hydrochloride is the result of Hsp104 inactivation. Mol Microbiol. 2001;40:1357–1369. doi: 10.1046/j.1365-2958.2001.02478.x. [DOI] [PubMed] [Google Scholar]
- Fowler DM, Kelly JW. Aggregating knowledge about prions and amyloid. Cell. 2009;137:20–22. doi: 10.1016/j.cell.2009.03.030. [DOI] [PubMed] [Google Scholar]
- Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009;284:12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganusova EE, Ozolins LN, Bhagat S, Newnam GP, Wegrzyn RD, Sherman MY, Chernoff YO. Modulation of prion formation, aggregation, and toxicity by the actin cytoskeleton in yeast. Mol Cell Biol. 2006;26:617–629. doi: 10.1128/MCB.26.2.617-629.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity SJ, Sivanathan V, Dong J, Lindquist S, Hochschild A. Conversion of a yeast prion protein to an infectious form in bacteria. Proc Natl Acad Sci U S A. 2010;107:10596–10601. doi: 10.1073/pnas.0913280107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch AP, Spellman PT, Kao CM, et al. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11:4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautschi M, Mun A, Ross S, Rospert S. A functional chaperone triad on the yeast ribosome. Proc Natl Acad Sci U S A. 2002;99:4209–4214. doi: 10.1073/pnas.062048599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebbink MF, Claessen D, Bouma B, Dijkhuizen L, Wosten HA. Amyloids--a functional coat for microorganisms. Nat Rev Microbiol. 2005;3:333–341. doi: 10.1038/nrmicro1127. [DOI] [PubMed] [Google Scholar]
- Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM, Anderson P. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol Biol Cell. 2004;15:5383–5398. doi: 10.1091/mbc.E04-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover JR, Lindquist S. Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell. 1998;94:73–82. doi: 10.1016/s0092-8674(00)81223-4. [DOI] [PubMed] [Google Scholar]
- Glover JR, Lum R. Remodeling of protein aggregates by Hsp104. Protein Pept Lett. 2009;16:587–597. doi: 10.2174/092986609788490087. [DOI] [PubMed] [Google Scholar]
- Gokhale KC, Newnam GP, Sherman MY, Chernoff YO. Modulation of prion-dependent polyglutamine aggregation and toxicity by chaperone proteins in the yeast model. J Biol Chem. 2005;280:22809–22818. doi: 10.1074/jbc.M500390200. [DOI] [PubMed] [Google Scholar]
- Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- Gong H, Romanova NV, Allen KD, et al. Polyglutamine toxicity is controlled by prion composition and gene dosage in yeast. PLoS Genet. 2012;8:e1002634. doi: 10.1371/journal.pgen.1002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishin AV, Rothenberg M, Downs MA, Blumer KJ. Mot3, a Zn finger transcription factor that modulates gene expression and attenuates mating pheromone signaling in Saccharomyces cerevisiae. Genetics. 1998;149:879–892. doi: 10.1093/genetics/149.2.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfmann R, Wright JR, Alberti S, Lindquist S, Rexach M. Prion formation by a yeast GLFG nucleoporin. Prion. 2012;6:391–399. doi: 10.4161/pri.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfmann R, Jarosz DF, Jones SK, Chang A, Lancaster AK, Lindquist S. Prions are a common mechanism for phenotypic inheritance in wild yeasts. Nature. 2012;482:363–368. doi: 10.1038/nature10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison CT, Gordon DB, Lee TI, et al. Transcriptional regulatory code of a eukaryotic genome. Nature. 2004;431:99–104. doi: 10.1038/nature02800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PM, Gerstein M. A method to assess compositional bias in biological sequences and its application to prion-like glutamine/asparagine-rich domains in eukaryotic proteomes. Genome Biol. 2003;4:R40. doi: 10.1186/gb-2003-4-6-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich SU, Lindquist S. Protein-only mechanism induces self-perpetuating changes in the activity of neuronal Aplysia cytoplasmic polyadenylation element binding protein (CPEB). Proc Natl Acad Sci U S A. 2011;108:2999–3004. doi: 10.1073/pnas.1019368108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsen CW, Glover JR. A new perspective on Hsp104-mediated propagation and curing of the yeast prion [PSI (+) ]. Prion. 2012;6:234–239. doi: 10.4161/pri.19913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsen CW, Glover JR. Insight into molecular basis of curing of [PSI+] prion by overexpression of 104-kDa heat shock protein (Hsp104). J Biol Chem. 2012;287:542–556. doi: 10.1074/jbc.M111.302869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Holmes DL, Lancaster AK, Lindquist S, Halfmann R. Heritable remodeling of yeast multicellularity by an environmentally responsive prion. Cell. 2013;153:153–165. doi: 10.1016/j.cell.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundley H, Eisenman H, Walter W, Evans T, Hotokezaka Y, Wiedmann M, Craig E. The in vivo function of the ribosome-associated Hsp70, Ssz1, does not require its putative peptide-binding domain. Proc Natl Acad Sci U S A. 2002;99:4203–4208. doi: 10.1073/pnas.062048399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iconomidou VA, Hamodrakas SJ. Natural protective amyloids. Curr Protein Pept Sci. 2008;9:291–309. doi: 10.2174/138920308784534041. [DOI] [PubMed] [Google Scholar]
- Ikeda F, Dikic I. Atypical ubiquitin chains: new molecular signals. ‘Protein Modifications: Beyond the Usual Suspects’ review series. EMBO Rep. 2008;9:536–542. doi: 10.1038/embor.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba M, Yamashita YM. Asymmetric stem cell division: precision for robustness. Cell Stem Cell. 2012;11:461–469. doi: 10.1016/j.stem.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Iwata A, Christianson JC, Bucci M, Ellerby LM, Nukina N, Forno LS, Kopito RR. Increased susceptibility of cytoplasmic over nuclear polyglutamine aggregates to autophagic degradation. Proc Natl Acad Sci U S A. 2005;102:13135–13140. doi: 10.1073/pnas.0505801102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P, Pfund C, Craig EA. Functional specificity among Hsp70 molecular chaperones. Science. 1997;275:387–389. doi: 10.1126/science.275.5298.387. [DOI] [PubMed] [Google Scholar]
- Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph SB, Kirkpatrick M. Effects of the [PSI+] prion on rates of adaptation in yeast. J Evol Biol. 2008;21:773–780. doi: 10.1111/j.1420-9101.2008.01515.x. [DOI] [PubMed] [Google Scholar]
- Jung G, Jones G, Masison DC. Amino acid residue 184 of yeast Hsp104 chaperone is critical for prion-curing by guanidine, prion propagation, and thermotolerance. Proc Natl Acad Sci U S A. 2002;99:9936–9941. doi: 10.1073/pnas.152333299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaganovich D, Kopito R, Frydman J. Misfolded proteins partition between two distinct quality control compartments. Nature. 2008;454:1088–1095. doi: 10.1038/nature07195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerscher O, Felberbaum R, Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol. 2006;22:159–180. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- Kiktev DA, Patterson JC, Muller S, Bariar B, Pan T, Chernoff YO. Regulation of chaperone effects on a yeast prion by cochaperone Sgt2. Mol Cell Biol. 2012;32:4960–4970. doi: 10.1128/MCB.00875-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU. Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem. 2013;82:323–355. doi: 10.1146/annurev-biochem-060208-092442. [DOI] [PubMed] [Google Scholar]
- Kirkin V, McEwan DG, Novak I, Dikic I. A role for ubiquitin in selective autophagy. Mol Cell. 2009;34:259–269. doi: 10.1016/j.molcel.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Kochneva-Pervukhova NV, Poznyakovski AI, Smirnov VN, Ter-Avanesyan MD. C-terminal truncation of the Sup35 protein increases the frequency of de novo generation of a prion-based [PSI+] determinant in Saccharomyces cerevisiae. Curr Genet. 1998;34:146–151. doi: 10.1007/s002940050379. [DOI] [PubMed] [Google Scholar]
- Kochneva-Pervukhova NV, Alexandrov AI, Ter-Avanesyan MD. Amyloid-mediated sequestration of essential proteins contributes to mutant huntingtin toxicity in yeast. PLoS One. 2012;7:e29832. doi: 10.1371/journal.pone.0029832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen M, Deriziotis P, Dimcheff DE, et al. Disease-associated prion protein oligomers inhibit the 26S proteasome. Mol Cell. 2007;26:175–188. doi: 10.1016/j.molcel.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Kryndushkin DS, Shewmaker F, Wickner RB. Curing of the [URE3] prion by Btn2p, a Batten disease-related protein. EMBO J. 2008;27:2725–2735. doi: 10.1038/emboj.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryndushkin DS, Engel A, Edskes H, Wickner RB. Molecular chaperone Hsp104 can promote yeast prion generation. Genetics. 2011;188:339–348. doi: 10.1534/genetics.111.127779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi H, Ishiwata M, Shibata S, Nakamura Y. A regulatory role of the Rnq1 nonprion domain for prion propagation and polyglutamine aggregates. Mol Cell Biol. 2008;28:3313–3323. doi: 10.1128/MCB.01900-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi H, Pack CG, Shibata S, Oishi K, Sako Y, Nakamura Y. [PSI(+)] aggregate enlargement in rnq1 nonprion domain mutants, leading to a loss of prion in yeast. Genes Cells. 2011;16:576–589. doi: 10.1111/j.1365-2443.2011.01511.x. [DOI] [PubMed] [Google Scholar]
- Kushnirov VV, Ter-Avanesyan MD. Structure and replication of yeast prions. Cell. 1998;94:13–16. doi: 10.1016/s0092-8674(00)81216-7. [DOI] [PubMed] [Google Scholar]
- Lacroute F. Non-Mendelian mutation allowing ureidosuccinic acid uptake in yeast. J Bacteriol. 1971;106:519–522. doi: 10.1128/jb.106.2.519-522.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman SW, Chernoff YO. Prions in yeast. Genetics. 2012;191:1041–1072. doi: 10.1534/genetics.111.137760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Larsson L, Caballero A, Hao X, Oling D, Grantham J, Nystrom T. The polarisome is required for segregation and retrograde transport of protein aggregates. Cell. 2010;140:257–267. doi: 10.1016/j.cell.2009.12.031. [DOI] [PubMed] [Google Scholar]
- Liu B, Larsson L, Franssens V, et al. Segregation of protein aggregates involves actin and the polarity machinery. Cell. 2011;147:959–961. doi: 10.1016/j.cell.2011.11.018. [DOI] [PubMed] [Google Scholar]
- Lowe J, Blanchard A, Morrell K, et al. Ubiquitin is a common factor in intermediate filament inclusion bodies of diverse type in man, including those of Parkinson's disease, Pick's disease, and Alzheimer's disease, as well as Rosenthal fibres in cerebellar astrocytomas, cytoplasmic bodies in muscle, and mallory bodies in alcoholic liver disease. J Pathol. 1988;155:9–15. doi: 10.1002/path.1711550105. [DOI] [PubMed] [Google Scholar]
- Ma J, Lindquist S. Wild-type PrP and a mutant associated with prion disease are subject to retrograde transport and proteasome degradation. Proc Natl Acad Sci U S A. 2001;98:14955–14960. doi: 10.1073/pnas.011578098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madania A, Dumoulin P, Grava S, et al. The Saccharomyces cerevisiae homologue of human Wiskott-Aldrich syndrome protein Las17p interacts with the Arp2/3 complex. Mol Biol Cell. 1999;10:3521–3538. doi: 10.1091/mbc.10.10.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maji SK, Perrin MH, Sawaya MR, et al. Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science. 2009;325:328–332. doi: 10.1126/science.1173155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A, Cesario WC, White-Grindley E, et al. Critical role of amyloid-like oligomers of Drosophila Orb2 in the persistence of memory. Cell. 2012;148:515–529. doi: 10.1016/j.cell.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Malinovska L, Kroschwald S, Munder MC, Richter D, Alberti S. Molecular chaperones and stress-inducible protein-sorting factors coordinate the spatiotemporal distribution of protein aggregates. Mol Biol Cell. 2012;23:3041–3056. doi: 10.1091/mbc.E12-03-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manogaran AL, Hong JY, Hufana J, Tyedmers J, Lindquist S, Liebman SW. Prion formation and polyglutamine aggregation are controlled by two classes of genes. PLoS Genet. 2011;7:e1001386. doi: 10.1371/journal.pgen.1001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo RU, Sharma SK, Priya S, Finka A, Goloubinoff P. Hsp110 is a bona fide chaperone using ATP to unfold stable misfolded polypeptides and reciprocally collaborate with hsp70 to solubilize protein aggregates. J Biol Chem. 2013;288:21399–21411. doi: 10.1074/jbc.M113.479253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur V, Taneja V, Sun Y, Liebman SW. Analyzing the birth and propagation of two distinct prions, [PSI+] and [Het-s](y), in yeast. Mol Biol Cell. 2010;21:1449–1461. doi: 10.1091/mbc.E09-11-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer RJ, Lowe J, Lennox G, Doherty F, Landon M. Intermediate filaments and ubiquitin: a new thread in the understanding of chronic neurodegenerative diseases. Prog Clin Biol Res. 1989;317:809–818. [PubMed] [Google Scholar]
- McGlinchey RP, Shewmaker F, McPhie P, Monterroso B, Thurber K, Wickner RB. The repeat domain of the melanosome fibril protein Pmel17 forms the amyloid core promoting melanin synthesis. Proc Natl Acad Sci U S A. 2009;106:13731–13736. doi: 10.1073/pnas.0906509106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meriin AB, Zhang X, He X, Newnam GP, Chernoff YO, Sherman MY. Huntington toxicity in yeast model depends on polyglutamine aggregation mediated by a prion-like protein Rnq1. J Cell Biol. 2002;157:997–1004. doi: 10.1083/jcb.200112104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meriin AB, Zhang X, Miliaras NB, et al. Aggregation of expanded polyglutamine domain in yeast leads to defects in endocytosis. Mol Cell Biol. 2003;23:7554–7565. doi: 10.1128/MCB.23.21.7554-7565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelitsch MD, Weissman JS. A census of glutamine/asparagine-rich regions: implications for their conserved function and the prediction of novel prions. Proc Natl Acad Sci U S A. 2000;97:11910–11915. doi: 10.1073/pnas.97.22.11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullally JE, Chernova T, Wilkinson KD. Doa1 is a Cdc48 adapter that possesses a novel ubiquitin binding domain. Mol Cell Biol. 2006;26:822–830. doi: 10.1128/MCB.26.3.822-830.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan V, Oksman A, Pal P, Lindquist S, Goldberg DE. Plasmodium falciparum heat shock protein 110 stabilizes the asparagine repeat-rich parasite proteome during malarial fevers. Nat Commun. 2012;3:1310. doi: 10.1038/ncomms2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayashiki T, Kurtzman CP, Edskes HK, Wickner RB. Yeast prions [URE3] and [PSI+] are diseases. Proc Natl Acad Sci U S A. 2005;102:10575–10580. doi: 10.1073/pnas.0504882102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namy O, Galopier A, Martini C, Matsufuji S, Fabret C, Rousset JP. Epigenetic control of polyamines by the prion [PSI+]. Nat Cell Biol. 2008;10:1069–1075. doi: 10.1038/ncb1766. [DOI] [PubMed] [Google Scholar]
- Ness F, Ferreira P, Cox BS, Tuite MF. Guanidine hydrochloride inhibits the generation of prion “seeds” but not prion protein aggregation in yeast. Mol Cell Biol. 2002;22:5593–5605. doi: 10.1128/MCB.22.15.5593-5605.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby GA, Lindquist S. Blessings in disguise: biological benefits of prion-like mechanisms. Trends Cell Biol. 2013;23:251–259. doi: 10.1016/j.tcb.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Newnam GP, Birchmore JL, Chernoff YO. Destabilization and recovery of a yeast prion after mild heat shock. J Mol Biol. 2011 doi: 10.1016/j.jmb.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newnam GP, Wegrzyn RD, Lindquist SL, Chernoff YO. Antagonistic interactions between yeast chaperones Hsp104 and Hsp70 in prion curing. Mol Cell Biol. 1999;19:1325–1333. doi: 10.1128/mcb.19.2.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osherovich LZ, Weissman JS. Multiple Gln/Asn-rich prion domains confer susceptibility to induction of the yeast [PSI(+)] prion. Cell. 2001;106:183–194. doi: 10.1016/s0092-8674(01)00440-8. [DOI] [PubMed] [Google Scholar]
- Osherovich LZ, Weissman JS. The utility of prions. Dev Cell. 2002;2:143–151. doi: 10.1016/s1534-5807(02)00118-1. [DOI] [PubMed] [Google Scholar]
- Park KW, Hahn JS, Fan Q, Thiele DJ, Li L. De novo appearance and “strain” formation of yeast prion [PSI+] are regulated by the heat-shock transcription factor. Genetics. 2006;173:35–47. doi: 10.1534/genetics.105.054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YN, Morales D, Rubinson EH, Masison D, Eisenberg E, Greene LE. Differences in the curing of [PSI+] prion by various methods of Hsp104 inactivation. PLoS One. 2012;7:e37692. doi: 10.1371/journal.pone.0037692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel BK, Gavin-Smyth J, Liebman SW. The yeast global transcriptional corepressor protein Cyc8 can propagate as a prion. Nat Cell Biol. 2009;11:344–349. doi: 10.1038/ncb1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Schwartz D, Elias JE, et al. A proteomics approach to understanding protein ubiquitination. Nat Biotechnol. 2003;21:921–926. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- Pickart CM, Fushman D. Polyubiquitin chains: polymeric protein signals. Curr Opin Chem Biol. 2004;8:610–616. doi: 10.1016/j.cbpa.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB. Cell biology. A unifying role for prions in neurodegenerative diseases. Science. 2012;336:1511–1513. doi: 10.1126/science.1222951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy M, Masison DC. Modulation and elimination of yeast prions by protein chaperones and co-chaperones. Prion. 2011;5:245–249. doi: 10.4161/pri.5.4.17749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy M, Miot M, Masison DC. Prokaryotic chaperones support yeast prions and thermotolerance and define disaggregation machinery interactions. Genetics. 2012;192:185–193. doi: 10.1534/genetics.112.142307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resende CG, Outeiro TF, Sands L, Lindquist S, Tuite MF. Prion protein gene polymorphisms in Saccharomyces cerevisiae. Mol Microbiol. 2003;49:1005–1017. doi: 10.1046/j.1365-2958.2003.03608.x. [DOI] [PubMed] [Google Scholar]
- Rikhvanov EG, Romanova NV, Chernoff YO. Chaperone effects on prion and nonprion aggregates. Prion. 2007;1:217–222. doi: 10.4161/pri.1.4.5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts BT, Moriyama H, Wickner RB. [URE3] prion propagation is abolished by a mutation of the primary cytosolic Hsp70 of budding yeast. Yeast. 2004;21:107–117. doi: 10.1002/yea.1062. [DOI] [PubMed] [Google Scholar]
- Rogoza T, Goginashvili A, Rodionova S, et al. Non-Mendelian determinant [ISP+] in yeast is a nuclear-residing prion form of the global transcriptional regulator Sfp1. Proc Natl Acad Sci U S A. 2010;107:10573–10577. doi: 10.1073/pnas.1005949107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer L, Scheibel T. The elaborate structure of spider silk: structure and function of a natural high performance fiber. Prion. 2008;2:154–161. doi: 10.4161/pri.2.4.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CD, McCarty BR, Hamilton M, Ben-Hur A, Ross ED. A promiscuous prion: efficient induction of [URE3] prion formation by heterologous prion domains. Genetics. 2009;183:929–940. doi: 10.1534/genetics.109.109322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saupe SJ. A short history of small s: a prion of the fungus Podospora anserina. Prion. 2007;1:110–115. doi: 10.4161/pri.1.2.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saupe SJ. The [Het-s] prion of Podospora anserina and its role in heterokaryon incompatibility. Semin Cell Dev Biol. 2011;22:460–468. doi: 10.1016/j.semcdb.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Schuldiner M, Metz J, Schmid V, et al. The GET complex mediates insertion of tail-anchored proteins into the ER membrane. Cell. 2008;134:634–645. doi: 10.1016/j.cell.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwimmer C, Masison DC. Antagonistic interactions between yeast [PSI(+)] and [URE3] prions and curing of [URE3] by Hsp70 protein chaperone Ssa1p but not by Ssa2p. Mol Cell Biol. 2002;22:3590–3598. doi: 10.1128/MCB.22.11.3590-3598.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sertil O, Kapoor R, Cohen BD, Abramova N, Lowry CV. Synergistic repression of anaerobic genes by Mot3 and Rox1 in Saccharomyces cerevisiae. Nucleic Acids Res. 2003;31:5831–5837. doi: 10.1093/nar/gkg792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyffer F, Kummer E, Oguchi Y, et al. Hsp70 proteins bind Hsp100 regulatory M domains to activate AAA+ disaggregase at aggregate surfaces. Nat Struct Mol Biol. 2012;19:1347–1355. doi: 10.1038/nsmb.2442. [DOI] [PubMed] [Google Scholar]
- Sharma D, Masison DC. Functionally redundant isoforms of a yeast Hsp70 chaperone subfamily have different antiprion effects. Genetics. 2008;179:1301–1311. doi: 10.1534/genetics.108.089458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D, Masison DC. Single methyl group determines prion propagation and protein degradation activities of yeast heat shock protein (Hsp)-70 chaperones Ssa1p and Ssa2p. Proc Natl Acad Sci U S A. 2011;108:13665–13670. doi: 10.1073/pnas.1107421108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter J. The mammalian disaggregase machinery: Hsp110 synergizes with Hsp70 and Hsp40 to catalyze protein disaggregation and reactivation in a cell-free system. PLoS One. 2011;6:e26319. doi: 10.1371/journal.pone.0026319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si K, Lindquist S, Kandel ER. A neuronal isoform of the aplysia CPEB has prion-like properties. Cell. 2003;115:879–891. doi: 10.1016/s0092-8674(03)01020-1. [DOI] [PubMed] [Google Scholar]
- Si K, Choi YB, White-Grindley E, Majumdar A, Kandel ER. Aplysia CPEB can form prion-like multimers in sensory neurons that contribute to long-term facilitation. Cell. 2010;140:421–435. doi: 10.1016/j.cell.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Sideri TC, Stojanovski K, Tuite MF, Grant CM. Ribosome-associated peroxiredoxins suppress oxidative stress-induced de novo formation of the [PSI+] prion in yeast. Proc Natl Acad Sci U S A. 2010;107:6394–6399. doi: 10.1073/pnas.1000347107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sideri TC, Koloteva-Levine N, Tuite MF, Grant CM. Methionine oxidation of Sup35 protein induces formation of the [PSI+] prion in a yeast peroxiredoxin mutant. J Biol Chem. 2011;286:38924–38931. doi: 10.1074/jbc.M111.272419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondheimer N, Lindquist S. Rnq1: an epigenetic modifier of protein function in yeast. Mol Cell. 2000;5:163–172. doi: 10.1016/s1097-2765(00)80412-8. [DOI] [PubMed] [Google Scholar]
- Song C, Kanthasamy A, Jin H, Anantharam V, Kanthasamy AG. Paraquat induces epigenetic changes by promoting histone acetylation in cell culture models of dopaminergic degeneration. Neurotoxicology. 2011;32:586–595. doi: 10.1016/j.neuro.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht S, Miller SB, Mogk A, Bukau B. Hsp42 is required for sequestration of protein aggregates into deposition sites in Saccharomyces cerevisiae. J Cell Biol. 2011;195:617–629. doi: 10.1083/jcb.201106037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spokoini R, Moldavski O, Nahmias Y, England JL, Schuldiner M, Kaganovich D. Confinement to organelle-associated inclusion structures mediates asymmetric inheritance of aggregated protein in budding yeast. Cell Rep. 2012;2:738–747. doi: 10.1016/j.celrep.2012.08.024. [DOI] [PubMed] [Google Scholar]
- Stefanovic S, Hegde RS. Identification of a targeting factor for posttranslational membrane protein insertion into the ER. Cell. 2007;128:1147–1159. doi: 10.1016/j.cell.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Stein KC, True HL. The [RNQ+] prion: a model of both functional and pathological amyloid. Prion. 2011;5:291–298. doi: 10.4161/pri.5.4.18213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki G, Tanaka M. Active conversion to the prion state as a molecular switch for cellular adaptation to environmental stress. Bioessays. 2013;35:12–16. doi: 10.1002/bies.201200121. [DOI] [PubMed] [Google Scholar]
- Suzuki G, Shimazu N, Tanaka M. A yeast prion, Mod5, promotes acquired drug resistance and cell survival under environmental stress. Science. 2012;336:355–359. doi: 10.1126/science.1219491. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Collins SR, Toyama BH, Weissman JS. The physical basis of how prion conformations determine strain phenotypes. Nature. 2006;442:585–589. doi: 10.1038/nature04922. [DOI] [PubMed] [Google Scholar]
- Taneja V, Maddelein ML, Talarek N, Saupe SJ, Liebman SW. A non-Q/N-rich prion domain of a foreign prion, [Het-s], can propagate as a prion in yeast. Mol Cell. 2007;27:67–77. doi: 10.1016/j.molcel.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessarz P, Schwarz M, Mogk A, Bukau B. The yeast AAA+ chaperone Hsp104 is part of a network that links the actin cytoskeleton with the inheritance of damaged proteins. Mol Cell Biol. 2009;29:3738–3745. doi: 10.1128/MCB.00201-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toret CP, Drubin DG. The budding yeast endocytic pathway. J Cell Sci. 2006;119:4585–4587. doi: 10.1242/jcs.03251. [DOI] [PubMed] [Google Scholar]
- Treusch S, Lindquist S. An intrinsically disordered yeast prion arrests the cell cycle by sequestering a spindle pole body component. J Cell Biol. 2012;197:369–379. doi: 10.1083/jcb.201108146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- True HL, Lindquist SL. A yeast prion provides a mechanism for genetic variation and phenotypic diversity. Nature. 2000;407:477–483. doi: 10.1038/35035005. [DOI] [PubMed] [Google Scholar]
- True HL, Berlin I, Lindquist SL. Epigenetic regulation of translation reveals hidden genetic variation to produce complex traits. Nature. 2004;431:184–187. doi: 10.1038/nature02885. [DOI] [PubMed] [Google Scholar]
- Tyedmers J, Madariaga ML, Lindquist S. Prion switching in response to environmental stress. PLoS Biol. 2008;6:e294. doi: 10.1371/journal.pbio.0060294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyedmers J, Treusch S, Dong J, McCaffery JM, Bevis B, Lindquist S. Prion induction involves an ancient system for the sequestration of aggregated proteins and heritable changes in prion fragmentation. Proc Natl Acad Sci U S A. 2010;107:8633–8638. doi: 10.1073/pnas.1003895107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman P, Wetzel R, Tanaka M, Nukina N, Goldberg AL. Eukaryotic proteasomes cannot digest polyglutamine sequences and release them during degradation of polyglutamine-containing proteins. Mol Cell. 2004;14:95–104. doi: 10.1016/s1097-2765(04)00151-0. [DOI] [PubMed] [Google Scholar]
- Verghese J, Abrams J, Wang Y, Morano KA. Biology of the heat shock response and protein chaperones: budding yeast (Saccharomyces cerevisiae) as a model system. Microbiol Mol Biol Rev. 2012;76:115–158. doi: 10.1128/MMBR.05018-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishveshwara N, Bradley ME, Liebman SW. Sequestration of essential proteins causes prion associated toxicity in yeast. Mol Microbiol. 2009;73:1101–1114. doi: 10.1111/j.1365-2958.2009.06836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishveshwara N, Liebman SW. Heterologous cross-seeding mimics cross-species prion conversion in a yeast model. BMC Biol. 2009;7:26. doi: 10.1186/1741-7007-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitrenko YA, Gracheva EO, Richmond JE, Liebman SW. Visualization of aggregation of the Rnq1 prion domain and cross-seeding interactions with Sup35NM. J Biol Chem. 2007;282:1779–1787. doi: 10.1074/jbc.M609269200. [DOI] [PubMed] [Google Scholar]
- Walker LC, LeVine H., 3rd Corruption and spread of pathogenic proteins in neurodegenerative diseases. J Biol Chem. 2012;287:33109–33115. doi: 10.1074/jbc.R112.399378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Meriin AB, Zaarur N, Romanova NV, Chernoff YO, Costello CE, Sherman MY. Abnormal proteins can form aggresome in yeast: aggresome-targeting signals and components of the machinery. FASEB J. 2009;23:451–463. doi: 10.1096/fj.08-117614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegrzyn RD, Bapat K, Newnam GP, Zink AD, Chernoff YO. Mechanism of prion loss after Hsp104 inactivation in yeast. Mol Cell Biol. 2001;21:4656–4669. doi: 10.1128/MCB.21.14.4656-4669.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer JM, Chattopadhyay S, Custer AW, Pearce DA. Elevation of Hook1 in a disease model of Batten disease does not affect a novel interaction between Ankyrin G and Hook1. Biochem Biophys Res Commun. 2005;330:1176–1181. doi: 10.1016/j.bbrc.2005.03.103. [DOI] [PubMed] [Google Scholar]
- Weisberg SJ, Lyakhovetsky R, Werdiger AC, Gitler AD, Soen Y, Kaganovich D. Compartmentalization of superoxide dismutase 1 (SOD1G93A) aggregates determines their toxicity. Proc Natl Acad Sci U S A. 2012;109:15811–15816. doi: 10.1073/pnas.1205829109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welchman RL, Gordon C, Mayer RJ. Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat Rev Mol Cell Biol. 2005;6:599–609. doi: 10.1038/nrm1700. [DOI] [PubMed] [Google Scholar]
- Werner-Washburne M, Stone DE, Craig EA. Complex interactions among members of an essential subfamily of hsp70 genes in Saccharomyces cerevisiae. Mol Cell Biol. 1987;7:2568–2577. doi: 10.1128/mcb.7.7.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner RB. [URE3] as an altered URE2 protein: evidence for a prion analog in Saccharomyces cerevisiae. Science. 1994;264:566–569. doi: 10.1126/science.7909170. [DOI] [PubMed] [Google Scholar]
- Wickner RB. A new prion controls fungal cell fusion incompatibility. Proc Natl Acad Sci U S A. 1997;94:10012–10014. doi: 10.1073/pnas.94.19.10012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner RB, Edskes HK, Bateman D, Kelly AC, Gorkovskiy A. The yeast prions [PSI+] and [URE3] are molecular degenerative diseases. Prion. 2011;5:258–262. doi: 10.4161/pri.5.4.17748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmund F, del Alamo M, Pechmann S, Chen T, Albanese V, Dammer EB, Peng J, Frydman J. The cotranslational function of ribosome-associated Hsp70 in eukaryotic protein homeostasis. Cell. 2013;152:196–209. doi: 10.1016/j.cell.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler J, Tyedmers J, Bukau B, Mogk A. Chaperone networks in protein disaggregation and prion propagation. J Struct Biol. 2012;179:152–160. doi: 10.1016/j.jsb.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Winkler J, Tyedmers J, Bukau B, Mogk A. Hsp70 targets Hsp100 chaperones to substrates for protein disaggregation and prion fragmentation. J Cell Biol. 2012;198:387–404. doi: 10.1083/jcb.201201074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Hong JY, Derkatch IL, Liebman SW. Heterologous gln/asn-rich proteins impede the propagation of yeast prions by altering chaperone availability. PLoS Genet. 2013;9:e1003236. doi: 10.1371/journal.pgen.1003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yedidia Y, Horonchik L, Tzaban S, Yanai A, Taraboulos A. Proteasomes and ubiquitin are involved in the turnover of the wild-type prion protein. EMBO J. 2001;20:5383–5391. doi: 10.1093/emboj/20.19.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Park YN, Todor H, Moomau C, Masison D, Eisenberg E, Greene LE. Sequestration of Sup35 by aggregates of huntingtin fragments causes toxicity of [PSI+] yeast. J Biol Chem. 2012;287:23346–23355. doi: 10.1074/jbc.M111.287748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Slaughter BD, Unruh JR, Eldakak A, Rubinstein B, Li R. Motility and segregation of Hsp104-associated protein aggregates in budding yeast. Cell. 2011;147:1186–1196. doi: 10.1016/j.cell.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Derkatch IL, Liebman SW. The relationship between visible intracellular aggregates that appear after overexpression of Sup35 and the yeast prion-like elements [PSI(+)] and [PIN(+)]. Mol Microbiol. 2001;39:37–46. doi: 10.1046/j.1365-2958.2001.02224.x. [DOI] [PubMed] [Google Scholar]