Abstract
Background and Aims
Young adults show the highest rates of escalating drinking, yet the neural risk mechanisms remain unclear. Heavy drinkers show variant functional magnetic resonance imaging (fMRI) blood oxygen level-dependent (BOLD) response to alcohol cues, which may presage increasing drinking. In this longitudinal study, we ascertained whether BOLD response to alcohol pictures predicted subsequent heavy drinking among college students.
Methods
Participants were forty-three 18- to 21-year-olds in the United States who underwent BOLD scanning and completed monthly substance use surveys over the following year. Participants were categorized according to baseline and follow-up drinking into 13 continuously moderate drinkers, 16 continuously heavy drinkers, and 14 transitioners who drank moderately at baseline but heavily by follow-up. During fMRI scanning at baseline, participants viewed alcohol and matched non-alcohol beverage images.
Results
We observed group differences in alcohol cue-elicited BOLD response in bilateral caudate, orbitofrontal cortex, medial frontal cortex/anterior cingulate and left insula (clusters>2619ml, voxel-wise F(2,40)>3.23, p<.05, whole-brain corrected p<.05), where transitioners hyperactivated compared with moderate and heavy drinkers (all Tukey p<.05). Exploratory factor analysis revealed a single brain network differentiating those who subsequently increased drinking. Exploratory regressions showed that, compared with other risk factors (e.g., alcoholism family history, impulsivity), BOLD response best predicted escalating drinking amount and alcohol-related problems.
Conclusions
Neural response to pictures of alcohol is substantially enhanced among United States college students who subsequently escalate drinking. Greater cue-reactivity is associated with larger increases in drinking and alcohol-related problems, regardless of other baseline factors. Thus, neural cue-reactivity could uniquely facilitate identifying individuals at greatest risk for future problematic drinking.
Keywords: alcoholism, imaging, cue reactivity, fMRI, development, adolescence
fMRI Response to Alcohol Pictures Predicts Subsequent Transition to Heavy Drinking in College Students
In the United States, 18–25-year-olds show the highest prevalence of escalating alcohol consumption and dependence (AD) (1). For instance, 16% of 21-year-olds disclose past-month heavy drinking, a 5-fold increase from age 17 (1), and 6% of 18–25-year-olds report past-year AD. It is critical to characterize the neurobiological features subserving accelerating drinking during this vulnerable period.
Functional magnetic resonance imaging (fMRI) studies suggest neural indicators of AD risk in reward-related and other brain regions. Non-dependent individuals with a family history of AD under-activate during monetary reward anticipation, demonstrating altered reward sensitivity that may underlie alcoholism liability (2, 3). Abnormal inhibition-related activation is associated with AD risk (4) and predicts subsequent escalating drinking in adolescents (5, 6). Impulsivity and reward sensitivity may interact in AD (7) further perpetuating risk. However, these may be general risks for addictive behaviors, rather than specific vulnerabilities for AD (8). Thus, exploring alcohol-related reward may provide additional insight into alcoholism risk.
In response to alcohol cues, individuals with AD activate cue-reactive networks including amygdala, anterior cingulate (ACC), ventromedial prefrontal cortex (VMPFC), ventral striatum/nucleus accumbens, dorsal striatum, and orbitofrontal cortex (OFC) (9, 10) and this feature may presage intensifying drinking. Our study of college students suggested that neural cue-reactivity reflects personal drinking experience rather than pre-existing risks such as alcoholism family history (11). Additionally, teenagers with AD exhibit similar cue-induced activation as adults with AD (12). Together, these findings could indicate that cue-reactivity develops early in the course of drinking, and may help predict accelerating use. Indeed, greater cue-elicited brain response was associated with relapse following treatment (13), highlighting the utility of cue-reactivity in predicting increasing drinking. Alternatively, others suggest that social drinkers show stronger activation to alcohol cues than non-dependent heavy drinkers (10, 14), signifying shifts in cue-reactivity as drinking progresses. Characterizing neural response to alcohol cues across different phases of drinking may improve models of addiction and provide a unique marker predicting escalating drinking.
In this longitudinal study, we ascertained whether blood oxygen level-dependent (BOLD) fMRI response to alcohol pictures predicted subsequent drinking among college students. We hypothesized that moderate drinkers who transitioned to heavy drinking (transitioners) would show greater baseline BOLD response to alcohol images than those who remained continuously moderate drinkers within cue-reactive networks, including dorsal and ventral striatum, VMPFC, and OFC. We also predicted that transitioners would show similar activation as individuals who were continuously heavy drinkers.
Method
Participants
Participants were first-year students from two local colleges, recruited via school email, flyers, and classroom visits to participate in the Brain and Alcohol Research in College Students (BARCS) study (11). Participants provided written informed consent, approved by the institutional review boards at Central Connecticut State University, Trinity College, Hartford Hospital, and Yale University. A representative subset of 411 individuals, all free from MRI contraindications, underwent neuroimaging, and 132 were randomly selected to undergo alcohol cue-reactivity BOLD imaging. As part of the ongoing study, participants completed online monthly substance use surveys. Participants in the current study were selected from 63 individuals who completed ≥5 surveys during the year following scanning and met additional exclusion criteria, including current schizophrenia or bipolar disorder, history of seizures or significant head injury, ≤10 lifetime experiences with alcohol, not meeting our criteria for drinking groups (described below), and excessive motion during scanning (>4.5 mm displacement).
Participants were divided into three groups according to baseline and 12-month follow-up drinking quantity and frequency, based on similar studies by our group and others (5, 11). Baseline heavy drinking comprised binge drinking (≥4 drinks/occasion for females, ≥5 drinks/occasion for males) ≥13 of the past 26 weeks, and averaging ≥30 drinks/month (7 drinks/week) in the 6 months before scanning. Baseline moderate drinking encompassed binge drinking <13 of the previous 26 weeks, averaging ≤30 drinks/month, and never meeting criteria for AD. Participants completed monthly online surveys detailing their alcohol use; data from the year following scanning determined follow-up group. Congruent with baseline designations, follow-up heavy drinking included ≥30 drinks and ≥4 binges in a month. The final sample included 43 participants: (i) 13 moderate drinkers who drank moderately at baseline and follow-up; (ii) 14 transitioners who drank moderately at baseline, but heavily at follow-up; and (iii) 16 heavy drinkers, who drank heavily at baseline and follow-up (Table 1).
Table 1.
Participant characteristics.
| Moderate Drinkers (n = 13) |
Transitioners (n = 14) |
Heavy Drinkers (n = 16) |
p-value | |||
|---|---|---|---|---|---|---|
| Demographics and Substance Use | Moderate vs. Transitio ners |
Transition ers vs. Heavy |
Moder ate vs. Heavy |
|||
| Age (range 18 – 21) | 18.54 (0.88) | 18.21 (0.43) | 18.69 (0.79) | .48 | .19 | .85 |
| Female | 61.5% | 50.0% | 50.0% | .70 | >.99 | .71 |
| Caucasian | 84.6% | 85.7% | 75.0% | >.99 | .66 | .66 |
| Family history of alcoholism | 33.3% | 50.0% | 43.8% | .45 | >.99 | .71 |
| History of psychiatric disorder | 7.7% | 0.00% | 37.5% | .48 | .019 | .09 |
| History of drug use disorder | 0.0% | 14.3% | 12.5% | .48 | >.99 | .49 |
| Cigarette smoker | 7.7% | 0.0% | 0.0% | .48 | - | .46 |
| Baseline STAI T-score | 47.79 (8.60) | 51.58 (9.78) | 51.55 (10.67) | .59 | >.99 | .59 |
| Baseline BDI score | 2.08 (2.39) | 2.36 (2.87) | 3.87 (4.41) | .98 | .47 | .38 |
| Maximum follow-up STAI T-score | 55.33 (11.53) | 55.60 (11.53) | 58.51 (12.07) | >.99 | .78 | .75 |
| Maximum follow-up BDI score | 5.77 (5.67) | 4.93 (6.65) | 5.77 (6.11) | .94 | .77 | .95 |
| Positive urine toxicology screen at scan§ | 23.1% | 21.4% | 43.8% | .64 | .26 | .43 |
| Marijuana use score per month during 12 months after scan (possible range 1–6)c§ | 1.41 (1.29) | 2.13 (1.57) | 3.59 (1.87) | .06 | .045 | .001 |
| Other drug use score per month during 12 months after scan (possible range 5–30)b, c § | 5.00 (0.00) | 5.09 (0.33) | 5.68 (1.03) | .34 | .008 | .002 |
| Used drugs other than marijuana in 12 months after scanb, c | 0% | 7.1% | 56.3% | >.99 | .007 | .001 |
|
Drinking Characteristics | ||||||
| Baseline drinking (6 months before scan) | ||||||
| Drinks per monthb, c | 3.59 (6.16) | 12.90 (8.82) | 81.27 (58.29) | .78 | <.001 | <.001 |
| Binge episodes per monthb, c | 0.54 (1.45) | 1.29 (1.50) | 7.71 (3.92) | .75 | <.001 | <.001 |
| Follow-up drinking (7–12 months after scan) | ||||||
| Drinks per monthb, c | 3.21 (3.78) | 34.51 (17.21) | 77.09 (61.41) | .11 | .013 | <.001 |
| Binge episodes per month a, b, c | 0.45 (0.72) | 3.46 (2.26) | 7.80 (4.65) | .044 | .002 | <.001 |
| Change in drinking, follow-up vs. baseline | ||||||
| Drinks per montha, b | −0.38 (6.44) | 21.61 (16.45)* | −4.17 (32.61) | .038 | .008 | .89 |
| Binge episodes per month | −0.09 (1.47) | 2.18 (2.29)* | −0.09 (3.90) | .11 | .12 | .98 |
| BYAACQ score per month during 12 months after scan (possible range 8–40)b, c, § | 8.16 (0.28) | 8.97 (0.77) | 12.52 (3.55) | .001 | <.001 | <.001 |
| Lifetime number of drinks | 25.31 (25.06) | 95.36 (77.32) | 556.88 (1209.54) | .97 | .22 | .15 |
| Age of first drink | 16.31 (1.89) | 15.50 (1.99) | 14.75 (2.60) | .61 | .16 | .63 |
| Maximum lifetime drinks in 24 hoursa, b, c | 4.35 (2.27) | 10.14 (4.20) | 16.50 (6.13) | .006 | <.001 | .002 |
|
Impulsivity Factor Scores | ||||||
| Self-reported behavioral activation | −0.48 (2.66) | 0.41 (2.18) | 0.14 (1.69) | .55 | .94 | .74 |
| Self-reported compulsivity and reward/punishment sensitivity | −1.00 (1.57) | 0.08 (2.33) | 0.79 (2.22) | .38 | .63 | .07 |
| Self-reported impulsivity | −0.75 (1.97) | −0.16 (1.75) | 0.98 (2.26) | .72 | .30 | .07 |
| Behavioral temporal discounting | 0.21 (1.36) | −0.20 (1.06) | 0.03 (1.39) | .69 | .88 | .93 |
| Behavioral risk-taking | −0.03 (0.78) | −0.18 (1.24) | 0.17 (1.20) | .93 | .88 | .66 |
Transitioners ≠ Moderate drinkers,
Transitioners ≠ Heavy drinkers,
Moderate drinkers ≠ Heavy drinkers (p value is Tukey-corrected or Fisher’s Exact Test except for
Non-parametric tests with Bonferroni correction),
Change score ≠ 0 (p < .05)
Measures
Baseline measures
At baseline, we ascertained lifetime and past 6-month drinking and dependence diagnoses using an in-house interview that incorporates questions from the SSAGA (15) and the alcohol use module of the SCID (16), current and past DSM-IV diagnoses of anxiety, psychotic, mood and other substance use disorders using the MINI (17), family history of AD using the Family History Assessment Module (FHAM) (18), cigarette smoking with the Fagerstrom Test of Nicotine Dependence (19), and handedness. Mood during the month of scanning was assessed using the Spielberger State-Trait Anxiety Index (STAI) (20) and Beck Depression Inventory (BDI) (21). At the scan, participants provided urine toxicology samples, negative breathalyzer screens, and negative pregnancy screens (females).
Follow-up measures
For one year following scanning, participants completed monthly online surveys assessing alcohol and other drug use in the preceding month, including number of drinking days, binges, and typical and maximum drinks/occasion. Drug use was reported on a 6-point scale from “never” to “≥20” uses for each of several drugs (marijuana, cocaine, LSD, sedatives, stimulant medications, pain medications). Alcohol-related consequences were assessed with items from the Brief Young Adult Alcohol Consequences Questionnaire (BYAACQ) (22) on a 4-point scale indicating the number of times an individual experienced hangovers, feeling sick, pass-outs, memory black-outs, missing class, waking in unexpected places, impaired schoolwork, and needing a drink upon waking. Participants also completed the STAI and BDI monthly.
Impulsivity is a significant risk for AD (8), and may influence cue-reactivity (23). We obtained impulsivity measures we have linked to alcoholism risk and substance dependence (2, 24–27). Based on our previous principal components analysis on a separate sample (24), we computed five factors: (i) “Self-reported behavioral activation” included the Behavioral Inhibition System/Behavioral Activation System (28) drive, fun, and reward; (ii) “Self-reported compulsivity and reward/punishment sensitivity” comprised the Padua Inventory of obsessive-compulsive symptoms (29) total and Sensitivity to Punishment and Sensitivity to Reward Questionnaire (30) punishment and reward scores; (iii) “Self-reported impulsivity” included the Barratt Impulsiveness scale (BIS-11) (31) attention, motor, and nonplanning and Sensation Seeking Scale-Form V (32) total; (iv) “Behavioral temporal discounting” included the Experiential Discounting Task (33) and Behavioral Inhibition System/Behavioral Activation System inhibition score; and (v) “Behavioral risk taking” included the Balloon Analog Risk Task (34) total pumps adjusted average.
Alcohol Pictures Task
The Alcohol Pictures Task (11, 35) comprised 22 alcohol and 22 non-alcohol beverage images matched on valence, arousal, image complexity, brightness, and hue, with each image presented twice (see Figure 1). There were 44 degraded stimuli serving as a visual baseline. Each picture appeared for 1750ms with intermittent fixation periods (250–4250ms). Total task length was 5:54. Participants indicated whether they liked, disliked, or felt neutral about each image; ratings and reaction times were logged via fiber-optic response box.
Figure 1.
Sample task stimuli.
Image Acquisition
Functional images were collected axially with a Siemens 3T Allegra scanner using an echoplanar image gradient-echo pulse sequence (TR/TE=1500/28ms, flip angle=65°, FOV=24×24cm, matrix=64×64, in-plane resolution=3.4×3.4mm, effective slice thickness=5mm, 30 slices, whole-brain coverage). A sagittal T1 MPRAGE structural image was also acquired (TR/TE/TI=2300/2.74/900msec, flip angle=8°, slab thickness=176mm, FOV=176×256mm, matrix=176×256×176, voxel size=1mm3, pixel band-width=190Hz, scan time=10:09).
Image Processing
Functional images were preprocessed and modeled in SPM5 (http://www.fil.ion.uCM.ac.uk/spm/software/spm5/). Images were slice-time corrected, realigned, spatially normalized to Montreal Neurological Institute (MNI) space, resampled to 3×3×3mm voxels, and smoothed with a 5mm full-width, half-maximum Gaussian filter. Participants with movement >4.5mm were excluded. Events were modeled using a synthetic hemodynamic response function comprising two gamma functions (36). BOLD response was modeled for alcohol images, non-alcohol beverage images, and degraded images while covarying for motion and linear trends. Individual datasets were transformed to Talairach space (37) in AFNI (38) for group analysis.
Statistical Analyses
Demographic and Behavioral Analyses
Continuous demographic, behavioral, drinking data and impulsivity factor scores were compared between groups using ANOVA and post-hoc pairwise comparisons, Tukey-corrected at α=.05. Categorical variables were examined using pairwise Fisher exact tests, Bonferroni-corrected at α=.05. Group differences in drinking at baseline (averaged over 6 months pre-scan) and follow-up (averaged over 7–12 months post-scan) were examined with repeated measures ANOVA examining drinking group, time (baseline vs. follow-up), and their interaction. We examined group differences in BYAACQ scores, marijuana use, and other drug use during the follow-up period (average score/month over all 12 months) with Kruskal-Wallis tests. To understand changes in marijuana or other drug use during follow-up, for each group, we compared use/month for post-scan months 1–6 vs. 7–12 using Wilcoxon signed rank tests. Responses to Alcohol Pictures Task stimuli were investigated with repeated measures ANOVA with picture type (alcohol, non-alcohol) and picture rating (like, dislike, neutral) as within-subjects factors, and group as the between-subjects factor. Reaction times were analyzed with repeated measures ANOVA modeling picture type and drinking group.
fMRI Analyses
Group comparisons of BOLD response contrast to alcohol vs. non-alcohol beverage images were conducted in AFNI (38) using whole-brain ANOVA. Multiple comparisons corrections used cluster volume and voxel thresholding, determined a priori through Monte Carlo simulation (39): significant clusters comprised 2619µl (97 voxels, voxel-wise F(2,40)>3.23, p<.05; whole-brain α=.05). To interpret ANOVA results, we extracted average BOLD response in each cluster for each participant, and conducted planned all-pairwise comparisons (Tukey p<.05) in SPSS. Post-hoc analyses of alcohol images vs. implicit baseline and non-alcohol beverage images vs. implicit baseline in SPSS further characterized group effects. Because our prior work indicated that degraded image contrasts did not aid interpretation (11), we did not include these contrasts in group comparisons. Within each group difference cluster, separate exploratory two-factor ANOVAs examined sex, family history of AD, or recent drug use (positive urine toxicology screen) as additional covariates.
Exploratory Analyses
Exploratory analyses characterized whether baseline BOLD response to alcohol pictures predicts subsequent escalating drinking or alcohol-related consequences, above and beyond other known risk factors. First, an exploratory factor analysis of group ANOVA BOLD results identified the underlying composition of brain networks involved, and reduced the number of variables and multicollinearity in subsequent analyses. For each participant, average BOLD response to alcohol vs. non-alcohol beverage images was extracted from each ANOVA cluster and analyzed in SPSS. We used principal axis factoring with varimax rotation and Kaiser normalization, retaining factors with eigenvalues >1 and variables with communalities >.5. Factor scores were computed for each participant. Next, bivariate correlations examined the relationships between change in drinks/month, follow-up BYAACQ scores, and several possible predictor variables selected given their roles in alcoholism risk (8, 40–42), including fMRI factor scores, baseline drinks/month, binges/month, maximum lifetime drinks/occasion, age of first drink, and impulsivity factor scores. Baseline variables that correlated to follow-up alcohol variables were examined in step-wise regressions determining the best predictors of subsequent drinking and alcohol-related consequences. These step-wise analyses were conducted in those who were moderate drinkers at baseline (n=27) because we wanted to identify features underlying the transition from moderate to heavy drinking.
Results
Demographic and Behavioral Results
Groups were statistically similar on age, sex, race, family history of AD, and history of substance use disorders, baseline and follow-up STAI and BDI scores, and impulsivity factor scores (Table 1). Heavy drinkers showed higher rates of past psychiatric disorders than transitioners (p=.019, non-significant at Bonferroni-corrected α=.05) and more marijuana and other drug use days/month during follow-up than moderate drinkers and transitioners. No group showed a change in marijuana (Wilcoxon p=.47, p=.09, p=.70 for moderate, transitioners, and heavy drinkers, respectively) or other drug (Wilcoxon p=1, p=.32, p=.11 for moderate, transitioners, and heavy drinkers) use during follow-up. Groups showed similar proportions of positive toxicology screens; all positives were for cannabinoids except two for prescribed medications. All but two participants were right handed.
There was a group×time interaction for drinks/month [F(2,40)=5.59, p=.007]: heavy drinkers showed more baseline and follow-up drinks/month than others (Tukey p<.001), and transitioners demonstrated a greater change in drinks/month between baseline and follow-up than others (Tukey p<.05, Table 1). Heavy drinkers showed more binges/month than others ([F(2,40)=32.35, p<.001, Tukey p<.05]. Paired sample t-tests indicated that transitioners showed significant increases in drinks/month [t(13)=4.91, p<.001] and binges/month [t(13)=3.56, p=.003], while other groups did not significantly change drinks/month or binges/month.
There was a picture type×rating×group interaction [F(4,80)=6.24, p<.001]: heavy drinkers “liked” alcohol images more than other groups, and moderate drinkers “disliked” alcohol images more than heavy drinkers (Tukey p<.05, Table 2). Groups did not differ on responses to non-alcohol beverage pictures. There were no reaction time effects of group, picture type, or their interaction.
Table 2.
Alcohol Pictures Task responses
| Continuously Moderate Drinkers M(SD) |
Transitioned to Heavy Drinkers M (SD) |
Continuously Heavy Drinkers M (SD) |
|
|---|---|---|---|
| Alcohol picture responses (number) | |||
| Likea, b | 6.69 (4.85) | 16.36 (12.69) | 26.44 (11.68) |
| Dislike a | 17.77 (13.08) | 14.64 (13.08) | 6.31 (5.91) |
| Neutral | 14.46 (11.68) | 9.64 (11.49) | 6.38 (6.49) |
| Non-alcohol picture responses (number) | |||
| Like | 25.46 (11.22) | 27.93 (9.81) | 24.44 (10.54) |
| Dislike | 8.85 (9.06) | 9.14 (9.08) | 8.75 (7.52) |
| Neutral | 6.08 (6.64) | 5.29 (5.85) | 5.69 (5.38) |
| Alcohol picture reaction time (ms) | 905.39 (76.76) | 902.49 (126.86) | 859.74 (109.05) |
| Non-alcohol picture responses (ms) | 897.75 (95.62) | 921.57 (114.98) | 860.68 (98.93) |
Continuously moderate drinkers ≠ Continuously heavy drinkers (Tukey p < .05)
Transitioners ≠ Continuously heavy drinkers (Tukey p < .05)
fMRI Results
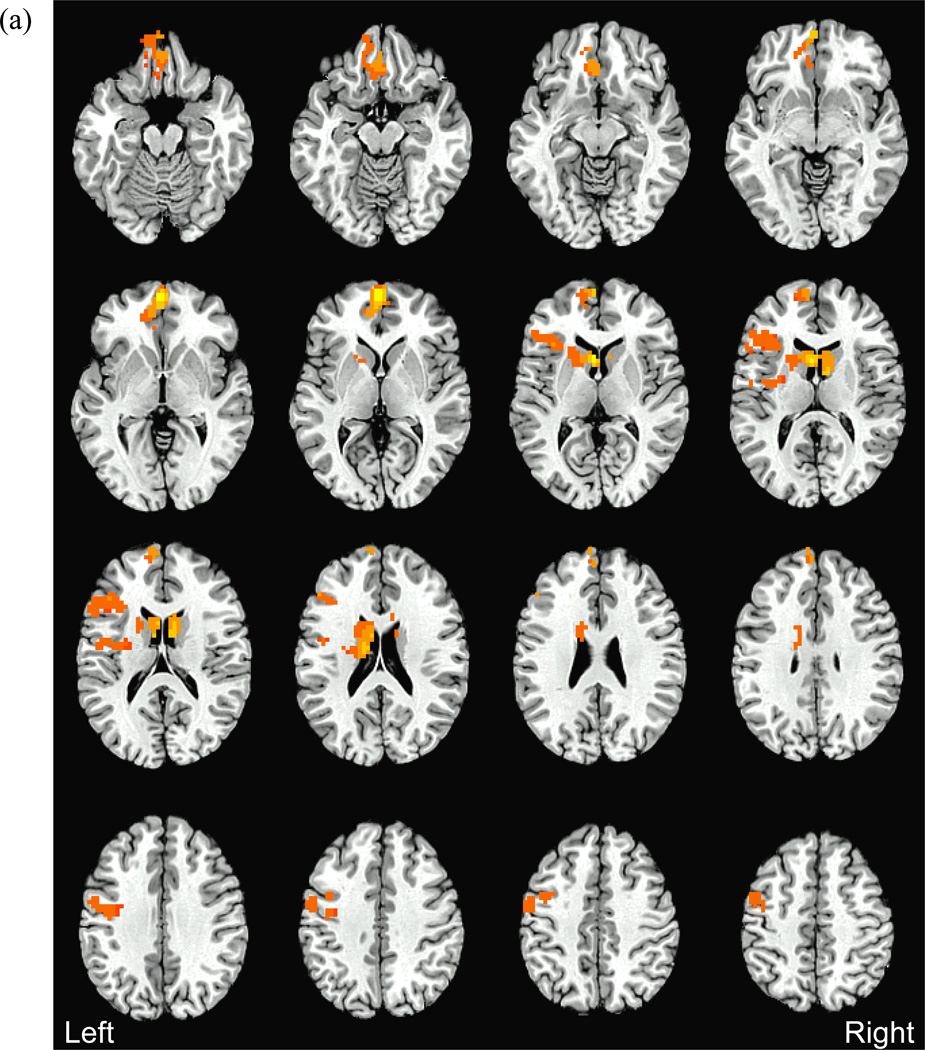
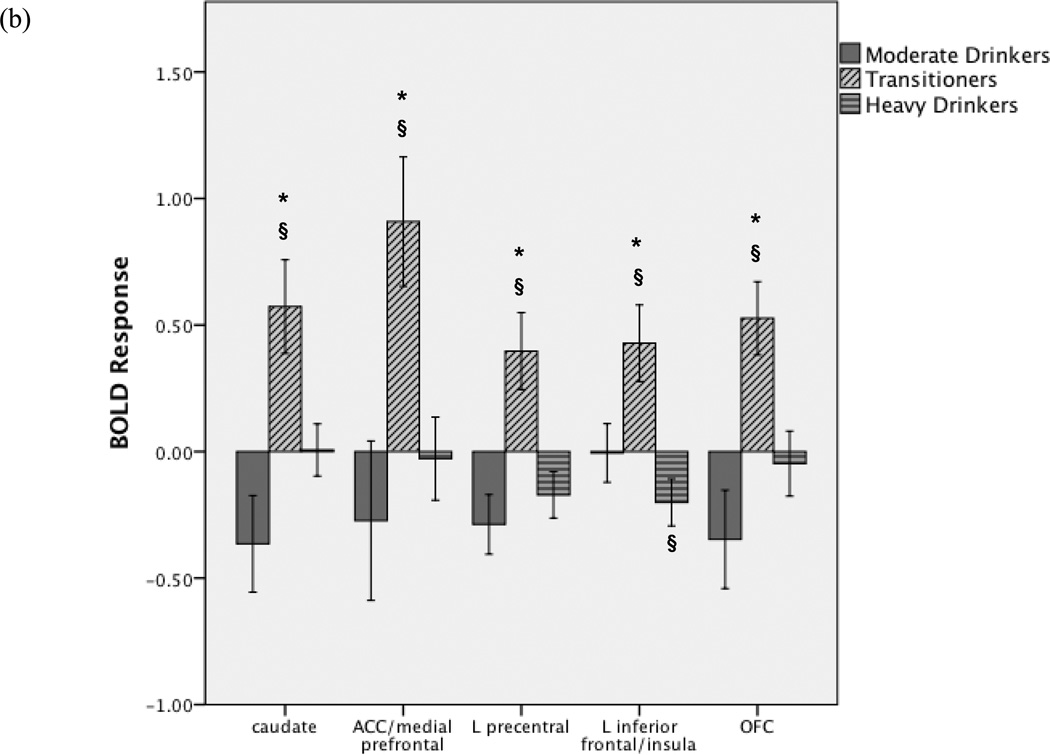
We observed group differences in BOLD response to alcohol vs. non-alcohol beverage images in VMPFC/ACC, bilateral OFC, bilateral caudate, left insula, and left precentral gyrus [F(2,40)>3.23, p<.05, Table 3, Figure 2]. In each region, transitioners showed greater BOLD response to alcohol vs. non-alcohol beverage images compared to other groups (Tukey p<.05). Follow-up tests confirmed this was not due to group differences in response to non-alcohol beverage images. In the left anterior insula/inferior frontal gyrus, although transitioners showed greater activation to alcohol relative to non-alcohol images, heavy drinkers showed greater response to non-alcohol beverages than to alcohol images, and greater response to non-alcohol images compared to other groups [F(2,40)=3.82, p<.03; Tukey p<.05]. Exploratory ANOVAs demonstrated no effect on BOLD response of the covariates sex, family history of AD, or positive urine toxicology screen (all ps>.10), and all group differences remained significant with these covariates included.
Table 3.
Regions showing significant group differences in BOLD response to alcohol vs. non-alcohol beverage images (clusters > 2619 µl, 97 voxels, F(2,40) > 3.23, p < .05 whole brain corrected).
| Anatomic Region (BA) |
Talairach Coordinates |
F(2,40) | Group Activation Mean (SD) | ||
|---|---|---|---|---|---|
| x, y, z | Continuously Moderate |
Transitioned to Heavy |
Continuously Heavy |
||
| Bilateral caudate, left thalamus, left mid-insula (13) | 3, −9, 9 | 8.39 | −0.37 (0.69) | 0.57 (0.69)*§ | 0.01 (0.41) |
| Bilateral medial frontal gyrus, anterior cingulate (10, 32) | 0, −57, 3 | 6.36 | −0.27 (1.14) | 0.91 (0.96)*§ | −0.03 (0.66) |
| Left precentral gyrus (6) | 45, 3, 51 | 8.88 | −0.29 § (0.42) | 0.40 (0.57)*§ | −0.17 (0.37) |
| Left anterior insula, inferior frontal gyrus (13) | 33, −21, 9 | 7.31 | −0.01 (0.42) | 0.43 (0.57)*§ | −0.20 § (0.37) |
| Left orbitofrontal cortex (11) | 0, −42, −18 | 7.86 | −0.35 (0.70) | 0.53 (0.54)*§ | −0.05 (0.52) |
Talairach coordinates refer to maximum intensity voxel within each cluster; F statistic and average activation refer to averages across each cluster. Positive BOLD response indicates greater response to alcohol vs. non-alcohol beverage images, negative BOLD response indicates greater response to non-alcohol vs. alcohol images.
For each cluster, Transitioners > Continuously moderate drinkers and Transitioners > Continuously heavy drinkers, p<.05
Group BOLD response (single sample t-test) significantly different from 0, p<.05
Figure 2.
(a) Between groups differences in BOLD response to alcohol vs. non-alcohol beverage images (clusters>2619µl, 97 voxels, voxel-wise F(2,40)>3.23, p<.05, whole-brain corrected p<.05). Post-hoc analyses revealed that in all clusters, transitioners showed greater response to alcohol vs. non-alcohol beverage images compared to continuously moderate and continuously heavy drinkers (Fig 2b). (b) Average BOLD response to alcohol vs. non-alcohol beverage images within each significant cluster in each group (OFC: orbitofrontal cortex, ACC: anterior cingulate cortex, L: left). Positive BOLD response indicates greater response to alcohol vs. non-alcohol beverage images, negative BOLD response indicates greater response to non-alcohol vs. alcohol images. *For each cluster, Transitioners > Continuously moderate drinkers and Transitioners > Continuously heavy drinkers, p<.05; §Group BOLD response (single sample t-test) significantly different from 0, p<.05. Error bars represent ±1 standard error.
Exploratory Results
The exploratory factor analysis included the five group ANOVA fMRI clusters. The Kaiser-Meyer-Olkin measure of sampling adequacy was 0.84 for the overall model (range=0.81–0.88 for each cluster), and Bartlett’s test of sphericity was significant (χ2(10)=161.69, p<.001), confirming the appropriateness of using factor analysis, despite the relatively small sample size. The factor structure resulted in sufficient communalities for each variable (range=0.60–0.77). All five fMRI regions loaded onto one factor with an eigenvalue of 3.54, accounting for 78% of the total variance. This fMRI factor score was utilized in the following step-wise regressions.
Bivariate correlations among moderate drinkers and transitioners revealed that change in drinks/month was related to fMRI factor scores [r=.72, p<.001] and to lifetime maximum drinks in 24 hours [r=.45, p=.019], but not to baseline drinks/month or binges/month, age of first drink, total lifetime drinks, or impulsivity factor scores. Step-wise regressions determined that fMRI factor scores accounted for 52% of the variance in change in drinks/month [F(1,25)=26.87, β=.72, p< .001] and maximum lifetime drinks was excluded from the model.
Follow-up BYAACQ scores were correlated to fMRI factor scores [r=.51, p=.006], baseline drinks/month [r=.52, p=.006], and the impulsivity factor “self-reported compulsivity and reward/punishment sensitivity” [r=.47, p=.014], but not to other tested variables. The final model contained two of these three predictors, and was reached in four steps (Table 4). fMRI factor scores uniquely accounted for 26% of the variance in subsequent BYAACQ scores, self-reported compulsivity and reward/punishment sensitivity accounted for 22%, and baseline drinks/month was excluded.
Table 4.
Step-wise regressions among continuously moderate drinkers and transitioned to heavy derinkers (n = 27). The dependent variable was follow-up alcohol-related consequences per month (BYAACQ score).
| Model Statistics | Change Statistics | Independent Variable Statistics | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | F | P | R2 | F | P | β | t | sr2 | p | |
| Model 1: | .27 | 9.23 | .006 | .27 | 9.23 | .006 | ||||
| 1) Baseline drinks per month | .52 | 3.04 | .27 | .006 | ||||||
| Model 2: | .44 | 9.41 | .001 | .17 | 7.27 | .013 | ||||
| 1) Baseline drinks per month | .43 | 2.75 | .18 | .011 | ||||||
| 2) fMRI factor score | .42 | 2.70 | .17 | .013 | ||||||
| Model 3: | .53 | 8.70 | <.001 | .09 | 4.52 | .045 | ||||
| 1) Baseline drinks per month | .26 | 1.58 | .05 | .127 | ||||||
| 2) fMRI factor score | .46 | 3.11 | .20 | .005 | ||||||
| 3) Self-reported compulsivity and sensitivity to reward/punishment factor score | .35 | 2.13 | .09 | .045 | ||||||
| Model 4: | .48 | 11.09 | <.001 | −.05 | 2.51 | .127 | ||||
| 1) fMRI factor score | .51 | 3.49 | .26 | .002 | ||||||
| 2) Self-reported compulsivity and sensitivity to reward/punishment factor score | .47 | 3.17 | .22 | .004 | ||||||
sr2 = squared semipartial correlation, representing the unique (unshared) variance accounted for by that independent variable
Discussion
This study characterized the relationship between neural response to alcohol picture cues and escalating drinking among college students. Participants underwent scanning during their first college year and completed monthly substance use surveys over the following year, capturing the period of greatest susceptibility to intensifying drinking (1). Compared to individuals who consistently remained moderate or heavy drinkers, those who increased from moderate to heavy drinking over the following year showed hyperactivation of networks associated with cue-reactivity in AD, including dorsal striatum, VMPFC, ACC, OFC, and insula (9, 10). Groups showed comparable activation to non-alcohol beverage images, indicating specific hyperactivation to alcohol cues, and not to appetitive stimuli in general. Moreover, greater cue-reactivity predicted larger increases in drinking and more alcohol-related problems, beyond other measured risk factors. Thus, cue-reactivity may provide a unique mechanism to identify individuals at greatest risk for subsequent problematic drinking.
Cue-reactivity and its associated circuitry may represent one feature within an addiction framework positing an imbalance among networks underlying reward, motivation, learning and memory, interoceptive awareness, and cognitive control (43). This model suggests that enhanced motivation for drug acquisition over-engages interconnected regions including dorsal striatum, medial OFC, ACC, and insula (43). In our factor analysis, activation of these regions contributed to a single factor, consistent with work indicating this circuitry in alcohol cue-reactivity (10). Our findings corroborate this model, demonstrating that hyper-reactivity of this network underlies subsequent escalating use.
Most notably, transitioners hyperactivated the caudate within the dorsal striatum, which mediates habit and compulsive drug-seeking (14, 44–46). Addiction models propose that caudate involvement in cue-reactivity may indicate shifting from initial reward-motivated phases of drug use to habit-driven stages (9, 11, 14). In the current study, enhanced caudate response predated heavy drinking, and may represent early stages of procedural learning and automatic processing contributing to compulsive use.
Transitioners showed augmented VMPFC/ACC response, which may underlie attention and motivation in alcohol cue-reactivity (9, 13, 47). Altered interactions between ACC and striatum may reflect shifting attention and motivation toward drug-related stimuli, contributing to addiction (43). Cue-induced VMPFC/ACC response may also link context and reward expectations (47). Consistent with this view, VMPFC/ACC cue-reactivity predicts craving (47) and relapse following treatment (13). Similarly, we previously suggested that VMPFC/ACC hyperactivation among heavy drinking college students may underlie greater attention and motivation for alcohol, which could subserve escalating drinking (11); our current results support this hypothesis.
In the context of cue-reactivity, OFC may signal drug availability and trigger anticipation of use, subserving drug-related decision-making (48). OFC cue-reactivity is stronger in non-treatment seeking individuals than in those in treatment, possibly reflecting intentions of use among current users (48). Similarly, among cocaine-dependent individuals, inhibiting craving corresponded to decreased cue-induced OFC metabolism, which could signify attempts to re-evaluate reinforcers and reduce expectancies of use (43). Thus, heightened OFC reactivity in transitioners could indicate cue-elicited expectation of consumption and biased decision-making (43, 48), contributing to increased drinking.
Finally, transitioners exhibited heightened insula activation, which underlies subjective interoceptive experiences, and relates to drug craving (49). Among methamphetamine users, insula response during decision-making was linked to relapse (50), highlighting its role in predicting drug use trajectories. Cue-induced insula activation may reflect conscious awareness of bodily and emotional states provoked by cues, contributing to stronger urges and drug seeking (49). Insula response to alcohol cues distinguishes non-dependent heavy drinkers from light drinkers, perhaps serving as a pre-diagnostic marker of maladaptive drinking (51). Indeed, our results suggest that insula cue-reactivity in moderate drinkers foreshadows the emergence of heavy drinking.
Heavy drinkers demonstrated less BOLD response than transitioners and similar activation as moderate drinkers. There may be several reasons for this. Heavy drinkers reported “liking” the alcohol images more than other groups did, and more than non-alcohol images; therefore, diminished cue-reactivity in heavy drinkers did not reflect poor subjective response to the images. Others have also reported stronger alcohol cue-elicited activation in social drinkers than heavy non-dependent drinkers (10, 14). Alcohol cue-induced BOLD response may reflect cue salience, motivation, and expectation of use following cue exposure (43). Although heavy drinkers here “liked” the alcohol images, the stimuli may not have engaged the same salience and motivational processes as in transitioners. Heavy drinkers may also be driven by different factors, including negative reinforcement, that are not captured by our paradigm. For instance, in AD adults, stress-induced BOLD response, rather than alcohol cue-elicited response, predicted alcohol craving and relapse following treatment (52). Additionally, heavy drinkers did not change their drinking overall, and our exploratory step-wise regressions demonstrated that cue-elicited BOLD response was more strongly associated with increasing subsequent alcohol involvement than baseline drinking. In support of this, others suggest that cue-reactivity predicts increased drinking following treatment, regardless of intake levels (13).
Our factor analysis demonstrated that several regions associated with cue-reactivity (9, 10) activated as a single factor that differentiated individuals who transitioned from moderate to heavy drinking and also was the primary predictor of change in drinking amount. This is of critical importance in identifying individuals at risk for escalating drinking, particularly as risk factors such as baseline drinking and impulsivity were unrelated to subsequent drinking. Further, the best model predicting alcohol-related consequences included fMRI response and the impulsivity factor, self-reported compulsivity and reward/punishment sensitivity. Our previous work in separate samples linked this same impulsivity factor to substance use risk and dependence (24–27) and to reward-related fMRI response in individuals at familial risk (2).
There are several strengths and limitations to this study. Despite the modest sample size, our novel longitudinal design linked neural cue-reactivity to subsequent drinking and alcohol-related problems. Baseline drinking was unrelated to alcoholism family history or impulsivity, two considerable risks for AD. Family history was unrelated to BOLD response or to change in drinking amount, nonetheless, this risk factor should be examined further in fMRI studies of alcoholism liability. Moreover, additional risk factors, including externalizing behavior, drinking motives, and other personality features, were not examined, but may moderate the relationship between cue-reactivity and drinking. As with any small preliminary study, replication and extension studies will be needed. Studies with multiple imaging time-points should characterize cue-reactivity throughout the course of initiation, escalation, maintenance, and decline of drinking. We hope that the current study lays the groundwork for such investigations.
In sum, we identified the first longitudinal evidence that amplified cue-elicited brain response predicts the emergence of heavy drinking in previously moderate college drinkers. This over-activation was observed in cue-reactive regions subserving habit formation, decision-making, motivation and attention, and was the most significant predictor of subsequent drinking and alcohol-related problems, consistent with models implicating these circuits in the development of addiction (43). Given that young adults are at elevated risk for escalating consumption and persistent future problem drinking, BOLD response to alcohol cues may help identify vulnerable individuals before the onset of maladaptive drinking.
Acknowledgements
This research was made possible by grant support from the National Institute on Alcohol Abuse and Alcoholism (AA016599 and AA19036, Pearlson) and the Alcohol Beverage Medical Research Foundation (Anderson). Portions of this work were presented at the International Conference on Applications of Neuroimaging to Alcoholism, February 2013. The authors thank Gregory Book, Meredith Ginley, Sharna Jamadar, Krishna Pancholi, Shashwath Meda, and Balaji Narayanan.
References
- 1.SAMHSA. NHSDA Series H-44. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012. Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. [Google Scholar]
- 2.Andrews MM, Meda SA, Thomas AD, Potenza MN, Krystal JH, Worhunsky P, Stevens MC, O'Malley S, Book GA, Reynolds B, Pearlson GD. Individuals family history positive for alcoholism show functional magnetic resonance imaging differences in reward sensitivity that are related to impulsivity factors. Biol Psychiatry. 2011;69:675–683. doi: 10.1016/j.biopsych.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yau WY, Zubieta JK, Weiland BJ, Samudra PG, Zucker RA, Heitzeg MM. Nucleus accumbens response to incentive stimuli anticipation in children of alcoholics: relationships with precursive behavioral risk and lifetime alcohol use. J Neurosci. 2012;32:2544–2551. doi: 10.1523/JNEUROSCI.1390-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silveri MM, Rogowska J, McCaffrey A, Yurgelun-Todd DA. Adolescents at risk for alcohol abuse demonstrate altered frontal lobe activation during Stroop performance. Alcohol Clin Exp Res. 2011;35:218–228. doi: 10.1111/j.1530-0277.2010.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend. 2011;119:216–223. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wetherill RR, Squeglia LM, Yang TT, Tapert SF. A longitudinal examination of adolescent response inhibition: neural differences before and after the initiation of heavy drinking. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-013-3198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck A, Schlagenhauf F, Wustenberg T, Hein J, Kienast T, Kahnt T, Schmack K, Hagele C, Knutson B, Heinz A, Wrase J. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 8.Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annu Rev Clin Psychol. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- 9.Heinz A, Beck A, Grusser SM, Grace AA, Wrase J. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addiction Biology. 2009;14:108–118. doi: 10.1111/j.1369-1600.2008.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol. 2013;18:121–133. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dager AD, Anderson BM, Stevens MC, Pulido C, Rosen R, Jiantonio-Kelly RE, Sisante J, Raskin SA, Tennen H, Austad CS, Wood RM, Fallahi CR, Pearlson GD. Influence of alcohol use and family history of alcoholism on neural response to alcohol cues in college drinkers. Alcoholism: Clinical and Experimental Research. 2013;37:E161–E171. doi: 10.1111/j.1530-0277.2012.01879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Archives of General Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- 13.Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- 14.Vollstadt-Klein S, Wichert S, Rabinstein J, Buhler M, Klein O, Ende G, Hermann D, Mann K. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction. 2010;105:1741–1749. doi: 10.1111/j.1360-0443.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- 15.Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- 16.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders - Research Version, Non-patient Edition (SCID-I/NP, 11/2002 revision) New York: Biometrics Research Department, New York State Psychiatric Institute; 2002. [Google Scholar]
- 17.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- 18.Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 19.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 20.Spielberger CD, Gorsuch RL, Lushene RE, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (Form Y) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 21.Beck AT. Beck Depression Inventory (BDI) San Antonio, TX: Psychological Corp; 1978. [Google Scholar]
- 22.Kahler CW, Strong DR, Read JP. Toward efficient and comprehensive measurement of the alcohol problems continuum in college students: the brief young adult alcohol consequences questionnaire. Alcohol Clin Exp Res. 2005;29:1180–1189. doi: 10.1097/01.alc.0000171940.95813.a5. [DOI] [PubMed] [Google Scholar]
- 23.Papachristou H, Nederkoorn C, Havermans R, van der Horst M, Jansen A. Can't stop the craving: the effect of impulsivity on cue-elicited craving for alcohol in heavy and light social drinkers. Psychopharmacology (Berl) 2012;219:511–518. doi: 10.1007/s00213-011-2240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meda SA, Stevens MC, Potenza MN, Pittman B, Gueorguieva R, Andrews MM, Thomas AD, Muska C, Hylton JL, Pearlson GD. Investigating the behavioral and self-report constructs of impulsivity domains using principal component analysis. Behav Pharmacol. 2009;20:390–399. doi: 10.1097/FBP.0b013e32833113a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmadi A, Pearlson GD, Meda SA, Dager A, Potenza MN, Rosen R, Austad CS, Raskin SA, Fallahi CR, Tennen H, Wood RM, Stevens MC. Influence of Alcohol Use on Neural Response to Go/No-Go Task in College Drinkers. Neuropsychopharmacology. 2013;38:2197–2208. doi: 10.1038/npp.2013.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hyatt CJ, Assaf M, Muska CE, Rosen RI, Thomas AD, Johnson MR, Hylton JL, Andrews MM, Reynolds BA, Krystal JH, Potenza MN, Pearlson GD. Reward-related dorsal striatal activity differences between former and current cocaine dependent individuals during an interactive competitive game. PLoS One. 2012;7:e34917. doi: 10.1371/journal.pone.0034917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devito EE, Meda SA, Jiantonio R, Potenza MN, Krystal JH, Pearlson GD. Neural Correlates of Impulsivity in Healthy Males and Females with Family Histories of Alcoholism. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- 29.Sanavio E. Obsessions and compulsions: the Padua Inventory. Behav Res Ther. 1988;26:169–177. doi: 10.1016/0005-7967(88)90116-7. [DOI] [PubMed] [Google Scholar]
- 30.Torrubia R, Avila C, Molto J, Caseras X. The sensitivity to punishment and sensitivity to reward questionnaire (SPSRQ) as a measure of Gray's anxiety and impulsivity dimensions. Personality and Individual Differences. 2001;31:837–862. [Google Scholar]
- 31.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 32.Zuckerman M, Neeb M. Sensation seeking and psychopathology. Psychiatry Res. 1979;1:255–264. doi: 10.1016/0165-1781(79)90007-6. [DOI] [PubMed] [Google Scholar]
- 33.Reynolds B, Schiffbauer R. Measuring state changes in human delay discounting: an experiential discounting task. Behav Processes. 2004;67:343–356. doi: 10.1016/j.beproc.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Strong DR, Brown RA. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) J Exp Psychol Appl. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- 35.Pulido C, Brown SA, Cummins K, Paulus MP, Tapert SF. Alcohol cue reactivity task development. Addictive Behaviors. 2010;35:84–90. doi: 10.1016/j.addbeh.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited--again. Neuroimage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- 37.Talairach J, Tournoux P. Coplanar stereotaxic atlas of the human brain. Three-dimensional proportional system: An approach to cerebral imaging. New York: Thieme; 1988. [Google Scholar]
- 38.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 39.Ward BD. Simultaneous Inference for FMRI Data. Milwaukee, WI: Biophysics Research Institute, Medical College of Wisconsin; 2000. [Google Scholar]
- 40.Saccone NL, Kwon JM, Corbett J, Goate A, Rochberg N, Edenberg HJ, Foroud T, Li TK, Begleiter H, Reich T, Rice JP. A genome screen of maximum number of drinks as an alcoholism phenotype. Am J Med Genet. 2000;96:632–637. doi: 10.1002/1096-8628(20001009)96:5<632::aid-ajmg8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 41.Jackson KM. Heavy episodic drinking: determining the predictive utility of five or more drinks. Psychol Addict Behav. 2008;22:68–77. doi: 10.1037/0893-164X.22.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Labouvie E, Bates ME, Pandina RJ. Age of first use: its reliability and predictive utility. J Stud Alcohol. 1997;58:638–643. doi: 10.15288/jsa.1997.58.638. [DOI] [PubMed] [Google Scholar]
- 43.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vollstadt-Klein S, Loeber S, Kirsch M, Bach P, Richter A, Buhler M, von der Goltz C, Hermann D, Mann K, Kiefer F. Effects of cue-exposure treatment on neural cue reactivity in alcohol dependence: a randomized trial. Biol Psychiatry. 2011;69:1060–1066. doi: 10.1016/j.biopsych.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 45.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 46.Claus ED, Ewing SW, Filbey FM, Sabbineni A, Hutchison KE. Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology. 2011;36:2086–2096. doi: 10.1038/npp.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- 48.Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garavan H. Insula and drug cravings. Brain Struct Funct. 2010;214:593–601. doi: 10.1007/s00429-010-0259-8. [DOI] [PubMed] [Google Scholar]
- 50.Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Archives of General Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- 51.Ihssen N, Cox WM, Wiggett A, Fadardi JS, Linden DE. Differentiating heavy from light drinkers by neural responses to visual alcohol cues and other motivational stimuli. Cereb Cortex. 2011;21:1408–1415. doi: 10.1093/cercor/bhq220. [DOI] [PubMed] [Google Scholar]
- 52.Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, Sinha R. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA psychiatry (Chicago, Ill) 2013;70:727–739. doi: 10.1001/jamapsychiatry.2013.762. [DOI] [PMC free article] [PubMed] [Google Scholar]