Abstract
Previous work in songbirds has suggested that testosterone increases neuronal recruitment and survival in HVC but does not affect neuronal proliferation in the ventricular zone and that males and females have similar rates of proliferation except at discrete locations. Many of these conclusions are however based on limited data or were inferred indirectly. Here we specifically tested the effects of testosterone on cellular proliferation in the ventricular zone of both male and female adult canaries. We implanted adult birds of both sexes with testosterone or empty implants for one week and injected them with BrdU. One day later, we collected their brains and quantified BrdU-positive cells in the ventricular zone (VZ) at different rostro-caudal levels of the brain, ranging from the level where the song nucleus Area X occurs through the caudal extent of HVC. Proliferation in the dorsal part of the VZ was low and unaffected by sex or testosterone treatment. In the ventral part of the VZ, females had more proliferating cells than males, but only at rostral levels, near Area X. Also in the ventral part of the VZ, testosterone increased proliferation in birds of both sexes, but only in the mid- to caudal-VZ, caudal to the level of Area X, around the septum and HVC. We thus demonstrate here that there is both an effect of testosterone and possibly a more subtle effect of sex on cellular proliferation in the adult songbird brain, and that these effects are specific to different levels of the brain.
Keywords: adult neurogenesis, testosterone, cell proliferation, songbird, sex difference
1. Introduction
Adult vertebrate brains continue to produce new neurons throughout life. This process is particularly prominent in songbirds where seasonal changes in numbers of new neurons in the song system nucleus HVC (used as a proper name; Reiner et al. 2004) during the breeding season are reflected by changes in the volume of the nucleus (Kirn et al., 1994). These new neurons are generated from the ventricular zone (VZ) around the lateral ventricles and migrate throughout the telencephalon (Alvarez-Buylla and Nottebohm, 1988; Scott and Lois, 2007; Balthazart et al., 2008; Vellema et al., 2010). Changes in HVC volume and neuron number take place across seasons based on studies in several species (Nottebohm et al., 1986; Kirn et al., 1994; Smith et al., 1997). Such changes can to some extent be reproduced by administering testosterone to adult female songbirds in species such as the canary (Goldman and Nottebohm, 1983; Rasika et al., 1994; Yamamura et al., 2011) indicating that gonadal hormone fluctuations contribute to the control of some aspects of neurogenesis in the adult canary brain even if the details of how and when these effects of testosterone take place are still partly unclear. Multiple studies have indeed demonstrated seasonal or testosterone-induced changes in the numbers of new neurons migrating to, incorporated or surviving in HVC (Rasika et al., 1994; Yamamura et al., 2011).
Because hormone treatment results in a change in neuron number in HVC, that is usually not observed in the surrounding telencephalic areas, it has been inferred that these changes reflect modifications in neuron recruitment or survival but not in proliferation in the VZ (Rasika et al., 1994; Yamamura et al., 2011). A few studies more directly tested this notion in female canaries and concluded that testosterone increases incorporation into HVC and survival of new neurons but has no effect on proliferation in the ventricle wall (e.g. Brown et al 1993, Rasika et al 1994). However in one study of adult male starlings it was found that testosterone increased the number of bromodeoxyuridine-immunoreactive (BrdU-ir) cells near the ventricle wall but since brains were collected weeks after BrdU injection, it is difficult to separate the effects of testosterone on proliferation from reduced cell migration away from the ventricles (Absil et al., 2003).
It has also been claimed that there is no sex difference in cell proliferation in the VZ (Alvarez-Buylla and Kirn, 1997) because neuron density or number in multiple telencephalic areas outside the song system do not differ between males and females. However, sex differences in proliferation have been reported in juvenile zebra finches in anatomically discrete brain regions, confined to the ventral and rostral part of the VZ at the level of area X (DeWulf and Bottjer 2002, 2005). Studies of 15 day old Bengalese finches also revealed localized sex differences in cell proliferation that the authors related to the development of sex differences in the morphology of the song nuclei HVC and area X (Zeng et al. 2007). Because adult females are often used to investigate cellular effects of testosterone on adult plasticity in songbirds, it is useful to confirm that cells in both sexes respond similarly to the steroid. There is to date no clear confirmation that this is the case in songbirds, whereas studies in mammals indicate that steroids affect proliferation of neural progenitor cells in the adult brain (recent reviews: Galea et al., 2006; Charalampopoulos et al., 2008) and do so in a sex-specific manner (Barker and Galea, 2008). There is evidence that progenitor cells in zebra finches respond differently to dehydroepiandrosterone in vitro, depending on whether they originated from a male or a female brain (Mirzatoni et al., 2010). These uncertainties led us to study in a quantitative manner the effects of testosterone on cell proliferation in the VZ of adult male and female canaries.
2. Materials and methods
2.1. Animals and in vivo treatments
Adult male (n=16) and female (n=14) canaries (Serinus canaria) were purchased from a local dealer (Noorderwijk, Belgium) during late summer and housed individually on a 14L:10D light schedule (lights on at 0700h). All experimental procedures complied with Belgian laws concerning the Protection and Welfare of Animals and the Protection of Experimental Animals, and experimental protocols were approved by the Ethics Committee for the Use of Animals at the University of Liège.
One week after arrival, birds were implanted under the skin between their shoulders with a Silastic™ capsule (12 mm × 0.76 mm ID, 1.65 mm OD; sealed on each end with a 1 mm cap of silicone glue) either packed with 10 mm crystalline testosterone (Sigma-Aldrich) or left empty (control). A recent study from our laboratories indicated that these capsules produce serum concentrations of approximately 1.5 ng/ml in both male and female canaries when measured by enzyme immunoassay one and three weeks after implantation (Madison F.N., Rouse L.L.Jr., Balthazart J. and Ball G.F. submitted for publication). These Silastic capsules of testosterone maintain elevated circulating concentrations of the steroid that are typical of adult sexually mature males for at least 3 weeks (Rasika et al., 1994; Madison F.N., Rouse L.L.Jr., Balthazart J. and Ball G.F. submitted for publication), and cause significant growth of HVC in female canaries (Nottebohm, 1980; Sartor et al., 2005; Boseret et al., 2006). Treatments and sex defined 4 experimental groups: control males and females (MC: n=9, FC: n=5) and testosterone-treated males and females (MT: n=7, FT: n=9).
One week later, birds were injected with BrdU (100 mg/kg) five times, every 2h on a single day starting at 0830h to ensure extensive labelling of cells replicating their DNA with minimal cellular damage. Previous studies used similar repeated injections of DNA replication markers (e.g. 6 injections of tritiated thymidine performed 8 hours apart in Goldman and Nottebohm 1983) and similar doses were used in many previous studies (e.g., Melleu et al. 2013; Briones & Wood 2011; Catlow et al. 2009; Jung et al. 2009; see Taupin 2007 for review). Previous work with BrdU actually showed that lower doses (e.g. 50 mg/kg) do not label all proliferating cells (Cameron and McKay, 2001). We also recently showed that a dose of 100 mg/kg BrdU is cleared from the canary blood within less than two hours (Barker et al. 2013) so that accumulation of the tracer in the blood that would reach cytotoxic concentrations is very unlikely.
Birds were killed by decapitation 24h after the third injection. The sex of each bird was confirmed by autopsy. Gonads and female oviduct were weighed as an indication of recent hormonal status. Brains were removed and fixed in 5% acrolein in 0.1M phosphate-buffered saline (PBS, pH 7.2) for 180 min, followed by two 30 min rinses on in PBS and cryoprotected in 30% glucose in PBS for 3 days. Brains were then frozen on dry ice and kept at −80°C until sectioning in the coronal plane on a cryostat (Leica).
All brains were mounted on the cryostat with the rostral tip up and the rostral part of the brain was trimmed until the point where the ventricle adopts a general “Y” shape (distinct lateral movement of the dorsal part of the ventricle) as opposed to the parallel orientation of the left and right ventricles seen in the more rostral sections. This level roughly corresponds to level A4.0 in the Stokes et al. (1974) atlas. In males, this is also the most rostral level where Area X appears. From that point on, all 30 μm thick sections were collected in 12 series of 11 sections until the caudal end of the telencephalon (approximately level P0.8-1.0 in Stokes et al.1974). One of these 12 series of sections was then stained for BrdU so that the periodicity of sampling was one section every 360 μm apart (12 × 30; i.e. there was 330 μm between two sections). The approximate level of these sections in the rostro-caudal axis is schematically indicated in figure 1. The position of the area X, septum and HVC is approximate on these sections and is only used as a rough indication of where each section was located in the brain. The absolute reference for locating effects is the number of the section running from 1 to 11 in the rostro-caudal direction.
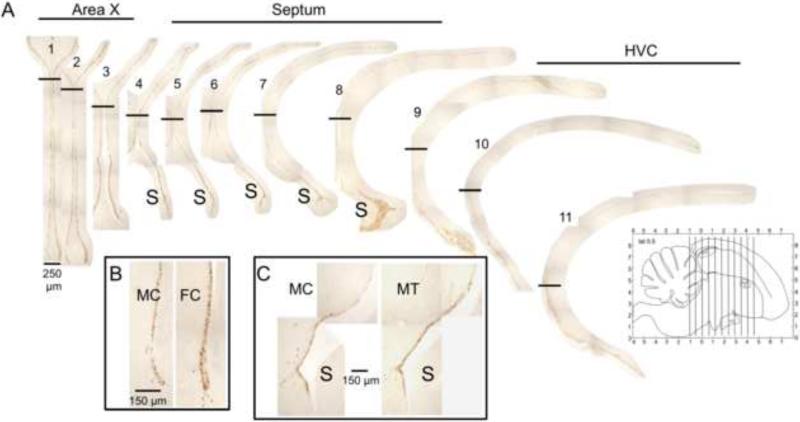
Fig. 1.
Representative photomicrographs of stained coronal sections from a control-treated male canary brain illustrating the BrdU labelling of the ventricle wall at successive rostro-caudal levels (from left to right). Levels corresponding to Area X (1-3), septum (4-8), and HVC (9-11) are indicated. Horizontal black bars show the separation between ‘dorsal’ (top) and ‘ventral’ (bottom) subregions of the lateral ventricles. Note the intensely-stained “hot spot” of proliferation in the ventricle wall around the septum (S). Insets illustrate at higher magnification the sex difference in control birds at a rostral level, at the level of Area X (B) and the effect of testosterone in males at a caudal level, at the level of the septum (C). The small figure in the lower right corner provides a schematic illustration of the position of the 11 sections that were stained and quantified reported on a sagittal view of the brain as provided in the Stokes et al (1974) canary brain atlas. Note that this view represents a parasagittal section at 0.5 mm of the mid-line; the full extension of the telencephalon and of nuclei HVC and area X are thus not represented.
Sections were not matched exactly to the stereotaxic coordinates in the Stokes et al. (1974) atlas because we did not implant markers at stereotaxic landmarks in the live birds and tissue shrinkage during processing modified the absolute size of objects. This is clearly reflected in the observation that the 12 series of 11 sections covered an area of approximately 3.96 mm (11 × 360 μm) and this corresponds roughly to 5 mm in the atlas (from A4.0 to P1.0). Tissue processing was however identical for all birds and experimental groups so that shrinkage was presumably similar. This ensured that we sampled homologous parts of the ventricle in the different experimental groups. Small differences (a few μm) that could exist between different subjects should be randomly distributed and could not be at the origin of the group differences that were detected. Sections were stored in antifreeze solution at −20°C until immunohistochemical staining.
2.2. Immunohistochemistry
Free-floating series of sections from each brain were immunostained for BrdU using 3,3′-diaminobenzidine (DAB). Tissue from 1-3 birds in each group (control males and females, testosterone-treated males and females) was included in each simultaneous staining run to avoid introducing any systematic bias during staining. Sections were sequentially incubated for 15 min in 0.1% sodium borohydride and in 0.6% hydrogen peroxide in phosphate-buffered saline (0.05M, 0.9% NaCl, pH 7.4; PBS). DNA was denatured by incubation in 2N HCl for 20 min at 37°C and then tissue was incubated for 10 min at room temperature in 0.1M sodium borate buffer (pH 8.5). Tissue was blocked with 10% normal horse serum in PBS with 0.2% Triton-X100 (PBS-T) and incubated overnight at 4°C with rat-anti-BrdU primary antibodies in PBS-T (1:2000; AbD Serotec nbr. OBT-0030, Kidlington, UK), followed by incubation for 2h at room temperature with biotinylated donkey-anti-rat secondary antibodies (1:2000; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) and then for 1.5h in ABC solution (Vectastain Elite PK-6100 kit; Vector Laboratories Inc., Burlingame, CA, USA) prepared as per the manufacturer's instructions. Tissue was then incubated for 12 min in 0.04% 3,3′-diaminobenzidine (DAB) and 0.012% hydrogen peroxide in PBS and mounted on Superfrost glass slides (Fisher Scientific) and coverslipped. Sections were rinsed 3x5 min in PBS between each step.
The rat anti-BrdU primary antibody is a rat monoclonal IgG2a antibody that has been broadly used in a variety of species (e.g., mouse: Encinas et al., 2006; rat: Sadgrove et al., 2006; quail: Nikolakopoulou et al., 2006; Bardet et al, 2012; and even canary: Balthazart et al., 2008; Vellema et al 2010). OBT-0030 recognizes BrdU incorporated into single-stranded DNA, attached to a protein carrier and free BrdU. OBT-0030 does not cross- react with thymidine but does react weakly with chlorodeoxyuridine (AbD Serotec Specifications). In canary, it never stained any structure in subjects that had not been injected with BrdU, thus demonstrating that it does not cross-react with endogenous thymidine or any other endogenous component in the brain.
2.3. Quantification of proliferating cells
The area in the VZ covered by DAB staining within 30 μm of the inner wall of the lateral ventricles was measured with ImageJ.v1.46o (National Institute of Health, USA). Individual images were captured with a digital camera (Scion Corporation, Meyer Instruments, Houston, TX) through a 10X objective on a light microscope (Olympus BH-2). When the entire ventricle from a single section would not fit in a single field of view, digital images were taken of multiple overlapping fields of view, overlaid manually and digitally merged using the GNU Image Manipulation Program (version 2.6). Assembled images were then processed in ImageJ. At each rostro-caudal level, the VZ was divided into a ‘dorsal’ and ‘ventral’ region, at the point where the ventricle is closest to the midline. The length of each of these regions at each section was also measured with ImageJ.
Images were converted to 8-bit greyscale, background staining was removed using ImageJ's Subtract Background function (light background, rolling ball radius of 10 pixels), and the image converted to a binary (black and white) image. The area covered by labelled cells (now black) was measured with ImageJ's Analyze Particles function, excluding particles <5μm2. Staining was quantified for each bird along the entire dorso-ventral extent of the VZ in a series of sections 360 μm apart, from the level of Area X (sections 1-3), through the septum (sections 4-8) and the extent of HVC (sections 9-11) (Fig. 1).
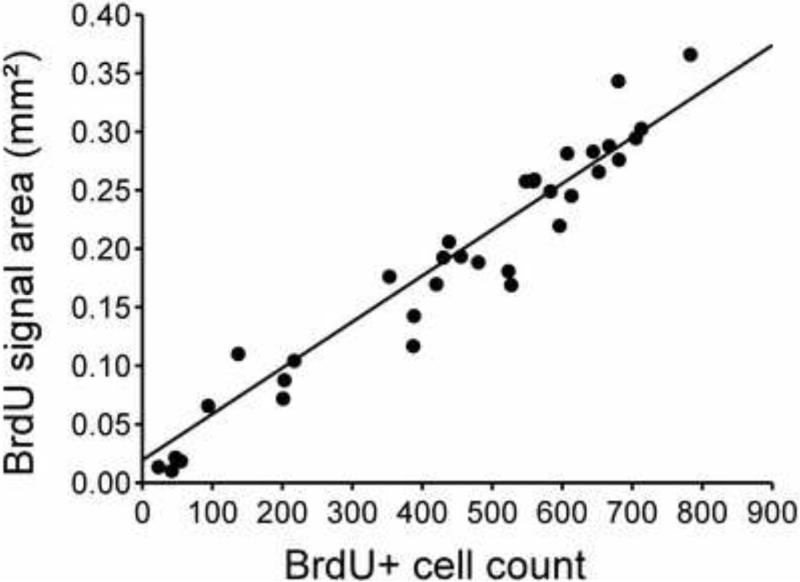
To validate the automated counting method, digital measurements of labeled area were evaluated against manual counts of cells. All stained cells in both the right and left VZ throughout an entire series of sections from one bird were counted manually under 40X objective. The same sections were then scored automatically using ImageJ as described above and resulting values compared to the manual cell counts. This identified a very close linear relationship between changes in the number of labeled nuclei along the rostro-caudal axis of a bird as quantified by manual cell counts and by estimating the positive signal area in digital images of the same sections (r=0.97, n=34, p<0.001; see Fig. 2).
Fig. 2.
Linear relationship between manual cell counts of BrdU-ir cell numbers and total area covered by the immunoreactive material as measured in digital images of the same sections. There was a strong linear relationship between results of the two quantification methods applied to 34 different sections from a same bird sampling the different rostro-caudal levels studied here.
This technical approach to the quantification of large numbers of labelled cell nuclei was previously used and validated in a study of immediate early genes (Fos and Zenk, also known as egr-1) where we showed that nearly identical changes in immediate early genes expression were measured by either counting Fos- or Zenk-positive nuclei or by quantifying the areas covered by the immunoreactive material (Charlier et al., 2005).
2.4. Statistical analysis
Regression analysis (Statistica, Statsoft, Tulsa, OK) was used to compare digital positive area measurement vs. manual cell counts. Sex and testosterone effects on proliferation were evaluated using a mixed design repeated-measures ANOVA, with the area covered by DAB-stained cells in the VZ as the dependent variable, the sex (male or female) and hormonal treatment (testosterone or control implant) as between-subjects independent factors, and the dorsal or ventral region and rostro-caudal level (11 levels per bird) as within-subjects repeated factor. Effects were considered significant at p<0.05. Significant interactions were further analyzed by the Fisher's Least significant difference (LSD) test.
3. Results
3.1. Morphology
Testosterone treatment resulted in a slight increase in the weight of the oviduct of non-breeding females (from 3.42±0.57 to 8.68±1.35 mg; F1,12=7.69, p=0.02; oviduct development still well below breeding condition; Steel et al., 1975), but did not affect ovarian weight (3.72±0.51 vs. 5.96±2.50 mg; F1,10 =0.43, p=0.53) nor testes weight in males (1.43±0.44 vs. 1.23 ± 0.11 mg; F1,14 =0.16, p=0.69).
3.2. Ventricle length
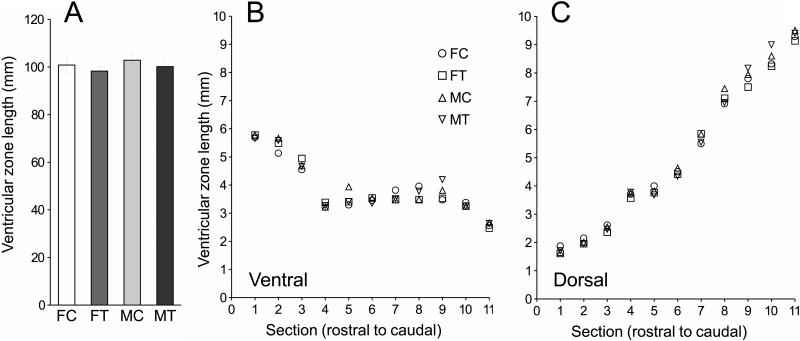
Ventricle length did not differ between sex or treatment groups (sex: F1,26=0.57, p=0.46; treatment: F1,26=1.03, p=0.32; all interactions involving sex and treatment all p>0.24) (Fig. 3A). Ventricle length varied between the dorsal and ventral extents of the ventricle (F1,26=141.64, p<0.0001) and as a function of rostro-caudal level (F10,260=107.49, p<0.0001). There was also a significant interaction between these two factors (F10,260=256.65, p<0.0001): ventricle length in the ventral region decreased from rostral to caudal levels, but increased in the dorsal region (Fig. 3B-C). We corrected for these changes in ventricle length in all data that are presented below by dividing the BrdU-stained area adjacent to the ventricle by the length of the ventricle in each section (= standardized positive area).
Fig. 3.
Ventricle length was affected by position in the brain, but not by sex nor by treatment. (A). Overall ventricle length was similar in the 4 groups of birds (F: female; M: male; T: testosterone; C: control) but differed between the ventral (panel B) and dorsal (panel C) regions and across rostrocaudal levels. Error bars were omitted for clarity but were at all data points smaller than 1-2 diameter(s) of the symbols and thus extensively overlapping.
3.3. Numbers of BrdU-immunoreactive cells
3.3.1. Main effects
The four way ANOVA of the standardized area covered by BrdU-ir cells identified significant main effects of the endocrine treatment (p<0.007) and of the position along the dorso-ventral (p<0.0001) and the rostro-caudal axis (p<0.0001) but there was no overall effect of sex (see Table 1 for detail of statistical results and Fig. 4). The position in the dorso-ventral region also interacted with the rostro-caudal level (p<0.0001).
Table 1.
Summary of the 4-way Analysis of variance analyzing the numbers of BrdU-immmunoreactive cells in the lateral ventricles of canaries as a function of their sex and endocrine treatment (2 independent factors) and position in the brain (2 repeated, matched factors).
| Effect | Sum of squares | df | F | P |
|---|---|---|---|---|
| Origin | 5919.777 | 1 | 1685.454 | 0.0000 |
| Sex | 1.004 | 1 | 0.286 | 0.5974 |
| Treatment (Trt) | 30.307 | 1 | 8.629 | 0.0068 |
| Sex * Trt | 2.660 | 1 | 0.757 | 0.3921 |
| Error | 91.319 | 26 | ||
| Dorso-Ventral Level (DV) | 421.321 | 1 | 201.458 | <0.0001 |
| DV * Sex | 0.519 | 1 | 0.248 | 0.6226 |
| DV * Trt | 15.418 | 1 | 7.372 | 0.0116 |
| DV * Sex * Trt | 2.684 | 1 | 1.283 | 0.2677 |
| Error | 54.375 | 26 | ||
| Rostro-caudal Level (RC) | 1366.959 | 10 | 261.280 | <0.0001 |
| RC * Sex | 4.129 | 10 | 0.789 | 0.6392 |
| RC * Trt | 3.487 | 10 | 0.666 | 0.7552 |
| RC * Sex * Trt | 4.072 | 10 | 0.778 | 0.6498 |
| Error | 136.026 | 260 | ||
| DV * RC | 327.304 | 10 | 71.223 | <0.0001 |
| DV * RC * Sex | 9.110 | 10 | 1.982 | 0.0355 |
| DV * RC * Trt | 9.095 | 10 | 1.979 | 0.0359 |
| DV * RC * Sex * Trt | 3.871 | 10 | 0.842 | 0.5882 |
| Error | 119.483 | 260 |
For each factor and interaction, the table presents the sums of squares, degrees of freedom (df), and the corresponding F and p values. Significant effects are highlighted in bold.
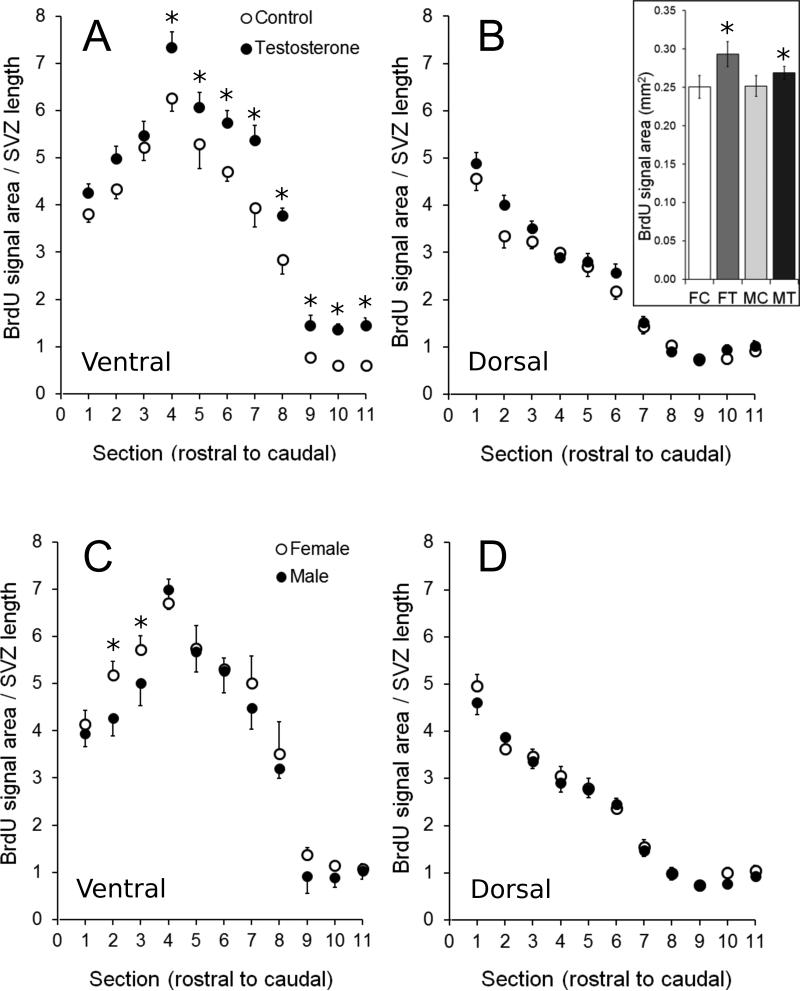
Fig. 4.
Effects of testosterone and sex on BrdU labelling of the ventricular zone (VZ) in the ventral (A,C) and dorsal (B,D) parts of the brain in the 4 experimental groups. There was a significant main effect of testosterone on proliferation in the ventricular zone of adult birds, independent of sex (A and insert in B) but this effect was localized only in the most caudal sections (levels 4-11). The insert in panel B illustrates the overall effect of testosterone in the 4 experimental groups (significant effect of the steroid similar in both sexes) averaged across the entire VZ (dorsal and ventral) in all sections in the rostral to caudal axis and thus ignoring the anatomical specificity of this effect. In addition, BrdU labelling was denser in females than in males (independent of steroid treatment) in the most rostral sections (levels 2-3). All data are means ± SEM. *= p < 0.05 vs. corresponding control (Panel A and insert of Panel B) or male (Panel C) values.
3.3.2. Interactions between sex differences and regions
There was no significant two-way interaction involving sex with location in the ventricle (sex × dorso-ventral region, sex × rostro-caudal level) or both sex and treatment (sex × treatment, sex × treatment × dorso-ventral region, sex × treatment × rostro-caudal level; Table 1). The four-way interaction (sex × treatment × dorso-ventral region × rostro-caudal level) was also not significant. However, we found a significant three-way sex × dorso-ventral region × rostro-caudal level interaction (p<0.04).
3.3.3. Interactions between treatments and regions
There was a significant two-way interaction between treatment and dorso-ventral region (p<0.02) but not between treatment and rostro-caudal level. There was also a significant three-way interaction treatment x dorso-ventral region x rostro-caudal level (p<0.04).
3.3.4. Post-hoc analyses
Since these main and two-way interactions were all contained within the significant three-way interactions (sex × dorso-ventral × rostro-caudal and treatment × dorso-ventral × rostro-caudal), we only analyzed the higher-order (three-way) effects using Fisher's LSD post-hoc tests, but ignored spurious comparisons (i.e. comparisons involving different levels of multiple variables).
The dorsal region of the VZ had less DAB labelling relative to its length than did the ventral region at rostral levels of the ventricles in both treatment groups (all comparisons through Area X and septum: p<0.01) and in both sexes (all comparisons through Area X and septum: p<0.006, except in males where labelling at one, mid-Area X level was similar in both regions, p=0.10; compare panels A and B or panels C and D). The difference between dorsal and ventral regions was maintained at the level of the most rostral extent of HVC in testosterone-treated birds (p=0.002) but not in control birds (p=0.96). It was also maintained at this level in females (p=0.01) but not in males (p=0.44). However, the difference was not significant at the level of the mid- to caudal HVC in either treatment group (control group: mid-HVC p=0.47, caudal HVC p=0.18; testosterone-treated group: mid-HVC p=0.08, caudal HVC p=0.08) or in either sex (females: mid-HVC p=0.57, caudal HVC p=0.93; males: mid-HVC p=0.60, caudal HVC p=0.65).
There were no significant effects on BrdU labelling in the dorsal region of the VZ (at any rostro-caudal level) of treatment (all comparisons p>0.09) (Fig. 4B), nor of sex (all comparisons p>0.25) (Fig. 4D).
A completely different situation was observed in the ventral region of the VZ. Here, both sex and treatment had significant effects on the extent of BrdU labelling at specific rostro-caudal levels. Females had higher densities of DAB labelling than males at the level of mid-Area X (p=0.005) and caudal Area X (p=0.03), but not in the rostral-most section examined (rostral Area X, p=0.52) and not at any level caudal to Area X (all comparisons with males: p>0.09; Fig. 4C). Conversely, testosterone had no significant effect at any of the rostral-most levels examined (rostral Area X: p=0.15, mid-Area X: p=0.06, caudal Area X: p=0.40), but the steroid significantly increased proliferation at every level caudal to Area X (i.e. at all levels including the septum and HVC; all comparisons with control: p<0.04; Fig. 4A).
4. Discussion
We present here evidence that, contrary to assumptions widely expressed in the literature, testosterone increases cell proliferation in caudal regions of the VZ of an adult songbird, the canary, and that females have more cell proliferation than males in limited parts of the rostral VZ. Past work on neurogenesis in adult songbirds demonstrated that while cell numbers in HVC increase with testosterone treatment, total cell numbers in regions outside of HVC do not change substantially (Rasika et al., 1994; Yamamura et al., 2011). This has been taken to mean that testosterone does not affect the production of new cells, but either enhances the recruitment of migrating cells into HVC or enhances the survival of cells that reach HVC. One study in female canaries specifically suggested that gonadal steroid treatment does not affect proliferation in the VZ (Brown et al., 1993). However, these results were not based on direct measures of proliferation alone; cells were counted days after BrdU injection, during which time some newly-produced cells may have died or begun to migrate. In addition cell proliferation at the ventricle wall was only quantified at two selected rostro-caudal levels. Therefore, the notion that changes in neuron numbers within HVC only relate to changes in recruitment and survival but not in cell proliferation at the ventricle wall does not seem to be established on firm experimental bases.
We demonstrate here that testosterone indeed increases proliferation in the VZ of the adult canary and does so similarly in both males and females. This observation is consistent with the use of adult females treated with testosterone as a model for changes in the song system that normally occur seasonally in males. However, since the fate of cells dividing at the level of the lateral ventricles was not established and some of these cells may die rapidly or differentiate into glia or other cell types, these data do not directly demonstrate that these cells will end up as neurons in HVC but they raise the possibility that changes in proliferations may contribute to changes in neuronal populations in HVC. This hypothesis should be experimentally investigated.
4.1. Anatomical specificity of testosterone effects
The finding that the rostral-most levels and the dorsal region of the VZ were not affected by testosterone suggests that VZ cells are divided into subpopulations that differ in their responsiveness to testosterone treatment. Evidence is sparse for a regional difference within the VZ that could mediate anatomically specialized effects of testosterone on proliferating cells, such as differential localization of the aromatase enzyme, estrogen receptor alpha (ER) or androgen receptors (AR), or steroid hormone receptor cofactors. However, there are some qualitative reports suggesting that there may be anatomical specializations of the neurochemical machinery mediating steroid hormone action. In adult zebra finches, ER mRNA (Jacobs et al., 1996) and aromatase mRNA (Shen et al., 1995) are present in a larger number of cells near the walls of the lateral ventricles in regions caudal to the anterior commissure, as compared to more rostral regions. At several rostral/caudal levels these cells also appear to be localized primarily around the ventral region of the ventricles rather than the dorsal region. A similar pattern of distribution is seen in developing zebra finches, in that cells containing ER mRNA around the lateral ventricles are sparse or absent from the rostral-most levels, but appear localized in the ventral region at more caudal levels (Gahr, 1996). It is however unclear whether this pattern of anatomical distribution observed in a non seasonal species would apply to a species with seasonal breeding such as the canary.
Cells containing ER also appear to be localized around the ventral region of the lateral ventricles, but not the dorsal region, in adult canaries again from the level of the anterior commissure and through more caudal levels (Gahr et al., 1987). We are unaware of any reports demonstrating their distribution around the ventricles at more rostral levels, for example at the level of Area X. As testosterone can be aromatized locally within the songbird brain to estradiol, the localization of ER to particular subregions of the VZ would allow for subregion-specific effects of testosterone treatment. The pattern of expression of AR mRNA is similar, with relatively localized concentrations around the ventral region of the ventricle throughout the most caudal levels of the telencephalon, but less localized labelling around the ventricles at more rostral levels (Kim et al., 2004). This pattern of distribution of aromatase and sex steroid receptors suggests that there could be localized effects of steroids on cell proliferation but this obviously needs to be tested directly.
4.2. Functional implications of the sex and testosterone-dependent changes in proliferation
In many species there are male-biased differences in the volume of song control nuclei that correspond to a reasonable degree with variation in how well and how much the male sings as compared to the female (MacDougall-Shackleton and Ball, 1999). There are also seasonal differences in the volume of key song control nuclei, with HVC being larger in the spring, when males are singing more stereotypical song, in part as a result of an increase in neuron number within HVC (Tramontin et al., 1998, Tramontin and Brenowitz 2000). Although the phenomenon of adult neurogenesis in songbirds was discovered by Fernando Nottebohm because of an interest in the seasonal changes in song system nuclei (Nottebohm 1989) it was discovered early on that adult neurogenesis is widespread throughout the telencephalon of apparently all avian species (Goldman 1988; Nottebohm 1989; Ling et al., 1997; Melleu et al., 2013). The function of this widespread pattern of adult neurogenesis is not well understood in birds though it is appreciated that birds exhibit marked changes in neurogenesis in response to brain injury (Ling et al., 1997; Chen et al., 2006; Saldanha et al., 2005). Adult neurogenesis may thus mediate neurological phenomena or behaviours not directly related to the production or perception of song.
The sex difference in HVC volume develops due to male-biased sex differences in neuronal incorporation and survival (Nordeen and Nordeen, 1988) and can be reversed by treatment of juvenile birds with an estrogen (Nordeen and Nordeen, 1989). These hormone effects on the development of the sex difference in volume are thought to be post-mitotic, i.e. involve events after neuron proliferation (Burek et al. 1995). Seasonal changes in HVC volume have been shown to involve changes in a number of cellular characteristics including changes in cell number (Smith et al. 1997; Tramontin and Brenowitz 2000). In seasonally breeding canaries, the peaks of new neuron incorporation into HVC occur in October and March and appear to correlate positively with the acquisition of new song syllables (Kirn et al., 1994). These times of peak new neuron incorporation follow periods of cell death in HVC and are coincident with increases in gonadal sex steroids suggesting that the steroids are facilitating the incorporation of new neurons in a seasonal context as well.
Based on this substantial background information about the regulation and significance of adult neurogenesis in songbirds what can we say about our findings? We found a suggestion of a localized female-biased sex difference in cellular proliferation in the ventral and very rostral part of the VZ at the level of area X among the song nuclei. Previously male biased sex differences in proliferation have been identified in developing and/or juvenile zebra finches (DeWulf and Bottjer 2002; 2005) and in juvenile Bengalese finches (Zeng et al., 2007). No female-biased sex differences in proliferation has been reported in zebra finches of any age and no sex differences of any sort in proliferation have been reported in adult zebra finches. However, it is unclear that previous studies quantified cell birth in enough detail to detect the localized differences we describe here. Importantly, female canaries, contrary to zebra fnches have a substantial area X, albeit still smaller in volume than the male area X (Nottebohm and Arnold, 1976). It is however difficult to relate this putative female-biased difference in proliferation with any aspect of the song control system. Alternatively one should thus consider that this sex difference in cell proliferation in adulthood could be related to an aspect of cellular plasticity that does not concern vocal behaviour nor the song system.
We also identified an effect of testosterone on cell proliferation in both males and females. In this case the effect is along the more ventral portions of the VZ in sections much more caudal to those where we observed the sex difference in proliferation. It could be tempting to relate this steroid hormone effect to the well-characterized hormone effects on HVC volume. However, if one assumes that new neurons incorporated in the seasonal/hormonal context are primarily HVC-RA projecting cells then our results are inconsistent with that view. Studies in juvenile zebra finches that labeled new neurons by employing oncoretroviral vectors to genetically label neuronal progenitors in different regions of the zebra finch lateral ventricle (Scott and Lois, 2007) suggest that HVC-RA projecting neurons are derived from the dorsal VZ adjacent to HVC where we have identified no hormone effects. Cells in the more ventral parts of the VZ where testosterone effects were observed in this study tend to give rise to inter-neurons (Scott and Lois, 2007). It has been claimed that in male zebra finches at least all new HVC neurons are HVC-RA projecting cells and not interneurons (Scotto-Lomassese et al., 2007). But this conclusion has been challenged by Walton et al. (2012) who argued that we still have not identified the cell type of all new HVC neurons detected by the BrdU method. It thus remains possible that the effects of testosterone on cell proliferation described in this paper relate to seasonal plasticity in the song system but alternatively they could relate to plasticity in any other telencephalic area related or not to song perception or production. We argue, however, that the effects documented in this study, although they do not have definitive functional interpretation, do raise important new questions about how adult neurogenesis is regulated in the songbird brain and should stimulate additional studies about the regulation and function of this phenomenon.
Ethical Statement.
This manuscript has been checked and approved by all authors. It has not been submitted for publication to any other journal and if accepted will be published exclusively in the Journal of Chemical Neuroanatomy.
Experimental protocols were approved by the Animal Care Committee of the University of Liege.
Highlights.
BrdU was used to quantify cellular proliferation in the adult canary brain
An intense cellular proliferation is present in the lateral ventricle wall of canaries
Proliferation is more intense in the ventral than in the dorsal ventricular zone
Proliferation appears more active in females than males at rostral levels
Testosterone increase proliferation at all levels caudal to the anterior commissure
Acknowledgements
Funding for this work was provided by NIH NINDS RO1-NS035467 and an Interuniversity Attraction Pole (IAP) grant number SSTC PAI P7/17 from the Belgian Science Policy Office (BELSPO) to JB and GFB and by a Postdoctoral Fellowship for Foreign Researchers from the University of Liège (JMB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest to declare.
References
- Absil P, Pinxten R, Balthazart J, Eens M. Effect of age and testosterone on autumnal neurogenesis in male European starlings (Sturnus vulgaris). Behav Brain Res. 2003;143:15–30. doi: 10.1016/s0166-4328(03)00006-8. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Kirn JR. Birth, migration, incorporation, and death of vocal control neurons in adult songbirds. J Neurobiol. 1997;33:585–601. [PubMed] [Google Scholar]
- Alvarez-Buylla A, Nottebohm F. Migration of young neurons in adult avian brain. Nature. 1988;335:353–354. doi: 10.1038/335353a0. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Boseret G, Konkle ATM, Hurley LL, Ball GF. Doublecortin as a marker of adult neuroplasticity in the canary song control nucleus HVC. Eur J Neurosci. 2008;27:801–817. doi: 10.1111/j.1460-9568.2008.06059.x. [DOI] [PubMed] [Google Scholar]
- Bardet SM, Mouriec K, Balthazart J. Birth of neural progenitors during the embryonic period of sexual differentiation in the japanese quail brain. J Comp Neurol. 2012;520:4226–4253. doi: 10.1002/cne.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Charlier TD, Ball GF, Balthazart J. A new method for in vitro detection of bromodeoxyuridine in serum: a proof of concept in a songbird species, the canary. PlosOne. 2013;8(5):e63692. doi: 10.1371/journal.pone.0063692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Galea LAM. Repeated estradiol administration alters different aspects of neurogenesis and cell death in the hippocampus of female, but not male, rats. Neuroscience. 2008;152:888–902. doi: 10.1016/j.neuroscience.2007.10.071. [DOI] [PubMed] [Google Scholar]
- Boseret G, Carere C, Ball GF, Balthazart J. Social context affects testosterone-induced singing and the volume of song control nuclei in male canaries (Serinus canaria). J Neurobiol. 2006;66:1044–1060. doi: 10.1002/neu.20268. [DOI] [PubMed] [Google Scholar]
- Briones TL, Wood J. Chemotherapy-induced cognitive impairment is associated with decreases in cell proliferation and histone modifications. BMC Neuroscience. 2011;12:124. doi: 10.1186/1471-2202-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burek MJ, Nordeen KW, Nordeen EJ. Estrogen promotes neuron addition to an avian song-control nucleus by regulating post-mitotic events. Dev Brain Res. 1995;85:220–224. doi: 10.1016/0165-3806(94)00215-l. [DOI] [PubMed] [Google Scholar]
- Brown S, Johnson F, Bottjer S. Neurogenesis in adult canary telencephalon is independent of gonadal hormone levels. J Neurosci. 1993;13:2024–2032. doi: 10.1523/JNEUROSCI.13-05-02024.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, McKay RDG. Adult neurogenesis produces large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Catlow BJ, Rowe AR, Clearwater CR, Mamcarz M, Arendash GW, Sanchez-Ramos J. Effects of environmental enrichment and physical activity on neurogenesis in transgenic PS1/APP mice. Brain Res. 2009;1256:173–9. doi: 10.1016/j.brainres.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier TD, Ball GF, Balthazart J. Sexual behaviour activates the expression of the immediate early genes c-Fos and Zenk (egr-1) in catecholaminergic neurons of male Japanese quail. Neuroscience. 2005;131:13–30. doi: 10.1016/j.neuroscience.2004.09.068. [DOI] [PubMed] [Google Scholar]
- Charalampopoulos I, Remboutsika E, Margioris AN, Gravanis A. Neurosteroids as modulators of neurogenesis and neuronal survival. Trends Endocrinol Metab. 2008;19:300–307. doi: 10.1016/j.tem.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Chen G, Bonder EM, Cheng MF. Lesion-induced neurogenesis in the hypothalamus is involved in behavioral recovery in adult ring doves. J Neurobiol. 2006;66:537–551. doi: 10.1002/neu.20247. [DOI] [PubMed] [Google Scholar]
- Dewulf V, Bottjer SW. Age and sex differences in mitotic activity within the zebra finch telencephalon. J Neurosci. 2002;22:4080–4094. doi: 10.1523/JNEUROSCI.22-10-04080.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWulf V, Bottjer SW. Neurogenesis within the juvenile zebra finch telencephalic ventricular zone, A map of proliferative activity. J Comp Neurol. 2005;481:70–83. doi: 10.1002/cne.20352. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci U S A. 2006;103:8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahr M. Developmental changes in the distribution of oestrogen receptor mRNA expressing cells in the forebrain of female, male and masculinized female zebra finches. Neuroreport. 1996;7:2469–2473. doi: 10.1097/00001756-199611040-00013. [DOI] [PubMed] [Google Scholar]
- Gahr M, Flügge G, Güttinger H-R. Immunocytochemical localization of estrogen-binding neurons in the songbird brain. Brain Res. 1987;402:173–177. doi: 10.1016/0006-8993(87)91063-8. [DOI] [PubMed] [Google Scholar]
- Galea LAM, Spritzer MD, Barker JM, Pawluski JL. Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus. 2006;16:225–232. doi: 10.1002/hipo.20154. [DOI] [PubMed] [Google Scholar]
- Goldman SA. Adult neurogenesis: from canaries to the clinic. J Neurobiol. 1998;36:267–286. [PubMed] [Google Scholar]
- Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EC, Arnold AP, Campagnoni AT. Zebra finch estrogen receptor cDNA: Cloning and mRNA expression. Journal Steroid Biochem Mol Biol. 1996;59:135–145. doi: 10.1016/s0960-0760(96)00096-9. [DOI] [PubMed] [Google Scholar]
- Jung SE, Lee S-T, Chu K, Park J-E, Lee S-U, Han T-R, Kim M. Cell proliferation and synaptogenesis in the cerebellum after focal cerebral ischemia. Brain Res. 2009;1284:180–190. doi: 10.1016/j.brainres.2009.05.051. [DOI] [PubMed] [Google Scholar]
- Katz A, Mirzatoni A, Zhen Y, Schlinger BA. Sex differences in cell proliferation and glucocorticoid responsiveness in the zebra finch brain. Eur J Neurosci. 2008;28:99–106. doi: 10.1111/j.1460-9568.2008.06303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-H, Perlman WR, Arnold AP. Expression of androgen receptor mRNA in zebra finch song system: Developmental regulation by estrogen. J Comp Neurol. 2004;469:535–547. doi: 10.1002/cne.11033. [DOI] [PubMed] [Google Scholar]
- Kirn J, O'Loughlin B, Kasparian S, Nottebohm F. Cell death and neuronal recruitment in the high vocal center of adult male canaries are temporally related to changes in song. Proc Natl Acad Sci USA. 1994;91:7844–7848. doi: 10.1073/pnas.91.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling CY, Zuo MX, Alvarez-Buylla A, Cheng MF. Neurogenesis in juvenile and adult ring doves. J Comp Neurol. 1997;379:300–312. [PubMed] [Google Scholar]
- MacDougall-Shackleton SA, Ball GF. Comparative studies of sex differences in the song-control system of songbirds. Trends Neurosci. 1999;22:432–436. doi: 10.1016/s0166-2236(99)01434-4. [DOI] [PubMed] [Google Scholar]
- Melleu FF, Santos TS, Lino-de-Oliveira C, Marino-Neto J. Distribution and characterization of doublecortin-expressing cells and fibers in the brain of the adult pigeon (Columba livia) J Chem Neuroanat. 2013;47:57–70. doi: 10.1016/j.jchemneu.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Mirzatoni A, Dong SM, Guerra M, Zhen Y, Katz A, Schlinger BA. Steroidal and gonadal effects on neural cell proliferation in vitro in an adult songbird. Brain Res. 2010;1351:41–49. doi: 10.1016/j.brainres.2010.07.027. [DOI] [PubMed] [Google Scholar]
- Nikolakopoulou AM, Parpas A, Panagis L, Zikopoulos B, Dermon CR. Early post-hatching sex differences in cell proliferation and survival in the quail telencephalic ventricular zone and intermediate medial mesopallium. Brain Res Bull. 2006;70:107–116. doi: 10.1016/j.brainresbull.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW. Sex and regional differences in the incorporation of neurons born during song learning in zebra finches. J Neurosci. 1988;8:2869–2874. doi: 10.1523/JNEUROSCI.08-08-02869.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW. Estrogen stimulates the incorporation of new neurons into avian song nuclei during adolescence. Dev Brain Res. 1989;49:27–32. doi: 10.1016/0165-3806(89)90056-4. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Testosterone triggers growth of brain vocal control nuclei in adult female canaries. Brain Res. 1980;189:429–436. doi: 10.1016/0006-8993(80)90102-x. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. From bird song to neurogenesis. Sci Am. 1989;260:74–79. doi: 10.1038/scientificamerican0289-74. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976;194:211–213. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Nottebohm MA, Crane L. Developmental and seasonal changes in canary song and their relation to changes in the anatomy of song-control nuclei. Behav Neural Biol. 1986;46:445–471. doi: 10.1016/s0163-1047(86)90485-1. [DOI] [PubMed] [Google Scholar]
- Rasika S, Nottebohm F, Alvarez-Buylla A. Testosterone increases the recruitment and/or survival of new high vocal center neurons in adult female canaries. Proc Natl Acad Sci USA. 1994;91:7854–7858. doi: 10.1073/pnas.91.17.7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Bruce L, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter GF, Wild M, Ball GF, Durand S, Güntürkün O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadgrove MP, Laskowski A, Gray WP. Examination of granule layer cell count, cell density, and single-pulse BrdU incorporation in rat organotypic hippocampal slice cultures with respect to culture medium, septotemporal position, and time in vitro. J Comp Neurol. 2006;497:397–415. doi: 10.1002/cne.21000. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Rohmann KN, Coomaralingam L, Wynne RD. Estrogen provision by reactive glia decreases apoptosis in the zebra finch (Taeniopygia guttata). J Neurobiol. 2005;64:192–201. doi: 10.1002/neu.20147. [DOI] [PubMed] [Google Scholar]
- Sartor JJ, Balthazart J, Ball GF. Coordinated and dissociated effects of testosterone on singing behavior and song control nuclei in canaries (Serinus canaria). Hormones Behav. 2005;47:467–476. doi: 10.1016/j.yhbeh.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Scott BB, Lois C. Developmental origin and identity of song system neurons born during vocal learning in songbirds. J Comp Neurol. 2007;502:202–214. doi: 10.1002/cne.21296. [DOI] [PubMed] [Google Scholar]
- Scotto-Lomassese S, Rochefort C, Nshdejan A, Scharff C. HVC interneurons are not renewed in adult male zebra finches. Eur J Neurosci. 2007;25:1663–1668. doi: 10.1111/j.1460-9568.2007.05418.x. [DOI] [PubMed] [Google Scholar]
- Shen P, Schlinger BA, Campagnoni AT, Arnold AP. An atlas of aromatase mRNA expression in the zebra finch brain. J Comp Neurol. 1995;360:172–184. doi: 10.1002/cne.903600113. [DOI] [PubMed] [Google Scholar]
- Smith GT, Brenowitz EA, Beecher MD, Wingfield JC. Seasonal changes in testosterone, neural attributes of song control nuclei, and song structure in wild songbirds. J.Neurosci. 1997;17:6001–6010. doi: 10.1523/JNEUROSCI.17-15-06001.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel E, Follett BK, Hinde RA. The role of short days in the termination of photorefractoriness in female canaries (Serinus canarius). J Endocrinol. 1975;64:451–464. doi: 10.1677/joe.0.0640451. [DOI] [PubMed] [Google Scholar]
- Stokes TM, Leonard CM, Nottebohm F. The telencephalon, diencephalon, and mesencephalon of the canary, Serinus canaria, in stereotaxic coordinates. J Comp Neurol. 1974;156:337–374. doi: 10.1002/cne.901560305. [DOI] [PubMed] [Google Scholar]
- Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: Paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Thompson CK, Brenowitz EA. Seasonal change in neuron size and spacing but not neuronal recruitment in a basal ganglia nucleus in the avian song control system. J Comp Neurol. 2005;481:276–283. doi: 10.1002/cne.20381. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Brenowitz EA. Seasonal plasticity in the adult brain. Trends Neurosci. 2000;23:251–258. doi: 10.1016/s0166-2236(00)01558-7. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Smith GT, Breuner CW, Brenowitz EA. Seasonal plasticity and sexual dimorphism in the avian song control system: Stereological measurement of neuron density and number. J Comp Neurol. 1998;396:186–192. doi: 10.1002/(sici)1096-9861(19980629)396:2<186::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Vellema M, Van der Linden A, Gahr M. Area-specific migration and recruitment of new neurons in the adult songbird brain. J Comp Neurol. 2010;518:1442–1459. doi: 10.1002/cne.22281. [DOI] [PubMed] [Google Scholar]
- Walton C, Pariser E, Nottebohm F. The zebra finch paradox: song is little changed, but number of neurons doubles. J Neurosci. 2012;32:761–774. doi: 10.1523/JNEUROSCI.3434-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura T, Barker JM, Balthazart J, Ball GF. Androgens and estrogens synergize to regulate the expression of doublecortin and enhance neuronal recruitment in the song system of adult female canaries. Eur J Neurosci. 2011;31:9649–9657. doi: 10.1523/JNEUROSCI.0088-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng SJ, Song K, Xu N, Zhang XW, Zuo MZ. Sex difference in cellular proliferation within the telencephalic ventricle zone of Bengalese finch. Neurosci Res. 2007;58:207–214. doi: 10.1016/j.neures.2007.02.001. [DOI] [PubMed] [Google Scholar]