Abstract
Hearing impairment in older adults is independently associated in longitudinal studies with accelerated cognitive decline and incident dementia, and in cross-sectional studies, with reduced volumes in the auditory cortex. Whether peripheral hearing impairment is associated with accelerated rates of brain atrophy is unclear. We analyzed brain volume measurements from magnetic resonance brain scans of individuals with normal hearing versus hearing impairment (speech-frequency pure tone average > 25 dB) followed in the neuroimaging substudy of the Baltimore Longitudinal Study of Aging for a mean of 6.4 years after the baseline scan (n = 126, age 56–86 years). Brain volume measurements were performed with semi-automated region-of-interest (ROI) algorithms, and brain volume trajectories were analyzed with mixed-effects regression models adjusted for demographic and cardiovascular factors. We found that individuals with hearing impairment (n = 51) compared to those with normal hearing (n = 75) had accelerated volume declines in whole brain and regional volumes in the right temporal lobe (superior, middle, and inferior temporal gyri, parahippocampus, p < .05). These results were robust to adjustment for multiple confounders and were consistent with voxel-based analyses, which also implicated right greater than left temporal regions. These findings demonstrate that peripheral hearing impairment is independently associated with accelerated brain atrophy in whole brain and regional volumes concentrated in the right temporal lobe. Further studies investigating the mechanistic basis of the observed associations are needed.
Keywords: Hearing loss, hearing impairment, MRI, brain volume, aging
1.0 Introduction
Two converging lines of evidence suggest that hearing impairment and alterations in peripheral auditory function could directly or indirectly lead to central effects on brain structure and function. Cross-sectional neuroimaging studies have demonstrated that peripheral hearing impairment is associated with reduced cortical volumes in the primary auditory cortex (Husain, Medina et al. 2010; Peelle, Troiani et al. 2011; Eckert, Cute et al. 2012) and variation in the integrity of central auditory white matter tracks (Chang, Lee et al. 2004; Lin, Wang et al. 2008). The basis of these associations remains unknown but may be related to alterations in the degree of neural activation provided by an impoverished auditory signal with subsequent structural changes in cortical reorganization and brain morphometry (Peelle, Troiani et al. 2011). Interestingly, degradation in the fidelity of peripheral encoding of sound likely results in recruitment and activation of broader neural networks needed for auditory processing (Wingfield and Grossman 2006; Davis and Johnsrude 2007; Peelle, Johnsrude et al. 2010), suggesting that peripheral hearing impairment may carry cascading consequences for other brain regions.
Broader functional implications of hearing impairment are suggested by epidemiologic studies investigating the association of hearing impairment with cognitive functioning. In these studies of older adults, peripheral hearing impairment was independently associated with poorer neurocognitive performance on both auditory and non-auditory tests (Lindenberger and Baltes 1994; Gussekloo, de Craen et al. 2005; Tay, Wang et al. 2006; Lin 2011; Lin, Ferrucci et al. 2011), accelerated rates of cognitive decline (Lin, Yaffe et al. 2013), and increased risk of incident all-cause dementia (Lin, Metter et al. 2011; Gallacher, Ilubaera et al. 2012). Hypothesized mechanisms to explain these associations include a shared neuropathologic etiology, cognitive load from the reallocation of brain resources for auditory processing (Wingfield, Tun et al. 2005; Tun, McCoy et al. 2009), and/or mediation through social isolation (Barnes, Mendes de Leon et al. 2004; Bennett, Schneider et al. 2006).
Whether peripheral hearing impairment is associated with regions outside the primary auditory cortex and with changes in brain volumes is unknown. A priori, we hypothesized that hearing impairment is associated with greater volume declines in regional brain volumes important for auditory and spoken language processing (superior, middle, and inferior temporal gyri) (Davis and Gaskell 2009; Adank 2012; Peelle 2012). Understanding the association of hearing impairment with structural brain volumes may provide insights into mechanistic pathways through which peripheral impairments in sensory function could contribute to brain aging.
2.0 Methods
2.1 Study Participants
Participants were followed in the neuroimaging substudy (Resnick, Goldszal et al. 2000) of the BLSA, an ongoing prospective study of the effects of aging that was initiated in 1958 and is conducted by the National Institute on Aging (Shock, Greulich et al. 1984). The present investigation is based on a longitudinal cohort of participants (n = 126, ages 56–86 at baseline) who were enrolled beginning in 1994 in the neuroimaging substudy of the BLSA and had audiometric assessments. Baseline was defined as the time at the first MRI scan. Individuals enrolled in the neuroimaging substudy were free of central nervous system disease (epilepsy, stroke, bipolar illness, prior diagnosis of dementia according to Diagnostic and Statistical Manual [DSM]-III-R criteria (Spitzer and Williams 1987)), severe cardiac disease (myocardial infarction, coronary artery disease requiring angioplasty or bypass surgery), pulmonary disease, or metastatic cancer. Two participants were later diagnosed with having mild cognitive impairment at baseline based on retrospective review of their baseline neurocognitive and clinical data. Audiometric testing was performed concurrently or before enrollment in the neuroimaging substudy, and the mean time from hearing assessment to the baseline magnetic resonance (MR) scan was 1.7 years (range 0 – 5 years). Neuroimaging data gathered at and following dementia diagnosis were excluded in 13 participants who developed incident dementia during follow-up. All longitudinal MR scans prior to dementia diagnosis were included in analyses, for a total of 872 imaging observations from 126 participants. The mean number of scans obtained on participants was 6 (range 1–10). The NIA and the Johns Hopkins School of Medicine Institutional Review Boards approved this study, and all participants gave informed consent.
2.2 Audiometry
Pure-tone audiometric testing is a measure of the sensitivity of the peripheral auditory system (Pickles 2008) and was performed using a semi- automated testing device (Virtual Equipment Co., Audiometer Model 320) in a sound-attenuating booth. A speech-frequency pure tone average (PTA) of air-conduction thresholds at 0.5, 1, 2, and 4 kHz was calculated for each ear. Hearing impairment was defined as a PTA > 25 dB in the better-hearing ear per the World Health Organization’s definition of hearing impairment (WHO) (the level at which hearing impairment begins to impair daily communication). All thresholds are expressed in dB HL (ANSI, 1989).
2.3 MRI acquisition
MR imaging was performed annually on a GE Signa 1.5T scanner (Milwaukee, WI) using a high-resolution volumetric spoiled-grass axial series (repetition time = 35 msec, echo time = 5 msec, field of view = 24 cm, flip angle = 45°, matrix = 256 × 256, number of excitations = 1, voxel dimensions 0.94 × 0.94 × 1.5 mm).
2.4 MRI analysis
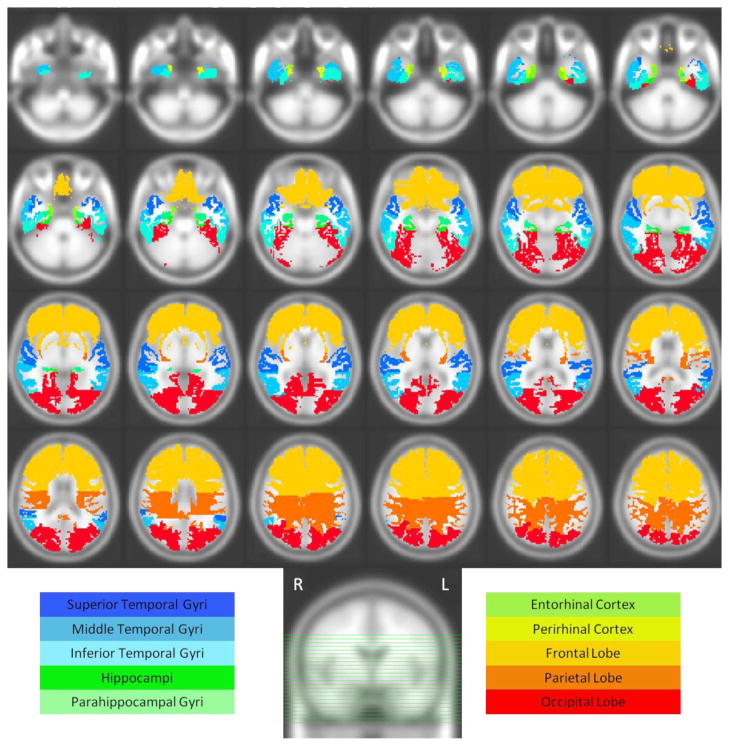
Image processing procedures have been previously validated and described (Goldszal, Davatzikos et al. 1998; Davatzikos, Genc et al. 2001; Resnick, Pham et al. 2003). Briefly, images are corrected for head tilt and rotation, and reformatted parallel to the anterior-posterior intercommissural plane. Extracranial tissue is removed using a semiautomated procedure followed by manual editing. Next, images are segmented into white matter (WM), gray matter (GM), and CSF. The final step involves stereotaxic normalization and tissue quantitation for specific regions of interest (ROI). A template-based deformation approach is employed, using the ICBM standard MRI (Montreal Neurologic Institute) as the template and a hierarchical elastic matching algorithm for ROI determination(Shen and Davatzikos 2002) (see Figure 1 for ROI locations used in this study). Voxel-based analysis utilizes our RAVENS approach (regional analysis of volumes examined in normalized space) (Goldszal, Davatzikos et al. 1998), whereby local values of tissue density maps (GM, WM, and CSF) reflect the amount of respective tissue in the vicinity of a voxel. Tissue densities are mathematical quantities measuring local tissue volumes and do not reflect any microstructural physical density of brain tissue. Intracranial volume (ICV) is determined using the template warping algorithm modified for head image registration. First, the ICV in the template is manually delineated by an expert. Then, the template with its ICV mask is warped to the space of each individual head to extract the ICV of the individual.
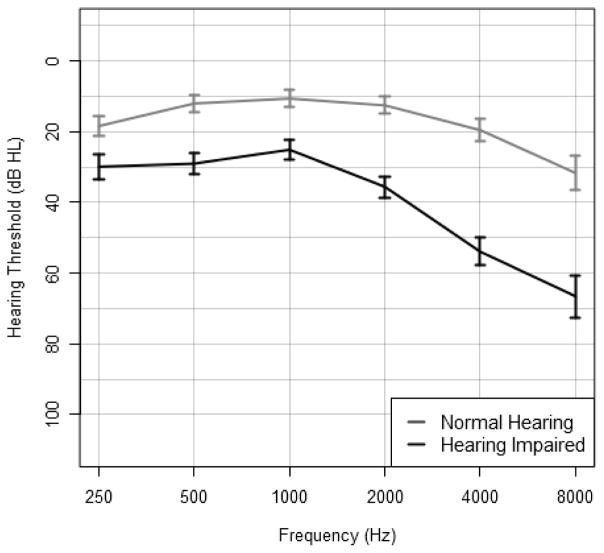
Figure 1. Mean audiograms of individuals with normal hearing (n = 75) and hearing impairment (n = 51).
Error bars denote 95% confidence intervals of the mean.
2.5 Other covariates
We adjusted for covariates such as cardiovascular (hypertension, smoking) and demographic risk factors (age, sex) that are known to be associated with hearing impairment (Lin, Thorpe et al. 2011) and that could potentially confound the association of hearing impairment with structural brain volumes. The diagnosis of hypertension was established based on a systolic blood pressure >140 and/or diastolic blood pressure >90 mmHg or treatment with antihypertensive medications. Smoking status (current, former, never) was based on self-report. A diagnosis of diabetes was established based on a fasting glucose >125 mg/dL, a pathologic oral glucose tolerance test, or a positive history of a physician diagnosis plus pharmacologic treatment. Hypertension, diabetes, and smoking history were defined at baseline.
2.6 Statistical analysis
Baseline characteristics were compared using t-test, chi-square, and/or Fisher exact tests as appropriate. For each MRI-defined brain volume, linear mixed effect (LME) models were used to investigate the association of hearing impairment as a time-invariant predictor with longitudinal trajectories of brain volumes over time. The dependent variable is volume of each MRI-defined brain region, and all models included covariates of intracranial volume (ICV), sex, baseline age, hypertension, smoking, hearing impairment, time (years of follow-up from baseline), and two-way interactions of time with hearing impairment, age, and sex. Race and diabetes were not included as covariates because few individuals were of non-white race or had diabetes in our cohort. The random effects included intercept and time, which allows individual-specific baseline brain volumes and rates of change to vary. Effect sizes of difference in rates of change between those with hearing impairment and normal hearing were calculated by dividing the estimated difference in annual rates of change by the standard deviation of the rates of change. Significance testing for ROI analyses was 2-sided with a type I error of 0.05. The statistical software used was SAS 9.2 (SAS Institute, Cary, North Carolina).
Voxel-based analyses were performed using R 2.15 (www.r-project.org). We used LME models and the RAVENS maps to investigate the association of baseline hearing impairment with longitudinal measures of RAVENS values. These models included age, sex, time, hearing impairment, and all two-way interactions with time as fixed effects. Individual-specific RAVENS baseline values and annual rates of change were modeled as random effects. In all LME models, an interaction term of hearing impairment × time was included to assess whether hearing impairment at baseline affected the rates of change in brain volumes or RAVENS values reflecting local volumes. All statistical map results were thresholded at p < 0.001, with a cluster size (spatial extent threshold) of at least 50 voxels, and projected onto the MNI template for visualization.
3.0 Results
Individuals with hearing impairment (n = 51) were more likely to be men, older, white, and smokers than individuals with normal hearing (n = 75) (Table 1). Mean audiograms for individuals with normal hearing vs. hearing impairment are presented in Figure 1. The majority of individuals with hearing impairment had impairments in the mild range (n = 40, 78%) rather than in the moderate (n = 9, 18%) or severe range (n = 2, 4%). Among participants with hearing impairment, 13 individuals (25.5%) reported hearing aid use. The mean follow-up time for study participants was 6.4 years (S.D. 2.8).
Table 1.
Baseline characteristics of study participants with normal hearing and hearing impairment
| Normal Hearing (n = 75) | Hearing Impairmenta (n = 51) | P-value | |
|---|---|---|---|
| Women | 39 (52.0) | 11 (21.6) | <.001 |
| Age, mean (SD), y | 67.0 (6.9) | 73.8 (7.3) | <.001 |
| Follow-up, mean (SD), y | 6.7 (2.6) | 6.1 (3.0) | .25 |
| White race | 67 (89.3) | 50 (98.0) | .08 |
| Education, mean (SD), y | 16.8 (2.3) | 15.6 (3.2) | .02 |
| Diabetes | 6 (8.0) | 4 (7.8) | .99 |
| Hypertension | 32 (42.7) | 19 (37.3) | .54 |
| Smoking | |||
| Never | 31 (41.3) | 15 (29.4) | .01 |
| Former | 38 (50.7) | 22 (43.1) | |
| Current | 6 (8.0) | 14 (27.5) | |
| Hearing impairment classificationb | |||
| Mild | 40 (78.4) | ||
| Moderate | 9 (17.6) | ||
| Severe | 2 (3.9) | ||
Values are expressed as number (percentage) unless otherwise indicated.
Hearing impairment is defined as a pure tone average (PTA) of air-conduction hearing thresholds at 0.5, 1, 2, and 4 kHz tones > 25 dB in the better hearing ear
Hearing impairment classification based on PTA in the better hearing ear: Mild (>25–40 dB), Moderate (>40–70 dB), and Severe (>70 dB).
For the ROI locations shown in Figure 2, we compared baseline brain volumes and rates of change in brain volumes of individuals with normal hearing and hearing impairment after adjusting for age, sex, ICV, hypertension, and smoking. At baseline, there were no significant differences in global and lobar brain volumes between the two groups (Table 2). We next investigated the association of hearing impairment with trajectories of brain volume change over time. Significant rates of atrophy over time were observed across all brain regions for both individuals with normal hearing and hearing impairment (Table 2). However, compared to individuals with normal hearing, individuals with baseline hearing impairment had accelerated rates of atrophy in whole brain and temporal lobe gray matter volumes (Table 2). On average, whole brain volumes declined by 8.4 cm3/year vs. 7.2 cm3/year, respectively, in those with hearing impairment versus normal hearing (p = .017). The association of hearing impairment with accelerated whole brain volume loss was not dependent on temporal lobe volume changes. Compared to individuals with normal hearing, those with hearing impairment had greater rates of whole brain volume decline even after excluding the temporal lobe from calculations of whole brain volume (Table 2).
Figure 2.
Axial slices displaying lobar volumes and temporal lobe ROIs used in this study
Table 2.
Adjusteda baseline brain volumes and annual rates of change in brain volume according to hearing status
| Baseline Volume (cm3)b | Annual Rate of Change (cm3/year) | |||||
|---|---|---|---|---|---|---|
| Normal Hearing Mean (95%CI) |
Hearing Impairment Mean (95%CI) |
Normal Hearing Mean (95%CI) |
Hearing Impairment Mean (95%CI) |
Differencec Mean (95%CI) |
Effect Sized | |
| Global measures | ||||||
| Whole brain | 979.6 (965.7 – 993.6) | 979.9 (962.3 – 997.5) | −7.22 (−7.78 – −6.66)** | −8.42 (−9.17 – −7.67) ** | −1.20 (− 2.17 – −0.22)+ p = .02 |
−0.68 |
| Whole brain (without temporal lobe) | 879.6 (866.8 – 892.4) | 879.8 (863.6 – 895.9) | −6.76 (−7.26 – −6.26)** | −7.72 (−8.38 – −7.05) ** | −0.96 (−1.83 – −0.08)+ p = .03 |
−0.63 |
| vCSF | 29.8 (25.7 – 34.0) | 30.8 (25.6 – 36.1) | 1.30 (1.10 – 1.51) ** | 1.29 (1.01 – 1.56) ** | 0.02 (−0.38 – 0.34) | −0.02 |
| Gray matter | 530.3 (521.4 – 539.2) | 535.1 (523.9 – 546.4) | −2.60 (−3.06 – −2.15) ** | −3.37 (−3.97 – −2.77) ** | −0.77 (−1.56 – 0.02) | −0.62 |
| White matter | 449.5 (439.7 – 459.2) | 444.9 (432.6 – 457.3) | −4.57 (−5.04 – −4.11) ** | −5.08 (−5.69 – −4/46) ** | −0.50 (−1.31 – 0.30) | −0.45 |
| Lobar measures | ||||||
| Gray matter | ||||||
| Frontal | 155.1 (151.9 – 158.3) | 156.9 (152.8 – 160.9) | −0.94 (−1.11 – −0.76) ** | −1.18 (−1.41 – −0.95) ** | −0.24 (−0.55 – 0.07) | −0.50 |
| Temporal | 114.2 (111.9 – 116.6) | 114.8 (111.8 – 117.8) | −0.37 (−0.51 – −0.22) ** | −0.66 (−0.85 – −0.46) ** | −0.29 (−0.54 – −0.04) + p = .02 |
−0.67 |
| Parietal | 85.5 (83.4 – 87.6) | 86.1 (83.5 – 88.7) | −0.74 (−0.84 – −0.63) ** | −0.77 (−0.91 – −0.63) ** | −0.03 (−0.21 – 0.15) | −0.14 |
| Occipital | 75.4 (73.6 –77.1) | 74.2 (72.1 – 76.5) | −0.45 (−0.53 – −0.36) ** | −0.36 (−0.47 – −0.24) ** | 0.09 (−0.06 – 0.24) | 0.55 |
| White Matter | ||||||
| Frontal | 173.6 (169.4 – 177.9) | 173.3 (167.9 – 178.7) | −1.86 (−2.06 – −1.66) ** | −2.09 (−2.36 – −1.83) ** | −0.24 (−0.58 – 0.11) | −0.51 |
| Temporal | 100.3 (97.5 – 103.0) | 99.9 (96.4 – 103.4) | −1.69 (1.84 – −1.54) ** | −1.68 (−1.87 – −1.48) ** | 0.01 (−0.24 – 0.27) | 0.03 |
| Parietal | 97.7 (95.3 – 100.0) | 96.9 (94.0 – 100.0) | −0.30 (−0.41 – −0.18) ** | −0.41 (−0.56 – −0.26) ** | −0.11 (−0.31 – 0.08) | −0.39 |
| Occipital | 45.2 (43.7 – 46.7) | 43.8 (41.9 – 45.7) | −0.13 (−0.24 – −0.02) + | −0.22 (−0.38 – −0.07) * | −0.09 (−0.29 – 0.10) | −0.39 |
Statistical significance:
p < .05,
p < .01,
p <.001
Abbreviations: 95% CI = 95% Confidence Interval; vCSF = ventricular CSF
All models included covariates of ICV, sex, baseline age, hypertension, smoking, hearing impairment, time (years of follow-up from baseline), and two-way interactions of time with hearing impairment, age, and sex
There were no significant differences in baseline brain volumes between individuals with normal hearing and hearing impairment after adjusting for the multiple covariates.
Difference is the (rate of change in hearing impairment) - (rate of change in normal hearing). Therefore, negative values indicate greater annual rates of atrophy in individuals with hearing impairment compared to those with normal hearing.
Effect size is calculated as the difference in rates of change between those with hearing impairment and normal hearing divided by the corresponding standard deviation of the rate of change.
We investigated whether hearing impairment was specifically associated with regional volumes in the right and left temporal lobe implicated in spoken language processing (superior [STG], middle [MTG], and inferior [ITG] temporal gyri). In these analyses (Table 3), we observed differential associations between hearing impairment and brain atrophy in the right versus left temporal regions (Table 3). Stronger associations between hearing impairment and rates of decline were observed in the right versus left temporal lobe. Specifically, compared to individuals with normal hearing, individuals with hearing impairment had accelerated volume declines in the STG (p = .003), MTG (p = .04), ITG (p = .01), and parahippocampal gyrus (p = .009) of the right but not the left temporal lobe. In analyses treating hearing impairment as a continuous variable to investigate for a “dose-response” effect in the entire cohort of individuals, we did not observe any significant associations between greater levels of hearing impairment and faster rates of brain atrophy in the right temporal lobe (data not shown).
Table 3.
Adjusteda baseline and annual rates of change in right versus left temporal lobe regional brain volumes according to hearing status
| Baseline Volume (cm3)b | Annual Rate of Change (cm3/year) | |||||
|---|---|---|---|---|---|---|
| Normal Hearing Mean (95%CI) |
Hearing Impairment Mean (95%CI) |
Normal Hearing Mean (95%CI) |
Hearing Impairment Mean (95%CI) |
Differencec Mean (95%CI)) |
Effect Sized | |
| Right | ||||||
| Superior | 13.9 (13.44 – 14.35) | 14.1 (13.5 – 14.6) | 0.01 (−0.02 – 0.05) | −0.08 (−0.13 – −0.03)* | −0.10 (−0.16 – −0.03)* p = 0.003 |
−1.02 |
| Middle | 21.8 (21.2 – 22.4) | 21.8 (21.1 – 22.6) | −0.03 (−0.08 – 0.03) | −0.13 (−0.20 – 0.06)** | −0.10 (−0.20 – −0.004)+ p = 0.04 |
−0.60 |
| Inferior | 7.74 (7.38 – 8.09) | 8.03 (7.57 – 8.48) | −0.02 (−0.04 – 0.003) | −0.07 (−0.09 – −0.04)** | −0.05 (−0.09 – −0.01)+ p = 0.01 |
−1.14 |
| Hippocampus | 3.22 (3.12 – 3.31) | 3.21 (3.09 – 3.33) | −0.01 (−0.02 – −0.003)** | −0.02 (−0.03 – −0.01)** | −0.01 (−0.02 – 0.01) | −0.20 |
| Parahippocampal | 2.03 (1.96 – 2.10) | 2.08 (1.98 – 2.17) | −0.002 (−0.01 – 0.003) | −0.02 (−0.02 – −0.01)** | −0.01 (−0.02 – −0.003)* p = 0.009 |
−1.18 |
| Entorhinal cortex | 1.35 (1.26 – 1.44) | 1.37 (1.26 – 1.48) | 0.01 (0.01 – 0.02)** | 0.01 (−0.002 – 0.02) | −0.01 (−0.02 – 0.01) | −0.34 |
| Perirhinal cortex | 1.27 (1.17 – 1.37) | 1.35 (1.22 – 1.47) | 0.001 (−0.01 – 0.01) | −0.01 (−0.02 – 0.01) | −0.01 (−0.02 – 0.01) | −0.26 |
| Left | ||||||
| Superior | 15.3 (14.9 – 15.7) | 15.7 (15.1 – 16.4) | −0.21 (−0.25 – −0.18)** | −0.24 (−0.29 – −0.19)** | −0.03 (−0.03 – 0.09) | −0.64 |
| Middle | 17.3 (16.8 −17.9) | 17.4 (16.7 – 18.1) | −0.13 (−0.16 – −0.10)** | −0.18 (−0.22 – −0.14)** | −0.04 (−0.01 – 0.10) | −1.48 |
| Inferior | 8.40 (8.04 – 8.75) | 8.40 (7.95 – 8.86) | −0.03 (−0.05 – −0.003)+ | −0.03 (−0.06 – −0.0003)+ | −0.005 (−0.04 – 0.05) | −0.09 |
| Hippocampus | 2.95 (2.86 – 3.04) | 2.90 (2.79 – 8.86) | −0.01 (−0.02 – −0.01)** | −0.01 (−0.02 – 0.00)+ | 0.001 (−0.01 – 0.01) | 0.09 |
| Parahippocampal | 1.54 (1.48 – 1.60) | 1.57 (1.50 – 1.65) | −0.001 (−0.01 – 0.003) | −0.004 (−0.01 – 0.002) | −0.003 (−0.01 – 0.004) | −0.48 |
| Entorhinal cortex | 0.97 (0.91 – 1.03) | 0.96 (0.89 – 1.04) | −0.0003 (−0.01 – 0.005) | −0.002 (−0.01 – 0.004) | −0.002 (−0.01 – 0.006) | −0.25 |
| Perirhinal cortex | 1.70 (1.59 – 1.81) | 1.66 (1.52 – 1.81) | −0.02 (−0.02 – −0.01)** | −0.02 (−0.03 – −0.01)* | −0.001 (−0.02 – 0.01) | −0.10 |
Statistical significance:
p < .05,
p < .01,
p < .001
All models included covariates of ICV, sex, baseline age, hypertension, smoking, hearing impairment, time (years of follow-up from baseline), and two-way interactions of time with hearing impairment, age, and sex.
There were no significant differences in baseline regional brain volumes between individuals with normal hearing and hearing impairment.
Difference is the (rate of change in hearing impairment) - (rate of change in normal hearing). Therefore, negative values indicate greater annual rates of atrophy in individuals with hearing impairment compared to those with normal hearing.
Effect size is calculated as the difference in rates of change between those with hearing impairment and normal hearing divided by the corresponding standard deviation of the rate of change.
Analyses of extratemporal regional brain volumes demonstrated no consistent associations with hearing impairment (Supplementary Table 1). We observed that hearing impairment was only associated with volume loss in two isolated extratemporal regions (decreased loss in the superior parietal lobule, and increased loss in the cingulate gyrus; Supplementary Table 1).
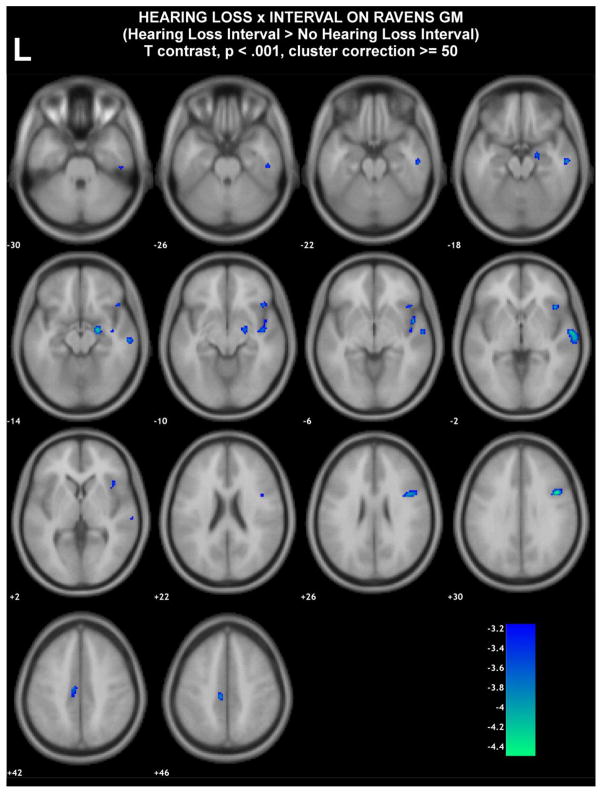
We conducted additional exploratory, voxel-based analyses to identify brain regions that could be associated with hearing impairment. In these exploratory analyses, we used a less stringent analytic model adjusting for fewer confounders in order to possibly identify other brain regions associated with hearing impairment. Models were adjusted for for age and sex but not for hypertension and smoking (factors not substantively associated with brain atrophy in ROI analyses). These analyses yielded results similar to ROI analyses with accelerated volume losses in the right temporal lobe being observed in those individuals with hearing impairment versus normal hearing (Figure 3 and Supplementary Table 2). These analyses, however, also demonstrated several other extratemporal areas, again primarily on the right side, associated with accelerated atrophy in individuals with hearing impairment.
Figure 3. Difference in average slopes of RAVENS gray matter maps between those with hearing impairment versus normal hearing.
Blue/green are regions in which individuals with hearing impairment compared to those with normal hearing had a higher rate of gray matter decrease. Color bars denote regression coefficient t-values (regression coefficient/standard error).
4.0 Discussion
In this study, hearing impairment in older adults was independently associated with accelerated rates of decline in regional brain volumes in the right temporal lobe (STG, MTG, ITG) critical for spoken language processing as well as whole brain volume over a mean follow-up period of 6.4 years. These results were robust to adjustment for multiple potential confounders, and the observation of specific vulnerability of the temporal lobe, particularly on the right side, was consistent in both region-of-interest and voxel-based analyses. The magnitude of the observed differences in the rates of brain atrophy associated with hearing impairment are comparable to differences previously observed between individuals developing incident mild cognitive impairment versus those maintaining normal cognition (Driscoll, Davatzikos et al. 2009).
Our findings extend the discussion in the literature on whether peripheral hearing impairment has broader implications for brain structure and function. Prior cross-sectional neuroimaging studies have demonstrated that greater audiometric hearing impairment is associated with reduced volumes in the primary auditory cortex and temporal lobe (Husain, Medina et al. 2010; Peelle, Troiani et al. 2011; Eckert, Cute et al. 2012). Other studies using diffusion-tensor imaging of the central auditory pathways have demonstrated decreased fractional anisotropy in the lateral lemniscus and inferior colliculus in individuals with hearing impairment versus normal hearing (Chang, Lee et al. 2004; Lin, Wang et al. 2008). These findings indicate underlying microstructural changes with possible loss of myelin and axonal fibers in central white matter auditory tracts. Our study builds on these prior results and has additional attributes of including repeated assessments of lobar and regional brain volumes in a well-characterized longitudinal cohort of participants.
The association of hearing impairment with regional brain atrophy over time was primarily observed in temporal lobe structures (STG, MTG, ITG) important for spoken language processing (Davis and Gaskell 2009; Adank 2012; Peelle 2012) consistent with our a priori hypotheses. Voxel-based analyses supported the more pronounced effects for temporal lobe structures and indicated greater right than left hemisphere involvement. The middle and inferior temporal gyri are of particular significance in view of observations that these regions are not only important for spoken language processing but are also involved in semantic memory, sensory integration, and in the early stages of mild cognitive impairment or early Alzheimer disease (Tranel, Damasio et al. 1997; Mesulam 1998; Kantarci and Jack 2004; Chetelat, Landeau et al. 2005). The association of hearing impairment with brain volume changes was specific to right temporal lobe regions, and we did not observe any consistent associations of hearing impairment with extratemporal brain regions in ROI analyses. Exploratory voxel-based analyses using a less stringent analytic model identified several extratemporal regions, again primarily on the right side, that were associated with hearing impairment. These identified regions are of unclear significance at present and could reflect chance effects from multiple comparisons. We did not perform specific adjustments for multiple comparisons for the main associations tested in the temporal lobe (Tables 2–3) given that these analyses were specifically based on our a priori hypotheses.
In analyses stratified by the right versus left temporal lobe, we observed substantially stronger associations of hearing impairment with accelerated volume losses in the right versus left temporal lobe. The basis of this differential association is unclear but may relate to laterality differences in language processing. Spoken language is predominantly processed in the STG, MTG, and ITG of the left temporal lobe (Davis and Gaskell 2009; Adank 2012; Peelle 2012) regardless of handedness (Knecht, Drager et al. 2000). Whether greater processing of auditory signals in the left temporal lobe could help maintain structural volumes of the left versus right temporal lobe in individuals with hearing impairment is plausible but speculative.
The basis of the observed associations between hearing impairment and accelerated brain atrophy is unknown. One possibility is a shared neuropathologic or intrinsic cellular aging process leading to both cochlear and brain aging. In the present analyses, we have adjusted for potential confounders (e.g., age, hypertension), and individuals selected to participate in the neuroimaging substudy did not have central nervous system at study entry. While it remains possible that unmeasured confounders (e.g., inflammatory mediators) could contribute to our findings, the specific perisylvian pattern of accelerated atrophy in brain regions critical for language processing, rather than mesial temporal lobe pattern characteristic of MCI and early Alzheimer disease, argues against a common neuropathologic process being the only basis for the observed associations. There could also be a possibility of reverse causation with hearing thresholds being affected by top-down processes associated with temporal lobe atrophy. However, hearing as measured with pure tone audiometry is generally considered to be primarily a measure of peripheral function because detection of a simple pure tone relies on cochlear transduction and neuronal afferents to brainstem nuclei without requiring significant higher auditory cortical processing (Pickles 2008). We are currently gathering additional measures of both peripheral and central auditory functioning in the BLSA that will allow us to further investigate the distinction between central and peripheral auditory function and its association with brain structure in future studies.
Hearing impairment could also be potentially associated with brain volume changes through reduced neural stimulation of the auditory cortex by impoverished auditory signals (Peelle, Troiani et al. 2011). In animal models, cochlear impairments are known to be associated with both tonotopic reorganization of the auditory cortex (Schwaber, Garraghty et al. 1993; Kakigi, Hirakawa et al. 2000; Cheung, Bonham et al. 2009) as well as morphologic changes in central neuronal structures (Groschel, Gotze et al. 2010). One possible explanation for our findings is that impoverished and degraded auditory signals associated with peripheral hearing impairment could lead to volume losses in regions of the temporal lobe important for auditory processing (STG, MTG, ITG) with cascading effects for semantic memory and cognitive processes dependent on these same regions. Whether these effects and the broader influence of hearing impairment on social isolation and stress (Cole, Hawkley et al. 2007; Cole, Hawkley et al. 2010) could mediate the observed association of hearing impairment with accelerated rates of whole brain volume (Sapolsky 1999; Radley and Morrison 2005; McEwen 2008) is plausible but speculative. Overall, our results suggest that impaired hearing has implications for cortical structures well beyond auditory cortex, and are generally consistent with epidemiologic data demonstrating broader functional implications of hearing impairment on cognitive performance.
In contrast to our longitudinal findings, we did not observe significant cross-sectional associations of hearing impairment with reduced brain volumes at baseline. This lack of association of hearing with inter-individual brain volumes at baseline may not be surprising given the expected heterogeneity in brain structural volumes between individuals. Therefore, detecting whether hearing impairment could affect brain structural volumes against a background of pronounced inter-individual variation in brain volumes requires longitudinal assessments of intra-individual brain structure over time. This observation underscores the importance of prospective studies with repeated measurements rather than relying on cross-sectional studies when analyzing neuroimaging data.
4.1 Limitations
Our study has several limitations. Measures of hearing were only available concurrent or before the baseline MRI scan rather than longitudinally over the course of the neuroimaging study. We, therefore, cannot ascertain the duration of hearing impairment in study participants. However, it is unlikely that this limitation would differentially bias our findings given that hearing impairment is a chronic condition that is not reversible, and hearing only gradually worsens with time. We also did not observe a dose-response effect between degree of hearing impairment and rates of temporal lobe atrophy, but these analyses were likely underpowered due to few individuals in our study with greater than mild hearing impairment. Alternatively, this observation may indicate that there is a non-linear association of hearing impairment with rates of brain atrophy such that greater levels of hearing impairment above a certain threshold are not necessarily associated with faster rates of atrophy. In future analyses incorporating a larger dataset, we plan to explore using a data-driven approach to identify whether other audiometric thresholds (rather than the 25dB cutoff defined a priori in this study) may hold significance in identifying individuals who differential rates of brain volume atrophy. The results from our study are also not fully generalizable given the high socioeconomic status of the volunteer BLSA cohort. This potential limitation to broad generalizability, however, may strengthen the internal validity of our findings given the relative homogeneity of the study cohort in both observed and likely unobserved characteristics.
In our present ROI analyses examining the association of hearing impairment with STG, MTG, and ITG volumes, we did not perform adjustment for multiple comparisons because these analyses were specifically performed based on our a priori hypothesis. The association of other brain regions with hearing were also investigated and presented (other temporal regions in Tables 2 and 3, extratemporal regions in Supplementary Table 1), but significant findings in these tables may need to be interpreted with caution in light of multiple comparisons. Our voxel-based analyses used a stringent threshold of p<.001 uncorrected with a cluster size >50. The use of cluster size thresholding avoids issues due to voxel-wise multiple comparisons based on random field theory (Friston, Worsley et al. 1993).
We were unable to explore whether hearing aid use could possibly moderate the association of hearing impairment with declines in brain volume because of the few number of participants with hearing impairment who reported use of a hearing aid (n=13). Importantly, the results of any such analyses from an observational study would also be markedly difficult to interpret given that data on other key variables (e.g. years of hearing aid use, type of hearing aid, hours worn per day, etc.) that would affect the success of hearing rehabilitative treatment and affect any possible association were not available. Individuals with hearing impairment choosing to use hearing aids versus those who do not also likely differ in multiple factors (e.g., education, health behaviors) that could possibly also affect this association in an observational study. Consequently, determining the possible role of hearing rehabilitative treatment in potentially mitigating brain volume declines will likely require a randomized controlled trial.
4.2 Conclusion
Our findings demonstrate that peripheral hearing impairment is independently associated with accelerated declines in whole brain volume and regional volumes concentrated in the right temporal lobe. Further studies investigating the mechanistic basis of the observed associations and whether rehabilitative interventions for hearing impairment could potentially affect brain aging are needed.
Supplementary Material
Acknowledgments
Funding/Support
This study was supported by the intramural research program of the National Institute on Aging. Dr. Lin was supported by NIH K23DC011279, a Triological Society/American College of Surgeons Clinician Scientist Award, and the Eleanor Schwartz Charitable Foundation.
Footnotes
Conflict of interest
Dr. Lin reports being a consultant to Cochear Limited, on the scientific advisory board of Pfizer and Autifony, and a speaker for Med El and Amplifon.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adank P. Design choices in imaging speech comprehension: an Activation Likelihood Estimation (ALE) meta-analysis. Neuroimage. 2012;63(3):1601–1613. doi: 10.1016/j.neuroimage.2012.07.027. [DOI] [PubMed] [Google Scholar]
- Barnes LL, Mendes de Leon CF, et al. Social resources and cognitive decline in a population of older African Americans and whites. Neurology. 2004;63(12):2322–2326. doi: 10.1212/01.wnl.0000147473.04043.b3. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, et al. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. 2006;5(5):406–412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- Chang Y, Lee SH, et al. Auditory neural pathway evaluation on sensorineural hearing loss using diffusion tensor imaging. Neuroreport. 2004;15(11):1699–1703. doi: 10.1097/01.wnr.0000134584.10207.1a. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Landeau B, et al. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. Neuroimage. 2005;27(4):934–946. doi: 10.1016/j.neuroimage.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Cheung SW, Bonham BH, et al. Realignment of interaural cortical maps in asymmetric hearing loss. J Neurosci. 2009;29(21):7065–7078. doi: 10.1523/JNEUROSCI.6072-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, et al. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc Natl Acad Sci U S A. 2010;108(7):3080–3085. doi: 10.1073/pnas.1014218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, et al. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8(9):R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davatzikos C, Genc A, et al. Voxel-based morphometry using the RAVENS maps: methods and validation using simulated longitudinal atrophy. Neuroimage. 2001;14(6):1361–1369. doi: 10.1006/nimg.2001.0937. [DOI] [PubMed] [Google Scholar]
- Davis MH, Gaskell MG. A complementary systems account of word learning: neural and behavioural evidence. Philos Trans R Soc Lond B Biol Sci. 2009;364(1536):3773–3800. doi: 10.1098/rstb.2009.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MH, I, Johnsrude S. Hearing speech sounds: top-down influences on the interface between audition and speech perception. Hear Res. 2007;229(1–2):132–147. doi: 10.1016/j.heares.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Davatzikos C, et al. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology. 2009;72(22):1906–1913. doi: 10.1212/WNL.0b013e3181a82634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Cute SL, et al. Auditory cortex signs of age-related hearing loss. J Assoc Res Otolaryngol. 2012;13(5):703–713. doi: 10.1007/s10162-012-0332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, et al. Assessing the significance of focal activations using their spatial extent. Human Brain Mapping. 1993;1(3):210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Gallacher J, Ilubaera V, et al. Auditory threshold, phonologic demand, and incident dementia. Neurology. 2012;79(15):1583–1590. doi: 10.1212/WNL.0b013e31826e263d. [DOI] [PubMed] [Google Scholar]
- Goldszal AF, Davatzikos C, et al. An image-processing system for qualitative and quantitative volumetric analysis of brain images. J Comput Assist Tomogr. 1998;22(5):827–837. doi: 10.1097/00004728-199809000-00030. [DOI] [PubMed] [Google Scholar]
- Groschel M, Gotze R, et al. Differential impact of temporary and permanent noise-induced hearing loss on neuronal cell density in the mouse central auditory pathway. J Neurotrauma. 2010;27(8):1499–1507. doi: 10.1089/neu.2009.1246. [DOI] [PubMed] [Google Scholar]
- Gussekloo J, de Craen AJ, et al. Sensory impairment and cognitive functioning in oldest-old subjects: the Leiden 85+ Study. Am J Geriatr Psychiatry. 2005;13(9):781–786. doi: 10.1176/appi.ajgp.13.9.781. [DOI] [PubMed] [Google Scholar]
- Husain FT, Medina RE, et al. Neuroanatomical changes due to hearing loss and chronic tinnitus: a combined VBM and DTI study. Brain Res. 2010;1369:74–88. doi: 10.1016/j.brainres.2010.10.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakigi A, Hirakawa H, et al. Tonotopic mapping in auditory cortex of the adult chinchilla with amikacin-induced cochlear lesions. Audiology. 2000;39(3):153–160. doi: 10.3109/00206090009073068. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Jack CR., Jr Quantitative magnetic resonance techniques as surrogate markers of Alzheimer’s disease. NeuroRx. 2004;1(2):196–205. doi: 10.1602/neurorx.1.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S, Drager B, et al. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123(Pt 12):2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Lin FR. Hearing loss and cognition among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66(10):1131–1136. doi: 10.1093/gerona/glr115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Ferrucci L, et al. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology. 2011;25(6):763–770. doi: 10.1037/a0024238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Metter EJ, et al. Hearing loss and incident dementia. Arch Neurol. 2011;68(2):214–220. doi: 10.1001/archneurol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Thorpe R, et al. Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66(5):582–590. doi: 10.1093/gerona/glr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Yaffe K, et al. Hearing loss and cognitive decline among older adults. JAMA Intern Med. 2013 doi: 10.1001/jamainternmed.2013.1868. Epublished January 21, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Wang J, et al. Diffusion tensor imaging of the auditory pathway in sensorineural hearing loss: changes in radial diffusivity and diffusion anisotropy. J Magn Reson Imaging. 2008;28(3):598–603. doi: 10.1002/jmri.21464. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: a strong connection. Psychol Aging. 1994;9(3):339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583(2–3):174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Peelle JE. “The hemispheric lateralization of speech processing depends on what speech” is: a hierarchical perspective. Front Hum Neurosci. 2012;6:309. doi: 10.3389/fnhum.2012.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, I, Johnsrude S, et al. Hierarchical processing for speech in human auditory cortex and beyond. Front Hum Neurosci. 2010;4:51. doi: 10.3389/fnhum.2010.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, et al. Hearing loss in older adults affects neural systems supporting speech comprehension. J Neurosci. 2011;31(35):12638–12643. doi: 10.1523/JNEUROSCI.2559-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles JO. An introduction to the physiology of hearing. Bingley, UK: Emerald Group Publishing; 2008. [Google Scholar]
- Radley JJ, Morrison JH. Repeated stress and structural plasticity in the brain. Ageing Res Rev. 2005;4(2):271–287. doi: 10.1016/j.arr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Goldszal AF, et al. One-year age changes in MRI brain volumes in older adults. Cereb Cortex. 2000;10(5):464–472. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, et al. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23(8):3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids, stress, and their adverse neurological effects: relevance to aging. Exp Gerontol. 1999;34(6):721–732. doi: 10.1016/s0531-5565(99)00047-9. [DOI] [PubMed] [Google Scholar]
- Schwaber MK, Garraghty PE, et al. Neuroplasticity of the adult primate auditory cortex following cochlear hearing loss. Am J Otol. 1993;14(3):252–258. [PubMed] [Google Scholar]
- Shen D, Davatzikos C. HAMMER: hierarchical attribute matching mechanism for elastic registration. IEEE Trans Med Imaging. 2002;21(11):1421–1439. doi: 10.1109/TMI.2002.803111. [DOI] [PubMed] [Google Scholar]
- Shock NW, Greulich RC, et al. Normal Human Aging: the Baltimore Longitudinal Study of Aging. Wasington, D.C: National Institutes of Health; 1984. [Google Scholar]
- Spitzer RL, Williams JB. Diagnostic and statistical manual of mental disorders DSM III-R. 3. Washington, DC: American Psychiatric Press; 1987. [Google Scholar]
- Tay T, Wang JJ, et al. Sensory and cognitive association in older persons: findings from an older Australian population. Gerontology. 2006;52(6):386–394. doi: 10.1159/000095129. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, et al. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia. 1997;35(10):1319–1327. doi: 10.1016/s0028-3932(97)00085-7. [DOI] [PubMed] [Google Scholar]
- Tun PA, McCoy S, et al. Aging, hearing acuity, and the attentional costs of effortful listening. Psychol Aging. 2009;24(3):761–766. doi: 10.1037/a0014802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann RF, Larson EB, et al. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. JAMA. 1989;261(13):1916–1919. [PubMed] [Google Scholar]
- WHO World Health Organization Prevention of Blindness and Deafness (PBD) Program. Prevention of Deafness and Hearing Impaired Grades of Hearing Impairment. < http://www.who.int/pbd/deafness/hearing_impairment_grades/en/index.html>.
- Wingfield A, Grossman M. Language and the aging brain: patterns of neural compensation revealed by functional brain imaging. J Neurophysiol. 2006;96(6):2830–2839. doi: 10.1152/jn.00628.2006. [DOI] [PubMed] [Google Scholar]
- Wingfield A, Tun PA, et al. Hearing loss in older adulthood - What it is and how it interacts with cognitive performance. Current directions in psychological science. 2005;14(3):144–148. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.