Abstract
Study Aim
Describe ECG abnormalities in the first year following transplant surgery.
Methods
Analysis of 12-lead ECGs from heart transplant subjects enrolled in an ongoing multicenter clinical trial.
Results
585 ECGs from 98 subjects showed few with abnormal cardiac rhythm (99% of ECGs were sinus rhythm/tachycardia). A majority of subjects (69%) had either right intraventricular conduction delay (56%) or right bundle branch block (13%). A second prevalent ECG abnormality was atrial enlargement (64% of subjects) that was more commonly left atrial (55%) than right (30%).
Conclusions
Right intraventricular conduction delay or right bundle branch block is prevalent in heart transplant recipients in the first year following transplant surgery. Whether this abnormality is related to acute allograph rejection or endomyocardial biopsy procedures is the subject of the ongoing clinical trial. Atrial enlargement ECG criteria (especially, left atrial) is also common and is likely due to transplant surgery with subsequent atrial remodeling.
Introduction
The effect of the denervated heart on the electrocardiogram (ECG) of heart transplant recipients is well documented to result in higher resting heart rate and reduced variation of heart rate over 24 hours.1–3 Less is known about other ECG abnormalities in subjects who have undergone a heart transplant within 1 year. This period is especially important to characterize as the heart remains denervated, and the subject is at highest risk for acute cellular rejection; the impact of which is unknown on the ECG. To characterize ECG abnormalities in the first year following transplant surgery, we performed a preliminary analysis of data from heart transplant subjects enrolled in an on-going multicenter clinical trial ending in 2016.4
Methods
Sample/Sites
Adult subjects who underwent heart transplantation were recruited from one of three centers: University of California Los Angeles, Cedars Sinai Medical Center in Los Angeles, and Columbia University-New York Presbyterian Medical Center in New York City.
ECG Analysis
All 12-lead ECGs acquired as part of routine clinical care during the first year following transplant were collected from each medical center’s ECG digital repository and uploaded via a secure network to the ECG Core Lab at the University of California San Francisco for analysis. Excluded from analysis were ECGs that may have been abnormal due to the initial recovery from transplant surgery (<7 days from surgery). All ECGs were interpreted manually onscreen with the aid of digital magnification using a standardized collection tool by a single reviewer (key ECG measurements in Table 1). The most recently published ECG criteria for myocardial ischemia /infarction were used.5
Table 1.
Definitions for selected ECG measurements
| ECG Measurement | Operational Definition |
|---|---|
| PR, QRS, QT, QTc intervals | Automated values after manual verification |
| Frontal plane axis (P, QRS, T) | Automated values after manual verification |
| Atrial or ventricular premature beats | Present/not present |
| Right intraventricular conduction delay | RSR' pattern in V1 with QRS 100–119 ms |
| Right bundle branch block (RBBB) | RSR’ pattern in V1 with a QRS duration ≥120 ms |
| Left intraventricular conduction delay | QS or rS pattern in V1 with a QRS duration 100–120 ms |
| Left bundle branch block (LBBB) | QS or rS pattern in V1 with a QRS duration >120 ms. |
| Anterior fascicular block | Left axis deviation with qR in aVL and onset to peak R ≥45 ms. |
| Posterior fascicular block | Right axis deviation with rS in I, aVL, and qR in III, aVF |
| Left atrial enlargement | Biphasic P wave in V1 with a large terminal negative component whose area ≥40 ms by −0.1 mV; or, notched P wave in II with inter-peak interval >40 ms |
| Right atrial enlargement | P wave amplitude in V1 or V2 ≥0.15 mV |
| Left ventricular hypertrophy | Sum of S wave in V1 + R in V5 or V6 ≥3.5 mV or R in aVL ≥0.9 mV for women or ≥1.1 mV for men |
| Right ventricular hypertrophy | Right axis deviation and R/S ratio in V1 ≥1 |
| ST elevation (2 contiguous leads) | J-point ST elevation with cutoff points in V2, V3 of ≥0.2 mV in men ≥40 years; ≥0.25 mV in men <40 years; ≥0.15 mV in women. |
| ST depression, T inversion (2 contiguous leads) | Horizontal or down-sloping ST depression of ≥0.05 mV and/or T wave inversion of ≥0.1 mV |
ms = millisecond; mV = millivolt
Results
Sample Characteristics
At the time of this report, 98 of the planned 325 subjects had been enrolled in the on-going clinical trial. These 98 subjects had a total of 585 ECGs available for analysis (mean, 6 ±5 per subject). The sample included 71 males (72%) and a mean age of 52 ±12 years (range, 22–75 years). Racial composition was 62% White, 24% Black, 12% Asian, 1% Native American or Pacific Islander. Seventy percent reported their ethnicity as being Non-Hispanic, 24% Hispanic, and 5% unknown.
Cardiac Rhythm
Of the total 585 ECGs, sinus rhythm or sinus tachycardia were present in 580 (99%); atrial fibrillation or flutter was present in 3 (0.5%), and junctional rhythm in 2 (0.3%). Mean heart rate was 94 ±12 bpm. Mean QRS amplitude in lead II was 0.9 ±0.4 mV. Neither heart rate nor QRS amplitude varied over time (r =−.067, p =.11& r =−.106, p =.01 [r2=0.01], respectively).
Frontal Plane Axis
Distributions for P, QRS, and T wave axis are shown in Table 2. Twenty three per cent of the ECGs had an abnormal QRS axis; right axis deviation was more common (13%) than left axis deviation (8%). Few ECGs had abnormal P wave ( 1%) or T wave (7%) axis.
Table 2.
Frontal plane axis in 585 ECGs from heart transplant patients
| Axis | P | QRS | T |
|---|---|---|---|
| Normal | 577 (99) | 452 (77) | 539 (92) |
| Right | 3 (>1) | 74 (13) | 24 (4) |
| Left | 0 (0) | 46 (8) | 5 (>1) |
| Superior | 1 (>1) | 9 (2) | 13 (2) |
ECG Intervals
Mean PR interval was 147 ±20 ms (range=92–218 ms) that had a minor correlation with time (r =.19, p<.05 [r2=0.035]). Disturbances in atrioventricular conduction were rare with just 4 ECGs (<0.5%) from 2 subjects revealing first degree block. Mean QRS duration was 91 ±18 ms (range=62–168 ms) which had a minor correlation with time (r =.12, p =.005 [r2=0.01]). However this correlation did not exist after excluding those with intraventricular conduction delays/blocks (r =−.046 p =.49). Mean QT interval was 355 ±27 ms in males and 375 ±38 ms in females; mean corrected QT interval (QTc) was 442 ±24 ms in males and 458 ±34 ms in females. These gender differences in QT and QTc were statistically significant (both p <0.000).
Intraventricular Conduction
Right intraventricular conduction delay (IVCD) was present in 50% of all ECGs (n=293) from 56% of subjects (Figure 1). Complete right bundle branch block (RBBB) was evident in 10% of ECGs (n=59) from 13% of subjects. Only 2 ECGs had evidence of left IVCD (<1%) and none had left bundle branch block. The onset of right IVCD/RBBB varied. For example, 30 (31%) subjects had right IVCD and 5 (5%) had RBBB from the first ECG analysed (>7 days post-surgery). After an initial normal ECG, 7 (7%) subjects developed right IVCD and 1 (1%) developed RBBB. In addition, 17 (17%) had initial right IVCD that changed to normal conduction and 2 (2%) subjects had initial RBBB that resolved. Criteria for fascicular blocks were uncommon with anterior fascicular block in 5% of subjects; posterior fascicular block in 4%).
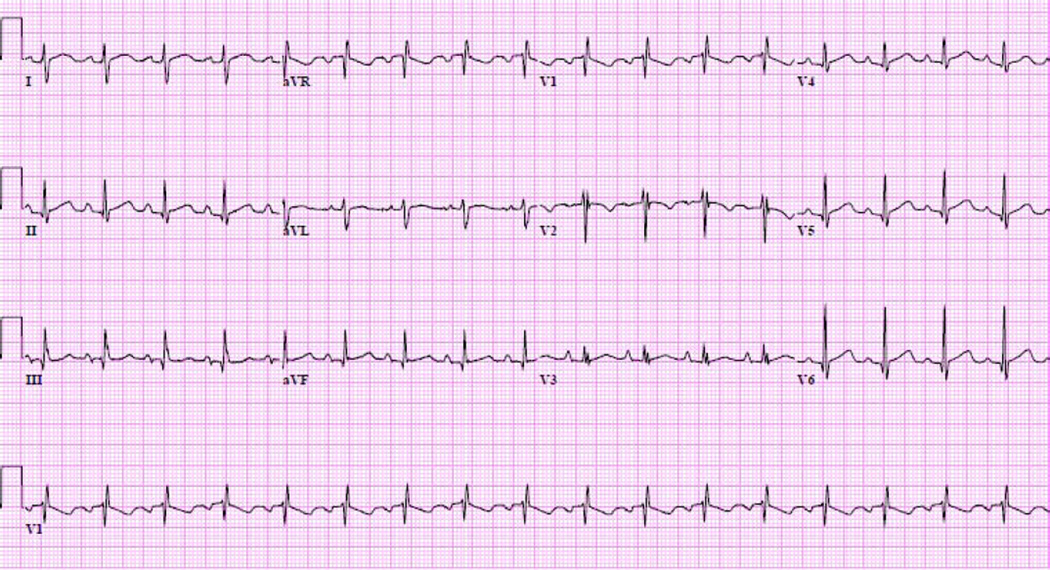
Figure 1.
Typical ECG findings in the first year following heart transplantation in a 30 year old female showing sinus tachycardia, rightward QRS axis, right intraventricular conduction delay, and left atrial enlargement.
Enlargement/Hypertrophy
Subjects were classified as having atrial enlargement or ventricular hypertrophy if the ECG criteria were evident in any one of a subject’s serial ECGs. Atrial enlargement criteria were present in 63 of the 98 patients (64%). Left atrial enlargement was more common (54 patients; 55% of subjects) than right atrial enlargement (29 patients; 30% of subjects). Left ventricular hypertrophy criteria were present in 7% of subjects; right ventricular hypertrophy criteria were present in 15% of subjects.
Criteria for myocardial ischemia/infarction
Distributions for ST elevation, ST depression, T wave inversion, and Q waves are shown in Table 3. Of the total 98 subjects, 23 had Q wave criteria for myocardial infarction, 13 had ST elevation, 11 had ST depression, and 21 had T wave inversion
Table 3.
ECG criteria for myocardial ischemia/infarction in 98 heart transplant recipients
| ECG Criteria | Inferior* | Anterior* | Lateral* | Any Location |
|---|---|---|---|---|
| Q wave | 17 | 8 | 8 | 23 |
| ST elevation | 2 | 13 | 3 | 13 |
| ST depression | 4 | 25 | 10 | 11 |
| T wave inversion | 5 | 12 | 19 | 21 |
Inferior: II, III, aVF; Anterior: V1-V4; Lateral: I, aVL, V5, V6
Discussion
Our findings show that sinus rhythm or sinus tachycardia predominate in the first year following heart transplantation. In our sample of 98 patients, supraventricular arrhythmias were rare (<1%) and ventricular arrhythmias or second/third degree AV block were non-existent.
We found that the most prevalent ECG abnormality was right intraventricular conduction delay or block, occurring in 69% of patients. The etiology and clinical significance of right IVCD and RBBB is unclear; it may be a sign of right ventricular strain and remodeling in patients who have had severe heart failure requiring transplantation. However, our cohort did not have irreversible pulmonary hypertension preoperatively because they were all isolated heart transplantation (i.e., we excluded patients with heart and lung transplants).
Some studies have related right IVCD and RBBB to increased mortality in the post-transplant population,6,7 whereas others found no such correlation.8–10 In a sub-analysis of heart transplant subjects with right IVCD, Gao and colleagues11 demonstrated higher intra-cardiac pressures in those with IVCD than without. However the pressures remained within acceptable ranges and thus, failed to adequately explain this phenomenon. Marcus, et al. conducted one of the larger studies to date with 322 heart transplant recipients followed for 9 ±3 years.10 They found that right IVCD and RBBB developed over time and therefore argued against a perioperative cause. However, in contrast, Jessen and colleagues9 found right IVCD and RBBB to be present immediately after surgery, providing the argument that geometric and rotational forces contributed to these conduction abnormalities. Other postulated mechanisms for the early development of right IVCD and RBBB are intra-operative factors such as increased graft ischemia times.12,13
Beyond confirming a high prevalence of IVCD, our data do not support one etiological hypothesis over another. In our ongoing clinical trial4 we will continue to investigate the etiologic and clinical significance of right IVCD/RBBB after heart transplantation, especially in relation to right ventricular biopsy procedures and cellular rejection grading. It might be hypothesized that intraventricular conduction delays/blocks were present in the donor prior to transplantation. However, an argument against our observed high prevalence of IVCD/RBBB originating with the donor is a recent analysis of 980 ECGs from the California Transplant Donor Network that reported 97% of donors had normal intraventricular conduction on their pre-transplant ECG.14
The second most prevalent ECG abnormality observed in our study was atrial enlargement that was present in 64% of subjects. A possible explanation for this finding may be related to the surgical technique in standard orthotopic heart transplantation15 that involves grafting the donor’s ventricles and a portion of the anterior atria to the native posterior and lateral walls of the recipient’s atria. This has been shown to result in enlarged atrial cavities of abnormal shape and produce a prominent suture line between the recipient and donor components.16 An explanation for the high prevalence of positive ECG criteria for atrial enlargement post-transplant may therefore be a combination of the native diseased atrial posterior walls and the surgical scars, rather than disease progression in the post-surgical transplant patient.17
In an alternate explanation, Cou and colleagues18 studied the relationship between cellular allograft rejection post cardiac transplant and changes in P terminal force criteria for left atrial enlargement in Lead V1. They concluded that abnormal left atrial depolarization was multi-faceted; changes in P terminal force were not correlated with either left atrial size or pressure, or systemic hypertension and thus were not indicative of atrial enlargement. These investigators found that only the degree of cellular rejection correlated to P terminal force. The proposed mechanism for this correlation is myocardial cell damage and derangement of myocardial fibres during cellular rejection, resulting in inhomogeneous conduction and P wave abnormalities. Although our preliminary analysis did not include data on acute allograph rejection, it is the subject of our ongoing clinical trial that aims to identify possible ECG markers for acute allograft rejection.
A third ECG abnormality that was present in 23% of patients was Q waves meeting the criteria for myocardial infarction. Delewi and colleagues19 compared 4 definitions of pathological Q waves that have been published over the years and found that the “classic” criteria of a Q wave ≥40 ms and/or a depth ≥25% of the R wave in the same lead showed the strongest correlation with infarct size as measured by cardiac magnetic resonance. Unfortunately, they did not evaluate the most recent third consensus criteria on universal myocardial infarction published in 2012 that we used in our study. So, it is unclear how our criteria would have compared to the "classic" criteria that Delewi found to correlate best with infarct size. Because the “classic” criteria require a Q wave depth criterion and a wider Q wave (≥40 ms versus ≥30 ms) than the criteria we used, it is likely that some of the 23% of patients in our cohort with Q waves are false positives. In addition, we agree with Goldberger, et al.20 that: a) not all Q waves are pathological, b) not all pathological Q waves are due to myocardial infarction, and c) there is no firm consensus for diagnosis of pathological Q waves. Finally, in the present analysis, we did not investigate the timing of Q waves and likely causes such as longer ischemic times; however, we will investigate this possible etiologic mechanism in our final sample of 325.
Limitations to this study are that it is unclear whether any of these ECG abnormalities were present in the heart donor prior to transplantation. In addition, we did not correlate the ECG abnormalities with other diagnostic tests such as echocardiograms for atrial/ventricular enlargement, or serum troponin for evidence of ST-T wave criteria for acute myocardial infarction.
Acknowledgments
Funding:
National Institutes of Health, NINR, RO1NR012003
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
David Pickham, University of California, San Francisco.
Kathleen Hickey, Columbia University.
Lynn Doering, University of California, Los Angeles.
Belinda Chen, University of California, Los Angeles.
Carmen Castillo, Columbia University.
Barbara J. Drew, University of California, San Francisco.
References
- 1.Villa AE, de Marchena EJ, Myerburg RJ, Castellanos A. Comparisons of paired orthotopic cardiac transplant donor and recipient electrocardiograms. American Heart Journal. 1994;127(1):70–74. doi: 10.1016/0002-8703(94)90511-8. [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulos D, Yusuf S, Johnston J, Bostock J, Sleight P, Yacoub M. The 24-Hour heart rate behavior in long-term survivors of cardiac transplantation. American Journal of Cardiology. 1988;61:880–884. doi: 10.1016/0002-9149(88)90363-3. [DOI] [PubMed] [Google Scholar]
- 3.Babuty D, Aupart M, Cosnay P, et al. Electrocardiographic and electrophysiologic properties of cardiac allografts. Journal of Cardiovascular Electrophysiology. 1994;5:1053–1063. doi: 10.1111/j.1540-8167.1994.tb01147.x. [DOI] [PubMed] [Google Scholar]
- 4.Doering L, Hickey K, Pickham D, Chen B, Drew B. Remote noninvasive allograft rejection monitoring for heart transplant recipients: study protocol for the novel evaluation with home electrocardiogram and remote transmission (NEW HEART) study. BMC Cardiovascular Disorders. 2012;12(14) doi: 10.1186/1471-2261-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Third Universal Definition of Myocardial Infarction. Circulation. 2012 Oct 16;126(16):2020–2035. doi: 10.1161/CIR.0b013e31826e1058. 2012. [DOI] [PubMed] [Google Scholar]
- 6.Osa A, Almenar L, Arnau MA, et al. Is the prognosis poorer in heart transplanted patients who develop a right bundle branch block? The Journal of Heart and Lung Transplantation. 2000;19(2):207–214. doi: 10.1016/s1053-2498(99)00122-9. [DOI] [PubMed] [Google Scholar]
- 7.Leonelli FM, Dunn JK, Young JB, Pacifico A. Natural history, determinants, and clinical relevance of conduction abnormalities following orthotopic heart transplantation. The American Journal of Cardiology. 1996;77(1):47–51. doi: 10.1016/s0002-9149(97)89133-3. [DOI] [PubMed] [Google Scholar]
- 8.Golshayan D, Seydoux C, Sillard Berguer D, et al. Incidence and prognostic value of electrocardiographic abnormalities after heart transplantation. Clinical Cardiology. 1998;21:680–684. doi: 10.1002/clc.4960210914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jessen ME, Olivari M-T, Wait MA, Meyer DM, Yancey CW, Jr, Ring WS. Frequency and significance of right bundle branch block after cardiac transplantation. The American Journal of Cardiology. 1994;73(13):1009–1011. doi: 10.1016/0002-9149(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 10.Marcus GM, Hoang KL, Hunt SA, Chun SH, Lee BK. Prevalence, Patterns of Development, and Prognosis of Right Bundle Branch Block in Heart Transplant Recipients. The American Journal of Cardiology. 2006;98(9):1288–1290. doi: 10.1016/j.amjcard.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Gao S-z, Hunt SA, Wiederhold V, Schroeder JS. Characteristics of serial electrocardiograms in heart transplant recipients. American Heart Journal. 1991;122(3, Part 1):771–774. doi: 10.1016/0002-8703(91)90524-l. [DOI] [PubMed] [Google Scholar]
- 12.Leonelli FM, Pacifico A, Young JB. Frequency and significance of conduction defects early after orthotopic heart transplantation. The American Journal of Cardiology. 1994;73(2):175–179. doi: 10.1016/0002-9149(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto S, Bergsland J, Michalek SM. EVOLUTION OF RIGHT BUNDLE BRANCH BLOCK AND OTHER INTRAVENTRICULAR CONDUCTION ABNORMALITIES IN THE TRANSPLANTED HUMAN HEART. Japanese circulation journal. 1990;54(9):1122–1129. doi: 10.1253/jcj.54.1122. 1990/09/20. [DOI] [PubMed] [Google Scholar]
- 14.Khush K, Menza R, Nguyen J, Goldstein B, Zaroff J, Drew B. Electrocardiographic characteristics of potential organ donors and associations with cardiac allograft use. Circulation Heart Failure. 2012;5:475–483. doi: 10.1161/CIRCHEARTFAILURE.112.968388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shumway N, Lower RS, RC Transplantation of the heart. Advanced Surgery. 1966;2:265–284. [PubMed] [Google Scholar]
- 16.Angermann C, Spes C, Tammen A, et al. Anatomic characteristics and valvular function of the transplanted heart: transthoracic versus transesophargeal echocardiographic findings. Journal of Heart Transplantation. 1990;9(4):331–338. [PubMed] [Google Scholar]
- 17.Young JB, Leon CA, Short HD, et al. Evolution of hemodynamics after orthotopic heart and heartlung transplantation: early restrictive patterns persisting in occult fashion. The Journal of heart transplantation. 1987;6(1):34–43. [PubMed] [Google Scholar]
- 18.Cui G, Kobashigawa J, Chung T, Sen L. Atrial conduction disturbance as an indicator of rejection after cardiac transplantation. Transplantation. 2000;70(1):223–227. [PubMed] [Google Scholar]
- 19.Delewi R, Ijff G, van de Hoef TP, et al. Pathological Q Waves in Myocardial Infarction in Patients Treated by Primary PCI. JACC: Cardiovascular Imaging. 2013;6(3):324–331. doi: 10.1016/j.jcmg.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Goldberger A. Pathogenesis and diagnosis of Q waves on the electrocardiogram. In: Mirvis D, editor. UpToDate. Waltham, MA: UpToDate; 2013. [Google Scholar]