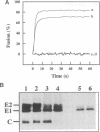
Abstract
Enveloped animal viruses, such as Semliki Forest virus (SFV), utilize a membrane fusion strategy to deposit their genome into the cytosol of the host cell. SFV enters cells through receptor-mediated endocytosis, fusion of the viral envelope occurring subsequently from within acidic endosomes. Fusion of SFV has been demonstrated before to be strictly dependent on the presence of cholesterol in the target membrane. Here, utilizing a variety of membrane fusion assays, including an on-line fluorescence assay involving pyrene-labeled virus, we demonstrate that low-pH-induced fusion of SFV with cholesterol-containing liposomal model membranes requires the presence of sphingomyelin or other sphingolipids in the target membrane. The minimal molecular characteristics essential for supporting SFV fusion are encompassed by a ceramide. The action of the sphingolipids is confined to the actual fusion event, cholesterol being necessary and sufficient for low-pH-dependent binding of the virus to target membranes. Complex formation of the sphingolipids with cholesterol is unlikely to be important for the induction of SFV--liposome fusion, as sphingolipids that do not interact appreciably with cholesterol, such as galactosylceramide, effectively support the process. The remarkably low levels of sphingomyelin required for half-maximal fusion (1-2 mole%) suggest that sphingolipids do not play a structural role in the SFV fusion process, but rather act as a cofactor, possibly activating the viral fusion protein in a specific manner.
Full text
PDF
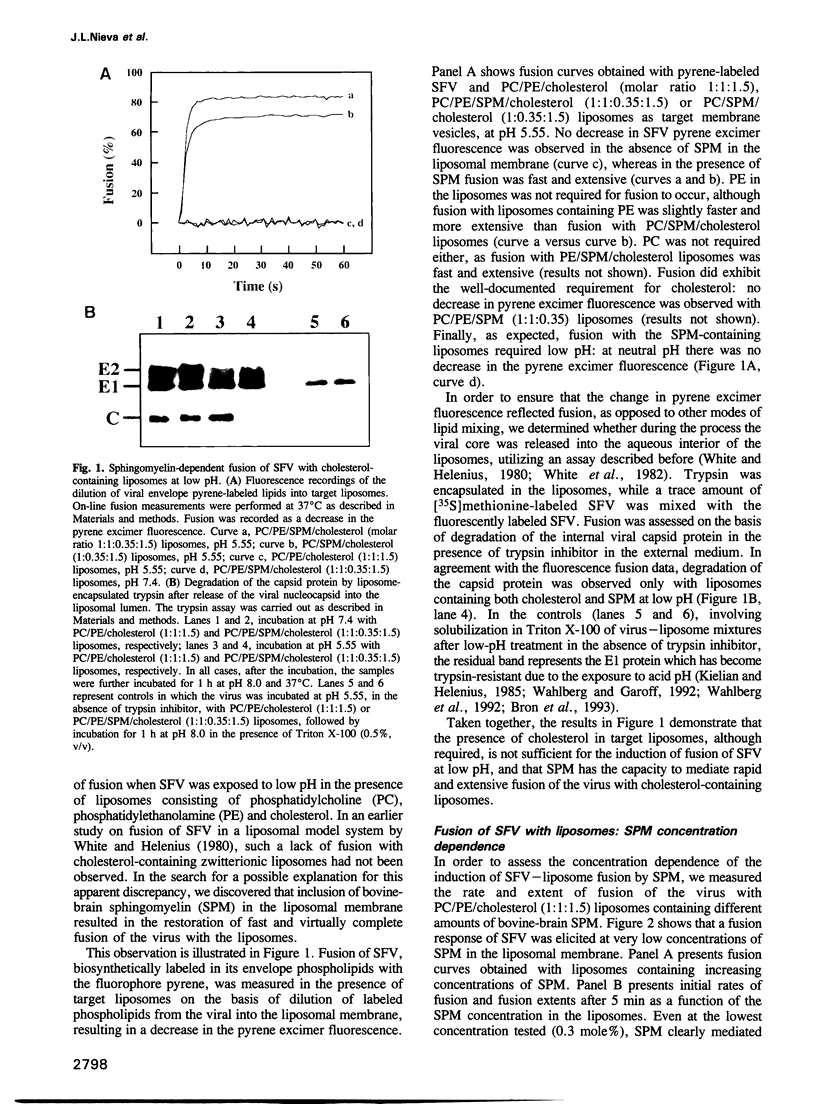
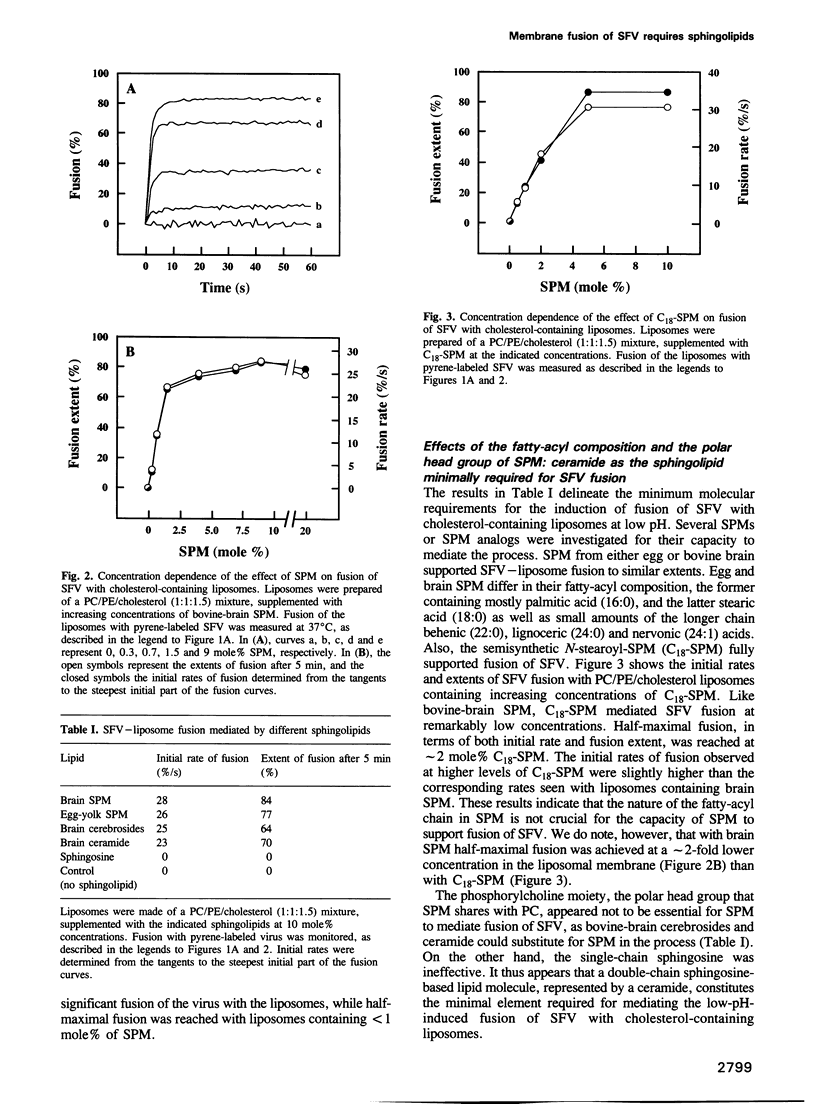
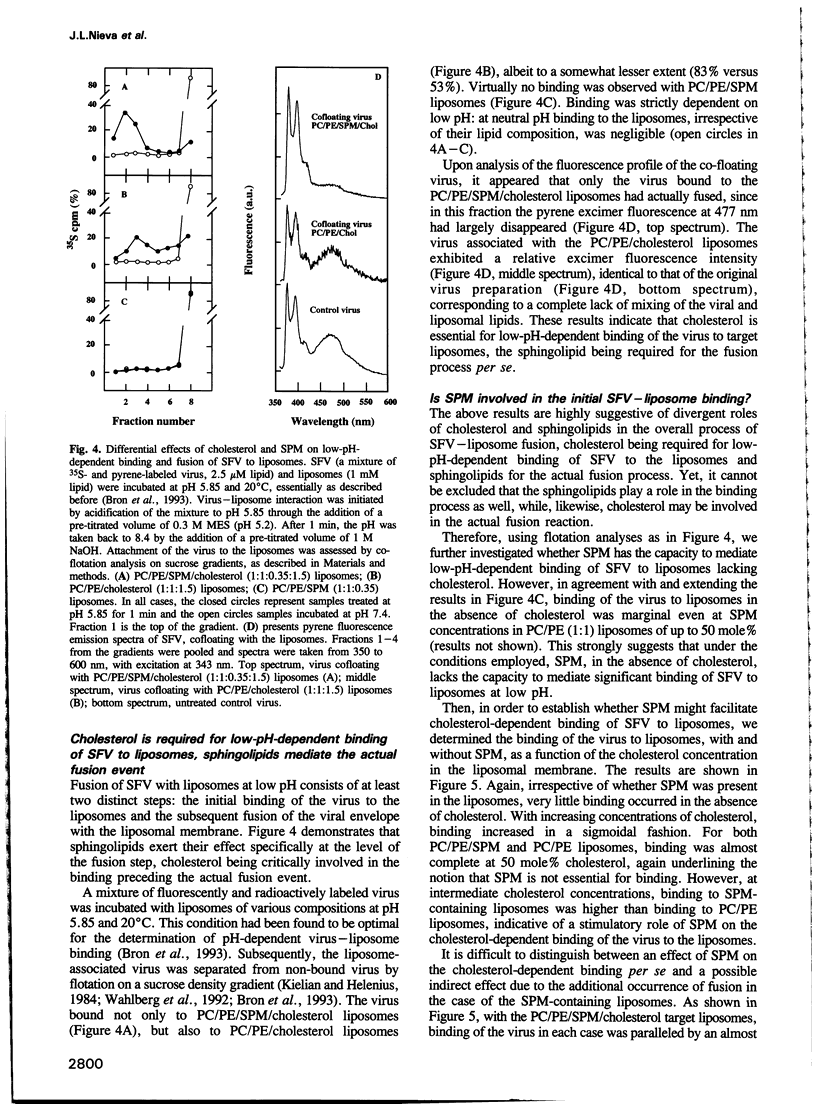



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloia R. C., Jensen F. C., Curtain C. C., Mobley P. W., Gordon L. M. Lipid composition and fluidity of the human immunodeficiency virus. Proc Natl Acad Sci U S A. 1988 Feb;85(3):900–904. doi: 10.1073/pnas.85.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloia R. C., Tian H., Jensen F. C. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5181–5185. doi: 10.1073/pnas.90.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bhat S., Spitalnik S. L., Gonzalez-Scarano F., Silberberg D. H. Galactosyl ceramide or a derivative is an essential component of the neural receptor for human immunodeficiency virus type 1 envelope glycoprotein gp120. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7131–7134. doi: 10.1073/pnas.88.16.7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron R., Wahlberg J. M., Garoff H., Wilschut J. Membrane fusion of Semliki Forest virus in a model system: correlation between fusion kinetics and structural changes in the envelope glycoprotein. EMBO J. 1993 Feb;12(2):693–701. doi: 10.1002/j.1460-2075.1993.tb05703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A. Interactions between GPI-anchored proteins and membrane lipids. Trends Cell Biol. 1992 Nov;2(11):338–343. [PubMed] [Google Scholar]
- Brown D. A., Rose J. K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992 Feb 7;68(3):533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Cervin M., Anderson R. Modulation of coronavirus-mediated cell fusion by homeostatic control of cholesterol and fatty acid metabolism. J Med Virol. 1991 Oct;35(2):142–149. doi: 10.1002/jmv.1890350213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chernomordik L. V., Melikyan G. B., Chizmadzhev Y. A. Biomembrane fusion: a new concept derived from model studies using two interacting planar lipid bilayers. Biochim Biophys Acta. 1987 Oct 5;906(3):309–352. doi: 10.1016/0304-4157(87)90016-5. [DOI] [PubMed] [Google Scholar]
- Devaux P. F. Static and dynamic lipid asymmetry in cell membranes. Biochemistry. 1991 Feb 5;30(5):1163–1173. doi: 10.1021/bi00219a001. [DOI] [PubMed] [Google Scholar]
- Fantini J., Cook D. G., Nathanson N., Spitalnik S. L., Gonzalez-Scarano F. Infection of colonic epithelial cell lines by type 1 human immunodeficiency virus is associated with cell surface expression of galactosylceramide, a potential alternative gp120 receptor. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2700–2704. doi: 10.1073/pnas.90.7.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugler L., Clejan S., Bittman R. Movement of cholesterol between vesicles prepared with different phospholipids or sizes. J Biol Chem. 1985 Apr 10;260(7):4098–4102. [PubMed] [Google Scholar]
- Galla H. J., Hartmann W. Excimer-forming lipids in membrane research. Chem Phys Lipids. 1980 Oct;27(3):199–219. doi: 10.1016/0009-3084(80)90036-5. [DOI] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. Nucleotide sequence of cdna coding for Semliki Forest virus membrane glycoproteins. Nature. 1980 Nov 20;288(5788):236–241. doi: 10.1038/288236a0. [DOI] [PubMed] [Google Scholar]
- Grönberg L., Ruan Z. S., Bittman R., Slotte J. P. Interaction of cholesterol with synthetic sphingomyelin derivatives in mixed monolayers. Biochemistry. 1991 Nov 5;30(44):10746–10754. doi: 10.1021/bi00108a020. [DOI] [PubMed] [Google Scholar]
- Grönberg L., Slotte J. P. Cholesterol oxidase catalyzed oxidation of cholesterol in mixed lipid monolayers: effects of surface pressure and phospholipid composition on catalytic activity. Biochemistry. 1990 Apr 3;29(13):3173–3178. doi: 10.1021/bi00465a003. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- Hansen J. E., Witzke N. M., Nielsen C., Mathiesen L. R., Teglbjaerg L. S., Nielsen C. M., Nielsen J. O. Derivatives of amphotericin inhibit infection with human immunodeficiency virus in vitro by different modes of action. Antiviral Res. 1990 Sep;14(3):149–159. doi: 10.1016/0166-3542(90)90031-2. [DOI] [PubMed] [Google Scholar]
- Harouse J. M., Bhat S., Spitalnik S. L., Laughlin M., Stefano K., Silberberg D. H., Gonzalez-Scarano F. Inhibition of entry of HIV-1 in neural cell lines by antibodies against galactosyl ceramide. Science. 1991 Jul 19;253(5017):320–323. doi: 10.1126/science.1857969. [DOI] [PubMed] [Google Scholar]
- Helenius A., Kartenbeck J., Simons K., Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980 Feb;84(2):404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasin M., Page K. A., Littman D. R. Glycosylphosphatidylinositol-anchored CD4/Thy-1 chimeric molecules serve as human immunodeficiency virus receptors in human, but not mouse, cells and are modulated by gangliosides. J Virol. 1991 Jan;65(1):440–444. doi: 10.1128/jvi.65.1.440-444.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justman J., Klimjack M. R., Kielian M. Role of spike protein conformational changes in fusion of Semliki Forest virus. J Virol. 1993 Dec;67(12):7597–7607. doi: 10.1128/jvi.67.12.7597-7607.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian M. C., Helenius A. Role of cholesterol in fusion of Semliki Forest virus with membranes. J Virol. 1984 Oct;52(1):281–283. doi: 10.1128/jvi.52.1.281-283.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian M., Helenius A. pH-induced alterations in the fusogenic spike protein of Semliki Forest virus. J Cell Biol. 1985 Dec;101(6):2284–2291. doi: 10.1083/jcb.101.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund-Katz S., Laboda H. M., McLean L. R., Phillips M. C. Influence of molecular packing and phospholipid type on rates of cholesterol exchange. Biochemistry. 1988 May 3;27(9):3416–3423. doi: 10.1021/bi00409a044. [DOI] [PubMed] [Google Scholar]
- Malvoisin E., Wild F. Effect of drugs which inhibit cholesterol synthesis on syncytia formation in vero cells infected with measles virus. Biochim Biophys Acta. 1990 Feb 23;1042(3):359–364. doi: 10.1016/0005-2760(90)90165-t. [DOI] [PubMed] [Google Scholar]
- Marquardt M. T., Phalen T., Kielian M. Cholesterol is required in the exit pathway of Semliki Forest virus. J Cell Biol. 1993 Oct;123(1):57–65. doi: 10.1083/jcb.123.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Bolzau E., Helenius A. Penetration of Semliki Forest virus from acidic prelysosomal vacuoles. Cell. 1983 Mar;32(3):931–940. doi: 10.1016/0092-8674(83)90078-8. [DOI] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Adsorptive endocytosis of Semliki Forest virus. J Mol Biol. 1980 Sep 25;142(3):439–454. doi: 10.1016/0022-2836(80)90281-8. [DOI] [PubMed] [Google Scholar]
- Marsh M., Wellsteed J., Kern H., Harms E., Helenius A. Monensin inhibits Semliki Forest virus penetration into culture cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5297–5301. doi: 10.1073/pnas.79.17.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L. D., Hope M. J., Cullis P. R., Janoff A. S. Solute distributions and trapping efficiencies observed in freeze-thawed multilamellar vesicles. Biochim Biophys Acta. 1985 Jul 11;817(1):193–196. doi: 10.1016/0005-2736(85)90084-7. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A., Needham D., Huang C. H. Structure and cohesive properties of sphingomyelin/cholesterol bilayers. Biochemistry. 1992 Feb 25;31(7):2012–2020. doi: 10.1021/bi00122a017. [DOI] [PubMed] [Google Scholar]
- Needham D., Nunn R. S. Elastic deformation and failure of lipid bilayer membranes containing cholesterol. Biophys J. 1990 Oct;58(4):997–1009. doi: 10.1016/S0006-3495(90)82444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar A., Koblet H. Semliki Forest virus particles containing only the E1 envelope glycoprotein are infectious and can induce cell-cell fusion. Virology. 1988 Sep;166(1):17–23. doi: 10.1016/0042-6822(88)90141-9. [DOI] [PubMed] [Google Scholar]
- Parrott D. T., Turner J. E. Mesophase formation by ceramides and cholesterol: a model for stratum corneum lipid packing? Biochim Biophys Acta. 1993 Apr 22;1147(2):273–276. doi: 10.1016/0005-2736(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Phalen T., Kielian M. Cholesterol is required for infection by Semliki Forest virus. J Cell Biol. 1991 Feb;112(4):615–623. doi: 10.1083/jcb.112.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkonen O., Käräinen L., Simons K., Gahmberg C. G. The lipid class composition of Semliki forest virus and plasma membranes of the host cells. Virology. 1971 Nov;46(2):318–326. doi: 10.1016/0042-6822(71)90033-x. [DOI] [PubMed] [Google Scholar]
- Sarin P. S., Gallo R. C., Scheer D. I., Crews F., Lippa A. S. Effects of a novel compound (AL 721) on HTLV-III infectivity in vitro. N Engl J Med. 1985 Nov 14;313(20):1289–1290. doi: 10.1056/NEJM198511143132011. [DOI] [PubMed] [Google Scholar]
- Siegel D. P. Energetics of intermediates in membrane fusion: comparison of stalk and inverted micellar intermediate mechanisms. Biophys J. 1993 Nov;65(5):2124–2140. doi: 10.1016/S0006-3495(93)81256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988 Aug 23;27(17):6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- Slotte J. P. Enzyme-catalyzed oxidation of cholesterol in mixed phospholipid monolayers reveals the stoichiometry at which free cholesterol clusters disappear. Biochemistry. 1992 Jun 23;31(24):5472–5477. doi: 10.1021/bi00139a008. [DOI] [PubMed] [Google Scholar]
- Slotte J. P., Ostman A. L., Kumar E. R., Bittman R. Cholesterol interacts with lactosyl and maltosyl cerebrosides but not with glucosyl or galactosyl cerebrosides in mixed monolayers. Biochemistry. 1993 Aug 10;32(31):7886–7892. doi: 10.1021/bi00082a008. [DOI] [PubMed] [Google Scholar]
- Wahlberg J. M., Bron R., Wilschut J., Garoff H. Membrane fusion of Semliki Forest virus involves homotrimers of the fusion protein. J Virol. 1992 Dec;66(12):7309–7318. doi: 10.1128/jvi.66.12.7309-7318.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlberg J. M., Garoff H. Membrane fusion process of Semliki Forest virus. I: Low pH-induced rearrangement in spike protein quaternary structure precedes virus penetration into cells. J Cell Biol. 1992 Jan;116(2):339–348. doi: 10.1083/jcb.116.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. M. Membrane fusion. Science. 1992 Nov 6;258(5084):917–924. doi: 10.1126/science.1439803. [DOI] [PubMed] [Google Scholar]
- White J., Helenius A. pH-dependent fusion between the Semliki Forest virus membrane and liposomes. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3273–3277. doi: 10.1073/pnas.77.6.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Kartenbeck J., Helenius A. Fusion of Semliki forest virus with the plasma membrane can be induced by low pH. J Cell Biol. 1980 Oct;87(1):264–272. doi: 10.1083/jcb.87.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Kartenbeck J., Helenius A. Membrane fusion activity of influenza virus. EMBO J. 1982;1(2):217–222. doi: 10.1002/j.1460-2075.1982.tb01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Blitterswijk W. J., van der Meer B. W., Hilkmann H. Quantitative contributions of cholesterol and the individual classes of phospholipids and their degree of fatty acyl (un)saturation to membrane fluidity measured by fluorescence polarization. Biochemistry. 1987 Mar 24;26(6):1746–1756. doi: 10.1021/bi00380a038. [DOI] [PubMed] [Google Scholar]
- van Meer G., Simons K., Op den Kamp J. A., van Deenen L. M. Phospholipid asymmetry in Semliki Forest virus grown on baby hamster kidney (BHK-21) cells. Biochemistry. 1981 Mar 31;20(7):1974–1981. doi: 10.1021/bi00510a037. [DOI] [PubMed] [Google Scholar]