Abstract
The outer membrane protein PupB of Pseudomonas putida WCS358 facilitates transport of iron complexed to the siderophores pseudobactin BN8 and pseudobactin BN7 into the cell. Its synthesis is induced by the presence of these specific siderophores under iron limitation. The signal transduction pathway regulating siderophore-dependent expression of pupB was shown to consist of two regulatory proteins, PupI and PupR, and the PupB receptor itself. Mutational analysis of the regulatory genes suggested that PupI acts as a positive regulator of pupB transcription, whereas PupR modifies PupI activity dependent on the presence of pseudobactin BN8. PupI and PupR do not share homology with the classical bacterial two-component systems but display significant similarity to the FecI and FecR proteins of Escherichia coli involved in regulation of ferric dicitrate transport. The function of the PupB receptor in pupB regulation was studied by the use of chimeric receptor proteins composed of PupB and the ferric pseudobactin 358 receptor PupA. This experiment revealed that PupB is involved in the initiation of the signal transduction pathway, implying a so far unique role for an outer membrane protein in signal transduction.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright L. M., Huala E., Ausubel F. M. Prokaryotic signal transduction mediated by sensor and regulator protein pairs. Annu Rev Genet. 1989;23:311–336. doi: 10.1146/annurev.ge.23.120189.001523. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Bitter W., Marugg J. D., de Weger L. A., Tommassen J., Weisbeek P. J. The ferric-pseudobactin receptor PupA of Pseudomonas putida WCS358: homology to TonB-dependent Escherichia coli receptors and specificity of the protein. Mol Microbiol. 1991 Mar;5(3):647–655. doi: 10.1111/j.1365-2958.1991.tb00736.x. [DOI] [PubMed] [Google Scholar]
- Bitter W., Tommassen J., Weisbeek P. J. Identification and characterization of the exbB, exbD and tonB genes of Pseudomonas putida WCS358: their involvement in ferric-pseudobactin transport. Mol Microbiol. 1993 Jan;7(1):117–130. doi: 10.1111/j.1365-2958.1993.tb01103.x. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Webb D., Godovac-Zimmermann J., Rosenberg H. Arg-220 of the PstA protein is required for phosphate transport through the phosphate-specific transport system in Escherichia coli but not for alkaline phosphatase repression. J Bacteriol. 1988 May;170(5):2283–2286. doi: 10.1128/jb.170.5.2283-2286.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C. R., Poole K. Expression of the ferric enterobactin receptor (PfeA) of Pseudomonas aeruginosa: involvement of a two-component regulatory system. Mol Microbiol. 1993 Jun;8(6):1095–1103. doi: 10.1111/j.1365-2958.1993.tb01654.x. [DOI] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd I. B., Egan J. B. Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res. 1990 Sep 11;18(17):5019–5026. doi: 10.1093/nar/18.17.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattermann D. R., Stacey G. Efficient DNA transformation of Bradyrhizobium japonicum by electroporation. Appl Environ Microbiol. 1990 Apr;56(4):833–836. doi: 10.1128/aem.56.4.833-836.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein S., Hantke K., Braun V. Citrate-dependent iron transport system in Escherichia coli K-12. Eur J Biochem. 1981 Jul;117(2):431–437. doi: 10.1111/j.1432-1033.1981.tb06357.x. [DOI] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Killmann H., Benz R., Braun V. Conversion of the FhuA transport protein into a diffusion channel through the outer membrane of Escherichia coli. EMBO J. 1993 Aug;12(8):3007–3016. doi: 10.1002/j.1460-2075.1993.tb05969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
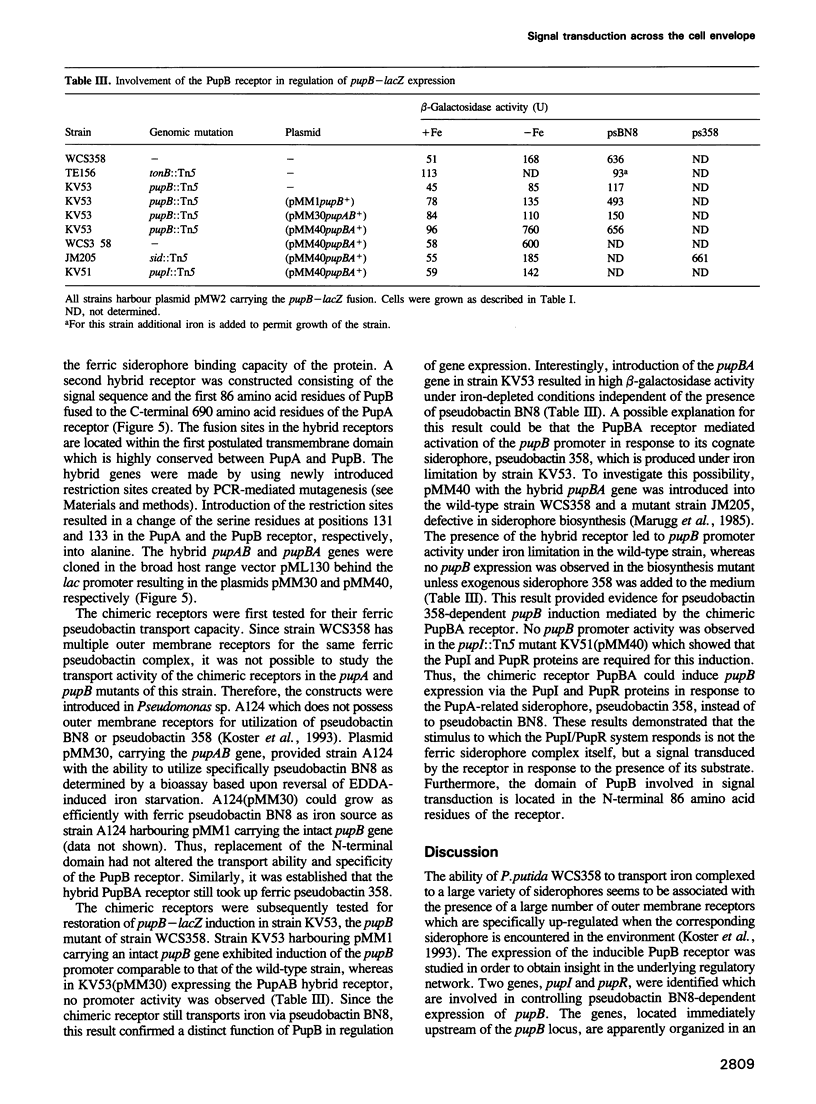
- Koster M., van de Vossenberg J., Leong J., Weisbeek P. J. Identification and characterization of the pupB gene encoding an inducible ferric-pseudobactin receptor of Pseudomonas putida WCS358. Mol Microbiol. 1993 May;8(3):591–601. doi: 10.1111/j.1365-2958.1993.tb01603.x. [DOI] [PubMed] [Google Scholar]
- Labes M., Pühler A., Simon R. A new family of RSF1010-derived expression and lac-fusion broad-host-range vectors for gram-negative bacteria. Gene. 1990 Apr 30;89(1):37–46. doi: 10.1016/0378-1119(90)90203-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marugg J. D., Nielander H. B., Horrevoets A. J., van Megen I., van Genderen I., Weisbeek P. J. Genetic organization and transcriptional analysis of a major gene cluster involved in siderophore biosynthesis in Pseudomonas putida WCS358. J Bacteriol. 1988 Apr;170(4):1812–1819. doi: 10.1128/jb.170.4.1812-1819.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marugg J. D., de Weger L. A., Nielander H. B., Oorthuizen M., Recourt K., Lugtenberg B., van der Hofstad G. A., Weisbeek P. J. Cloning and characterization of a gene encoding an outer membrane protein required for siderophore-mediated uptake of Fe3+ in Pseudomonas putida WCS358. J Bacteriol. 1989 May;171(5):2819–2826. doi: 10.1128/jb.171.5.2819-2826.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marugg J. D., van Spanje M., Hoekstra W. P., Schippers B., Weisbeek P. J. Isolation and analysis of genes involved in siderophore biosynthesis in plant-growth-stimulating Pseudomonas putida WCS358. J Bacteriol. 1985 Nov;164(2):563–570. doi: 10.1128/jb.164.2.563-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Parkinson J. S. Signal transduction schemes of bacteria. Cell. 1993 Jun 4;73(5):857–871. doi: 10.1016/0092-8674(93)90267-t. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole K., Young L., Neshat S. Enterobactin-mediated iron transport in Pseudomonas aeruginosa. J Bacteriol. 1990 Dec;172(12):6991–6996. doi: 10.1128/jb.172.12.6991-6996.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle K. TonB and the gram-negative dilemma. Mol Microbiol. 1990 Dec;4(12):2019–2025. doi: 10.1111/j.1365-2958.1990.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Pressler U., Staudenmaier H., Zimmermann L., Braun V. Genetics of the iron dicitrate transport system of Escherichia coli. J Bacteriol. 1988 Jun;170(6):2716–2724. doi: 10.1128/jb.170.6.2716-2724.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutz J. M., Liu J., Lyons J. A., Goranson J., Armstrong S. K., McIntosh M. A., Feix J. B., Klebba P. E. Formation of a gated channel by a ligand-specific transport protein in the bacterial outer membrane. Science. 1992 Oct 16;258(5081):471–475. doi: 10.1126/science.1411544. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hove B., Staudenmaier H., Braun V. Novel two-component transmembrane transcription control: regulation of iron dicitrate transport in Escherichia coli K-12. J Bacteriol. 1990 Dec;172(12):6749–6758. doi: 10.1128/jb.172.12.6749-6758.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturi V., Ottevanger C., Leong J., Weisbeek P. J. Identification and characterization of a siderophore regulatory gene (pfrA) of Pseudomonas putida WCS358: homology to the alginate regulatory gene algQ of Pseudomonas aeruginosa. Mol Microbiol. 1993 Oct;10(1):63–73. doi: 10.1111/j.1365-2958.1993.tb00904.x. [DOI] [PubMed] [Google Scholar]
- Viebrock A., Zumft W. G. Molecular cloning, heterologous expression, and primary structure of the structural gene for the copper enzyme nitrous oxide reductase from denitrifying Pseudomonas stutzeri. J Bacteriol. 1988 Oct;170(10):4658–4668. doi: 10.1128/jb.170.10.4658-4668.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. C., Abdelal A. T. Unorthodox expression of an enzyme: evidence for an untranslated region within carA from Pseudomonas aeruginosa. J Bacteriol. 1990 Feb;172(2):630–642. doi: 10.1128/jb.172.2.630-642.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. C., Leong J. Structure of pseudobactin 7SR1, a siderophore from a plant-deleterious Pseudomonas. Biochemistry. 1984 Jul 17;23(15):3534–3540. doi: 10.1021/bi00310a023. [DOI] [PubMed] [Google Scholar]
- Zimmermann L., Hantke K., Braun V. Exogenous induction of the iron dicitrate transport system of Escherichia coli K-12. J Bacteriol. 1984 Jul;159(1):271–277. doi: 10.1128/jb.159.1.271-277.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorenzo V., Wee S., Herrero M., Neilands J. B. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol. 1987 Jun;169(6):2624–2630. doi: 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weger L. A., van Boxtel R., van der Burg B., Gruters R. A., Geels F. P., Schippers B., Lugtenberg B. Siderophores and outer membrane proteins of antagonistic, plant-growth-stimulating, root-colonizing Pseudomonas spp. J Bacteriol. 1986 Feb;165(2):585–594. doi: 10.1128/jb.165.2.585-594.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Membrane protein structure prediction. Hydrophobicity analysis and the positive-inside rule. J Mol Biol. 1992 May 20;225(2):487–494. doi: 10.1016/0022-2836(92)90934-c. [DOI] [PubMed] [Google Scholar]