Summary
Dagda et al. showed in this issue that cytosolic PINK1 released from the mitochondrion promotes dendritic outgrowth. This neurite-promoting activity of PINK1 is associated with the kinase activities of protein kinase A and is impaired by a pathogenic mutation in PINK1. The study by Dagda et al. has unravelled a novel mechanism underlying neurodegeneration caused by PINK1 mutation.
The etiology of Parkinson’s disease (PD) is multifactorial, and the pathogenic pathways involved are complex. Mitochondrial dysfunction has been linked to PD by the discovery of parkinsonism in patients who mistakenly took synthetic meperidine contaminated with the selective mitochondrial toxicant 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) (Langston et al., 1983). The subsequent discovery of PD-linked gene mutations further fueled the long-standing interest in the role of mitochondria in PD. Among these PD-linked genes, identification of mutations in the PTEN-induced putative kinase 1 (pink1) provided the first genetic evidence to support the involvement of mitochondrial dysfunction in PD. PINK1 has a mitochondrion-targeting signal at its N-terminus, and mutations in the pink1 gene have been found in autosomal recessive PD (Valente et al., 2004). Since these discoveries were made, the vital roles of PINK1 in mitochondrial dynamics, mitochondrial function, and mitophagy have been extensively studied. Whereas PINK1 is mainly localized to mitochondria, a fraction of the protein is found in the cytosol (Meissner et al., 2011). After PINK1 is imported to mitochondria, it is cleaved by the mitochondrial intra-membrane protease presenilin-associated rhomboid-like protein (PARL), to release most of the cleaved PINK1 into the cytosol (Meissner et al., 2011). However, under pathological conditions where mitochondrial membrane potential is disrupted, PINK1 remains uncleaved in the mitochondria (Lin and Kang, 2008). Despite the two pools of PINK1, the cytosolic form has been less examined than its mitochondrial counterpart. In this issue of Journal of Neurochemistry, Dagda and colleagues provide substantial evidence that cytosolic rather than mitochondrial PINK1 promotes dendritic outgrowth (Dagda et al., 2013).
The authors first examined the effects of cleaved and uncleaved PINK1 on neurite outgrowth in both immortalized cells and primary neurons. In human SH-SY5Y cells and mouse cortical neurons, overexpression of full-length PINK1 caused morphological changes resembling dendritic outgrowth, and this morphological change required PINK1 kinase activity. Upon closer examination, these cells expressed higher levels of dendritic and postsynaptic markers. To tease out whether these changes were induced by mitochondrial or cytosolic PINK1, they generated PINK1 with an outer mitochondrial membrane-targeting sequence (OMM) in the N-terminus (OMM-PINK1) and another without OMM (ΔN111-PINK1) that remained in the cytosolic compartment. Full-length PINK1 exhibits both cytosolic and mitochondrial localization. They observed that full-length and cytosolic PINK1, but not mitochondrial PINK1, promoted dendritic outgrowth. This outgrowth effect was specific to dendrites, as axonal length did not change. It would be informative to determine if cytosolic PINK1 also affects axonal terminals, which were not quantified in this study. In contrast to the effects of PINK1 overexpression, PINK1 deficiency reduced the dendritic length of primary neurons isolated from PINK1-knockout mice. They next examined the effects of cytosolic PINK1 on mitochondrial mobility. Cytosolic but not mitochondrial PINK1 enhanced anterograde mitochondrion movement in dendrites, resulting in increased mitochondrial density in the dendrites. The authors also observed that cytosolic PINK1 prevented SH-SY5Y cells from PINK1 knockdown-induced apoptosis, a cell-protective effect previously reported in an MPTP mouse model (Haque et al., 2008). Like its mitochondrial partner, cytosolic PINK1 has neuroprotective effects. Based on their evidence, Dagda et al. proposed a mechanism by which cytosolic PINK1 promotes dendritic outgrowth. Because PINK1 knockdown selectively blocked neuronal differentiation in cAMP-treated SH-SY5Y cells but not in those treated with retinoic acid (a commonly used compound to differentiate this cell type), the authors reasoned that protein kinase A (PKA) is involved in PINK1’s dendritic outgrowth effect. Their hypothesis is verified by the observation that both PKA activity and nuclear phosphorylated cAMP response element-binding protein (CREB) levels were reduced in PINK1-knockdown cells. Furthermore, PINK1 lost its ability to promote dendritic outgrowth after PKA activity was blocked. Interestingly, Chang and Blackstone reported that activating cAMP-dependent PKA leads to the phosphorylation of dynamin-related protein 1 (Drp1), a key regulator of mitochondrial fission (Chang and Blackstone, 2007). Phosphorylated Drp1 loses its GTPase activity and thus its ability to promote mitochondrial fission. Inhibiting Drp1 activity has been shown to block cytochrome C release and apoptosis and promote the exchange of mitochondrial contents between functional and defective mitochondria, which attenuates their negative impact. PINK1 prevents Drp1 translocation from the cytosol to the mitochondria (Zhao et al., 2013), inhibiting Drp1-promoted mitochondrial fission. Taken together, the evidence indicates that cytosolic PINK1 promotes dendritic outgrowth and contributes to mitochondrial fusion in mammalian cells, likely through PKA.
The study by Dagda and colleagues highlights a novel role of cytosolic PINK1 that has not yet received adequate attention as compared to mitochondrial PINK1. It also raises some important issues related to emerging concepts in PD research. One of these is synaptic dysfunction in PD. In recent years, synaptic dysfunction was found to be an early pathology prior to neurodegeneration in different neurological disorders, including PD (Picconi et al., 2012). Thus, restoring synaptic function at both pre- and post-synaptic sites has been suggested as an early therapeutic strategy for PD. In genetic animal studies, PINK1, parkin, DJ-1, α-synuclein, and LRRK2 have all been reported to play roles in striatal pre-synaptic dopamine (DA) release. Relevant to Dagda’s study, acute striatal slices prepared from PINK1-knockout mice exhibit impaired DA release (Kitada et al., 2007). Because striatal DA modulates corticostriatal glutamatergic input at striatal medium spiny neurons, a reduction in striatal DA levels results in reduced activation of post-synaptic D1 and D2 receptors, which in turn impairs long-term potentiation and long-term depression in these animals. Precisely how a loss of PINK1 function would lead to DA release deficits is not completely understood. Mitochondrial function is likely implicated in impaired DA release. Since the first ultrastructural analysis of synapses half a century ago, it is now clear that mitochondria are enriched in the synapses and play a critical role in pre-synaptic functions (Hollenbeck, 2005). For example, they regulate cytosolic Ca2+ levels during neurotransmission and supply energy to synaptic terminals to maintain synaptic membrane potential, ionic gradient restoration following synaptic transmission, and the reloading of synaptic vesicles with neurotransmitters. The correlation between PINK1, mitochondrial function, and synaptic function is another important research topic inspired by Dagda’s study.
In summary, combined with evidence from other reports, the study by Dagda et al. has a significant implication regarding the effects of PINK1 on synaptic function. PINK1 is crucial to pre-synaptic DA release because it maintains mitochondrial function, morphology, and integrity. At postsynaptic sites, cytosolic PINK1 maintains dendritic mitochondrial density and promotes dendritic outgrowth. By improving synaptic function at both pre- and post-synaptic levels, PINK1 ensures proper synaptic transmission and signaling. However, additional studies are required to validate these novels roles of PINK1. For example, Dagda et al. performed their study in cultured cells. It is important to extend their findings to an in vivo model. Perhaps by using virus-mediated gene delivery, additional insights will be gained into how mitochondrial and cytosolic PINK1 modulate pre- and post-synaptic activities in the nigrostriatal pathway of an intact model system. In addition, Dagda et al. demonstrated that cytosolic PINK1 enhanced the anterograde transport of mitochondria in the dendrites of cultured mammalian cells (Dagda et al., 2013); however, an opposite effect of PINK1 on mitochondrial mobility was observed in Drosophila larval motor neurons (Liu et al., 2012). Overexpression of Drosophila PINK1 inhibits both the antero- and retrograde transport of mitochondria in motor neuron axons (Liu et al., 2012). Because the effect of PINK1 on axonal mitochondrion transport was not examined or compared with the observations in dendrites (Dagda et al., 2013), it is not known whether PINK1 (or cytosolic PINK1 alone) has differential effects on mitochondrial mobility in axons and dendrites in mammalian neurons under physiological conditions. These interesting questions need to be resolved with future studies.
Acknowledgments
This work is supported by the National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (NS072113 and NS084089 to X.G.X) and by the Plymouth University Peninsula Schools of Medicine and Dentistry (K. T). The authors thank Laura Hausmann for preparing the figure.
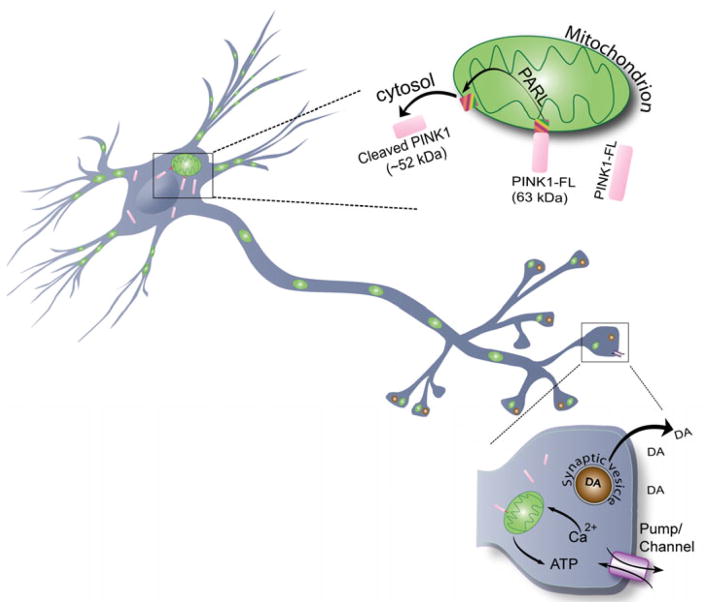
Figure. PINK1 promotes synaptic activity.
Under normal conditions, PINK1 is present both in the mitochondria and the cytosol. Full-length PINK1 is cleaved by presenilin-associated rhomboid-like protein (PARL) and released to the cytosol. The results by Dagda et al. (2013) indicate that it is cytosolic rather than mitochondrial PINK1 that promotes anterograde mitochondrial transport in dendrites, leading to increased dendritic mitochondrial density and dendritic outgrowth. Under pathological conditions, PINK1 remains uncleaved and neuronal differentiation is blocked. At the axonal terminals, a loss of PINK1 function has been reported by other studies to impair pre-synaptic dopamine (DA) release. It is possible that PINK1 maintains presynaptic release of neurotransmitters by maintaining proper mitochondrial function to meet much needed energy demand by synaptic terminals for various homeostasis functions. Together, PINK1 appears to play an important role in synaptic function at both pre- and post-synaptic levels.
Footnotes
The authors have no conflicts of interest to declare.
References
- Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem. 2007;282:21583–21587. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- Dagda RK, Pien I, RW, Zhu J, Wang KZQ, callio J, Chu CT. Beyond the mitochondrion: cytosolic PINK1 remodels dendrites through Protein Kinase A. J Neurochem. 2013 doi: 10.1111/jnc.12494. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque ME, Thomas KJ, D’Souza C, Callaghan S, Kitada T, Slack RS, Fraser P, Cookson MR, Tandon A, Park DS. Cytoplasmic Pink1 activity protects neurons from dopaminergic neurotoxin MPTP. Proc Natl Acad Sci U S A. 2008;105:1716–1721. doi: 10.1073/pnas.0705363105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck PJ. Mitochondria and neurotransmission: evacuating the synapse. Neuron. 2005;47:331–333. doi: 10.1016/j.neuron.2005.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Pisani A, Porter DR, Yamaguchi H, Tscherter A, Martella G, Bonsi P, Zhang C, Pothos EN, Shen J. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci U S A. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka CA, Locke MN, Gallagher PS, Pham N, Hart MP, Walker CJ, Gitler AD, Gardner RG. A yeast model for polyalanine-expansion aggregation and toxicity. Mol Biol Cell. 2011;22:1971–1984. doi: 10.1091/mbc.E11-01-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Lin W, Kang UJ. Characterization of PINK1 processing, stability, and subcellular localization. J Neurochem. 2008;106:464–474. doi: 10.1111/j.1471-4159.2008.05398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Sawada T, Lee S, Yu W, Silverio G, Alapatt P, Millan I, Shen A, Saxton W, Kanao T, Takahashi R, Hattori N, Imai Y, Lu B. Parkinson’s disease-associated kinase PINK1 regulates Miro protein level and axonal transport of mitochondria. PLoS Genet. 2012;8:e1002537. doi: 10.1371/journal.pgen.1002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner C, Lorenz H, Weihofen A, Selkoe DJ, Lemberg MK. The mitochondrial intramembrane protease PARL cleaves human Pink1 to regulate Pink1 trafficking. J Neurochem. 2011;117:856–867. doi: 10.1111/j.1471-4159.2011.07253.x. [DOI] [PubMed] [Google Scholar]
- Picconi B, Piccoli G, Calabresi P. Synaptic dysfunction in Parkinson’s disease. Adv Exp Med Biol. 2012;970:553–572. doi: 10.1007/978-3-7091-0932-8_24. [DOI] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. Epub 2004 Apr 1115. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Chen F, Chen S, Liu X, Cui M, Dong Q. The Parkinson’s disease-associated gene PINK1 protects neurons from ischemic damage by decreasing mitochondrial translocation of the fission promoter Drp1. J Neurochem. 2013 doi: 10.1111/jnc.12340. [DOI] [PubMed] [Google Scholar]