Abstract
Objective
To estimate the prevalence of both dementia and depression among community-dwelling older Americans, and to determine if hospitalization is independently associated with dementia or depression in this population.
Method
This cross-sectional study utilized data from a nationally representative, population-based sample of 7,197 community-dwelling adults ≥ 65 years old interviewed in 2011 as part of the National Health and Aging Trends Study. Information on hospitalizations was obtained from self or proxy-report. Possible and probable dementia was assessed according to a validated algorithm. Depressive symptoms were assessed with the Patient Health Questionnaire-2.
Results
An estimated 3.1 million community-dwelling older Americans may have dementia, and approximately 5.3 million may have substantial depressive symptoms. After adjusting for demographic and social characteristics, medical diagnoses, smoking history, serious falls, and pain symptoms, being hospitalized in the previous year was independently associated with greater odds of probable dementia (odds ratio [OR]: 1.42, 95% confidence interval[95%CI]: 1.16, 1.73) and substantial depressive symptoms (OR: 1.60, 95%CI: 1.29, 1.99).
Conclusions
Dementia and depression are common in community-dwelling older Americans, and hospitalization is associated with these conditions. Additional research increasing understanding of the bi-directional relationship between hospitalizations, dementia, and depression, along with targeted interventions to reduce hospitalizations, are needed.
Keywords: dementia, depression, hospitalization
INTRODUCTION
As the population ages and the burdens of chronic illnesses such as coronary artery disease and diabetes increase [1, 2], hospitalizations among older adults are of growing concern to healthcare providers and healthcare systems. Hospitalizations among older adults are costly to the American healthcare system [3], and the Centers for Medicare and Medicaid Services (CMS) is actively incentivizing efforts to reduce rehospitalizations among older adults in order to reduce costs and improve quality of care [4].
In addition to financial strain on the healthcare system, an emerging body of literature has identified that hospitalizations for a wide range of medical illnesses among older adults may increase the risks of dementia and depression [5-8]. The potential for these outcomes following hospitalizations for older adults is an important public health problem since both dementia and depression are substantial contributors to disability, and are both independently associated with increased healthcare costs and early mortality [9-14].
Despite the adverse outcomes associated with dementia and depression among older adults, these disorders remain under-recognized [15, 16]. In addition, recent prevalence estimates of dementia and depression in older Americans have included residents of skilled nursing and assisted-living facilities, populations known to have extremely high rates of these disorders [17]. Recognition of dementia and depression in community-dwelling older adults is especially important in order to preserve functional independence, particularly in-light of existing evidence-based interventions [18, 19]. Furthermore, although hospitalizations among older adults may be associated with increased risks of cognitive impairment and depression, it remains unclear if these risks are independent of chronic medical comorbidity or other associated factors such as falls [20-23], a potential indication of overall frailty [24].
The present investigation utilizes data from a new, nationally representative cohort of Americans 65 and older, the National Health and Aging Trends Study (NHATS), to estimate the prevalence of dementia and depression among community-dwelling older Americans, as well as to examine if hospitalization among community-dwelling older adults is independently associated with dementia or depression after adjusting for demographic and social characteristics, medical comorbidity, pain, and falls.
METHODS
Participants
The present investigation is a cross-sectional study comprised of community-dwelling participants in the first wave of the NHATS, Beta Release 1.0. The NHATS is the successor to the National Long Term Care Survey, and is a panel study of Medicare beneficiaries ages 65 and older [25]. The NHATS used a stratified three-stage sample design with sampling based on U.S. county and residential zip code as well as age [26, 27], and the final sample included participants from every state except Alaska, Hawaii, and Puerto Rico [26]. Participants and/or their proxies were interviewed in-person in 2011, and the first wave response rate was 71% [27], with annual re-interviews planned. The NHATS protocol was approved by the Johns Hopkins University Institutional Review Board and all participants provided informed consent.
Primary Independent Variable
The primary independent variable in our analyses was whether a NHATS participant was hospitalized in the previous year. This information was provided by participants or their proxies [27].
Covariates of Interest
All data on covariates of interest in our analyses were obtained from the NHATS interviews of participants and/or their proxies. The interview included questions on demographic and social characteristics, including age, gender, race/ethnicity, education, income, marital status, having adult children, and having living siblings [27]. Participants or their proxies were also asked if they had been diagnosed with any of the following medical conditions: myocardial infarction, other cardiovascular disease, hypertension, osteoarthritis, osteoporosis, diabetes, respiratory disease, stroke, cancer, hip fracture, other long-bone fracture, or another disease(s), as well as about smoking history, whether they had a fall in the previous year, and the number of body regions with pain symptoms [27]. NHATS participants (or their proxies) were also asked about impairments in any of 6 activities of daily living (ADLs): walking, dressing, bathing, eating, getting into/out of bed, and toileting, or 6 instrumental ADLs (IADLs): preparing a hot meal, grocery shopping, making telephone calls, doing laundry, taking medicines, and managing money.
Outcomes of Interest
Our outcomes of interest were the presence of possible or probable dementia, and the presence of substantial depressive symptoms, assessed at the NHATS first wave interview.
Possible and Probable Dementia
Cognition in the NHATS was assessed in multiple ways. Participants (or their proxies) were asked if they had been diagnosed with dementia by a physician. Proxies (if needed) completed the AD8, a validated proxy-report assessment of dementia [28]. Finally, participants were administered a battery of cognitive tests including the Modified Telephone Interview for Cognitive Status (TICSm) and the Clock-Drawing Test [29, 30]. The TICSm has been validated against a 3-4 hour neuropsychiatric interview in the Aging, Demographics, and Memory Study (ADAMS) and found to have a weighted accuracy of 74.1% in correct classification of individuals as either having normal cognition, cognitive impairment without dementia, or dementia [29]. The Clock-Drawing Test, a brief test of executive functioning, has been shown to have a mean sensitivity of 85% and a mean specificity of 85% for the diagnosis of dementia [31].
Probable dementia was defined in the NHATS by the presence of any of the following: 1) a self-report or proxy-report of a diagnosis of dementia; 2) a proxy-reported score on the AD8 of ≥ 2 [28]; or 3) a score of ≤ 1.5 standard deviations below the mean on at least two of three of the following cognitive functioning domains tested with the TICSm and Clock-Drawing Test: orientation, memory, and executive functioning [32]. Possible dementia was defined by a score of ≤ 1.5 standard deviations below the mean on at least one of the three cognitive functioning domains. The NHATS definitions of possible and probable dementia were validated against dementia cases identified in the ADAMS [32].
Depression
Depression was assessed in the NHATS using the Patient Health Questionnaire-2 (PHQ-2) [33]. The PHQ-2 is a validated two question screening questionnaire that inquires about the frequency of depressed mood and anhedonia during the preceding two weeks [33]. Each item is scored with a Likert scale ranging from 0 to 3 with a maximum score of 6. We used a PHQ-2 score ≥ 3 to define substantial depressive symptoms because this threshold has been found to have a sensitivity of 100% and a specificity of 77% for the diagnosis of major depression among older adults versus structured interview [34]. The PHQ-2 has also been validated amongst older adults with cognitive impairment [35].
Statistical Analysis
Descriptive data are presented as proportions or means and standard deviations (SDs). To estimate the prevalence of possible and probable dementia as well as substantial depressive symptoms among community-dwelling older Americans, we multiplied the prevalence of each condition among community-dwelling NHATS participants by the population of community-dwelling adults 65 or older based on statistics obtained from the U.S. Administration on Aging in the Department of Health and Human Services [36], using the tracker sampling weights provided by the study investigators to adjust for sampling design [37]. Estimation was done using svy linearized in STATA 12 (Stata Corporation, College Station, TX).
We used ordinal logistic regression models to examine the association of hospitalization in the previous year and odds of probable dementia, with the dependent variable defined as an ordinal variable with 0 = normal cognition, 1 = possible dementia, and 2 = probable dementia. We initially fitted an unadjusted regression model. We then sequentially adjusted for our covariates of interest in three groups: 1) demographic (age categorized as 65-75, 76-85, and ≥ 86 years old; gender; race categorized as white versus non-white; education categorized as less than high school graduate versus high school graduate or greater; and income categorized by the median annual income of NHATS participants) and social characteristics (marital/partnered status categorized as married/partnered versus single/widowed; having living adult children; and having living siblings); 2) comorbid medical diagnoses and smoking history; and 3) a fall in the previous year and the number of body regions with pain symptoms. We also estimated the mean number of impairments in ADLs and IADLs among those with probable dementia who were hospitalized in the previous year versus those without a hospitalization.
We used binary logistic regression models to examine the association of hospitalization in the previous year and odds of substantial depressive symptoms, initially fitting an unadjusted regression model and then sequentially adjusting for the same series of covariates as in our analyses of probable dementia.
To account for the complex sampling design of the NHATS in variance estimation, we used the analytic weights provided by the study investigators [37]. We implemented our regression analyses using svy ologit for ordinal logistic regression models and svy logistic for binary logistic regression models in STATA 12. We used two-sided significance tests for all analyses with statistical significance set at P = 0.05.
Since cognitive impairment can be a prominent feature of severe depression in older adults [38], we conducted a sensitivity analysis for examination of probable dementia in which we also adjusted for the total PHQ-2 score as a continuous variable. In addition, because late-life depression could be part of the prodrome of a dementing illness [39], we conducted a sensitivity analysis for our examination of substantial depressive symptoms in which we also adjusted for participant performance on NHATS cognitive functioning tests as a continuous variable. Furthermore, as a secondary analysis, we repeated our fully adjusted regression models stratifying by age deciles (e.g., 65-75, 76-85, ≥ 86).
RESULTS
A total of 7,197 community-dwelling Medicare recipients ages 65 or older participated in the first wave of the NHATS (Figure). Table 1 describes the demographic, social and clinical characteristics of the entire cohort as well as by possible dementia, probable dementia, and substantial depressive symptom status. Over half of the cohort was female and nearly one-third were racial/ethnic minorities. The most prevalent medical diagnoses among the entire cohort of community-dwelling NHATS first wave respondents were hypertension, osteoarthritis, and diabetes. Nearly one-quarter had fallen at least once in the previous year.
Figure. National Health and Aging Trends Study Wave 1 Community-Dwelling Participants.
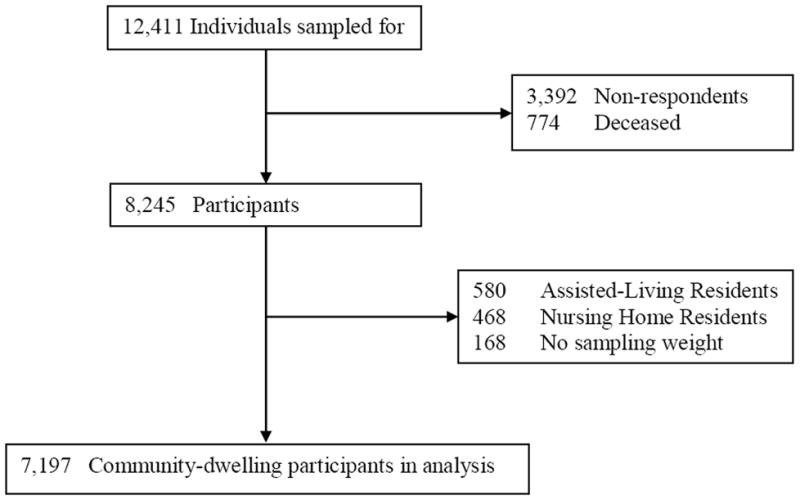
Table 1.
Demographic and clinical characteristics of community-dwelling participants in the first wave of the National Health and Aging Trends Study
| Variable | Total (n = 7,197) | Possible dementia (n = 1,024) | Probable dementia (n = 810) | Substantial depressive symptoms (n = 1,123) |
|---|---|---|---|---|
| Age (years) | ||||
| 65-75 | 2,933 (41%) | 284 (28%) | 109 (14%) | 420 (37%) |
| 76-85 | 2,883 (40%) | 459 (45%) | 327 (40%) | 455 (41%) |
| ≥ 86 | 1,381 (19%) | 281 (27%) | 374 (46%) | 248 (22%) |
| Female | 4,147 (58%) | 535 (52%) | 511 (63%) | 691 (62%) |
| Race | ||||
| White | 4,844 (68%) | 631 (62%) | 399 (50%) | 636 (57%) |
| Non white | 2,269 (32%) | 384 (38%) | 401 (50%) | 471 (43%) |
| < High school graduate | 1,947 (27%) | 441 (43%) | 415 (52%) | 461 (42%) |
| Marital status | ||||
| Married/partnerd | 3,710 (52%) | 454 (44%) | 288 (36%) | 483 (43%) |
| Single/widowed | 3,480 (48%) | 570 (56%) | 520 (64%) | 640 (57%) |
| Annual incomea | ||||
| < $48,000 | 5,204 (72%) | 863 (84%) | 726 (90%) | 955 (85%) |
| ≥ $48,000 | 1,993 (28%) | 161 (16%) | 84 (10%) | 168 (15%) |
| Living children | 6,253 (87%) | 876 (86%) | 645 (80%) | 944 (84%) |
| Living siblings | 6,278 (87%) | 882 (86%) | 647 (80%) | 944 (84%) |
| Myocardial infarction | 1,087 (15%) | 209 (20%) | 170 (21%) | 238 (21%) |
| Cardiovascular diseaseb | 1,328 (18%) | 223 (22%) | 209 (26%) | 305 (27%) |
| Hypertension | 4,841 (67%) | 705 (69%) | 558 (69%) | 845 (75%) |
| Arthritis | 3,996 (56%) | 595 (58%) | 499 (62%) | 782 (70%) |
| Osteoporosis | 1,462 (20%) | 183 (18%) | 200 (25%) | 291 (26%) |
| Diabetes | 1,818 (25%) | 319 (31%) | 218 (27%) | 407 (36%) |
| Respiratory disease | 1,098 (15%) | 163 (16%) | 138 (17%) | 241 (21%) |
| Stroke | 823 (11%) | 146 (14%) | 193 (24%) | 217 (19%) |
| Cancer | 1,843 (26%) | 257 (25%) | 171 (21%) | 278 (25%) |
| Hip fracture | 339 (5%) | 45 (4%) | 90(11%) | 85 (8%) |
| Other fracture | 1,412 (20%) | 187 (18%) | 177 (22%) | 272 (24%) |
| Other disease | 2,069 (29%) | 261 (26%) | 240 (30%) | 391 (35%) |
| Smoking status | ||||
| Never smoked | 3,535 (49%) | 494 (48%) | 462 (57%) | 554 (49%) |
| Former smoker | 3,088 (43%) | 441 (43%) | 303 (37%) | 451 (40%) |
| Current smoker | 568 (8%) | 89 (9%) | 44 (5%) | 117(10%) |
| Fall in the last year | 1,449 (23%) | 231 (26%) | 198 (32%) | 303 (34%) |
| Number of body regions with pain symptoms | 2.9 (2.1) | 3.0 (2.1) | 3.3 (2.4) | 3.8 (2.5) |
| Had proxy | 386 (5%) | 19 (2%) | 268 (33%) | 138 (12%) |
| Hospitalized in the previous year | 1,649 (23%) | 300 (29%) | 286 (35%) | 392 (35%) |
| Number of hospitalizations in the previous yearc | 1.7 (1.8) | 1.7 (1.5) | 2.0 (2.9) | 1.9 (1.9) |
All values are N(%) or mean (SD).
The mean annual income for wave 1 participants was $48,000.
Cardiovascular disease other than myocardial infarction.
Number of hospitalizations in the previous year among hospitalized.
Prevalence of Possible Dementia, Probable Dementia, and Substantial Depressive Symptoms
Among community-dwelling NHATS participants, the prevalence of probable dementia was 8.2% and the prevalence of possible dementia was 12.6%. The prevalence of substantial depressive symptoms was 14.1%. This translates to an estimated 3.1 million (95% confidence interval [95%CI]: 2.9 million, 3.3 million) community-dwelling older Americans with probable dementia, 4.8 million (95%CI: 4.5 million, 5.1 million) with possible dementia, and 5.3 million (95%CI: 5.0 million, 5.7 million) with substantial depressive symptoms.
Associations of Hospitalization in the Previous Year with Probable Dementia and Depression
In unadjusted regression analyses, being hospitalized in the previous year was associated with greater odds of probable dementia (odds ratio [OR]: 2.12, 95%CI: 1.85, 2.43) and substantial depressive symptoms (OR: 2.21, 95%CI: 1.89, 2.59).
Table 2 presents the results of sequentially adjusted ordinal logistic regression models testing the association of hospitalization in the previous year with probable dementia at the time of the NHATS interview. Hospitalization in the previous year was found to be independently associated with odds of probable dementia even after adjusting for demographic and social characteristics, medical diagnoses, smoking status, having a fall in the previous year, and number of body regions with pain symptoms (OR: 1.42, 95%CI: 1.16, 1.73). This association remained significant after a sensitivity analysis adjusting for participant PHQ-2 score (OR: 1.36, 95%CI: 1.11, 1.67). Community-dwelling NHATS participants with probable dementia who were hospitalized in the previous year had a mean of 5.4 ADL/IADL impairments (95%CI: 4.7, 5.0) compared to 3.4 ADL/IADL impairments (95%CI: 3.0, 3.8) among those without a hospitalization in the previous year (P < 0.001 by one-way analysis of variance).
Table 2.
Adjusted associations of hospitalization in the previous year with odds of probable dementia among community-dwelling older adults participating in the first wave of the National Health and Aging Trends Study
| Adjusted for demographics and personal characteristics | Adjusted for comorbid medical conditions and smoking | Adjusted for falls and number of pain symptoms | |
|---|---|---|---|
|
| |||
| Odds Ratio (95% Confidence Interval) | |||
| Hospitalization in the previous year | 1.63 (1.40,1.89)‡ | 1.52 (1.29,1.78)‡ | 1.42 (1.16,1.73)† |
| Age (years) | |||
| 76-85 | 2.18 (1.86,2.56)‡ | 2.14 (1.82,2.53)‡ | 2.04 (1.64,2.53)‡ |
| ≥ 86 | 5.72 (4.75, 6.90)‡ | 5.60 (4.61,6.81)‡ | 4.79 (3.71,6.18)‡ |
| Female | 0.72 (0.62, 0.83)‡ | 0.71 (0.60,0.81)‡ | 0.65 (0.52, 0.82)‡ |
| Non-white | 1.91 (1.64,2.23)‡ | 1.87 (2.60,2.19)‡ | 1.91 (1.56,2.34)‡ |
| < High school graduate | 2.85 (2.45,3.31)‡ | 2.80 (2.40, 3.27)‡ | 3.06 (2.51, 3.74)‡ |
| Single/widowed | 1.12 (0.96, 1.30) | 1.08 (0.93, 1.26) | 1.13 (0.93, 1.39) |
| < $48,000 annual incomea | 1.93 (1.60,2.32)‡ | 1.86 (1.53,2.25)‡ | 1.71 (1.32,2.20)‡ |
| Living children | 0.82 (0.67, 1.00) | 0.83 (0.68, 1.01) | 0.77 (0.59, 1.01) |
| Living siblings | 0.97 (0.94, 1.01) | 0.97 (0.94, 1.00) | 0.97 (0.93, 1.01) |
| Myocardial infarction | 1.19 (0.99, 1.44) | 1.20 (0.95, 1.53) | |
| Cardiovascular diseaseb | 1.16 (0.97, 1.38) | 1.09 (0.88, 1.34) | |
| Hypertension | 0.85 (0.73, 0.99)* | 0.86 (0.70, 1.05) | |
| Arthritis | 0.97 (0.84, 1.13) | 0.95 (0.78, 1.17) | |
| Osteoporosis | 1.14 (0.95, 1.37) | 1.21 (0.97, 1.51) | |
| Diabetes | 1.26 (1.08, 1.48)† | 1.14 (0.93, 1.40) | |
| Respiratory disease | 0.97 (0.81, 1.17) | 0.97 (0.76, 1.22) | |
| Stroke | 1.87 (1.53,2.29)‡ | 1.73 (1.33,2.25)‡ | |
| Cancer | 0.86 (0.73, 1.01) | 0.94(0.77, 1.16) | |
| Hip fracture | 1.55 (1.15, 2.08)† | 1.65 (1.15, 2.37)† | |
| Other fracture | 0.88 (0.74, 1.04) | 0.77 (0.61, 0.96)* | |
| Other disease | 0.98 (0.85, 1.15) | 1.00 (0.82, 1.20) | |
| Smoking status | |||
| Former smoker | 0.86 (0.74, 0.99)* | 0.72 (0.59, 0.87)† | |
| Current smoker | 0.96 (0.74,1.25) | 0.98 (0.07, 1.37) | |
| Fall in last year | 1.28 (1.06, 1.56)* | ||
| Number of pain symptoms | 1.04 (1.00, 1.09) | ||
P < 0.05
P < 0.01
P < 0.001
The mean annual income for wave 1 participants was $48,000.
Cardiovascular disease other than myocardial infarction.
Table 3 presents the results of sequentially adjusted binary logistic regression models testing the association of hospitalization in the previous year with substantial depressive symptoms during the two weeks prior to the NHATS interview. As with probable dementia, hospitalization in the previous year was found to be independently associated with odds of substantial depressive symptoms (OR: 1.60, 95%CI: 1.29, 1.99). This association remained significant after a sensitivity analysis adjusting for participant total score on cognitive testing (OR: 1.55, 95%CI: 1.23, 1.96).
Table 3.
Adjusted associations of hospitalization in the previous year with odds of substantial depressive symptoms among community-dwelling older adults participating in the first wave of the National Health and Aging Trends Study
| Adjusted for demographics and personal characteristics | Adjusted for comorbid medical conditions and smoking | Adjusted for falls and number of pain symptoms | |
|---|---|---|---|
|
| |||
| Odds Ratio (95% Confidence Interval) | |||
| Hospitalization in the previous year | 1.98 (1.68, 2.34)‡ | 1.58 (1.33,1.88)‡ | 1.60 (1.29,1.99)‡ |
| Age (years) | |||
| 76-85 | 0.93 (0.78, 1.10) | 0.93 (0.78, 1.11) | 0.93 (0.74, 1.17) |
| ≥ 86 | 0.98 (0.79, 1.22) | 1.02 (0.80, 1.28) | 1.11 (0.83, 1.50) |
| Female | 1.01 (0.85, 1.19) | 0.93 (0.77, 1.13) | 0.76 (0.55, 0.97)* |
| Non-white | 1.27 (1.07, 1.51)† | 1.34(1.12, 1.60)‡ | 1.47 (1.17, 1.84)† |
| < High school graduate | 1.72 (1.45, 2.05)‡ | 1.54 (1.29, 1.83)‡ | 1.60 (1.28, 2.01)‡ |
| Single/widowed | 1.17 (0.98, 1.39) | 1.07 (0.89, 1.29) | 0.92 (0.73, 1.15) |
| < $48,000 annual incomea | 1.85 (1.49, 2.29)‡ | 1.71 (1.37, 2.13)‡ | 1.54 (1.17, 2.03)† |
| Living children | 0.80 (0.64, 1.00) | 0.75 (0.60, 0.95)* | 0.76 (0.56, 1.02) |
| Living siblings | 1.00 (0.97, 1.04) | 1.01 (0.97, 1.05) | 1.02 (0.97, 1.07) |
| Myocardial infarction | 1.13 (0.91, 1.40) | 1.04 (0.80, 1.34) | |
| Cardiovascular diseaseb | 1.27 (1.04, 1.54)* | 1.03 (0.81, 1.31) | |
| Hypertension | 1.10 (0.92, 1.32) | 1.14 (0.90, 1.44) | |
| Arthritis | 1.33 (1.12, 1.58)† | 0.99 (0.79, 1.25) | |
| Osteoporosis | 1.15 (0.95, 1.40) | 1.15 (0.95, 1.46) | |
| Diabetes | 1.54 (1.34, 1.89)‡ | 1.44 (1.16, 1.78)† | |
| Respiratory disease | 1.36 (1.11, 1.66)† | 1.26 (0.98, 1.63) | |
| Stroke | 1.77 (1.43, 2.20)‡ | 1.78 (1.35, 2.33)‡ | |
| Cancer | 0.94 (0.78, 1.12) | 1.02 (0.81, 1.28) | |
| Hip fracture | 1.38 (1.01, 1.88)* | 1.03 (0.68, 1.54) | |
| Other fracture | 1.23 (1.02, 1.48)* | 1.19 (0.94, 1.50) | |
| Other disease | 1.38 (1.17, 1.63)‡ | 1.27 (1.03, 1.56)* | |
| Smoking status | |||
| Former smoker | 0.91 (0.76, 1.08) | 0.83 (0.67, 1.04) | |
| Current smoker | 1.34 (1.01, 1.79)* | 1.28 (0.89, 1.85) | |
| Fall in last year | 1.53 (1.23, 1.89)‡ | ||
| Number of pain symptoms | 1.17 (1.12, 1.23)‡ | ||
P < 0.05
P < 0.01
P < 0.001
The mean annual income for wave 1 participants was $48,000.
Cardiovascular disease other than myocardial infarction.
Certain demographic and clinical characteristics were also found to be independently associated both with odds of probable dementia and substantial depressive symptoms. In the analyses examining odds of probable dementia, increasing age (76-85 years old: OR: 2.04, 95%CI: 1.64, 2.53; ≥ 86 years old: OR: 4.79, 95%CI: 3.71, 6.18), non-white race/ethnicity (OR: 1.91, 95%CI: 1.56, 2.34), less education (OR: 3.06, 95%CI: 2.51, 3.74), lower income (OR: 1.71, 95%CI: 1.32, 2.20), prior stroke (OR: 1.73, 95%CI: 1.33, 2.25), prior hip fracture (OR: 1.65, 95%CI: 1.15, 2.37), and having a fall in the prior year (OR: 1.28, 95%CI: 1.06, 1.56) were all independently associated with greater odds of probable dementia. Female sex (OR: 0.65, 95%CI: 0.52, 0.82) and being a former smoker (OR: 0.72, 95%CI: 0.59, 0.87) were associated with decreased odds of probable dementia. In analyses of substantial depressive symptoms, non-white race (OR: 1.47, 95%CI: 1.17, 1.84), less education (OR: 1.60, 95%CI: 1.28, 2.01), less income (OR: 1.54, 95%CI: 1.17, 2.03), diabetes (OR: 1.44, 95%CI: 1.16, 1.78), prior stroke (OR: 1.78, 95%CI: 1.35, 2.33), other disease (s) (OR: 1.27, 95%CI: 1.03, 1.56), having a fall in the prior year (OR: 1.53, 95%CI: 1.23, 1.89), and a greater number of body regions with pain symptoms (OR: 1.17, 95%CI: 1.12, 1.23) were all independently associated with greater odds of substantial depressive symptoms.
In our secondary analyses where we stratified by age deciles, hospitalization in the previous year remained independently associated with increased odds of probable dementia among 76-85 year olds (OR: 1.40, 95%CI: 1.06, 1.85) as well as increased odds of substantial depressive symptoms among 65-75 year olds (OR: 1.92, 95%CI: 1.36, 2.70). Although the magnitudes of the associations between hospitalization in the previous year and our outcomes of interest were similar among the other age deciles, our estimates lacked precision (OR for probable dementia among 65-75 year olds: 1.47, 95%CI: 0.98, 2.19; OR for probable dementia among ≥ 86 years old: 1.32, 95%CI: 0.91, 1.90; OR for substantial depressive symptoms among 76-85 year olds: 1.35, 95%CI: 0.96, 1.90; OR for substantial depressive symptoms among ≥ 86 years old: 1.33, 95%CI: 0.85, 2.09).
DISCUSSION
In this nationally representative sample, we have identified that dementia and depression are alarmingly common among community-dwelling older Americans. Our results suggest that nearly one in twelve community-dwelling Americans 65 or older may have dementia, and nearly one in seven may have substantial depressive symptoms. The prevalence of probable dementia and substantial depressive symptoms among community-dwelling Americans 65 or older presented here are similar to previous studies of these conditions in older Americans despite differences in case ascertainment [14, 40, 41], suggesting reliability in our findings. We also found that being hospitalized in the previous year was independently associated with probable dementia and substantial depressive symptoms, even after adjusting for potential confounders such as chronic medical conditions and indicators of frailty.
In addition to hospitalization, we found that non-white race and indicators of lower socioeconomic status, such as not graduating from high school and lower income, were independently associated with odds of both probable dementia and substantial depressive symptoms among NHATS participants. These findings are in line with previous work [14, 42-44], and suggest the need for enhanced efforts to prevent disparities in dementia and depression prevention and treatment among diverse groups of community-dwelling older adults. Although we found that female NHATS participants appeared to have significantly lower odds of probable dementia, this result was driven primarily by women in this sample having 25% lower odds of possible dementia compared to men, in line with prior work suggesting older men are at higher risk of mild cognitive impairment than women [45].
While interpretation of our results regarding the direction of causality between hospitalizations and risk of probable dementia or depression among community-dwelling older adults is limited by the cross-sectional nature of our study, the implications of our findings are nonetheless important. Dementia and depression, particularly among older adults with comorbid medical conditions, may increase the risk of hospitalization [20, 46, 47], which in turn could lead to further cognitive decline and depression [5-8], and subsequent risk of early rehospitalization [48, 49], precipitating a vicious cycle. Due to the bi-directional nature of the associations between dementia, depression, and hospitalization, early recognition of these conditions at any point of this complex cascade could be crucial in order to potentially break this cycle.
Hospitalizations for a wide range of medical illnesses or injuries could lead to the development of cognitive impairment or depression through several mechanisms. Pneumonia or congestive heart failure, both common causes of hospitalization among older adults [3], could contribute to the development of cognitive decline through associated hypoxia [50]. Increased systemic inflammation, a hallmark of critical illnesses such as severe sepsis [6, 51], could also lead to the development of cognitive impairment [51], as well as depressive episodes in vulnerable older patients such as those with a prior history of depression [52], both potentially mediated by the development of delirium [53, 54]. In addition, immobility during prolonged hospitalizations could exacerbate age-related muscle atrophy and worsen muscle fiber and neuronal degradation [55, 56], leading to physical disability and associated depression [8, 52, 57].
Conversely, for older adults depression and dementia could increase the risk of hospitalizations for both behavioral and psychobiologic reasons. Major depression is associated with obesity, sedentary lifestyle, and tobacco use, all of which increase the risk of chronic medical illnesses [20]. Depression and dementia can also lead to non-adherence with treatment for chronic conditions and difficulty with care coordination, leading to further complications [20, 46]. Depression is also associated with increased systemic inflammation [20], further potentiating the development of medical-surgical complications.
If depression and/or dementia do play a key role in a bi-directional pathway with hospitalizations among older adults, then increased efforts aimed at screening for these disorders and improving access to evidence-based treatments are needed. Collaborative care interventions based in primary care settings have demonstrated improvements in depression in older adults [18, 58], including those with substantial medical comorbidity [59]. Similar interventions have also decreased neuropsychiatric symptoms and behavioral disturbances in older adults with dementia [19]. Additional research is needed to examine if aspects of these interventions could be implemented in the acute care setting for hospitalized older adults, possibly in combination with interventions that target improving the quality of care transition from the hospital back to primary care [60], in order to reduce early rehospitalizations and maintain independent functioning in older adults.
Our study has several potential limitations. In addition to not being able to infer the direction of causality between hospitalizations and risk of dementia or depression as well as the possibility that the nature of some associations found here changing with analyses of data from subsequent NHATS waves, we lack information on prior cognitive functioning or psychiatric history as well as previous healthcare utilization. However, prior research has shown that hospitalizations for acute medical conditions are associated with cognitive decline, and in some cases depression, even after controlling for premorbid state [6, 8], limiting the impact of this limitation on the interpretation of our results. Since our outcome was obtained from self-report, we lack data on the specific diagnoses resulting in hospitalization as well as on specific interventions received during the hospitalization. Although our ability to diagnose major depression was limited by our use of a questionnaire and not a diagnostic interview, the PHQ-2 has been specifically validated in our study population [34], as well as in older adults with cognitive impairment [35]. Finally, residual confounding remains a possibility, as in any observational study.
In conclusion, using a nationally representative sample of community-dwelling older Americans, we found that probable dementia and substantial depressive symptoms are common in this population. Furthermore, we identified that being hospitalized in the previous year was associated with greater odds of both probable dementia and substantial depressive symptoms. Future research that increases understanding of the complex, bi-directional relationship between hospitalizations for medical illnesses and neuropsychiatric syndromes such as dementia and depression, as well as the development of targeted interventions that reduce hospitalizations in this high risk group, are needed in order to help maintain quality of life and independence in older adults.
Acknowledgments
We appreciate the expert programming of Laetitia Shapiro, A.M. at the University of Michigan, Vicki A. Freedman, Ph.D., at the University of Michigan for assistance with use of the National Health and Aging Trends Study sampling weights, and Judith D. Kasper, Ph.D., at Johns Hopkins University for assistance with the National Health and Aging Trends Study dementia classifications.
This work was supported by grants KL2 TR000421 and U01 AG032947 from the National Institutes of Health. The National Health and Aging Trends Study is performed at the Johns Hopkins University School of Public Health.
Sponsor’s Role: The sponsor had no role in the design, methods, subject recruitment, data collection, analysis, or preparation of the manuscript.
Footnotes
Potential Conflicts of Interest: The authors have no relevant potential conflicts of interest to disclose.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs, the National Institutes of Health, or the US government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aronow WS. Heart disease and aging. Med Clin North Am. 2006;90:849–862. doi: 10.1016/j.mcna.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Caspersen CJ, Thomas GD, Boseman LA, Beckles GLA, Albright AL. Aging, diabetes, and the public health system in the United States. Am J Public Health. 2012;102:1482–1497. doi: 10.2105/AJPH.2011.300616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 4.Kocher RP, Adashi EY. Hospital readmissions and the Affordable Care Act: paying for coordinated quality care. JAMA. 2011;306:1794–1795. doi: 10.1001/jama.2011.1561. [DOI] [PubMed] [Google Scholar]
- 5.Ehlenbach WJ, Hough CL, Crane PK, et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303:763–770. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson RS, Hebert LE, Scherr PA, et al. Cognitive decline after hospitalization in a community population of older persons. Neurology. 2012;78:950–956. doi: 10.1212/WNL.0b013e31824d5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davydow DS, Hough CL, Levine DA, Langa KM, Iwashyna TJ. Functional disability, cognitive impairment, and depression after hospitalization for pneumonia. Am J Med. 2013;126:615–624. doi: 10.1016/j.amjmed.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Burden of Disease Collaborators. The state of U.S. health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan A, Lucas M, Sun Q, et al. Increased mortality risk in women with depression and diabetes mellitus. Arch Gen Psychiatry. 2011;68:42–50. doi: 10.1001/archgenpsychiatry.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larson EB, Shadlen MF, Wang L, et al. Survival after initial diagnosis of Alzheimer disease. Ann Intern Med. 2004;140:501–509. doi: 10.7326/0003-4819-140-7-200404060-00008. [DOI] [PubMed] [Google Scholar]
- 13.Katon W, Lin E, Russo J, Unützer J. Increased medical costs of a population-based sample of depressed elderly patients. Arch Gen Psychiatry. 2003;60:897–903. doi: 10.1001/archpsyc.60.9.897. [DOI] [PubMed] [Google Scholar]
- 14.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368:1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rait G, Walters K, Bottomley C, et al. Survival of people with clinical diagnosis of dementia in primary care: cohort study. BMJ. 2010;341:c3584. doi: 10.1136/bmj.c3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cepoiu M, McCusker J, Cole MG, et al. Recognition of depression by non-psychiatric physicians – a systematic literature review and meta-analysis. J Gen Intern Med. 2008;23:25–36. doi: 10.1007/s11606-007-0428-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seitz D, Purandare N, Conn D. Prevalence of psychiatric disorders among older adults in long-term care homes: a systematic review. Int Psychogeriatr. 2010;22:1025–1039. doi: 10.1017/S1041610210000608. [DOI] [PubMed] [Google Scholar]
- 18.Unützer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288:2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 19.Callahan CM, Boustani MA, Unverzagt FW, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295:2148–2157. doi: 10.1001/jama.295.18.2148. [DOI] [PubMed] [Google Scholar]
- 20.Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biol Psychiatry. 2003;54:216–226. doi: 10.1016/s0006-3223(03)00273-7. [DOI] [PubMed] [Google Scholar]
- 21.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5(1):64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 22.Eggermont LH, Penninx BW, Jones RN, Leveille SG. Depressive symptoms, chronic pain, and falls in older community-dwelling adults: the MOBILIZE Boston Study. J Am Geriatr Soc. 2012;60:230–237. doi: 10.1111/j.1532-5415.2011.03829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welmerink DB, Longstreth WT, Jr, Lyles MF, Fitzpatrick AL. Cognition and the risk of hospitalization for serious falls in the elderly: results from the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2010;65:1242–1249. doi: 10.1093/gerona/glq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence of a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M157. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 25.Freedman VA, Kasper JD, Cornman JC, et al. Validation of new measures of disability and functioning in the National Health and Aging Trends Study. J Gerontol A Biol Sci Med Sci. 2011;66:1013–1021. doi: 10.1093/gerona/glr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montaquila J, Freedman VA, Edwards B, Kasper JD. National Health and Aging Trends Study Round 1 Sample Design and Study Selection. NHATS Technical Paper #1. Baltimore, MD: Johns Hopkins University School of Public Health; May 10, 2012. [Google Scholar]
- 27.Kasper JD, Freedman VA. Baltimore, MD: Johns Hopkins University School of Public Health; May 14, 2012. National Health and Aging Trends Study Round 1 User Guide: Beta Release 1.0. [Google Scholar]
- 28.Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65:559–564. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- 29.Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of Cognition Using Surveys and Neuropsychological Assessment: The Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci. 2011;66B(Supplement 1):i162–i171. doi: 10.1093/geronb/gbr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schretlen DJ, Testa SM, Pearlson GD. Lutz, FL: Psychological Assessment Resources; 2010. Clock-drawing test scoring approach from the Calibrated Neuropsychological Normative System. [Google Scholar]
- 31.Shulman KI. Clock-drawing: is it the ideal cognitive screening test? Int J Geriatr Psychiatry. 2000;15:548–561. doi: 10.1002/1099-1166(200006)15:6<548::aid-gps242>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 32.Kasper JD, Freedman VA, Spillman BC. Classification of persons by dementia status in the National Health and Aging Trends Study. NHATS Technical Paper #5. Baltimore, MD: Johns Hopkins University School of Public Health; Jul 23, 2013. [Google Scholar]
- 33.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: Validity of a two-item depression screener. Med Care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 34.Li C, Friedman B, Conwell Y, Fiscella K. Validity of the Patient Health Questionnaire 2 (PHQ-2) in identifying major depression in older people. J Am Geriatr Soc. 2007;55:596–602. doi: 10.1111/j.1532-5415.2007.01103.x. [DOI] [PubMed] [Google Scholar]
- 35.Boyle LL, Richardson TM, He H, et al. How do the PHQ-2, PHQ-9 perform in aging services clients with cognitive impairment. Int J Geriatr Psychiatry. 2011;26:952–960. doi: 10.1002/gps.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Administration on Aging. A Profile of Older Americans: 2011. U.S Department of Health and Human Services; Washington, D.C.: 2011. [Google Scholar]
- 37.Montaquila J, Freedman VA, Spillman B, Kasper JD. National Health and Aging Trends Study Round 1 Development of Round 1 Survey Weights. NHATS Technical Paper #2. Baltimore, MD: Johns Hopkins University School of Public Health; Nov 29, 2012. [Google Scholar]
- 38.Dobie DJ. Depression, dementia, and pseudodementia. Semin Clin Neuropsychiatry. 2002;7:170–186. doi: 10.1053/scnp.2002.0070170. [DOI] [PubMed] [Google Scholar]
- 39.Li G, Wang LY, Shofer JB, et al. Temporal relationship between depression and dementia: findings from a large community-based 15-year follow-up study. Arch Gen Psychiatry. 2011;68:970–977. doi: 10.1001/archgenpsychiatry.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zivin K, Llewellyn DJ, Lang IA, et al. Depression among older adults in the United States and England. Am J Geriatr Psychiatry. 2010;18:1036–1044. doi: 10.1097/JGP.0b013e3181dba6d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zivin K, Pirraglia PA, McCammon RJ, Langa KM, Vijan S. Trends in depressive symptom burden among older adults in the United States from 1998 to 2008. J Gen Intern Med. 2013 Jul 9; doi: 10.1007/s11606-013-2533-y. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chin AL, Negash S, Hamilton R. Diversity and disparity in dementia: the impact of ethnoracial differences in Alzheimer’s disease. Alzheimer Dis Assoc Disord. 2011;25:187–195. doi: 10.1097/WAD.0b013e318211c6c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharp ES, Gatz M. Relationship between education and dementia: an updated systematic review. Alzheimer Dis Assoc Disord. 2011;25:289–304. doi: 10.1097/WAD.0b013e318211c83c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lorant V, Deliège D, Eaton W, et al. Socioeconomic inequalities in depression: a meta-analysis. Am J Epidemiol. 2003;157:98–112. doi: 10.1093/aje/kwf182. [DOI] [PubMed] [Google Scholar]
- 45.Roberts RO, Geda YE, Knopman DS, et al. The incidence of MCI differs by subtype and is higher in men: the Mayo Clinic Study of Aging. Neurology. 2012;78:342–351. doi: 10.1212/WNL.0b013e3182452862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phelan EA, Borson S, Grothaus L, Balch S, Larson EB. Association of incident dementia with hospitalizations. JAMA. 2012;307:165–172. doi: 10.1001/jama.2011.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davydow DS, Katon WJ, Lin EHB, et al. Depression and risk of hospitalizations for ambulatory care-sensitive conditions in patients with diabetes. J Gen Intern Med. 2013;28:921–929. doi: 10.1007/s11606-013-2336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Callahan CM, Arling G, Tu W, et al. Transitions in care for older adults with and without dementia. J Am Geriatr Soc. 2012;60:813–820. doi: 10.1111/j.1532-5415.2012.03905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitchell SE, Paasche-Orlow MK, Forsythe SR, et al. Post-discharge hospital utilization among adult medical inpatients with depressive symptoms. J Hosp Med. 2010;5:378–384. doi: 10.1002/jhm.673. [DOI] [PubMed] [Google Scholar]
- 50.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacob A, Brorson JR, Alexander JJ. Septic encephalopathy: inflammation in man and mouse. Neurochem Int. 2011;58:472–476. doi: 10.1016/j.neuint.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 52.Davydow DS, Hough CL, Langa KM, Iwashyna TJ. Symptoms of depression in survivors of severe sepsis: a prospective cohort study of older Americans. Am J Geriatr Psychiatry. 2013;21:887–897. doi: 10.1016/j.jagp.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38:1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davydow DS. Symptoms of depression and anxiety after delirium. Psychosomatics. 2009;50:309–316. doi: 10.1176/appi.psy.50.4.309. [DOI] [PubMed] [Google Scholar]
- 55.Carmeli E, Reznick AZ. The physiology and biochemistry of skeletal muscle atrophy as a function of age. Proc Soc Exp Biol Med. 1994;206:103–113. doi: 10.3181/00379727-206-43727. [DOI] [PubMed] [Google Scholar]
- 56.Schweickert WD, Hall J. ICU-acquired weakness. Chest. 2007;131:1541–1549. doi: 10.1378/chest.06-2065. [DOI] [PubMed] [Google Scholar]
- 57.Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, et al. Depressive symptoms and impaired physical function after acute lung injury: a 2-year longitudinal study. Am J Respir Crit Care Med. 2012;185:517–524. doi: 10.1164/rccm.201103-0503OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bruce ML, Ten Have TR, Reynolds CF, 3, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004291:1081–1091. doi: 10.1001/jama.291.9.1081. [DOI] [PubMed] [Google Scholar]
- 59.Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363:2611–2620. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coleman EA, Parry C, Chalmers S, Min SJ. The Care Transitions Intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166:1822–1828. doi: 10.1001/archinte.166.17.1822. [DOI] [PubMed] [Google Scholar]


