Abstract
Herpes simplex virus (HSV) infection of adult humans occasionally results in life-threatening herpes simplex encephalitis (HSE) for reasons that remain to be defined. An animal system that could prove useful to model HSE could be miR-155 knockout mice (miR-155KO). Thus we observe that mice with a deficiency of miR-155 are highly susceptible to HSE with a majority of animals (75–80%) developing HSE after ocular infection with HSV-1. The lesions appeared to primarily represent the destructive consequences of viral replication and animals could be protected from HSE by acyclovir treatment provided 4 days after ocular infection. The miR-155KO animals were also more susceptible to develop zosteriform lesions, a reflection of viral replication and dissemination within the nervous system. One explanation for the heightened susceptibility to HSE and zosteriform lesions could be because miR-155KO animals develop diminished CD8 T cell responses when the numbers, functionality and homing capacity of effector CD8 T cell responses were compared. Indeed, adoptive transfer of HSV-immune CD8 T cells to infected miR-155KO mice at 24 hours post infection provided protection from HSE. Deficiencies in CD8 T cell numbers and function also explained the observation that miR-155KO animals were less able than control animals to maintain HSV latency. Our observations may be the first to link miR-155 expression with increased susceptibility of the nervous system to virus infection.
Introduction
Infections with herpes simplex virus (HSV) usually cause lesions at body surfaces such as the skin, mucosal surface and the eye. Characteristically, after primary infection HSV establishes a non-replicating persistent (latent) infection in neuronal tissue, which can break down periodically and give rise to recurrent lesions at primary lesion sites (1). A rare yet often tragic manifestation of HSV infection is dissemination to the brain with resultant herpes simplex encephalitis (HSE) (2). In adult humans HSE is usually caused by HSV-1 and can occur in persons whom are seropositive and latently infected with virus (2). Additionally, infants can develop encephalitis if seronegative and incur primary infection usually with HSV-2 (2). A rare form of HSE also occurs in children with genetic defects in innate immune defenses (3–5). Once virus enters the brain, the lesions that follow are considered to either be the consequence of viral replication in critical cells (3, 6) and/or be caused by an inflammatory response to the infection (7–9). Support for the latter ideas comes mainly from studies in rodents. For example, mild lesions occur in gene knockout animals that lack the expression of some innate immune receptors involved in causing inflammatory responses (7, 8). Further support for the inflammation hypothesis came from studies showing that whereas antiviral therapy had no effect on disease outcome inflammatory cell depletion markedly diminished HSE (9). Conceivably, the pathogenesis of herpes encephalitis could differ in the natural host from that studied in animal model systems.
MicroRNAs regulate gene expression post transcriptionally and are implicated in some immunoinflammatory diseases in humans and in various mouse models of human diseases (10, 11). For example, animals deficient in miR-155 are relatively resistant to develop autoimmune disease, such as EAE an animal model for the human disease multiple sclerosis (12, 13). MicroRNA-155 also plays a critical role in the pathogenesis of human rheumatoid arthritis with miR-155 being upregulated in the synovial membrane cells and assumed to function by promoting inflammatory cytokine production (14, 15). Mouse studies have indicated that miR-155 influences inflammatory disease by both promoting the expansion of pro-inflammatory Th1 and Th17 cells as well as amplifying effects on inflammatory gene expression in macrophages and T cells (12, 14).
Few studies have evaluated the role of miRNAs in the pathogenesis of virus infections. In the present report, we have evaluated the susceptibility of animals with a deficiency for miR-155 because of gene knockout to ocular and intradermal infection with HSV-1. We demonstrate that miR-155KO mice show heightened susceptibility to HSV ocular infection, with the majority of animals succumbing to HSE under conditions where wild type (WT) animals remained normal. miR-155KO mice were also markedly more susceptible than WT to develop zosteriform lesions upon intradermal infection, a lesion that reflects viral dissemination into the nervous system (16). Additionally, ganglionic latent infection with HSV-1 reactivated more abundantly from miR-155KO than WT latently infected ganglia upon ex-vivo culture. One explanation for the observations was that miR-155KO animals developed diminished virus specific CD8 T cell responses, particularly those that were functionally effective. Other mechanistic explanations were also discussed.
Materials and methods
Mice
Female 5–6wks old C57BL/6 mice were purchased from Harlan Sprague Dawley Inc. (Indianapolis, Indiana, USA). Breeder pair’s of miR-155KO mice on C57BL/6 background were obtained from Jackson laboratories (Bar Harbor, ME) and additional mice were bred in the Walters Life Sciences animal facility at the University of Tennessee, Knoxville. HSV-specific TCR transgenic mice (gBT-I.3-referred to in the text as gBT mice) were produced in the laboratory of Francis Carbone (University of Melbourne, Melbourne, Australia). The animals were housed in American Association of Laboratory Animal Care-approved facilities at the University of Tennessee, Knoxville. All investigations followed guidelines of the institutional animal care and use committee.
Virus
Three different strains of virus were used. HSV-1 Tumpey (obtained from Dr. Robert Lausch, University of South Alabama), HSV-1 RE (obtained from Dr. Robert Hendricks, University of Pittsburgh) and HSV-1 KOS (obtained from Dr. David Knipe, Harvard University) were used. All strains were propagated and titrated on monolayers of Vero cells (ATCC CCL81) using standard protocols. All virus stocks were aliquoted and stored at −80°C.
Infection of mice
Infections of all mice groups (5–8 week old) were conducted under deep anesthesia with avertin (Tribromoethanol). For corneal infection, the mice were scarified on their corneas with a 27-gauge needle, and a 3 μl drop containing 104 PFU of HSV-1 Tumpey was applied to one eye and was used to monitor the development of encephalitis. In experiments involving HSV reactivation, mice were infected with 105 PFU of HSV-RE for corneal infection. The zosterifrom infection was used in some of the experiments. The zosteriform infection was performed as described earlier (16). Briefly, hair was clipped on each left flank and depilated with Veet hair removal cream after anesthetizing the mice using avertin intraperitoneal injection. A small area of skin (1cm2) near the top of the spleen was scarified with a 27 gauge needle, and 20 μl of HSV-1 Tumpey containing 106 PFU of virus was applied to hair-depleted area of the skin and massaged. Additionally, in some experiments HSV footpad model was used. Mice were injected subcutaneously in each hind footpad (FP) with 4×105 PFU HSV-1 KOS in 30μl of phosphate-buffered saline (PBS). Mice were sacrificed at day 5 pi, and the PLN were isolated for analysis.
Adoptive transfer of HSV-immune CD8+ T cells
To generate HSV-immune CD8+ T cells, gBT mice were scarified on their corneas with a 27-gauge needle, and a 3μl drop containing 104 PFU of HSV-1 Tumpey was applied to one eye. Single-cell suspensions of pooled spleens and popliteal lymph nodes were prepared from immunized mice 7–8 days later, and CD8+ T cells were purified using a mouse CD8 T cell isolation kit from miltenyl biotec. By flow cytometry analysis, the purified population consisted of 85% CD8+ T cells. Ocularly infected miR-155KO animals received an intra venous injection of 20 × 106 purified cells at 24 hours pi.
Immunohistochemistry
Groups of miR-155KO mice and WT mice were ocularly infected with 106 PFU of HSV-1 Tumpey and mice showing signs of encephalitis from each group (day 8 pi) were anesthetized with avertin and transcardially perfused with isotonic sucrose solution; sucrose perfusion was followed by perfusion with a solution of 4% paraformaldehyde (PFA). Post fixation of the brain samples were done by immersion of the skull in the same 4% PFA fixative for 1 day. After brain extraction from the skull, cryoprotection was done in 10% glycerol on day 1 and 20% glycerol on day 2. Mouse brains were embedded within a single gelatin matrix, freeze cut into 35μm coronal sections, and collected into 24 series (Neuroscience Associates Knoxville, TN). Each 12th section was then stained as free-floating section. High-sensitivity immunohistochemistry on multibrain sections was performed essentially following the protocol described by Osmand et al. and Hoffman et al. (17, 18) This involved treatment with sodium borohydride, blocking with 0.5% Triton X-100, and overnight incubation in a solution of primary antibody at a predetermined optimal concentration, followed by exposure to biotinylated species-specific secondary antibody and enzymatic detection using a 1:500 dilution of reagents A and B from the ABC Elite reagent (Vector Laboratories) and Ni–DAB–glucose-glucose oxidase (19). Sections were mounted and cover slipped without the use of counter stains.
Abs and reagents
APC-conjugated anti-mouse CD8a (53–6.7), FITC-conjugated anti-mouse TNF-α, allophycocyanin-conjugated anti-mouse IFN-γ, FITC-conjugated anti-mouse CD49d, FITC-conjugated anti-mouse CD44 and Golgi transport inhibitor (brefeldin A) were purchased from BD Biosciences. Allophycocyanin-conjugated and PE-conjugated H-2Kb/gB498–505 (SSIEFARL) tetramers were provided by the National Institutes of Health Tetramer Core Facility (Emory University, Atlanta, GA). Recombinant mouse Gal-9 was provided by GalPharma, Japan. CD8 T cell isolation kit was obtained from Miltenyi Biotec. Primary antibodies Rat Anti-Mouse CD8a and Rabbit Anti-Glial Fibrillary Acidic Protein (GFAP) for immunohistochmeistry staining were purchased from BD Biosceince and DAKO respectively. The secondary antibodies Donkey Anti-Rat IgG (H+L) and Donkey Anti Rabbit IgG (H+L) were purchased from Jackson Immunoresearch.
Preparation of TG single-cell suspensions
At 14 days after HSV-1 RE ocular infection, mice were anaesthetized and euthanized by exsanguinations (20). TGs were excised and subjected to collagenase type I treatment (Sigma-Aldrich, St. Louis, MO) at a concentration of 3 mg/ml for 90 min at 37°C. After incubation, the TGs were dispersed into single cells by trituration. Each single cell suspension was then plated in 48-well tissue culture plates. The cells were cultured in DMEM with 10% FCS and 10 U/ml recombinant murine IL-2 (R&D Systems) as described (20).
Ex vivo reactivation experiments
Each TG sample isolated from miR155KO mice was divided into 2 aliquots. One aliquot was left unmanipulated and the other aliquot received 1×105 CD8 T cells isolated at day 8 pi from lymph nodes of HSV-1 infected WT mice. Similarly, each WT TG was divided into 2 aliquots and one aliquot was left unmanipulated whereas, the other aliquot received 1μM rGal-9 a procedure shown in a previous report to block CD8 T cell function and result in viral reactivation (21). TG cultures were incubated in DMEM in a 5% CO2 humidified incubator at 37°C for a 10 day period and culture supernatant samples were collected at 24-h intervals and assayed for infectious virus by plaque titrations on Vero cells. Gal-9 (1μM) and IL-2 (10U/ml) concentrations were constantly maintained throughout the culture period.
Flow Cytometry
Single-cell suspensions isolated from draining cervical lymph nodes, and TG samples of mice ocularly infected with HSV-1 were collected at different time points. Additionally in separate experiments were foot infection was used; PLN were isolated and made into single cell suspensions after HSV-1 footpad infection. Aliquots of the above single-cell suspensions were stained for CD8 and Kb-gB tetramer cell surface markers. To enumerate the functionality of CD8 T cell, intracellular staining was performed with freshly isolated DLN, PLN or TG suspensions from WT and miR-155KO mice. The cells were cultured in U-bottom 96-well plates and left untreated or stimulated with gB498–505 (SSIEFARL) peptide (1 μg/ml) and incubated for 6 h at 37°C in 5% CO2. Brefeldin A (5μg/ml) was added for the duration of the culture period to facilitate intracellular cytokine accumulation. After this period, cell surface staining was performed, followed by intracellular cytokine staining using a Cytofix/Cytoperm kit (BD Pharmingen) to enumerate the number of IFN-γ and TNF-α producing CD8 T cells as previously described (22). Finally, the cells were washed three times and re-suspended in 1% para-formaldehyde. The stained samples were acquired with a FACS Calibur (BD Biosciences) and the data were analyzed using the FlowJo software.
Viral plaque assay
Virus titers were measured in the brain, TG and skin of HSV infected mice as described previously by others (9, 21, 23). Additionally, mouse corneas were swabbed with sterile swabs (Fisher HealthCare, USA) at 6 days after ocular infection. Virus titers in all samples were measured using standard plaque assay as described previously (24).
Statistics
Mortality data were analyzed by log-rank testing (taking into account both time of death and final mortality). The statistical significance between two groups was determined using unpaired two-tailed student’s t test. One-way ANOVA with Bonferroni’s post hoc test was used to calculate the level of significance for some experiments. P ≤ 0.001 (***), P ≤ 0.01 (**), P ≤ 0.05 (*) were considered as significant and results are expressed as mean ± SEM. For all statistical analysis, GraphPad Prism software was used.
Results
Differential susceptibility of miR-155KO and WT mice to ocular infection with HSV
Upon ocular infection with HSV, mice develop a T cell orchestrated immnoinflammatory lesion in the cornea (stromal keratitis (SK)) and susceptible strains may succumb to encephalitis (25, 26). The latter outcome has also been advocated to represent an immunoinflammatory reaction to virus replication (8, 9). Since miR-155KO animals express higher resistance than WT animals to the induction of some immunoinflammatory diseases (12, 13), we anticipated that miR-155KO animals would be more refractory than WT animals to both SK and HSE. We did observe significantly heightened resistance to SK (these data will be documented in a separate manuscript), but unexpectedly miR-155KO animals were markedly more susceptible to HSE than were the WT animals. Thus under infectious conditions with a strain of HSV-1 virus which failed to cause detectable illness or symptoms of encephalitis in WT animals, 75–80% (in three separate experiments) of miR-155KO animals developed encephalitis and most had to be terminated by 9 days post infection (pi) (Figure 1A). By 6 days pi, affected animals became lethargic, lost weight, showed ruffled fur, hunched appearance and signs of incoordination. To cause encephalitis with the same virus strain in WT required a virus dose that was 1000 times greater, and then fewer than 20% developed encephalitis. Brains were collected from encephalitic miR-155KO animals, both to investigate pathological changes as well as to quantify levels of virus present. High virus levels of HSV were detectable in brain homogenates in all showing signs of encephalitis by day 9 pi, although none had detectable virus in ocular swabs at day 6 pi (Figure 1B and C). Virus could not be detected in the brains at day 9 pi or in the ocular tissue at day 6 pi in the WT animals when infected at the low virus dose that caused encephalitis in the miR-155KO animals (Figure 1C).
Figure 1. miR-155 knockout mice are highly susceptible to encephalitis after HSV-1 infection and have elevated viral titers in the brain but no difference in the cornea.
Groups of WT and miR-155KO (KO) animals were ocularly infected with 1×104 HSV-1 Tumpey. (A) Survival of age matched WT and miR-155 KO was established over 9 days. (B) Brains were harvested from WT and miR-155 KO mice at day 9 pi. Brains were homogenized and centrifuged, and supernatants were tested for virus titers. (C) The presence of virus in the cornea was measured at day 6 pi by swabbing the HSV infected eye with a sterile swab and assaying for the virus by plaque assay. The level of significance was determined by a Student t test (unpaired). Error bars represent means ± SEM (n = 5–8 mice/group). Experiments were repeated at least three times. *p ≤ 0.05, **p ≤ 0.005, ***p ≤ 0.001.
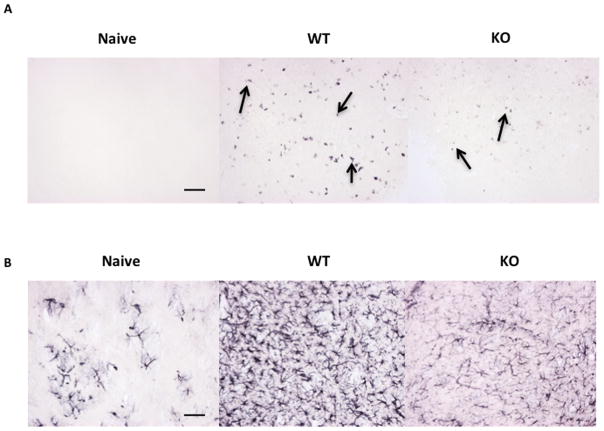
Brain sections from miR-155KO and WT animals examined 8 days pi and showing signs of encephalitis revealed differences in the nature of pathological changes. Thus the density of CD8 T cell infiltration in the posterior temporal lobe was notably more abundant in the WT animals than in the miR-155KO animals (Figure 2A). There was also marked differences in the extent of astrocytosis indicative of inflammatory reactions to infection with the response more abundant in WT animals (Figure 2B).
Figure 2. Infiltration of CD8 T cells and astrocytosis by immunohistochemistry of HSV-infected WT and miR-155KO mice.
Brains were collected from WT and miR-155KO mice that showed signs of encephalitis after ocular infection with 1×106 HSV-1 Tumpey at day 8 pi. Brains were embedded in gelatin and sections were stained with different antibodies. (A) Posterior temporal lobe staining of naïve (Left) infected WT (center) and infected miR-155KO (right) mice for CD8 T cells (black arrows) (15.03 ± 4.713 arbitrary units, p ≤ 0.05) (B) Striatum staining of naïve (left) and infected WT (center) and infected miR-155KO (right) mice for astrocytes (39.10 ± 7.804 arbitrary units, p ≤ 0.005). The figure shows the pictures of the sections taken at 20X magnification. Bar = 50μ. Statistical analysis was performed by gray scaling and inverting the images and then calculating the staining intensities using Photoshop. Statistical significance was determined by a Student t test (unpaired). (n = 3–4 mice/group).
The above observations are consistent with the viewpoint that the CNS damage in the miR-155KO animals was likely the consequence of the direct effects of virus infection rather than an immunopathological response to infection. Further support for this notion also came from experiments which showed that ocularly infected miR-155KO animals could be protected from developing encephalitis if treated with acyclovir starting at 4 days pi (Figure 3A and B). Moreover animals killed 5 days after treatment expressed minimal levels of virus in brain extracts compared to untreated animals (Figure 3C). In separate experiments we could recover infectious virus from the brains of both miR-155KO and WT mice one day before acyclovir treatment. However, higher viral titers were evident at day 4 pi in the miR-155KO animals (Figure 3D).
Figure 3. Viral replication in the brain is the cause of encephalitis in the miR-155 KO mice.
(A) WT and miR-155KO mice ocularly infected with 104 PFU HSV-1 Tumpey were either given 1mg acyclovir or vehicle intraperitonealy (i.p.) daily starting day 4 pi until day 8 pi. (B) Survival for all the groups of mice was established over a 9 day period. (C) Brains were harvested and homogenized from these different groups of mice at day 9 pi and the supernatants were measured for virus titer. (D) In separate experiments WT and miR-155KO mice were ocularly infected with 104 PFU HSV-1 Tumpey and brains were harvested and homogenized at days 1,2,3 and 4 pi and supernatants were measured for virus titer. Data are representative of three independent experiments and show mean values ± SEM (n = 4–8mice/group). Statistical levels of significance were analyzed by one-way ANOVA test with Tukeys post test settings. *p ≤ 0.05, **p ≤ 0.005, ***p ≤ 0.001
Our results are consistent with the notion that miR-155KO animals succumb to encephalitis with lesions in the brains likely the direct consequence of viral infection rather than representing the result of an inflammation reaction to infection, as some advocate accounts for encephalitis in WT mice (9).
miR-155 is required for optimal CD8 T cell responses
To investigate whether or not miR-155 influences the nature of HSV-1 specific CD8 T cell responses, miR-155KO and WT mice were infected intradermally in the hind footpads with HSV-1 strain KOS and effector CD8 T cell responses were measured in the draining popliteal lymph nodes (PLN) at day 5 pi when responses are at their peak (27, 28). The results show that the total numbers of HSV gB tetramer specific CD8 T cells per lymph node were significantly reduced (~3 fold) in miR-155KO mice compared to WT control animals (Figure 4A). We also investigated the homing capacity of CD8 T cells in the miR-155KO animals. Analyzing expression of the homing molecules VLA-4 and CD44, we found ~1.5–3 fold reduced expression in the infected miR-155KO animals compared to the WT animals (Figure 4 B and C). Additionally, no differences were evident in the expression of the homing molecule LFA-1 between the infected WT and miR-155KO animals (data not shown). When cell numbers were compared using the intracellular cytokine staining (ICS) assay to detect virus specific IFN-γ producing cells, differences between miR-155KO and WT responses were of even greater magnitude (average of 5 fold) (Figure 4D). As an additional measure of functional responses, numbers of CD8 T cells that produced both IFN-γ and TNF-α or a single cytokine alone were compared in the two groups. This approach revealed that dual cytokine producing CD8 T cells were reduced ~10 fold in miR-155KO compared to WT (Figure 4E), a result we take to indicate that the CD8 virus specific response in miR-155KO mice was functionally impaired. In additional experiments this trend was also seen in the DLN at day 9 after ocular infection with the HSV-1 strain that caused encephalitis in miR-155KO mice (data not shown).
Figure 4. miR-155 KO mice mount weaker CD8 T cell responses compared to WT mice after HSV-1 footpad infection.
Groups of wild type (WT) and miR-155KO (KO) animals were infected in the hind footpads with HSV-1 strain KOS. PLN were collected at day 5 pi and aliquots of single-cell suspensions were stained for cell surface markers CD8 and Kb-gB tetramer (A) Representative FACS plots, frequencies and numbers of tetramer specific CD8 T cells in WT versus miR-155KO mice. To measure Kb-gB tetramer specific CD8 T cell responses aliquots of single cell suspension of PLN’s were stimulated for 6 hours with HSV-1 gB peptide in the presence of brefeldin A. After stimulation the cells were stained for the surface marker CD8 and cytokines IFN-γ and TNF-α. (B) Representative FACS plots, frequencies and numbers depicting IFN-y producing CD8 T cells and (C) IFN-γ and TNF-α double producing CD8 T cells in WT versus miR-155KO mice. The level of significance was determined by a Student t test (unpaired). Error bars represent means ± SEM (n = 3 – 5 mice/group). Experiments were repeated at least three times. *p ≤ 0.05, **p ≤ 0.005, ***p ≤ 0.001.
In separate experiments, WT and miR-155KO mice were infected with another strain of HSV-1 (HSV-1 RE) that did not cause HSE in miR-155KO mice. In such experiments, trigeminal ganglia (TG) were collected 14 days pi and processed either for viral reactivation experiments (described in a subsequent section) or to recover T cells to measure virus specific CD8 T cell responses by using both tetramer and the ICS assay to quantify cytokine producers. The total numbers of gB tetramer specific CD8 T cells were ~2 fold higher in WT compared to miR-155KO mice (Figure 5A). The number of total CD8 T cells that produced IFN-γ in the WT group was ~4 fold higher compared with miR-155KO animals. Additionally, the dual cytokine (IFN-γ and TNF-α)-producing cells were ~4.5 fold more frequent in WT mice as compared with miR-155KO mice (Figure 5B and C).
Figure 5. miR155 KO mice mount impaired CD8+ T cell responses in TG compared with WT mice.

Kb-gB tetramer-specific CD8 T cell responses in TG of HSV-1– ocularly infected mice were compared among age- and gender-matched HSV-1 infected WT and miR155 KO animals on day 14 pi. TGs were excised and individual TGs (n=8) from WT and miR155 KO mice were dispersed into single cell suspensions and stained for cell surface markers CD8 and Kb-gB tetramer (A) Representative numbers of tetramer specific CD8+ T cells in WT versus miR-155KO mice. To measure Kb-gB tetramer specific CD8+ T cell responses single cell suspension of TG’s were stimulated for 6 hours with HSV-1 gb peptide in the presence of brefeldin A. After incubation, the cells were stained for surface marker CD8 and cytokines IFN-γ and TNF-α by ICS and analyzed by flow cytometry. (B) Representative FACS plots, frequencies and numbers depicting IFN-γ producing CD8 T cells and (C) IFN-γ and TNF-α double producing CD8+ T cells in WT versus miR-155KO mice. The level of significance was determined by a Student t test (unpaired). Error bars represent means ± SEM (n = 3–5 mice/group). Experiments were repeated at least three times. *p ≤ 0.05, **p ≤ 0.005, ***p ≤ 0.001
Taken together the above data demonstrate that the absence of miR155 results in diminished CD8 T cell response, which is particularly evident when using assays that measure numbers of functional CD8 T cells.
HSV-immune CD8+ T cells from gBT mice protect miR-155O animals from lethal herpetic encephalitis
To see if the reduced number and function of CD8 T cells is one of the reasons for HSE, we carried out adoptive transfer experiments. Infected miR-155KO mice were given HSV-immune CD8+ T cell transfers from gBT mice at 24h pi, and recipients were monitored clinically over the next 9 days. 80% of the miR-155KO mice succumbed to death by day 9 pi, however 100% of the miR-155KO mice that received HSV-immune CD8 T cells at 24h pi survived (Figure 6A). Animals were subsequently sacrificed at day 9 pi and brains were collected to quantify levels of virus present. High virus levels were detectable in the brain homogenates in all miR-155KO animals showing signs of encephalitis by day 9 pi, although none had detectable virus in the group of animals that received CD8 T cell adoptive transfers (Figure 6B).
Figure 6. Survival in miR-155KO mice after receiving HSV immune CD8 T cells.
miR-155KO (KO) animals were ocularly infected with 1×104 HSV-1 Tumpey and were divided in two groups. One group (n=8) received adoptive transfer of HSV immune CD8+ T cells via tail vein at 24 h pi (A) while other group served as a control. Survival of age matched miR-155ko mice and miR-155 KO animals that received CD8 T cell adoptive transfer was established over 9 days. (B) Brains were harvested from mir-155KO animals and miR-155 KO mice that received CD8 T cell adoptive transfer mice at day 9 pi. Brains were homogenized and centrifuged, and supernatants were tested for virus levels.
Virus reactivation differences between latently infected miR-155KO and WT mice
In additional experiments, WT and miR-155KO mice were infected with a strain of HSV-1 (HSV-1RE) that did not cause HSE in KO mice. In such experiments TG were collected 14 days pi and processed to induce viral reactivation ex vivo (20, 21). In these experiments, multiple TG cultures from individual miR-155KO and WT infected mice were established 14 days pi and aliquots were exposed to different treatments. The culture supernatants were tested daily to detect infectious virus over a 10 day period. Unmanipulated cultures revealed differences in the viral reactivation pattern between miR-155KO and WT TG. Whereas ~15% of WT cultures showed reactivation, ~ 90% of the miR-155KO cultures reactivated (Figure 7). Infectious virus was detectable in the miR-155KO culture supernatants by day 2 post culture but not until day 3 in the WT cultures that reactivated. Although the majority of WT cultures did not reactivate all were judged to be latently infected since the addition of 1mM rGal-9 (a procedure shown previously to cause ex-vivo reactivation (21)) caused virus reactivation in all cultures (Figure 7).
Figure 7. Comparison of ex vivo HSV-1 reactivation between miR155 KO and WT mice.

Individual TGs excised on day 14 pi from HSV-1 (RE) infected WT and miR155KO mice were dispersed into single-cell suspensions and the cultures were established in a 48 well plate. Each TG (n=6) sample from miR155 KO mice was divided into 2 aliquots. One aliquot was left unmanipulated and the other aliquot received 1×105 CD8 T cells isolated at day 8 pi from lymph nodes of HSV-1 infected WT mice. Similarly, each WT TG was divided into 2 aliquots and one aliquot was left unmanipulated whereas, the other aliquot received 1mM rGal-9. TG cultures were incubated in DMEM medium for a 10 day period and samples of culture supernatant were collected at 24-h intervals and assayed for infectious virus by plaque titrations on Vero cells. Bar graph represents the percentage of virus reactivation from various experimental combinations. The experiment has been repeated 3 times.
With the miR-155KO cultures CD8 T cells isolated from the lymph nodes of WT HSV infected mice were added to culture aliquots to determine the effect on virus reactivation. This procedure prevented virus reactivation in all cultures (Figure 7). Accordingly, our results demonstrate that viral reactivation from latency occurs far more readily with cultures from miR-155KO animals than WT and this observation might be attributed at least in part to differences in CD8 T cell function.
Differential susceptibility of miR-155KO and WT mice to intradermal infection with HSV
Animals infected in the scarified skin with HSV develop so called zosteriform skin lesions which as first demonstrated by Nash and colleagues, reflect the consequence of viral entrance into sensory nerve endings followed by viral replication in the dorsal root ganglia and subsequent spread to the dermatome (16). When groups of WT and mir-155KO were infected intra-dermally with identical viral dosage of HSV, the outcome was significantly different in the development of zosteriform lesions. Thus a greater proportion of miR-155KO mice developed lesions compared to WT mice. By day 6 pi, 100 % of the miR-155KO mice had developed lesions compared to only 25 % in the WT mice. In addition, miR-155KO mice exhibited lesions that were far larger in size than in those in WT that developed lesions (Figure 8A). In addition whereas, by day 7 pi, the majority of the miR-155KO mice developed hind limb paralysis all of the WT mice remained free from any neurological signs (Figure 8B). In some experiments, test mice were terminated at day 6 pi and virus levels were assayed in the skin encompassing the inoculation site as well as in the brain. In such experiments, it was only possible to detect virus in the brains and skin isolated from miR-155KO animals (Figure 8C and D). Thus our results demonstrate a marked increase in susceptibility of miR-155KO to HSV infection in a model that reflects spread within the nervous system.
Figure 8. Zosteriform lesions are more severe in miR-155KO mice compared to WT mice and miR-155KO mice show impaired viral clearance from both brain and skin.
A group of WT and miR-155KO mice was infected with 1×106 HSV-1 Tumpey using flank scarification. (A) Mice were examined on day 6 pi for secondary lesion development. (B) Mice were observed for lesion development for up to 7 days pi and scored as following: scores of 1 or 2: local site lesions, characterized by ulceration and swelling around the site of infection; Score of 3: progression of vesicles and then ulcers (Score of 4 or 5) down the flank of the mouse; Score of 6: significant morbidity, characterized by partial immobility (C) Brains were harvested from WT and miR-155 KO mice at day 6 pi. Brains were homogenized and centrifuged, and supernatants were tested for virus titers using plaque assay. (D) The primary site of skin inoculation was examined for viral titers at day 6 pi using plaque assay. The level of significance was determined by a Student t test (unpaired). Error bars represent means ± SEM (n=4–8 mice/group). Experiments were repeated at least three times. *p ≤ 0.05, **p ≤ 0.005, ***p ≤ 0.001
Discussion
Herpes simplex virus infection usually causes lesions at body surface sites but occasionally the virus spreads to the brain inducing life threatening encephalitis (2). We show in this report that mice unable to produce miR-155 may develop HSE following ocular infection with the lesion primarily the direct consequence of virus replication in the CNS. Affected animals could be protected from HSE by acyclovir treatment commenced 4 days after infection and pathological features in the CNS were consistent with direct viral destructive effects. miR-155KO animals were also more susceptible to develop zosteriform lesions, a reflection of viral replication and dissemination within the nervous system. One explanation for the heightened susceptibility to HSE and zosteriform lesions could be because miR-155KO animals develop diminished CD8 T cell responses especially when the numbers of functional effector CD8 T cell responses were compared. Indeed, adoptive transfer of HSV-immune CD8 T cells into infected miR-155KO mice provided protection from HSE. Deficiencies in CD8 T cell numbers, function and homing capacity may also explain the observation that miR-155KO animals were less able than WT animals to maintain latency upon ex-vivo culture. Our observations may be the first to link miR-155 expression with susceptibility of the nervous system to virus infection.
HSE is a rare manifestation of HSV infection and can be a devastating disease especially if not treated promptly (2). Most cases in adult humans are caused by HSV-1 and these usually occur in latently infected persons whose previous clinical consequences of infection were either not observed, or were only mild surface lesions. Little is understood regarding the triggers that cause reactivated virus to traffic to the brain or the pathogenic mechanisms involved at causing the brain damage. Occasional cases of human HSE can occur in children with genetic defects in TLR3 dependent interferon responses (3–5), but in the great majority of HSE cases genetic defects in immune function have not been demonstrated (2). Moreover, even profound immunosuppression, as can occur during AIDS or immunosuppressive therapy, very rarely results in HSE. In HSE in humans, encephalitis appears to be largely the consequence of virus replicating in and destroying cells, an idea supported by the success that can be achieved using antiviral drug therapy (2). However, others advocate that an inflammatory reaction to the brain infection can also contribute or perhaps be mainly responsible for the encephalitis (9). Enthusiasm for the later idea has mainly come from experimental studies in mice where innate immune signaling dependent activation of PMN and macrophages and the production of inflammatory mediators in response to HSV were shown necessary for the development of fulminate lesions of encephalitis (7, 8). Other studies indicate that encephalitis in susceptible mouse strains may represent an immunopathological response since it fails to respond to antiviral therapy but is controllable by procedures that diminish inflammatory cells (9). More than likely, the pathogenesis of HSE involves multiple mechanisms with studies in mice not accurately reflecting the pathogenesis of the natural human disease. We advocate, however that the miR-155KO mice could represent a more appropriate model than other mouse systems to understand the pathogenesis of human HSE and to evaluate novel therapies. Accordingly, the encephalitis in miR-155KO animals appeared to represent primarily the consequences of viral replication events. Thus the disease was readily controllable with antiviral therapy even when this was begun 4 days pi, a time point when HSV was readily detectable in the brains of miR-155KO animals and presumably could be inducing an inflammatory response. Immunohistochemical analysis of brain lesions of miR-155KO animals revealed lesser T cell inflammatory infiltrates in affected areas along with less reactive astrocytosis as compared to WT animals with encephalitis. We interpret this to mean that the nature of lesions in miR-155KO animals are less immunoinflammatory than those in the WT animals.
We suspect that one reason miR-155KO animals readily developed HSE was because of their reduced virus specific T cell responses to infection. Another might relate to the role that miR-155 could play in susceptibility of neural tissue to HSV infection (discussed subsequently). It is well known that the CD8 T cell response plays a critical role in protecting both the CNS and peripheral nervous tissues (PNS) from HSV infection (20, 29, 30). Particularly strong evidence for the protective effects of CD8 T cells in the PNS has come from the Hendricks and Carbone laboratories (20, 23, 31). In addition, our own past studies showed how CD8 T cells are needed to protect the CNS (29). The present observations showed that miR-155KO mice had significantly diminished virus specific CD8 T cell responses, especially when numbers of functionally competent CD8 T cells were compared where differences could be as much as 10 fold. This is consistent with the recent observations made by other groups who noted compromised CD8 T cell responses in miR-155KO animals in response to LCMV and influenza virus infection, as well as in some tumor models (32–35). Additionally, it is conceivable that brain homing capacity of CD8 T cells differed between KO and WT animals. In support of this we could show that KO CD8 T cells showed diminished levels of VLA-4 and CD44 both shown in other systems to influence brain homing of T cells (36, 37). We suspect that the diminished protective CD8 T cell response permitted virus to traffic effectively to the brain and PNS and that once there fewer protective CD8 T cells were around to abort infection. This is consistent with the previous reports showing that CD8 deficient animals failed to control HSV in the brain and developed encephalitis (30). This argument was also supported by the adoptive transfer experiments where HSV immune CD8 T cells adoptively transferred to miR-155KO mice were shown to be fully protective. However further experiments are needed to clarify if the apparent defect in miR-155KO CD8 T cells is a problem with priming, effector cytokine production, homing defects or additional events such as the numbers of cells that can access the nervous system. Furthermore although we favor the idea that differences in CD8 T cell activity accounted for the difference in outcome in miR-155KO and WT mice other explanations merit exploration such as differences in NK cell homeostasis or levels of interferon induced which have both been implicated as providing protection in herpetic encephalitis (7, 38–40).
A diminished protective CD8 response in miR-155KO animals was also demonstrated using two models that reflect the activity of CD8 T cells. First in a food pad infection model we could show that miR-155KO animals generated lesser numbers of HSV specific CD8 T cells than WT animals in draining lymph nodes which was especially evident when IFN-γ producing cell responses were compared. CD8 T cells are required to contain HSV replication in ganglia and they orchestrate this response largely by IFN-γ production and the release of granzyme B in HSV infected neurons (20, 41, 42). In studies reported herein, we could show that ganglionic virus specific CD8 T cells were diminished and less polycytokine producers in miR-155KO animals compared to WT which might account for their more rapid and abundant reactivation.
In addition to encephalitis we also observed that miR-155KO mice were more susceptible than the WT animals to develop zosteriform lesions, an event that requires dissemination of virus within the nervous system (16). Accordingly, with doses of virus that produced barely noticeable lesions in WT, almost all miR-155KO animals developed overt lesions and many had to be killed because of hind limb paralysis. The miR-155KO animals failed to control HSV and virus was easily detectable in the brains of miR-155KO animals, but could not be demonstrated in the brains of WT animals.
Currently it is not clear how miR-155 influences the magnitude and functionality of CD8 T cell responses, but there are several possibilities. Firstly it might result from the fact that miR-155KO mice also generate impaired helper T cell responses (12, 13), and optimum CD8 T cell responses are known to require signals from CD4 helper T cells (43, 44). It is also conceivable that miR-155 plays a direct role during CD8 T cell differentiation. Thus some have observed that in the absence of miR-155 type 1 interferon driven proliferative responses of CD8 T cell are defective (33, 34) while others suggest that CD8 T cells survive less well and show defective responses to PI3K/AKT signaling (34). It has also been suggested that in the absence of miR-155, SOCS1 is upregulated which expresses suppressive effects on T cell function (32). Further studies are clearly needed to clarify how miR-155 expression influences the CD8 T cell response.
Our results also raise the issue as to whether miR-155 expression somehow influences the dissemination of HSV to and replication within the nervous system. Thus miRNAs could influence expression of proteins involved in axon transport but this point has not been investigated to our knowledge. Alternatively miRNAs could influence the infectivity and replication efficiency in target cells within the nervous system. It is known for example that miR-155 regulates microglia immune responses by targeting SOCS-1 and promoting cytokine and nitric oxide production (45, 46). So it is conceivable that the glial cells in miR-155KO mice could be defective in cytokine and nitric oxide production, a possibility we are currently investigating. We are also investigating if different cell types taken from miR-155KO and WT mice show differential susceptibility to HSV replication events.
In conclusion our report makes the novel observation that deficiency of a single species of miRNA can result in enhanced susceptibility of the nervous system to a virus infection. Our observations lead us to wonder if miRNA defects could be involved in some cases of human HSE. Moreover, it is also curious to note that glucocorticoids which are upregulated during stressful situations that cause herpes reactivation may selectively inhibit miR-155 expression (10, 47). Thus the relationship of miR-155 expression to changing events in HSV pathogenesis merits further investigation.
Acknowledgments
We thank Ujjaldeep Jaggi, Pranay Dogra, Sujata Agarwal, and Nancy Nielsen for assistance during research and manuscript preparation. We also thank H. Penny McWilliams-Koeppen in helping us with immunohistochemistry staining.
This work was supported by National Institutes of Health Grant EY 005093.
Abbreviations
- HSV
Herpes simplex virus
- HSE
Herpes simplex encephalitis
- miR-155KO
microRNA-155 knockout
- TG
Trigeminal ganglia
- WT
Wild type
- SK
Stromal keratitis
- DLN
Draining lymph node
- PLN
Popliteal lymph node
- PMN
Polymorphonuclear leukocytes
- rGal-9
Recombinant Galectin-9
References
- 1.Roizman B, Knipe DM, Whitley R. Herpes Simplex Viruses. In: Knipe DM, Howley PM, et al., editors. Fields Virology. 5. Lippincott, Williams & Wilkins; Philadelphia, PA: 2007. pp. 2501–601. [Google Scholar]
- 2.Whitley RJ. Herpes simplex encephalitis: adolescents and adults. Antiviral Res. 2006;71:141–148. doi: 10.1016/j.antiviral.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Zhang SY, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, Picard C, Chapgier A, Plancoulaine S, Titeux M, Cognet C, von Bernuth H, Ku CL, Casrouge A, Zhang XX, Barreiro L, Leonard J, Hamilton C, Lebon P, Héron B, Vallée L, Quintana-Murci L, Hovnanian A, Rozenberg F, Vivier E, Geissmann F, Tardieu M, Abel L, Casanova JL. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- 4.Guo Y, Audry M, Ciancanelli M, Alsina L, Azevedo J, Herman M, Anguiano E, Sancho-Shimizu V, Lorenzo L, Pauwels E, Philippe PB, Pérez de Diego R, Cardon A, Vogt G, Picard C, Andrianirina ZZ, Rozenberg F, Lebon P, Plancoulaine S, Tardieu M, Doireau Valérie, Jouanguy E, Chaussabel D, Geissmann F, Abel L, Casanova JL, Zhang SY. Herpes simplex virus encephalitis in a patient with complete TLR3 deficiency: TLR3 is otherwise redundant in protective immunity. J Exp Med. 2011;208:2083–2098. doi: 10.1084/jem.20101568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang SY, Herman M, Ciancanelli MJ, Pérez de Diego R, Sancho-Shimizu V, Abel L, Casanova JL. TLR3 immunity to infection in mice and humans. Curr Opin Immunol. 2013;25:19–33. doi: 10.1016/j.coi.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitley RJ. Herpes simplex viruses. In: Fields BN, KDM, Howley PM, editors. Fields virology. Lippenscott-Raven; Philadelphia, Pa: 1990. pp. 1843–1845. [Google Scholar]
- 7.Wang JP, Bowen GN, Zhou S, Cerny A, Zacharia A, Knipe DM, Finberg RW, Kurt-Jones EA. Role of specific innate immune responses in herpes simplex virus infection of the central nervous system. J Virol. 2012;86:2273–2281. doi: 10.1128/JVI.06010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurt-Jones EA, Chan M, Zhou S, Wang J, Reed G, Bronson R, Arnold MM, Knipe DM, Finberg RW. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci U S A. 2004;101:1315–1320. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lundberg P, Ramakrishna C, Brown J, Tyszka JM, Hamamura M, Hinton DR, Kovats S, Nalcioglu O, Weinberg K, Openshaw H, Cantin EM. The immune response to herpes simplex virus type 1 infection in susceptible mice is a major cause of central nervous system pathology resulting in fatal encephalitis. J Virol. 2008;82:7078–7088. doi: 10.1128/JVI.00619-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulik S, Bhela S, Rouse BT. Potential function of miRNAs in herpetic stromal keratitis. Invest Ophthalmol Vis Sci. 2013;54:563–573. doi: 10.1167/iovs.12-11094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Connell RM, Rao DS, Baltimore D. microRNA regulation of inflammatory responses. Annu Rev Immunol. 2012;30:295–312. doi: 10.1146/annurev-immunol-020711-075013. [DOI] [PubMed] [Google Scholar]
- 12.O’Connell RM, Kahn D, Gibson WS, Round JL, Scholz RL, Chaudhuri AA, Kahn ME, Rao DS, Baltimore D. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity. 2010;33:607–619. doi: 10.1016/j.immuni.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murugaiyan G, Beynon V, Mittal A, Joller N, Weiner HL. Silencing microRNA-155 ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2011;187:2213–2221. doi: 10.4049/jimmunol.1003952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurowska-Stolarska M, Alivernini S, Ballantine LE, Asquith DL, Millar NL, Gilchrist DS, Reilly J, Ierna M, Fraser AR, Stolarski B, McSharry C, Hueber AJ, Baxter D, Hunter J, Gay S, Liew FY, McInnes IB. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc Natl Acad Sci U S A. 2011;108:11193–11198. doi: 10.1073/pnas.1019536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blüml S, Bonelli M, Niederreiter B, Puchner A, Mayr G, Hayer S, Koenders MI, van den Berg WB, Smolen J, Redlich K. Essential role of microRNA-155 in the pathogenesis of autoimmune arthritis in mice. Arthritis Rheum. 2011;63:1281–1288. doi: 10.1002/art.30281. [DOI] [PubMed] [Google Scholar]
- 16.Simmons A, Nash AA. Zosteriform spread of herpes simplex virus as a model of recrudescence and its use to investigate the role of immune cells in prevention of recurrent disease. J Virol. 1984;52:816–821. doi: 10.1128/jvi.52.3.816-821.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osmand AP, Berthelier V, Wetzel R. Imaging polyglutamine deposits in brain tissue. Methods Enzymol. 2006;412:106–122. doi: 10.1016/S0076-6879(06)12008-X. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman GE, Le WW, Sita LV. The importance of titrating antibodies for immunocytochemical methods. Curr Protoc Neurosci. 2008;Chapter 2(Unit 2.12) doi: 10.1002/0471142301.ns0212s45. [DOI] [PubMed] [Google Scholar]
- 19.Llewellyn-Smith IJ, MJ, Pilowsky PM. Methods in Neurosciences. Vol. 8. Academic Press; San Diego: 1992. Retrograde tracing with cholera toxin B-gold or with immunocytochemically detected cholera toxin B in central nervous system; pp. 180–201. [Google Scholar]
- 20.Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med. 2000;191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy PB, Sehrawat S, Suryawanshi A, Rajasagi NK, Mulik S, Hirashima M, Rouse BT. Influence of galectin-9/Tim-3 interaction on herpes simplex virus-1 latency. J Immunol. 2011;187:5745–5755. doi: 10.4049/jimmunol.1102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sehrawat S, Reddy PB, Rajasagi N, Suryawanshi A, Hirashima M, Rouse BT. Galectin-9/TIM-3 interaction regulates virus-specific primary and memory CD8 T cell response. PLoS Pathog. 2010;6:e1000882. doi: 10.1371/journal.ppat.1000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Lint A, Ayers M, Brooks AG, Coles RM, Heath WR, Carbone FR. Herpes simplex virus-specific CD8+ T cells can clear established lytic infections from skin and nerves and can partially limit the early spread of virus after cutaneous inoculation. J Immunol. 2004;172:392–397. doi: 10.4049/jimmunol.172.1.392. [DOI] [PubMed] [Google Scholar]
- 24.Mulik S, Xu J, Reddy PB, Rajasagi NK, Gimenez F, Sharma S, Lu PY, Rouse BT. Role of miR-132 in angiogenesis after ocular infection with herpes simplex virus. Am J Pathol. 2012;181:525–534. doi: 10.1016/j.ajpath.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez C. Genetics of natural resistance to herpesvirus infections in mice. Nature. 1975;258:152–153. doi: 10.1038/258152a0. [DOI] [PubMed] [Google Scholar]
- 26.Biswas PS, Rouse BT. Early events in HSV keratitis--setting the stage for a blinding disease. Microbes Infect. 2005;7:799–810. doi: 10.1016/j.micinf.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Coles RM, Mueller SN, Heath WR, Carbone FR, Brooks AG. Progression of armed CTL from draining lymph node to spleen shortly after localized infection with herpes simplex virus 1. J Immunol. 2002;168:834–838. doi: 10.4049/jimmunol.168.2.834. [DOI] [PubMed] [Google Scholar]
- 28.Pfizenmaier K, Jung H, Starzinski-Powitz A, Röllinghoff M, Wagner H. The role of T cells in anti-herpes simplex virus immunity. I. Induction of antigen-specific cytotoxic T lymphocytes. J Immunol. 1977;119:939–944. [PubMed] [Google Scholar]
- 29.Banerjee K, Biswas PS, Kumaraguru U, Schoenberger SP, Rouse BT. Protective and pathological roles of virus-specific and bystander CD8+ T cells in herpetic stromal keratitis. J Immunol. 2004;173:7575–7583. doi: 10.4049/jimmunol.173.12.7575. [DOI] [PubMed] [Google Scholar]
- 30.Lang A, Nikolich-Zugich J. Development and migration of protective CD8+ T cells into the nervous system following ocular herpes simplex virus-1 infection. J Immunol. 2005;174:2919–2925. doi: 10.4049/jimmunol.174.5.2919. [DOI] [PubMed] [Google Scholar]
- 31.Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, Carbone FR, Mueller SN. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477:216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 32.Dudda JC, Salaun B, Ji Y, Palmer DC, Monnot GC, Merck E, Boudousquie C, Utzschneider DT, Escobar TM, Perret R, Muljo SA, Hebeisen M, Rufer N, Zehn D, Donda A, Restifo NP, Held W, Gattinoni L, Romero P. MicroRNA-155 is required for effector CD8+ T cell responses to virus infection and cancer. Immunity. 2013;38:742–753. doi: 10.1016/j.immuni.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gracias DT, Stelekati E, Hope JL, Boesteanu AC, Doering TA, Norton J, Mueller YM, Fraietta JA, Wherry EJ, Turner M, Katsikis PD. The microRNA miR-155 controls CD8(+) T cell responses by regulating interferon signaling. Nat Immunol. 2013;14:593–602. doi: 10.1038/ni.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lind EF, Elford AR, Ohashi PS. Micro-RNA 155 is required for optimal CD8+ T cell responses to acute viral and intracellular bacterial challenges. J Immunol. 2013;190:1210–1216. doi: 10.4049/jimmunol.1202700. [DOI] [PubMed] [Google Scholar]
- 35.Huffaker TB, Hu R, Runtsch MC, Bake E, Chen X, Zhao J, Round JL, Baltimore D, O’Connell RM. Epistasis between microRNAs 155 and 146a during T cell-mediated antitumor immunity. Cell Rep. 2012;2:1697–1709. doi: 10.1016/j.celrep.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brocke S, Piercy C, Steinman L, Weissman IL, Veromaa T. Antibodies to CD44 and integrin alpha4, but not L-selectin, prevent central nervous system inflammation and experimental encephalomyelitis by blocking secondary leukocyte recruitment. Proc Natl Acad Sci U S A. 1999;96:6896–6901. doi: 10.1073/pnas.96.12.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irani DN, Griffin DE. Regulation of lymphocyte homing into the brain during viral encephalitis at various stages of infection. J Immunol. 1996;156:3850–3857. [PubMed] [Google Scholar]
- 38.Adler H, Beland JL, Del-Pan NC, Kobzik L, Sobel RA, Rimm IJ. In the absence of T cells, natural killer cells protect from mortality due to HSV-1 encephalitis. J Neuroimmunol. 1999;93:208–213. doi: 10.1016/s0165-5728(98)00236-7. [DOI] [PubMed] [Google Scholar]
- 39.Wang P, Hou J, Lin L, Wang C, Liu X, Li D, Ma F, Wang Z, Cao X. Inducible microRNA-155 feedback promotes type I IFN signaling in antiviral innate immunity by targeting suppressor of cytokine signaling 1. J Immunol. 2010;185:6226–6233. doi: 10.4049/jimmunol.1000491. [DOI] [PubMed] [Google Scholar]
- 40.Zawislak CL, Beaulieu AM, Loeb GB, Karo J, Canner D, Bezman NA, Lanier LL, Rudensky AY, Sun JC. Stage-specific regulation of natural killer cell homeostasis and response against viral infection by microRNA-155. Proc Natl Acad Sci U S A. 2013;110:6967–6972. doi: 10.1073/pnas.1304410110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008;322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Decman V, Kinchington PR, Harvey SA, Hendricks RL. Gamma interferon can block herpes simplex virus type 1 reactivation from latency, even in the presence of late gene expression. J Virol. 2005;79:10339–10347. doi: 10.1128/JVI.79.16.10339-10347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajasagi NK, Kassim SH, Kollias CM, Zhao X, Chervenak R, Jennings SR. CD4+ T cells are required for the priming of CD8+ T cells following infection with herpes simplex virus type 1. J Virol. 2009;83:5256–5268. doi: 10.1128/JVI.01997-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cardoso AL, Guedes JR, Pereira de Almeida L, Pedroso de Lima MC. miR-155 modulates microglia-mediated immune response by down-regulating SOCS-1 and promoting cytokine and nitric oxide production. Immunology. 2012;135:73–88. doi: 10.1111/j.1365-2567.2011.03514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ponomarev ED, Veremeyko T, Weiner HL. MicroRNAs are universal regulators of differentiation, activation, and polarization of microglia and macrophages in normal and diseased CNS. Glia. 2013;61:91–103. doi: 10.1002/glia.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng Y, Xiong S, Jiang P, Liu R, Liu X, Qian J, Zheng X, Chu Y. Glucocorticoids inhibit lipopolysaccharide-mediated inflammatory response by downregulating microRNA-155: a novel anti-inflammation mechanism. Free Radic Biol Med. 2012;52:1307–1317. doi: 10.1016/j.freeradbiomed.2012.01.031. [DOI] [PubMed] [Google Scholar]