Abstract
Peroxisomes are often dismissed as the cellular hoi polloi, relegated to cleaning up reactive oxygen chemical debris discarded by other organelles. However, their functions extend far beyond hydrogen peroxide metabolism. Peroxisomes are intimately associated with lipid droplets and mitochondria, and their ability to carry out fatty acid oxidation and lipid synthesis, especially the production of ether lipids, may be critical for generating cellular signals required for normal physiology. Here we review the biology of peroxisomes and their potential relevance to human disorders including cancer, obesity-related diabetes, and degenerative neurologic disease.
Introduction
Peroxisomes are multifunctional organelles present in virtually all eukaryotic cells. In addition to being ubiquitous, they are also highly plastic, responding rapidly to cellular or environmental cues by modifying their size, number, morphology, and function (Schrader et al., 2013). Early ultrastructural studies of kidney and liver cells revealed cytoplasmic particles enclosed by a single membrane containing granular matrix and a crystalline core (Rhodin, 1958). These particles were linked with the term “peroxisome” by Christian de Duve, who first identified the organelle in mammalian cells when enzymes such as oxidases and catalases involved in hydrogen peroxide metabolism co-sedimented in equilibrium density gradients (De Duve and Baudhuin, 1966). Based on these studies, it was originally thought that the primary function of these organelles was the metabolism of hydrogen peroxide. Characterization of peroxisomes (also called microbodies in the early literature) was greatly facilitated by the development of a cytochemical staining procedure using 3,3’-diaminobenzadine (DAB), which permits visualization of these organelles based on the peroxidative activity of catalase at alkaline pH (Fahimi, 1969; Novikoff and Goldfischer, 1969). Using this staining method, Novikoff and colleagues observed a large number of peroxisomes in tissues active in lipid metabolism such as liver, brain, intestinal mucosa, and adipose tissue (Novikoff and Novikoff, 1982; Novikoff et al., 1980). Peroxisomes in different tissues vary greatly in shape and size, ranging from 0.1-0.5 μM in diameter. In adipocytes, peroxisomes tend to be small in size and localized in the vicinity of lipid droplets. Notably, a striking increase in the number of peroxisomes was observed during differentiation of adipogenic cells in culture (Novikoff and Novikoff, 1982). These findings suggest that peroxisomes may be involved in lipid metabolism.
Beevers and colleagues implicated peroxisomes in lipid metabolism by demonstrating that enzymes involved in fatty acid oxidation are co-localized in plant peroxisome-like organelles called glyoxysomes, which are capable of converting fatty acids to metabolic intermediates for carbohydrate synthesis (Cooper and Beevers, 1969). Based on the finding that the fibrate class of hypolipidemic drugs promotes peroxisome proliferation, Lazarow and de Duve hypothesized that peroxisomes in animal cells were capable of carrying out fatty acid oxidation. This was confirmed when they showed that purified rat liver peroxisomes contained fatty acid oxidation activity that was robustly increased by treatment of animals with clofibrate (Lazarow and De Duve, 1976). In a series of experiments, Hajra and colleagues discovered that peroxisomes were also capable of lipid synthesis (Hajra and Das, 1996). Over the past three decades, multiple lines of evidence have solidified the concept that peroxisomes play fundamentally important roles in lipid metabolism. In addition to removal of reactive oxygen species, metabolic functions of peroxisomes in mammalian cells include β-oxidation of very long chain fatty acids, α-oxidation of branched chain fatty acids, and synthesis of ether-linked phospholipids as well as bile acids (Figure 1). β-oxidation also occurs in mitochondria, but peroxisomal β-oxidation involves distinctive substrates and complements mitochondrial function; the processes of α-oxidation and ether lipid synthesis are unique to peroxisomes and important for metabolic homeostasis.
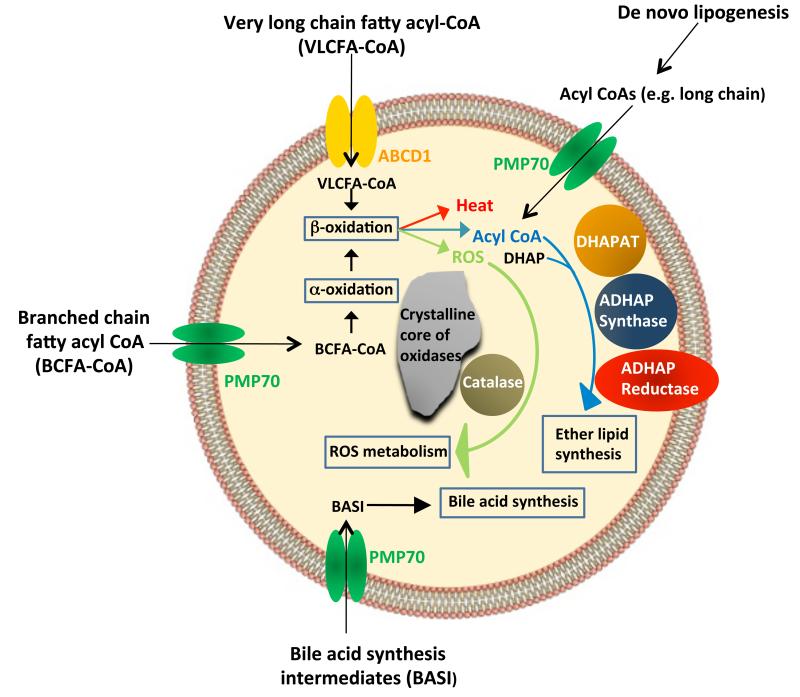
Figure 1. Structure and functions of peroxisomes.
The peroxisome is a single membrane-enclosed organelle that plays an important role in metabolism. The main metabolic functions of peroxisomes in mammalian cells include β-oxidation of very long chain fatty acids, α-oxidation of branched chain fatty acids, synthesis of bile acids and ether-linked phospholipids and removal of reactive oxygen species. Peroxisomes in many, but not all, cell types contain a dense crystalline core of oxidative enzymes.
Here we highlight the established role of peroxisomes in lipid metabolism and their emerging role in cellular signaling relevant to metabolism. We describe the origin of peroxisomes and factors involved in their assembly, division, and function. We address the interaction of peroxisomes with lipid droplets and implications of this interaction for lipid metabolism. We consider fatty acid oxidation and lipid synthesis in peroxisomes and their importance in brown and white adipose tissue (sites relevant to lipid oxidation and synthesis) and disease pathogenesis. Finally, we review what is known about the mechanisms underlying human peroxisomal disorders.
Peroxisomal biogenesis
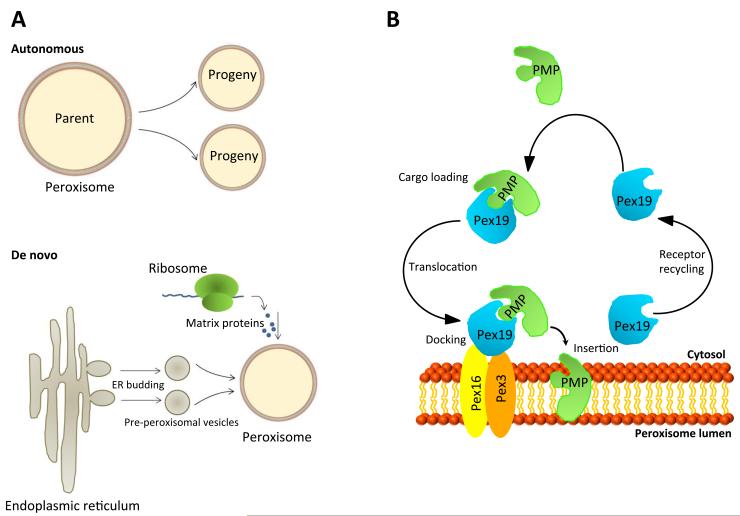
Despite two decades of research on the process, the origin of peroxisomes remains controversial (Dimitrov et al., 2013). In principle, organelles can be derived from 1) growth and fission of pre-existing organelles, 2) templated assembly using an existing copy of the organelle, or 3) de novo generation (Lowe and Barr, 2007). For peroxisomes, two alternative theories of biogenesis have been proposed (Figure 2A). According to the classical model of peroxisome biogenesis, peroxisomes are autonomous organelles that arise from pre-existing peroxisomes through growth and division (Lazarow and Fujiki, 1985). This model (Figure 2A, top) was supported by the observation that some peroxisomes in histologic sections of liver are visualized as dumbbell-shaped interconnected structures. Such structures were more abundant following induction of peroxisome proliferation by pharmacological means or by partial hepatectomy (Legg and Wood, 1970; Reddy and Svoboda, 1971; Rigatuso et al., 1970). This model was generally accepted for over 20 years. However, subsequent studies suggested that peroxisomes could arise de novo. In yeast cells lacking detectable peroxisomes due to a single gene mutation, reintroduction of the wild-type version of the gene complements the defect and restores peroxisome formation (Baerends et al., 1996; Hohfeld et al., 1991; Wiemer et al., 1996), consistent with the idea that peroxisomes may not be exclusively autonomous.
Figure 2.
A. Potential pathways to peroxisomal biogenesis. Peroxisomes are generated autonomously through division of pre-existing organelles (top) or through a de novo process involving budding from the ER followed by import of matrix proteins (bottom). B. Peroxisomal membrane protein import. Peroxisomal membrane proteins (PMPs) are imported post-translationally to the peroxisomal membrane. Pex19 is a soluble chaperone that binds to PMPs and transports them to the peroxisomal membrane, where it docks with a complex containing Pex16 and Pex3. Following insertion of the PMP, Pex19 is recycled back to the cytosol.
An alternative model (Figure 2A, bottom) holds that peroxisomes are semiautonomous organelles deriving their matrix proteins from the cytosol and their membrane proteins and lipids from the endoplasmic reticulum (ER) (Tabak et al., 2013). A potential ER origin for peroxisomes is supported by classic morphology studies showing peroxisomes as clusters surrounded by smooth ER in absorptive cells of guinea pig intestine (Novikoff and Novikoff, 1972). Numerous contacts between peroxisomes and smooth ER were observed, suggesting that peroxisomes arise from dilated regions of ER. These regions were thought to contain high concentrations of peroxisomal proteins, such as catalase (Novikoff and Novikoff, 1972; Reddy and Svoboda, 1971). Although the existence of direct contact and luminal continuity between peroxisomes and ER has been challenged (Lazarow and Fujiki, 1985), recent studies suggest that pre-peroxisomal vesicles, precursors of peroxisomes, bud off of the ER, thus obviating the need for luminal continuity to allow transport of peroxisomal contents (van der Zand et al., 2012). Using a bi-molecular fluorescence complementation assay, these authors monitored peroxisomal assembly in real time. This assay is based on reconstitution of a functional fluorescent protein mediated by interactions between two peptides fused to two different non-fluorescent halves of the fluorescent protein (Kerppola, 2008). van der Zand and colleagues used this technology to show that two distinct pre-peroxisomal vesicles, each carrying half of a peroxisomal translocon complex, undergo heterotypic fusion, forming a functional translocon that permits uptake of matrix proteins from the cytosol (van der Zand et al., 2012).
Factors involved in peroxisomal biogenesis
Regardless of their origin, peroxisomes require a group of proteins called peroxins for their assembly, division, and inheritance. The budding yeast Saccharomyces cerevisae is a particularly apt model for studying peroxisomal biogenesis due to its ease of genetic manipulation and its robust peroxisomal proliferation induced by oleate-containing medium (Subramani, 1998). Over 30 peroxins, encoded by Pex genes, have been identified in yeast (Dimitrov et al., 2013). At least a dozen of these proteins are conserved in mammals, where they regulate various aspects of peroxisomal biogenesis, including factors that control assembly of the peroxisomal membrane, factors that interact with peroxisomal targeting sequences allowing proteins to be shuttled to peroxisomes, and factors that act as docking receptors for peroxisomal proteins.
At least three peroxins (Pex3, Pex16 and Pex19) appear to be critical for assembly of the peroxisomal membrane and import of peroxisomal membrane proteins (PMPs) (Figure 2B). Pex19 is a soluble chaperone and import receptor for newly synthesized PMPs (Jones et al., 2004). Pex3 buds from the ER in a pre-peroxisomal vesicle and functions as a docking receptor for Pex19 (Fang et al., 2004). Pex16 acts as a docking site on the peroxisomal membrane for recruitment of Pex3 (Matsuzaki and Fujiki, 2008). Peroxisomal matrix proteins are translated on free ribosomes in the cytoplasm prior to their import. These proteins have specific peroxisomal targeting sequences (PTS) located either at the carboxyl (PTS1) or amino (PTS2) terminus (Gould et al., 1987; Swinkels et al., 1991). Recently, cryptic PTS1 motifs were identified in several glycolytic enzymes, such as glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and 3-phosphoglycerate kinase (PGK), in various fungal species. These motifs are activated by post-translational processes and may direct bona fide cytoplasmic proteins to peroxisomes under certain metabolic conditions (Freitag et al., 2012). The import receptor for PTS1-containing peroxisomal matrix proteins is Pex5 (Dodt and Gould, 1996), whereas Pex7 functions as the import receptor for PTS2-containing proteins (Braverman et al., 1997). These receptors bind cargo in the cytoplasm and transport it to docking sites at the peroxisomal membrane. Once their cargo is delivered to the peroxisomal lumen, the receptors recycle back to the cytoplasm (Liu et al., 2012b). The recycling of Pex5 requires ubiquitination (Platta et al., 2007).
Peroxisome proliferator activated receptors (PPARs) in peroxisome biogenesis and function
PPARs are relevant to peroxisomal lipid oxidation and synthesis and how these processes impact disease pathogenesis. Fibrates, used to lower lipids in humans, and certain xenobiotics promote proliferation of peroxisomes in cells presumably due to de novo biogenesis. This property, as well as the capacity of these compounds to modulate gene expression of the peroxisomal fatty acid oxidation machinery (Reddy et al., 1986), led to the hypothesis that these chemicals activate a nuclear receptor by a mechanism analogous to that used by steroid hormones (Issemann and Green, 1990). Issemann and Green used reporter assays to demonstrate that peroxisome proliferating chemicals activated a PPAR (now referred to as PPARα) (Issemann and Green, 1990). Subsequently, two related nuclear receptors, PPARγ and PPARδ were cloned (Dreyer et al., 1992; Kliewer et al., 1994).
PPARs are recognized as fundamentally important regulators of lipid metabolism (Wang, 2010). They act as ligand-activated transcription factors that form obligate heterodimers with retinoid X receptors (RXR) and bind to specific DNA sequences known as PPAR response elements (PPREs) located in promoter regions of target genes. In the absence of ligand, PPARs are associated with co-repressors. Ligand binding promotes a conformation change in PPARs, resulting in replacement of repressors with co-activators and subsequent activation of target gene expression. The three members of the PPAR family manifest distinct tissue distribution patterns and target gene expression profiles. PPARα is the target of fibrate drugs used to treat lipid disorders in humans. It is highly enriched in liver and brown adipose tissue (BAT), where it is a key regulator of fatty acid oxidation. PPARδ has significant functional overlap with PPARα, but is more widely expressed. PPARγ is the target of glitazone drugs used to treat diabetes in humans. It is enriched in adipose tissue, and is absolutely required for adipogenesis (Ahmadian et al., 2013; Tontonoz and Spiegelman, 2008).
PPARα is known to be important for peroxisome proliferation. PPARα activation increases not only the expression of fatty acid oxidation genes, but also the abundance of peroxisomes in liver (Schrader et al., 2012). Its activation induces expression of the peroxin Pex11 (Cornwell et al., 2004), which may be involved in peroxisomal biogenesis by promoting peroxisome division (Li and Gould, 2002) as depicted in Figure 2A. Less is known about how PPARδ and PPARγ affect proliferation. Recently, high fat feeding of mice was shown to increase PPARγ activation and peroxisome proliferation in the hypothalamus. Increased peroxisome abundance was thought to decrease levels of neuronal free radicals, which modulate feeding behavior through effects on the melanocortin system (Diano et al., 2011). PPARγ activation may be involved in cold-induced proliferation of peroxisomes in BAT (Guardiola-Diaz et al., 1999). The transcriptional co-activator PGC1α, critical for thermogenesis (Puigserver et al., 1998), promotes cold-induced peroxisome biogenesis in BAT in a PPARα independent manner, implicating PPARγ or PPARδ in this effect (Bagattin et al., 2010). Our preliminary studies indicate that treatment of 3T3-L1 preadipocytes with rosiglitazone substantially increases the expression of several Pex genes, suggesting that PPARγ promotes peroxisomal biogenesis in adipocytes (Lodhi and Semenkovich, unpublished). Collectively, these findings suggest that PPARs coordinate both peroxisomal biogenesis and integrative lipid metabolism.
Crosstalk between peroxisomes and lipid droplets
Intracellular lipid storage provides protection against toxic effects of excessive lipid concentrations, a source of energy for cellular growth and metabolic processes, and a survival advantage during starvation. Lipid droplets are omnipresent subcellular organelles that store lipids. Their structure resembles that of circulating lipoproteins in mammals, consisting of a phospholipid monolayer surrounding a core of neutral lipids such as triglycerides and cholesteryl esters. Although most prominent in adipocytes, which store excess energy as triglyceride, lipid droplets are present in most cell types. Lipid droplets vary greatly in size, ranging from less than 1 μm to ~100 μm in diameter. Mature white adipocytes have large unilocular lipid droplets that occupy the majority of the cytosol. Brown adipocytes have small multilocular lipid droplets. In most other cell types, lipid droplets are less than 1 μm in diameter (Reue, 2011). Like peroxisomes, lipid droplets are thought to be derived from the ER. According to the prevailing model of lipid droplet biogenesis, neutral lipids are incorporated into the interspace of the bilayer leaflets of the ER membrane, where several enzymes involved in triglyceride synthesis are localized. Once a certain threshold of triglyceride accumulation is reached, the outer leaflet of the ER membrane probably bulges into the cytosol through a budding process, forming a new lipid droplet that is released from the ER (Farese and Walther, 2009). Cytosolic lipid droplets increase their volume through localized de novo lipogenesis, transport of extracellular lipids into lipid droplets, or fusion with other lipid droplets.
Several lines of evidence suggest that peroxisomes are closely associated with lipid droplets. In yeast grown in oleate, peroxisomes stably adhere to lipid droplets (Binns et al., 2006). This interaction appears to be more than a simple juxtaposition; peroxisomes extend processes, referred to as pexopodia, into the lipid droplet core. Proteomic analysis of these cells shows that lipid droplets are enriched in peroxisomal β-oxidation enzymes (Binns et al., 2006). Whether peroxisomes and lipid droplets intermingle in animal cells is unknown. However, several studies have reported close associations between peroxisomes and lipid droplets in various mammalian cells. Live cell imaging in Cos-7 and HepG2 cells expressing GFP-PTS1 revealed tubulo-reticular clusters of peroxisomes in close association with lipid droplets (Schrader, 2001). In 3T3-L1 adipocytes and mouse epididymal white adipose tissue, immunogold staining with an antibody against catalase showed numerous small dumbbell-shaped peroxisomes at the periphery of lipid droplets (Blanchette-Mackie et al., 1995), confirming the observation of Novikoff and colleagues using DAB staining (Novikoff and Novikoff, 1982; Novikoff et al., 1980). Recently, several protein-protein interactions involving lipid droplet resident proteins and peroxisomal markers were identified using a bi-molecular fluorescence complementation assay (Pu et al., 2011).
Because peroxisomes and lipid droplets each control lipid metabolic flux, the close interaction between the two organelles suggests a coordinated regulation of metabolism and lipid trafficking across their boundaries. When an organism requires energy, very long chain fatty acids stored as triglycerides in lipid droplets can be mobilized and rapidly transported to peroxisomes for initiation of fatty acid oxidation. However, it is unlikely that lipid trafficking between droplets and peroxisomes is unidirectional. As discussed below, peroxisomes are important in the synthesis of alkyl ether equivalents of acylglycerols. These glycerides may be differentially compartmentalized as compared to those generated by acylation of glycerol-3-phosphate in the ER. Mass spectrometry-based lipidomic analyses show that up to 20% of neutral lipids in lipid droplets are present as an ether lipid equivalent of triglyceride, monoalk(en)yl diacylglycerol (MADAG). MADAG is undetectable in lipid droplets from cells lacking peroxisomes (Bartz et al., 2007). These studies support the existence of bidirectional lipid trafficking between peroxisomes and lipid droplets, constituting crosstalk between the two subcellular compartments to balance energy synthesis and utilization depending on metabolic context.
Peroxisomal fatty acid oxidation
Peroxisomes in all eukaryotic organisms are thought to have the ability to oxidize fatty acids. In plants and most fungi, peroxisomes are the only site of fatty acid oxidation (Poirier et al., 2006). In animal cells, fatty acid oxidation takes place in both mitochondria and peroxisomes. It is becoming increasingly clear that peroxisomes have a role in fatty acid oxidation that cannot be replaced by mitochondria. Peroxisomes in animal cells were originally thought to play only an ancillary role in fatty acid oxidation, participating in oxidation only when the mitochondrial oxidative capacity was exceeded. This notion was challenged by the observation that patients with certain peroxisomal disorders (discussed below) have increased serum levels of very long chain (>22 carbon atoms) and branched chain fatty acids (Brown et al., 1982; Poulos et al., 1986). Subcellular fractionation studies with liver tissue confirmed that β-oxidation of very long chain fatty acids preferentially occurs in peroxisomes as compared to mitochondria (Singh et al., 1984). The accumulation of branched chain fatty acids in patients with peroxisomal disorders suggested a requirement for peroxisomes in α-oxidation (Wanders et al., 2001). Branched chain fatty acids, such as phytanic acid, have a methyl group on the third carbon atom (γ position), which prevents β-oxidation. These fatty acids first undergo oxidative decarboxylation (α-oxidation) in peroxisomes to remove the terminal carboxyl group as CO2. This shortened fatty acid now has a methyl group on the second carbon and can be further oxidized in peroxisomes or mitochondria. Unlike 3-methyl branched fatty acids, 2-methyl fatty acids can undergo β-oxidation with release of a propionyl CoA group, instead of acetyl CoA, after the first round of β-oxidation (Verhoeven et al., 1998).
Most fatty acids are metabolized by β-oxidation, which involves the removal of two carbons at the carboxyl terminus of the molecule and is carried out in peroxisomes and mitochondria. As noted above, branched chain fatty acids are subject to α-oxidation, which involves the removal of one carbon at the carboxyl terminus. This process is thought to occur in peroxisomes but not in mitochondria; the adaptive rationale for lacking α-oxidative capacity in mitochondria is not known. Long chain and very long (greater than 22 carbons) chain fatty acids can be metabolized by ω-oxidation, which involves the removal of one carbon at the end of the molecule farthest from the carboxyl terminus. ω-oxidation, thought to be a minor pathway under most physiological circumstances, occurs in the smooth endoplasmic reticulum in a process dependent on the cytochrome P450 CYP4A subfamily. Dicarboxylic acids, the product of ω-oxidation, can then be metabolized by β-oxidation in peroxisomes (Reddy and Hashimoto, 2001), suggesting a link between peroxisomal and microsomal fatty acid metabolism in addition to the well-characterized link between peroxisomal and mitochondrial fatty acid metabolism.
Similarities and differences between peroxisomal and mitochondrial fatty acid oxidation
Although there are some fundamental differences in the biochemistry of mitochondrial and peroxisomal β-oxidation (Wanders and Waterham, 2006), the overall mechanism is essentially the same and involves four consecutive enzymatic steps: 1) dehydrogenation that removes two hydrogen molecules and introduces a trans double bond between the α and β carbons of the fatty acyl CoA molecule; 2) hydration across the double bond, forming a 3-L-hydroxyacyl-CoA; 3) dehydrogenation of the 3-L-hydroxyacyl-CoA, forming 3-ketoacyl CoA; and 4) thiolytic cleavage of the terminal acetyl-CoA group, forming a new acyl-CoA molecule that is shorter by two carbons.
Despite these similarities, there are several differences between mitochondrial and peroxisomal fatty acid oxidation (Wanders and Tager, 1998). First, the enzymes involved in the two pathways are entirely different. For example, the first step of mitochondrial β-oxidation is catalyzed by the flavoenzyme acyl CoA dehydrogenase to produce FADH2, whose electrons are utilized to produce 2 molecules of ATP. In contrast, the first step of peroxisomal β-oxidation reaction is catalyzed by a different flavoenzyme, acyl CoA oxidase. Because peroxisomes lack a respiratory chain, electrons from FADH2 are transferred directly to O2 (creating H2O2), and energy is released as heat, instead of being used to generate ATP. Thus, peroxisomal fatty acid oxidation is less favorable in terms of energy storage than mitochondrial fatty acid oxidation. Because fatty acid oxidation in peroxisomes is not carried to completion (Osumi and Hashimoto, 1980; Vanhove et al., 1993), this process is thought to shorten very long chain fatty acids, which can then be further oxidized in mitochondria. Consistent with this concept, the substrate specificities of peroxisomal and mitochondrial β-oxidation systems are different. Fatty acids of 26 carbon chain length or greater are thought to be oxidized exclusively in peroxisomes, although the number of peroxisomal oxidation cycles utilized for these molecules is unknown. Shorter fatty acids can be oxidized in either organelle (Wanders et al., 2010), but the quantitative contributions of each are not well defined since metabolism varies depending on substrates and physiological context.
Mitochondrial and peroxisomal β-oxidation systems also differ in terms of fatty acyl-CoA transport from the cytosol. Uptake of fatty acyl CoAs across the mitochondrial membrane requires a carnitine exchange system, consisting of 2 carnitine palmitoyltransferases (CPT1 and CPT2) and the transporter protein carnitine-acylcarnitine translocase (CACT). CPT1 is associated with the outer mitochondrial membrane. It exchanges carnitine for CoA, resulting in a fatty acid-carnitine, which is transported across the inner mitochondrial membrane by CACT. Once inside the matrix, fatty acid carnitine is converted back to fatty acyl CoA by CPT2. Carnitine does not appear to be required for fatty-acyl CoA uptake into peroxisomes. However, peroxisomes do have carnitine acyltransferase activity, which may be required for converting acyl-CoAs to acylcarnitines so that they can be transferred to mitochondria for further oxidation (Wanders, 2013). Fatty acyl-CoA uptake into peroxisomes requires three ATP binding cassette transporter D subfamily proteins that are localized to the peroxisomal membrane: ABCD1, ABCD2 and ABCD3 (Wanders et al., 2007). Each can form a functional homodimeric or heterodimeric transporter by partnering with another ABCD protein. Each subunit contains a transmembrane domain as well as an ATP binding cassette. ABCD1 (also called ALDP) is mutated in the human disease adrenoleukodystrophy. As a homodimer, ABCD1 is involved in transporting very long chain fatty acids from the cytosol to the peroxisomal lumen for β–oxidation (van Roermund et al., 2008). ABCD2 is particularly abundant in adipose tissue, where it may import dietary erucic acid (C22:1) into peroxisomes (Liu et al., 2012a). ABCD3 (also called PMP70) may import long chain fatty acids and branched chain acyl-CoAs into peroxisomes for fatty acid oxidation (Imanaka et al., 1999).
Although PPARα is required for transcriptional regulation of both pathways (Aoyama et al., 1998), acute regulation of mitochondrial fatty acid oxidation is different from that of peroxisomes. Unlike peroxisomal fatty acid oxidation, the mitochondrial pathway is exquisitely regulated by malonyl-CoA at the level of CPT1 (McGarry et al., 1978; McGarry et al., 1977). Whether there are analogous mechanisms for rapid control of peroxisomal fatty acid oxidation is unknown.
Physiological significance of peroxisomal fatty acid oxidation
It is likely that peroxisomal fatty acid oxidation is more complex than simply shortening chains of very long chain fatty acids that cannot be directly oxidized in mitochondria. The biochemical basis underlying the metabolism of very long chain fatty acids in peroxisomes but not in mitochondria is known. Peroxisomal acyl CoA oxidases and mitochondrial acyl CoA dehydrogenases manifest different chain length specificities. However, the physiological basis for this organelle-specific functional difference is not known. Perhaps mitochondrial toxicities associated with the presence of very long chain fatty acids favored the evolutionary selection of peroxisomes for very long chain metabolism in mammals. Alternatively, peroxisome-dependent generation of heat (see below) may provide tissue-specific benefits such as maintenance of membrane structure, preservation of regional blood flow to ensure oxygenation, or control of optimum temperature to ensure enzymatic functions required for tissue regeneration or repair.
Ether lipids (which encompass alkyl-acylphospholipids and alkenyl-acylphospholipids, also known as plasmalogens, Figure 3) have unique physical properties that impact many aspects of cell biology (discussed below). Several studies suggest that the synthesis of ether lipids is linked to peroxisomal β oxidation in a manner that is physiologically relevant. Injection of [1-14C]lignoceric acid (C24:0) into clofibrate-treated rats results in incorporation of the radiolabel into ethanolamine plasmalogens, suggesting that peroxisomal fatty acid oxidation may preferentially channel acetyl-CoAs toward ether lipid synthesis (Hayashi and Oohashi, 1995). Consistent with this possibility, humans and mice with mutations in ABCD1 have decreased levels of ethanolamine plasmalogens, coupled with accumulation of very long chain fatty acids in brain white matter (Khan et al., 2008). ABCD1 was recently shown to interact with several lipogenic enzymes, including ATP citrate lyase (ACLY), acetyl CoA carboxylase (ACC), and fatty acid synthase (FAS) (Hillebrand et al., 2012). FAS is a large multifunctional enzyme that primarily synthesizes palmitate (C16:0) using malonyl CoA as a two carbon source (Lodhi et al., 2011). We recently demonstrated increased fatty acid oxidation in subcutaneous white fat in mice with adipose-specific knockout of FAS (Lodhi et al., 2012). It possible that this increased fatty acid oxidation was primarily peroxisomal since FAS inactivation results in accumulation of malonyl-CoA, which inhibits mitochondrial fatty acid oxidation (McGarry et al., 1977).
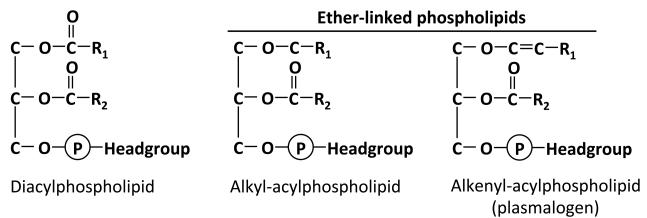
Figure 3. Chemical structures of diacyl- and ether-linked phospholipids.
In conventional diacyl phospholipids, fatty acyl side chains are linked to the sn-1 and sn-2 positions of the glycerol backbone by ester bonds. Ether-linked phospholipids are a special class of glycerophospholipids that have an alkyl chain attached to the sn-1 position by an ether bond. The sn-2 substituent of ether lipids is generally an ester-linked acyl chain as in diacylphospholipids. The headgroup of ether phospholipids is usually choline or ethanolamine. Some of the ether linked phospholipids have a cis double bond adjacent to the ether bond, and are referred to as alkenyl-acylphospholipids or more commonly as plasmalogens. According to convention, plasmalogen form of phospholipids have the prefix “plasmenyl”, as in plasmenylcholine. Alkyl-acylphospholipids have the prefix “plasmanyl”.
Lipid biosynthesis in peroxisomes
In 1977, Amiya Hajra and colleagues discovered that dihydroxyacetone phosphate acyltransferase (DHAPAT), an enzyme required for the synthesis of acyl DHAP, a precursor for glycerol ether lipids, was localized to peroxisomes (Jones and Hajra, 1977). This was a surprising discovery because it suggested that peroxisomes were not only involved in catabolic reactions, but also the synthesis of phospholipids. In the more common diacyl phospholipids, fatty acyl side chains are linked to the sn-1 and sn-2 positions of the glycerol backbone by ester bonds. In ether lipids (such as alkyl ether phospholids and plasmalogens), the sn-1 substituent is attached to the glycerol backbone by an ether bond (Figure 3). In the subsequent 20 years, the Hajra laboratory identified several other components of a peroxisomal ether lipid synthetic pathway (Hajra and Das, 1996), while others found that enzymes involved in bile acid synthesis were also localized to peroxisomes (Pedersen et al., 1997). These studies firmly established peroxisomes as a participant in lipid biosynthesis.
Synthesis of ether phospholipids
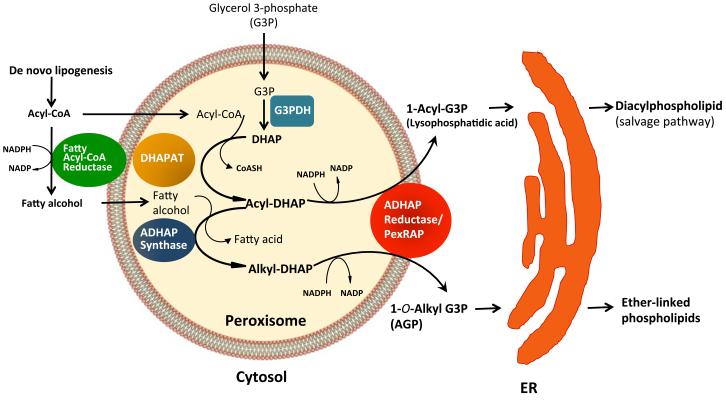
Ether phospholipid synthesis requires a series of enzymes associated with the peroxisomal membrane (Figure 4). This pathway is an alternative route for lysophosphatidic acid (LPA) synthesis (as opposed to direct acylation of glycerol 3-phosphate) and is obligatory for synthesis of precursors of all ether lipids in mammals, including platelet activating factors (PAFs) and plasmalogens (Hajra and Das, 1996). The pathway uses dihydroxacetone phosphate (DHAP), produced by dehydrogenation of glycerol 3-phophate (G3P), as a building block for the synthesis of phospholipids. Fatty acids derived from FAS–mediated de novo lipogenesis or other sources are activated to fatty acyl CoA by a peroxisome-membrane associated acyl CoA synthetase (ACS). Acyl CoA is used by DHAPAT to acylate DHAP or is reduced to a fatty alcohol by fatty acyl CoA reductase 1 (FAR1) in an NADPH-dependent reaction. A fatty alcohol is used by alkyl DHAP synthase (ADHAPS) to convert acyl DHAP to alkyl DHAP. Acyl DHAP or alkyl DHAP can be reduced to 1-Acyl G3P (LPA) or 1-O-Alkyl G3P (AGP), respectively, by Acyl/Alkyl DHAP reductase (ADHAPR). ADHAPR was purified from guinea pig liver and biochemically characterized (LaBelle and Hajra, 1974), but the gene encoding this protein was not known in mammals (MycIntyre et al, 2008). We recently identified this gene and renamed the protein it encodes PexRAP (for Peroxisomal Reductase Activating PPARγ) (Lodhi et al., 2012). Subsequent steps of phospholipid synthesis, such as acylation of the sn-2 position and addition of the head group (e.g. CDP-choline or CDP-ethanolamine) take place in the ER (Hajra and Das, 1996).
Figure 4. Acyl DHAP pathway of phospholipid synthesis.
This pathway is obligatory for synthesis of ether-linked phospholipids and is also an alternative route for synthesis of diacylphospholipids. Phospholipid synthesis begins in peroxisomes and is completed in the ER. The pathway uses dihydroxacetone phosphate (DHAP), generated by glycerol 3-phosphate dehydrogenase (G3PDH)-mediated dehydrogenation of G3P, as a building block for the synthesis of phospholipids. Fatty acyl CoA produced by de novo lipogenesis is used by DHAPAT (DHAP acyltransferase) to acylate DHAP, or is reduced to a fatty alcohol by a peroxisomal membrane-associated fatty acyl CoA reductase in an NADPH-dependent reaction. The fatty alcohol is used by alkyl DHAP synthase (ADHAPS) to convert acyl DHAP to alkyl DHAP. Acyl DHAP or alkyl DHAP can be reduced to 1-acyl G3P (lysophosphatidic acid) or its ether lipid equivalent, 1-O-alkyl G3P (AGP), respectively, by acyl/alkyl DHAP reductase (also called PexRAP). The subsequent steps of phospholipid synthesis, including acylation at the sn-2 position, occur in the ER.
Properties and functions of ether phospholipids
Ether lipids, such as plasmalogens, make up approximately 20% of the total phospholipid mass in humans. Tissue levels of these lipids vary greatly. Ether phospholipids are particularly abundant in the brain, heart, and white blood cells. Up to two thirds of the ethanolamine phospholipid in the brain and 12% of the total myelin phospholipid pool is plasmalogen (Nagan and Zoeller, 2001). In neutrophils, up to 46% of the phosphatidylcholine pool is in the plasmanyl form (Brautigam et al., 1996; Mueller et al., 1984; Mueller et al., 1982). In contrast, intracellular ether lipids are scant in the liver (Braverman and Moser, 2012). This is surprising, because many of the enzymes in the ether lipid synthetic pathway were originally purified from liver. Studies by Vance and colleagues suggest that liver synthesizes ether phospholipids, but does not store them. Instead, hepatocytes preferentially secrete ether phospholipids lipids in the form of lipoproteins. Up to 30% of phosphatidylethanolamine in lipoproteins is in the form of plasmalogen (Vance, 1990). Others have confirmed significant levels of plasmalogens in human serum (Brautigam et al., 1996). The precise molecular function of intracellular and circulating ether lipids is unknown. In brain, several lines of evidence suggest that ether lipids in myelin may be critical for modulating ion transport, and similar effects may be at play in heart (Ford and Hale, 1996). In white blood cells such as neutrophils, ether lipids may create a sink for polyunsaturated fatty acids such as arachidonic acid utilized for inflammatory signaling through the generation of eicosanoids (Nagan and Zoeller, 2001). Regardless of the precise processes driven by ether lipids, dramatic developmental abnormalities in humans and mice with defective ether lipid synthesis suggest that these lipids are responsible for fundamental cellular tasks that cannot be carried out by diacyl phospholipids.
Phospholipids are the predominant component of plasma membranes and intracellular membranes. The presence of ether-linked alkyl chains in phospholipids alters their physical properties and modulates membrane dynamics. This effect is presumably due to the lack of a carbonyl oxygen at the sn-1 position, which affects hydrophilic headgroup interactions (Lohner, 1996; Nagan and Zoeller, 2001). Model semi-synthetic membranes enriched in ethanolamine plasmalogens form non-lamellar structures at around 30° C, but diacylphospholipids form these non-bilayer structures at much higher temperatures (~68° C). This property of plasmalogens is thought to increase membrane permeability and promote membrane-membrane fusion (Lohner, 1996). Plasmalogens are also important for the organization of detergent resistant microdomains, cholesterol-rich membrane regions that are thought to be involved in cell signaling. Plasmalogen-deficient mice lacking DHAPAT (encoded by gnpat) show evidence of disruption of these membrane microdomains; cholesterol is inappropriately localized to a perinuclear compartment and flotillin-1 is decreased in fractions of neurological tissue from these mice (Rodemer et al., 2003).
Ether lipids may be involved in cancer pathogenesis. It has been known for decades that cancer cells have high levels of alkyl ether lipids (Albert and Anderson, 1977; Howard et al., 1972; Roos and Choppin, 1984; Snyder and Wood, 1969). Roos and Choppin determined the tumorigenicity of mouse fibroblast cell lines differing in ether lipid content. One such line, F40, with 30-fold higher ether lipid content as compared to its parental line, required 1000-fold fewer cells to form tumors in irradiated mice and these tumors were aggressive (Roos and Choppin, 1984). The expression of the ether lipid synthetic enzyme ADHAPS (also called alkylgyceronephosphate synthase, AGPS) is increased in various cancer cell lines and primary tumors (Benjamin et al., 2013). AGPS knockdown impaired experimental cancer pathogenesis, including cell survival, migration, and invasion. Conversely, AGPS overexpression increased ether lipids and promoted tumor growth. While its precise role in cancer progression is unclear, AGPS may generate structural and signaling lipids that promote carcinogenicity (Benjamin et al., 2013).
Emerging evidence suggests that alkyl ether phospholipids may be important in cellular signaling. Recent studies with DHAPAT knockout mice demonstrate that ether lipids likely serve as self ligands to activate invariant natural killer T (iNKT) cells (Facciotti et al., 2012). As discussed below, results from our laboratory and others suggest that peroxisome-derived lipids may also be involved in activating the nuclear receptor PPARγ.
Peroxisomes in adipose tissue development and function
Adipose tissue is an important metabolic organ that regulates whole body energy balance. Two major types of adipose tissue are found in mammals, white fat and brown fat. Both types store energy as triglyceride in intracellular lipid droplets and secrete a host of hormones, called adipokines, which influence metabolic homeostasis. Whereas white adipose tissue (WAT) primarily stores fat, which can be mobilized in times of need, brown adipose tissue (BAT) transforms the chemical energy in food into heat through uncoupled respiration. Because of their contribution to lipid metabolism, peroxisomes may regulate adipose tissue development and function (Figure 5).
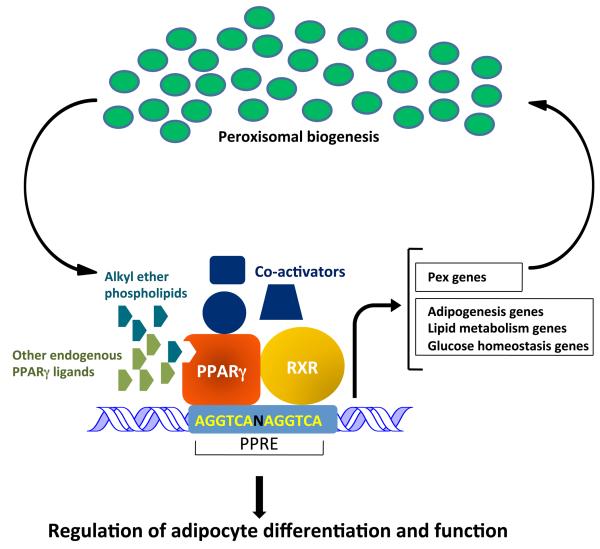
Figure 5. Relationship between peroxisomes and PPARγ.
PPARγ is a key regulator of adipocyte differentiation as well as function and is activated by multiple endogenous ligands, including alkyl ether phospholipids, which are synthesized in peroxisomes. PPARγ exists as a heterodimer with RXR and regulates expression of a large number of genes harboring PPAR response elements (PPRE), including genes involved in adipogenesis, lipid metabolism, and glucose homeostasis. Emerging studies indicate that PPARγ also regulates the expression of genes involved in peroxisomal biogenesis, suggesting a feed-forward mechanism of PPARγ activation.
Peroxisome proliferation in brown and white adipocytes
Peroxisomes in adipocytes are small in size and belong to a subclass of the organelle referred to as microperoxisomes. Microperoxisomes are difficult to detect by electron microscopy because they lack a crystalline nucleoid found in larger peroxisomes, such as those in hepatocytes (Novikoff and Novikoff, 1982). Nevertheless, these small peroxisomes are abundantly present in adipocytes, and closely associated with lipid droplets. Using histochemical or immunogold staining for catalase, a large number of microperoxisomes were observed in 3T3-L1 adipocytes and mice epididymal WAT (Blanchette-Mackie et al., 1995; Novikoff et al., 1980).
A large number of catalase-positive particles were also observed in rat BAT and increased dramatically with cold exposure, suggesting the presence of physiologically relevant microperoxisomes in brown adipocytes (Ahlabo and Barnard, 1971). Subsequent studies showed that a hydrogen peroxide-producing oxidase was present in these particles together with catalase, consistent with their identification as bona fide peroxisomes (Pavelka et al., 1976). Pex genes involved in peroxisomal biogenesis are significantly increased during differentiation of brown adipocytes in culture and in BAT of mice exposed to cold temperatures in a manner dependent on PGC1α (Bagattin et al., 2010).
Peroxisomal metabolism may be related to heat production in brown adipose tissue. Acetyl CoAs generated by peroxisomal fatty acid oxidation may enter the tricarboxylic acid cycle in brown fat mitochondria to fuel thermogenesis mediated by UCP1, a mitochondrial uncoupling protein that allows protons to return to the mitochondrial matrix without synthesizing ATP. Alternatively, because peroxisomal β-oxidation itself generates heat instead of ATP due to the lack of a peroxisomal electron transport system, peroxisomes may be involved in adaptive thermogenesis independent of UCP1.
Together, these studies indicate that peroxisomes affect white and brown adipocyte differentiation and function.
Peroxisome-derived phospholipids in PPARγ signaling and adipocyte differentiation
The nuclear receptor PPARγ is required for adipocyte differentiation and function. Studies in mice and cultured cells have established PPARγ as both necessary and sufficient for adipocyte differentiation. Moreover, PPARγ regulates whole-body lipid metabolism and insulin sensitivity through its transcriptional control of various adipocyte activities, including fat storage, lipogenesis, adipokine production, and thermogenesis (Tontonoz and Spiegelman, 2008). Genome-wide mapping of PPARγ regulatory elements using chromatin immunoprecipitation (ChIP) assays combined with microarrays revealed several thousand distinct PPARγ binding sites in adipocytes (Lefterova et al., 2008). How does PPARγ regulate such a large number of genes in adipocytes? One possibility is that PPARγ is activated by multiple endogenous ligands that in turn recruit different transcriptional co-activators controlling unique subsets of genes. PPARγ has a large ligand binding pocket capable of engaging a variety of agonists (Xu and Li, 2008). Originally identified as the target of the diabetes drugs thiazolidinediones, PPARγ binds several different lipids, with variable effects on transcriptional activity (Schupp and Lazar, 2010). Identification of specific endogenous ligands of PPARγ has been difficult. A study using a PPARγ-dependent reporter assay suggested that endogenous ligands for PPARγ could be produced during early phase adipogenesis, but these ligands remain unidentified (Tzameli et al., 2004).
Our previous studies suggested that de novo lipogenesis mediated by FAS is involved in generating an endogenous ligand for PPARα in liver (Chakravarthy et al., 2009). To determine if FAS is also involved in endogenous activation of PPARγ in adipocytes, we generated mice with adipose-specific deletion of FAS. Studies with these mice and embryonic fibroblasts derived from these animals suggested that FAS is part of a lipogenic pathway that promotes PPARγ activation in adipose tissue. Using a mass spectrometry-based approach, we identified several FAS-dependent alkyl ether-linked phosphatidylcholine species that were associated with PPARγ in a rosiglitazone-displaceable manner. Treatment of cells with one of these lipid species promoted PPARγ-dependent luciferase reporter activity, suggesting that it could activate PPARγ (Lodhi et al., 2012). As noted above, the peroxisomal acyl-DHAP pathway of lipid synthesis produces ether phospholipids. Activities of various enzymes in this pathway increase during adipogenesis (Hajra et al., 2000). Knockdown of PexRAP, the terminal enzyme in this pathway (Figure 4), impairs PPARγ activation and adipogenesis in cultured cells. Antisense oligonucleotide-mediated knockdown of PexRAP in mice decreases expression of PPARγ–dependent genes, reduces fat mass, increases leanness, and improves insulin sensitivity (Lodhi et al., 2012).
We identified alkyl ether phospholipids as potential endogenous ligands of PPARγ in an unbiased screen, and there is precedent for PPARγ activation by this class of lipids. Azelaoyl phosphatidylcholine (1-O-hexadecyl-2-O-(9-carboxyoctanoyl)-sn-glyceryl-3-phosphocholine), an oxiditatively shortened phospholipid in LDL, was reported to be a high affinity ligand (equipotent to rosiglitazone) for PPARγ (Davies et al., 2001). An alkyl ether analog of LPA, 1-O-alkyl glycerol 3-phosphate (AGP), a direct product of the PexRAP-catalyzed reaction (see Figure 4), has been shown to be a high affinity partial agonist for PPARγ (McIntyre et al., 2003; Tsukahara et al., 2006; Zhang et al., 2004). Since PPARγ can be activated by a variety of lipids, it is possible that endogenous ligands of this nuclear receptor in general exhibit partial agonism, thus permitting selective recruitment of co-activators to regulate different subsets of genes.
A direct requirement for peroxisome-derived lipids in adipose tissue development was suggested by studies in Pex7 knockout mice that lack plasmalogens (Brites et al., 2011; Brites et al., 2009). These mice have severely reduced body fat. This decreased adiposity was not due to a significant difference in food intake and could be rescued by feeding a diet enriched in alkyl-glycerol, which restored plasmalogen levels (Brites et al., 2011). Exogenously added alkyl-glycerol enters the ether lipid synthetic pathway downstream of the peroxisomal steps. Because endogenous ether phospholipids are present at low abundance in adipose tissue (our unpublished observation), it is possible that ether phospholipids have a signaling function. Whether adipose tissue deficiency in Pex7 knockout mice is due to defective PPARγ signaling remains to be determined. Recently, an unbiased mass spectrometry-based metabolomics screen showed that endogenous synthesis of alkyl-glycerol ether lipids increases early during 3T3-L1 adipogenesis and that treatment with these lipids promotes adipocyte differentiation (Homan et al., 2011).
Peroxisomal disorders
Some degree of peroxisome function is required for health in humans since there are several devastating genetic disorders caused by impaired peroxisomal activity or lack of peroxisomes due to defective peroxisomal biogenesis (Aubourg and Wanders, 2013). Peroxisomal disorders are clinically heterogeneous. However, they are consistently associated with impaired peroxisomal lipid metabolism, resulting in the accumulation of VLCFAs and phytanic acid, and defective synthesis of ether lipids and bile acids.
Peroxisomal biogenesis disorders
Diseases caused by defects in peroxisomal biogenesis include Zellweger spectrum disorders and rhizomelic chondrodysplasia punctata type 1. Zellweger spectrum disorders affect 1 in 30,000 infants and include cerebrohepatorenal Zellweger syndrome, neonatal adrenoleukodystrophy, and infantile Refsum disease. These diseases result from mutations in one of the dozen Pex genes involved in peroxisomal biogenesis. Mutations in Pex3, Pex16 and Pex19, which result in the complete absence of peroxisomes, cause the most severe phenotypes. Mutations in other Pex genes result in ghost peroxisomes. Features of Zellweger syndrome, which is the most severe end of the clinical spectrum, include craniofacial dysmorphism, hepatomegaly, and neurological abnormalities such as disruption of normal development, hypotonia, seizures, glaucoma, retinal degeneration, and deafness. Most Zellweger infants do not survive past one year of age due to respiratory compromise, gastrointestinal bleeding, and liver failure. They seldom reach appropriate developmental milestones. The features of neonatal adrenoleukodystrophy and infantile Refsum disease are similar to those of Zellweger syndrome, but these disorders progress more slowly. Children with neonatal adrenoleukodystrophy usually die between the age of two and three years. Patients with infantile Refsum disease can live into early adulthood (Aubourg and Wanders, 2013).
Rhizomelic chondrodysplasia punctata type 1 (RCDP1) is clinically and genetically distinct from Zellweger spectrum disorders. It is characterized by distinctive facial features, including a prominent forehead, hypertelorism (widely set eyes), and anteverted nares (nostrils that face anteriorly due to an upturned nose). These patients also suffer from growth failure, developmental delay, seizures, and congenital cataracts. Most die in early childhood. RCDP1 is caused by mutations in Pex7, a chaperone for PTS2-containing peroxisomal matrix proteins, including ADHAPS. A mouse model of RCDP1 has been generated by knocking out the Pex7 gene. Feeding these mice a diet enriched in alkyl-glycerol partially rescues their phenotype, implicating defective ether lipid synthesis inthe pathophysiology (Brites et al., 2011).
Disorders caused by an impaired peroxisomal function
Defects in a single peroxisomal protein in the setting of intact peroxisomal structure result in adult Refsum disease (ARD), X-linked adrenoleukodystrophy (X-ALD), RCDP2, and RCDP3. ARD is characterized biochemically by accumulation of phytanic acid, which is found in dairy products. Phytanic acid, a branched chain fatty acid, must first undergo α oxidation in peroxisomes prior to β oxidation in mitochondria. The primary defect in ARD is a mutation in the peroxisomal enzyme phytanoyl-CoA hydroxylase that catalyzes the first step in α–oxidation. Features of ARD include night blindness, loss of peripheral vision, and ataxia. Symptoms evolve slowly and usually appear in early adulthood. X-ALD is a disorder of peroxisomal β–oxidation, caused by mutations in ABCD1. It is characterized by loss of myelin in the central nervous system and disruption of the adrenal cortex. Major features include learning disabilities, seizures, visual disturbances, and hearing loss. RCDP2 and RCDP3 are related to RCDP1 (described above). They are caused by defects in ether lipid synthesis due to mutations in DHAPAT (GNPAT) and ADHAPS (AGPS), respectively.
A peroxisome database website (http://www.peroxisomedb.org/) summarizes the genes involved in peroxisomal disorders.
Future directions
As an organelle, peroxisomes get little respect. This may change given increasing appreciation for the complexity of their interactions with lipid droplets, the manner in which they complement metabolic functions of mitochondria, and the critical part they play in the generation of ether lipids. Several aspects of peroxisomal biology are ripe for novel translational pathways to human disease.
Ether lipids have been known for decades to be increased in a variety of cancers, although the precise implications of this finding are poorly understood. FAS is linked to ether lipid production, FAS expression is known to be increased in certain cancers, and ether lipids appear to be increased in particularly aggressive cancers (Benjamin et al., 2013). It is possible that targeted inhibition of a peroxisomal lipogenic pathway leading to ether lipid production specifically in cancer cells could treat malignancies without inducing developmental abnormalities seen with congenital loss of ether lipid synthesis. Mitochondrial function is altered in insulin resistant states such as obesity and diabetes. Based on the relationships between peroxisomes and mitochondria, modulating ether lipid synthesis in the heart (a site commonly affected in diabetes), white blood cells (which may propagate diabetes complications through effects on inflammation), nerves (neuropathy is a common and inadequately treated complication of diabetes), and other tissues could affect mitochondrial endpoints potentially beneficial in obesity-related diabetes. Defects in ether lipid synthesis cause protean neurologic disorders, and elevations of certain ether lipids are found in neurogenerative disorders such as Alzheimer’s disease (Pettegrew et al., 2001). Dietary interventions to alter peroxisomal activities could impact the clinical course of neurologic diseases.
Peroxisomes are still mysterious. Uncovering their signaling mechanisms and how they leverage relationships with lipid droplets and mitochondria might lead to new approaches to disease and prompt us to view peroxisomes as intracellular patricians instead of plebians.
Acknowledgements
This work was supported by NIH grants DK076729, DK088083, T32 DK007120, DK20579, DK56341, and K99 DK094874.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlabo I, Barnard T. Observations on peroxisomes in brown adipose tissue of the rat. J Histochem Cytochem. 1971;19:670–675. doi: 10.1177/19.11.670. [DOI] [PubMed] [Google Scholar]
- Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. PPARgamma signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19:557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert DH, Anderson CE. Ether-linked glycerolipids in human brain tumors. Lipids. 1977;12:188–192. doi: 10.1007/BF02533292. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez FJ. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha) J Biol Chem. 1998;273:5678–5684. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- Aubourg P, Wanders R. Peroxisomal disorders. Handbook Clin Neurol. 2013;113:1593–1609. doi: 10.1016/B978-0-444-59565-2.00028-9. [DOI] [PubMed] [Google Scholar]
- Baerends RJ, Rasmussen SW, Hilbrands RE, van der Heide M, Faber KN, Reuvekamp PT, Kiel JA, Cregg JM, van der Klei IJ, Veenhuis M. The Hansenula polymorpha PER9 gene encodes a peroxisomal membrane protein essential for peroxisome assembly and integrity. J Biol Chem. 1996;271:8887–8894. doi: 10.1074/jbc.271.15.8887. [DOI] [PubMed] [Google Scholar]
- Bagattin A, Hugendubler L, Mueller E. Transcriptional coactivator PGC-1alpha promotes peroxisomal remodeling and biogenesis. Proc Natl Acad Sci U S A. 2010;107:20376–20381. doi: 10.1073/pnas.1009176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz R, Li WH, Venables B, Zehmer JK, Roth MR, Welti R, Anderson RG, Liu P, Chapman KD. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J Lipid Res. 2007;48:837–847. doi: 10.1194/jlr.M600413-JLR200. [DOI] [PubMed] [Google Scholar]
- Benjamin DI, Cozzo A, Ji X, Roberts LS, Louie SM, Mulvihill MM, Luo K, Nomura DK. Ether lipid generating enzyme AGPS alters the balance of structural and signaling lipids to fuel cancer pathogenicity. Proc Natl Acad Sci U S A. 2013;110:14912–14917. doi: 10.1073/pnas.1310894110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binns D, Januszewski T, Chen Y, Hill J, Markin VS, Zhao Y, Gilpin C, Chapman KD, Anderson RG, Goodman JM. An intimate collaboration between peroxisomes and lipid bodies. J Cell Biol. 2006;173:719–731. doi: 10.1083/jcb.200511125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette-Mackie EJ, Dwyer NK, Barber T, Coxey RA, Takeda T, Rondinone CM, Theodorakis JL, Greenberg AS, Londos C. Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. J Lipid Res. 1995;36:1211–1226. [PubMed] [Google Scholar]
- Brautigam C, Engelmann B, Reiss D, Reinhardt U, Thiery J, Richter WO, Brosche T. Plasmalogen phospholipids in plasma lipoproteins of normolipidemic donors and patients with hypercholesterolemia treated by LDL apheresis. Atherosclerosis. 1996;119:77–88. doi: 10.1016/0021-9150(95)05632-7. [DOI] [PubMed] [Google Scholar]
- Braverman N, Steel G, Obie C, Moser A, Moser H, Gould SJ, Valle D. Human PEX7 encodes the peroxisomal PTS2 receptor and is responsible for rhizomelic chondrodysplasia punctata. Nat Genet. 1997;15:369–376. doi: 10.1038/ng0497-369. [DOI] [PubMed] [Google Scholar]
- Braverman NE, Moser AB. Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta. 2012;1822:1442–1452. doi: 10.1016/j.bbadis.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Brites P, Ferreira AS, da Silva TF, Sousa VF, Malheiro AR, Duran M, Waterham HR, Baes M, Wanders RJ. Alkyl-glycerol rescues plasmalogen levels and pathology of ether-phospholipid deficient mice. PLoS One. 2011;6:e28539. doi: 10.1371/journal.pone.0028539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brites P, Mooyer PA, El Mrabet L, Waterham HR, Wanders RJ. Plasmalogens participate in very-long-chain fatty acid-induced pathology. Brain. 2009;132:482–492. doi: 10.1093/brain/awn295. [DOI] [PubMed] [Google Scholar]
- Brown FR, 3rd, McAdams AJ, Cummins JW, Konkol R, Singh I, Moser AB, Moser HW. Cerebro-hepato-renal (Zellweger) syndrome and neonatal adrenoleukodystrophy: similarities in phenotype and accumulation of very long chain fatty acids. Johns Hopkins Med J. 1982;151:344–351. [PubMed] [Google Scholar]
- Chakravarthy MV, Lodhi IJ, Yin L, Malapaka RR, Xu HE, Turk J, Semenkovich CF. Identification of a physiologically relevant endogenous ligand for PPARalpha in liver. Cell. 2009;138:476–488. doi: 10.1016/j.cell.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper TG, Beevers H. Beta oxidation in glyoxysomes from castor bean endosperm. J Biol Chem. 1969;244:3514–3520. [PubMed] [Google Scholar]
- Cornwell PD, De Souza AT, Ulrich RG. Profiling of hepatic gene expression in rats treated with fibric acid analogs. Mutation Res. 2004;549:131–145. doi: 10.1016/j.mrfmmm.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Davies SS, Pontsler AV, Marathe GK, Harrison KA, Murphy RC, Hinshaw JC, Prestwich GD, Hilaire AS, Prescott SM, Zimmerman GA, McIntyre TM. Oxidized alkyl phospholipids are specific, high affinity peroxisome proliferator-activated receptor gamma ligands and agonists. J Biol Chem. 2001;276:16015–16023. doi: 10.1074/jbc.M100878200. [DOI] [PubMed] [Google Scholar]
- De Duve C, Baudhuin P. Peroxisomes (microbodies and related particles) Physiol Rev. 1966;46:323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- Diano S, Liu ZW, Jeong JK, Dietrich MO, Ruan HB, Kim E, Suyama S, Kelly K, Gyengesi E, Arbiser JL, Belsham DD, Sarruf DA, Schwartz MW, Bennett AM, Shanabrough M, Mobbs CV, Yang X, Gao XB, Horvath TL. Peroxisome proliferation-associated control of reactive oxygen species sets melanocortin tone and feeding in diet-induced obesity. Nat Med. 2011;17:1121–1127. doi: 10.1038/nm.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov L, Lam SK, Schekman R. The role of the endoplasmic reticulum in peroxisome biogenesis. Cold Spring Harb Perspect Biol. 2013;5:a013243. doi: 10.1101/cshperspect.a013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodt G, Gould SJ. Multiple PEX genes are required for proper subcellular distribution and stability of Pex5p, the PTS1 receptor: evidence that PTS1 protein import is mediated by a cycling receptor. J Cell Biol. 1996;135:1763–1774. doi: 10.1083/jcb.135.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992;68:879–887. doi: 10.1016/0092-8674(92)90031-7. [DOI] [PubMed] [Google Scholar]
- Facciotti F, Ramanjaneyulu GS, Lepore M, Sansano S, Cavallari M, Kistowska M, Forss-Petter S, Ni G, Colone A, Singhal A, Berger J, Xia C, Mori L, De Libero G. Peroxisome-derived lipids are self antigens that stimulate invariant natural killer T cells in the thymus. Nature Immunol. 2012;13:474–480. doi: 10.1038/ni.2245. [DOI] [PubMed] [Google Scholar]
- Fahimi HD. Cytochemical localization of peroxidatic activity of catalase in rat hepatic microbodies (peroxisomes) J Cell Biol. 1969;43:275–288. doi: 10.1083/jcb.43.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Morrell JC, Jones JM, Gould SJ. PEX3 functions as a PEX19 docking factor in the import of class I peroxisomal membrane proteins. J Cell Biol. 2004;164:863–875. doi: 10.1083/jcb.200311131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese RV, Jr., Walther TC. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell. 2009;139:855–860. doi: 10.1016/j.cell.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford DA, Hale CC. Plasmalogen and anionic phospholipid dependence of the cardiac sarcolemmal sodium-calcium exchanger. FEBS Lett. 1996;394:99–102. doi: 10.1016/0014-5793(96)00930-1. [DOI] [PubMed] [Google Scholar]
- Freitag J, Ast J, Bolker M. Cryptic peroxisomal targeting via alternative splicing and stop codon read-through in fungi. Nature. 2012;485:522–525. doi: 10.1038/nature11051. [DOI] [PubMed] [Google Scholar]
- Gould SG, Keller GA, Subramani S. Identification of a peroxisomal targeting signal at the carboxy terminus of firefly luciferase. J Cell Biol. 1987;105:2923–2931. doi: 10.1083/jcb.105.6.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardiola-Diaz HM, Rehnmark S, Usuda N, Albrektsen T, Feltkamp D, Gustafsson JA, Alexson SE. Rat peroxisome proliferator-activated receptors and brown adipose tissue function during cold acclimatization. J Biol Chem. 1999;274:23368–23377. doi: 10.1074/jbc.274.33.23368. [DOI] [PubMed] [Google Scholar]
- Hajra AK, Das AK. Lipid biosynthesis in peroxisomes. Ann N Y Acad Sci. 1996;804:129–141. doi: 10.1111/j.1749-6632.1996.tb18613.x. [DOI] [PubMed] [Google Scholar]
- Hajra AK, Larkins LK, Das AK, Hemati N, Erickson RL, MacDougald OA. Induction of the peroxisomal glycerolipid-synthesizing enzymes during differentiation of 3T3-L1 adipocytes. Role in triacylglycerol synthesis. J Biol Chem. 2000;275:9441–9446. doi: 10.1074/jbc.275.13.9441. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Oohashi M. Incorporation of acetyl-CoA generated from peroxisomal beta-oxidation into ethanolamine plasmalogen of rat liver. Biochim Biophys Acta. 1995;1254:319–325. doi: 10.1016/0005-2760(94)00194-4. [DOI] [PubMed] [Google Scholar]
- Hillebrand M, Gersting SW, Lotz-Havla AS, Schafer A, Rosewich H, Valerius O, Muntau AC, Gartner J. Identification of a new fatty acid synthesis-transport machinery at the peroxisomal membrane. J Biol Chem. 2012;287:210–221. doi: 10.1074/jbc.M111.272732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohfeld J, Veenhuis M, Kunau WH. PAS3, a Saccharomyces cerevisiae gene encoding a peroxisomal integral membrane protein essential for peroxisome biogenesis. J Cell Biol. 1991;114:1167–1178. doi: 10.1083/jcb.114.6.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan EA, Kim YG, Cardia JP, Saghatelian A. Monoalkylglycerol ether lipids promote adipogenesis. J Am Chem Soc. 2011;133:5178–5181. doi: 10.1021/ja111173c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard BV, Morris HP, Bailey JM. Ether-lipids, -glycerol phosphate dehydrogenase, and growth rate in tumors and cultured cells. Cancer Res. 1972;32:1533–1538. [PubMed] [Google Scholar]
- Imanaka T, Aihara K, Takano T, Yamashita A, Sato R, Suzuki Y, Yokota S, Osumi T. Characterization of the 70-kDa peroxisomal membrane protein, an ATP binding cassette transporter. J Biol Chem. 1999;274:11968–11976. doi: 10.1074/jbc.274.17.11968. [DOI] [PubMed] [Google Scholar]
- Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- Jones CL, Hajra AK. The subcellular distribution of acyl CoA: dihydroxyacetone phosphate acyl transferase in guinea pig liver. Biochem Biophys Res Commun. 1977;76:1138–1143. doi: 10.1016/0006-291x(77)90974-3. [DOI] [PubMed] [Google Scholar]
- Jones JM, Morrell JC, Gould SJ. PEX19 is a predominantly cytosolic chaperone and import receptor for class 1 peroxisomal membrane proteins. J Cell Biol. 2004;164:57–67. doi: 10.1083/jcb.200304111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerppola TK. Bimolecular fluorescence complementation (BiFC) analysis as a probe of protein interactions in living cells. Annu Rev Biophys. 2008;37:465–487. doi: 10.1146/annurev.biophys.37.032807.125842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Singh J, Singh I. Plasmalogen deficiency in cerebral adrenoleukodystrophy and its modulation by lovastatin. J Neurochem. 2008;106:1766–1779. doi: 10.1111/j.1471-4159.2008.05513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, Umesono K, Evans RM. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci U S A. 1994;91:7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBelle EF, Jr., Hajra AK. Purification and kinetic properties of acyl and alkyl dihydroxyacetone phosphate oxidoreductase. J Biol Chem. 1974;249:6936–6944. [PubMed] [Google Scholar]
- Lazarow PB, De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci U S A. 1976;73:2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow PB, and Fujiki Y. Biogenesis of peroxisomes. Annu Rev Cell Biol. 1985;1:489–530. doi: 10.1146/annurev.cb.01.110185.002421. [DOI] [PubMed] [Google Scholar]
- Lefterova MI, Zhang Y, Steger DJ, Schupp M, Schug J, Cristancho A, Feng D, Zhuo D, Stoeckert CJ, Jr., Liu XS, Lazar MA. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008;22:2941–2952. doi: 10.1101/gad.1709008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legg PG, Wood RL. New observations on microbodies. A cytochemical study on CPIB-treated rat liver. J Cell Biol. 1970;45:118–129. doi: 10.1083/jcb.45.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Gould SJ. PEX11 promotes peroxisome division independently of peroxisome metabolism. J Cell Biol. 2002;156:643–651. doi: 10.1083/jcb.200112028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liang S, Liu X, Brown JA, Newman KE, Sunkara M, Morris AJ, Bhatnagar S, Li X, Pujol A, Graf GA. The absence of ABCD2 sensitizes mice to disruptions in lipid metabolism by dietary erucic acid. J Lipid Res. 2012a;53:1071–1079. doi: 10.1194/jlr.M022160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ma C, Subramani S. Recent advances in peroxisomal matrix protein import. Curr Opin Cell Biol. 2012b;24:484–489. doi: 10.1016/j.ceb.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodhi IJ, Wei X, Semenkovich CF. Lipoexpediency: de novo lipogenesis as a metabolic signal transmitter. Trends Endocrinol Metab. 2011;22:1–8. doi: 10.1016/j.tem.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodhi IJ, Yin L, Jensen-Urstad AP, Funai K, Coleman T, Baird JH, El Ramahi MK, Razani B, Song H, Fu-Hsu F, Turk J, Semenkovich CF. Inhibiting adipose tissue lipogenesis reprograms thermogenesis and PPARgamma activation to decrease diet-induced obesity. Cell Metab. 2012;16:189–201. doi: 10.1016/j.cmet.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohner K. Is the high propensity of ethanolamine plasmalogens to form non-lamellar lipid structures manifested in the properties of biomembranes? Chem Phys Lipids. 1996;81:167–184. doi: 10.1016/0009-3084(96)02580-7. [DOI] [PubMed] [Google Scholar]
- Lowe M, Barr FA. Inheritance and biogenesis of organelles in the secretory pathway. Nat Rev Mol Cell Biol. 2007;8:429–439. doi: 10.1038/nrm2179. [DOI] [PubMed] [Google Scholar]
- Matsuzaki T, Fujiki Y. The peroxisomal membrane protein import receptor Pex3p is directly transported to peroxisomes by a novel Pex19p- and Pex16p-dependent pathway. J Cell Biol. 2008;183:1275–1286. doi: 10.1083/jcb.200806062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry JD, Leatherman GF, Foster DW. Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. J Biol Chem. 1978;253:4128–4136. [PubMed] [Google Scholar]
- McGarry JD, Mannaerts GP, Foster DW. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest. 1977;60:265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre TM, Pontsler AV, Silva AR, St Hilaire A, Xu Y, Hinshaw JC, Zimmerman GA, Hama K, Aoki J, Arai H, Prestwich GD. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc Natl Acad Sci U S A. 2003;100:131–136. doi: 10.1073/pnas.0135855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller HW, O'Flaherty JT, Greene DG, Samuel MP, Wykle RL. 1-O-alkyl-linked glycerophospholipids of human neutrophils: distribution of arachidonate and other acyl residues in the ether-linked and diacyl species. J Lipid Res. 1984;25:383–388. [PubMed] [Google Scholar]
- Mueller HW, O'Flaherty JT, Wykle RL. Ether lipid content and fatty acid distribution in rabbit polymorphonuclear neutrophil phospholipids. Lipids. 1982;17:72–77. doi: 10.1007/BF02535178. [DOI] [PubMed] [Google Scholar]
- Nagan N, Zoeller RA. Plasmalogens: biosynthesis and functions. Prog Lipid Res. 2001;40:199–229. doi: 10.1016/s0163-7827(01)00003-0. [DOI] [PubMed] [Google Scholar]
- Novikoff AB, Goldfischer S. Visualization of peroxisomes (microbodies) and mitochondria with diaminobenzidine. J Histochem Cytochem. 1969;17:675–680. doi: 10.1177/17.10.675. [DOI] [PubMed] [Google Scholar]
- Novikoff AB, Novikoff PM. Microperoxisomes and peroxisomes in relation to lipid metabolism. Ann N Y Acad Sci. 1982;386:138–152. doi: 10.1111/j.1749-6632.1982.tb21412.x. [DOI] [PubMed] [Google Scholar]
- Novikoff AB, Novikoff PM, Rosen OM, Rubin CS. Organelle relationships in cultured 3T3-L1 preadipocytes. J Cell Biol. 1980;87:180–196. doi: 10.1083/jcb.87.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff PM, Novikoff AB. Peroxisomes in absorptive cells of mammalian small intestine. J Cell Biol. 1972;53:532–560. doi: 10.1083/jcb.53.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osumi T, Hashimoto T. Purification and properties of mitochondrial and peroxisomal 3-hydroxyacyl-CoA dehydrogenase from rat liver. Arch Biochem Biophys. 1980;203:372–383. doi: 10.1016/0003-9861(80)90189-7. [DOI] [PubMed] [Google Scholar]
- Pavelka M, Goldenberg H, Huttinger M, Kramar R. Enzymic and morphological studies on catalase positive particles from brown fat of cold adapted rats. Histochemistry. 1976;50:47–55. doi: 10.1007/BF00492785. [DOI] [PubMed] [Google Scholar]
- Pedersen JI, Eggertsen G, Hellman U, Andersson U, Bjorkhem I. Molecular cloning and expression of cDNA encoding 3alpha,7alpha,12alpha-trihydroxy-5beta-chole stanoyl-CoA oxidase from rabbit liver. J Biol Chem. 1997;272:18481–18489. doi: 10.1074/jbc.272.29.18481. [DOI] [PubMed] [Google Scholar]
- Pettegrew JW, Panchalingam K, Hamilton RL, McClure RJ. Brain membrane phospholipid alterations in Alzheimer's disease. Neurochem Res. 2001;26:771–782. doi: 10.1023/a:1011603916962. [DOI] [PubMed] [Google Scholar]
- Platta HW, El Magraoui F, Schlee D, Grunau S, Girzalsky W, Erdmann R. Ubiquitination of the peroxisomal import receptor Pex5p is required for its recycling. J Cell Biol. 2007;177:197–204. doi: 10.1083/jcb.200611012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier Y, Antonenkov VD, Glumoff T, Hiltunen JK. Peroxisomal beta-oxidation--a metabolic pathway with multiple functions. Biochim Biophys Acta. 2006;1763:1413–1426. doi: 10.1016/j.bbamcr.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Poulos A, van Crugten C, Sharp P, Carey WF, Robertson E, Becroft DM, Saudubray JM, Poll-The BT, Christensen E, Brandt N. Prenatal diagnosis of Zellweger syndrome and related disorders: impaired degradation of phytanic acid. Eur J Pediatr. 1986;145:507–510. doi: 10.1007/BF02429053. [DOI] [PubMed] [Google Scholar]
- Pu J, Ha CW, Zhang S, Jung JP, Huh WK, and Liu P. Interactomic study on interaction between lipid droplets and mitochondria. Protein Cell. 2011;2:487–496. doi: 10.1007/s13238-011-1061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Reddy J, Svoboda D. Proliferation of microbodies and synthesis of catalase in rat liver. Induction in tumor-bearing host by CPIB. Am J Pathol. 1971;63:99–108. [PMC free article] [PubMed] [Google Scholar]
- Reddy JK, Goel SK, Nemali MR, Carrino JJ, Laffler TG, Reddy MK, Sperbeck SJ, Osumi T, Hashimoto T, Lalwani ND, et al. Transcription regulation of peroxisomal fatty acyl-CoA oxidase and enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase in rat liver by peroxisome proliferators. Proc Natl Acad Sci U S A. 1986;83:1747–1751. doi: 10.1073/pnas.83.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy JK, Hashimoto T. Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: an adaptive metabolic system. Ann Rev Nutr. 2001;21:193–230. doi: 10.1146/annurev.nutr.21.1.193. [DOI] [PubMed] [Google Scholar]
- Reue K. A thematic review series: lipid droplet storage and metabolism: from yeast to man. J Lipid Res. 2011;52:1865–1868. doi: 10.1194/jlr.E020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodin J. Electron microscopy of the kidney. Am J Med. 1958;24:661–675. doi: 10.1016/0002-9343(58)90373-5. [DOI] [PubMed] [Google Scholar]
- Rigatuso JL, Legg PG, Wood RL. Microbody formation in regenerating rat liver. J Histochem Cytochem. 1970;18:893–900. doi: 10.1177/18.12.893. [DOI] [PubMed] [Google Scholar]
- Rodemer C, Thai TP, Brugger B, Kaercher T, Werner H, Nave KA, Wieland F, Gorgas K, Just WW. Inactivation of ether lipid biosynthesis causes male infertility, defects in eye development and optic nerve hypoplasia in mice. Hum Mol Gen. 2003;12:1881–1895. doi: 10.1093/hmg/ddg191. [DOI] [PubMed] [Google Scholar]
- Roos DS, Choppin PW. Tumorigenicity of cell lines with altered lipid composition. Proc Natl Acad Sci U S A. 1984;81:7622–7626. doi: 10.1073/pnas.81.23.7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader M. Tubulo-reticular clusters of peroxisomes in living COS-7 cells: dynamic behavior and association with lipid droplets. J Histochem Cytochem. 2001;49:1421–1429. doi: 10.1177/002215540104901110. [DOI] [PubMed] [Google Scholar]
- Schrader M, Bonekamp NA, Islinger M. Fission and proliferation of peroxisomes. Biochim Biophys Acta. 2012;1822:1343–1357. doi: 10.1016/j.bbadis.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Schrader M, Grille S, Fahimi HD, Islinger M. Peroxisome interactions and cross-talk with other subcellular compartments in animal cells. Subcell Biochem. 2013;69:1–22. doi: 10.1007/978-94-007-6889-5_1. [DOI] [PubMed] [Google Scholar]
- Schupp M, Lazar MA. Endogenous ligands for nuclear receptors: digging deeper. J Biol Chem. 2010;285:40409–40415. doi: 10.1074/jbc.R110.182451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh I, Moser AE, Goldfischer S, Moser HW. Lignoceric acid is oxidized in the peroxisome: implications for the Zellweger cerebro-hepato-renal syndrome and adrenoleukodystrophy. Proc Natl Acad Sci U S A. 1984;81:4203–4207. doi: 10.1073/pnas.81.13.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder F, Wood R. Alkyl and alk-1-enyl ethers of glycerol in lipids from normal and neoplastic human tissues. Cancer Res. 1969;29:251–257. [PubMed] [Google Scholar]
- Subramani S. Components involved in peroxisome import, biogenesis, proliferation, turnover, and movement. Physiol Rev. 1998;78:171–188. doi: 10.1152/physrev.1998.78.1.171. [DOI] [PubMed] [Google Scholar]
- Swinkels BW, Gould SJ, Bodnar AG, Rachubinski RA, Subramani S. A novel, cleavable peroxisomal targeting signal at the amino-terminus of the rat 3-ketoacyl-CoA thiolase. EMBO J. 1991;10:3255–3262. doi: 10.1002/j.1460-2075.1991.tb04889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak HF, Braakman I, van der Zand A. Peroxisome formation and maintenance are dependent on the endoplasmic reticulum. Annu Rev Biochem. 2013;82:723–744. doi: 10.1146/annurev-biochem-081111-125123. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- Tsukahara T, Tsukahara R, Yasuda S, Makarova N, Valentine WJ, Allison P, Yuan H, Baker DL, Li Z, Bittman R, Parrill A, Tigyi G. Different residues mediate recognition of 1-O-oleyllysophosphatidic acid and rosiglitazone in the ligand binding domain of peroxisome proliferator-activated receptor gamma. J Biol Chem. 2006;281:3398–3407. doi: 10.1074/jbc.M510843200. [DOI] [PubMed] [Google Scholar]
- Tzameli I, Fang H, Ollero M, Shi H, Hamm JK, Kievit P, Hollenberg AN, Flier JS. Regulated production of a peroxisome proliferator-activated receptor-gamma ligand during an early phase of adipocyte differentiation in 3T3-L1 adipocytes. J Biol Chem. 2004;279:36093–36102. doi: 10.1074/jbc.M405346200. [DOI] [PubMed] [Google Scholar]
- van der Zand A, Gent J, Braakman I, Tabak HF. Biochemically distinct vesicles from the endoplasmic reticulum fuse to form peroxisomes. Cell. 2012;149:397–409. doi: 10.1016/j.cell.2012.01.054. [DOI] [PubMed] [Google Scholar]
- van Roermund CW, Visser WF, Ijlst L, van Cruchten A, Boek M, Kulik W, Waterham HR, Wanders RJ. The human peroxisomal ABC half transporter ALDP functions as a homodimer and accepts acyl-CoA esters. FASEB J. 2008;22:4201–4208. doi: 10.1096/fj.08-110866. [DOI] [PubMed] [Google Scholar]
- Vance JE. Lipoproteins secreted by cultured rat hepatocytes contain the antioxidant 1-alk-1-enyl-2-acylglycerophosphoethanolamine. Biochim Biophys Acta. 1990;1045:128–134. doi: 10.1016/0005-2760(90)90141-j. [DOI] [PubMed] [Google Scholar]
- Vanhove GF, Van Veldhoven PP, Fransen M, Denis S, Eyssen HJ, Wanders RJ, Mannaerts GP. The CoA esters of 2-methyl-branched chain fatty acids and of the bile acid intermediates di- and trihydroxycoprostanic acids are oxidized by one single peroxisomal branched chain acyl-CoA oxidase in human liver and kidney. J Biol Chem. 1993;268:10335–10344. [PubMed] [Google Scholar]
- Verhoeven NM, Roe DS, Kok RM, Wanders RJ, Jakobs C, Roe CR. Phytanic acid and pristanic acid are oxidized by sequential peroxisomal and mitochondrial reactions in cultured fibroblasts. J Lipid Res. 1998;39:66–74. [PubMed] [Google Scholar]
- Wanders RJ. Peroxisomes in human health and disease: metabolic pathways, metabolite transport, interplay with other organelles and signal transduction. Subcell Biochem. 2013;69:23–44. doi: 10.1007/978-94-007-6889-5_2. [DOI] [PubMed] [Google Scholar]
- Wanders RJ, Ferdinandusse S, Brites P, Kemp S. Peroxisomes, lipid metabolism and lipotoxicity. Biochim Biophys Acta. 2010;1801:272–280. doi: 10.1016/j.bbalip.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Wanders RJ, Tager JM. Lipid metabolism in peroxisomes in relation to human disease. Mol Aspects Med. 1998;19:69–154. doi: 10.1016/s0098-2997(98)00003-x. [DOI] [PubMed] [Google Scholar]
- Wanders RJ, Visser WF, van Roermund CW, Kemp S, Waterham HR. The peroxisomal ABC transporter family. Pflugers Arch. 2007;453:719–734. doi: 10.1007/s00424-006-0142-x. [DOI] [PubMed] [Google Scholar]
- Wanders RJ, Vreken P, Ferdinandusse S, Jansen GA, Waterham HR, van Roermund CW, Van Grunsven EG. Peroxisomal fatty acid alpha- and beta-oxidation in humans: enzymology, peroxisomal metabolite transporters and peroxisomal diseases. Biochem Soc Trans. 2001;29:250–267. doi: 10.1042/0300-5127:0290250. [DOI] [PubMed] [Google Scholar]
- Wanders RJ, Waterham HR. Biochemistry of mammalian peroxisomes revisited. Annu Rev Biochem. 2006;75:295–332. doi: 10.1146/annurev.biochem.74.082803.133329. [DOI] [PubMed] [Google Scholar]
- Wang YX. PPARs: diverse regulators in energy metabolism and metabolic diseases. Cell Res. 2010;20:124–137. doi: 10.1038/cr.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemer EA, Luers GH, Faber KN, Wenzel T, Veenhuis M, Subramani S. Isolation and characterization of Pas2p, a peroxisomal membrane protein essential for peroxisome biogenesis in the methylotrophic yeast Pichia pastoris. J Biol Chem. 1996;271:18973–18980. doi: 10.1074/jbc.271.31.18973. [DOI] [PubMed] [Google Scholar]
- Xu HE, Li Y. Ligand-dependent and -independent regulation of PPAR gamma and orphan nuclear receptors. Sci Signal. 2008;1:pe52. doi: 10.1126/scisignal.148pe52. [DOI] [PubMed] [Google Scholar]
- Zhang C, Baker DL, Yasuda S, Makarova N, Balazs L, Johnson LR, Marathe GK, McIntyre TM, Xu Y, Prestwich GD, Byun HS, Bittman R, Tigyi G. Lysophosphatidic acid induces neointima formation through PPARgamma activation. J Exp Med. 2004;199:763–774. doi: 10.1084/jem.20031619. [DOI] [PMC free article] [PubMed] [Google Scholar]