Abstract
Fatigue is the most common symptom related to cytotoxic chemotherapeutic treatment of cancer. Peripheral inflammation associated with cytotoxic chemotherapy is likely a causal factor of fatigue. The neural mechanisms by which cytotoxic chemotherapy associated inflammation induces fatigue behavior are not known. This lack of knowledge hinders development of interventions to reduce or prevent this disabling symptom. Infection induced fatigue/lethargy in rodents is mediated by suppression of hypothalamic orexin activity. Orexin is critical for maintaining wakefulness and motivated behavior. Though there are differences between infection and cytotoxic chemotherapy in some symptoms, both induce peripheral inflammation and fatigue. Based on these similarities we hypothesized that cytotoxic chemotherapy induces fatigue by disrupting orexin neuron activity. We found that a single dose of a cytotoxic chemotherapy cocktail (cyclophosphamide, adriamycin, 5-fluorouracil—CAF) induced fatigue/lethargy in mice and rats as evidenced by a significant decline in voluntary locomotor activity measured by telemetry. CAF induced inflammatory gene expression—IL-1R1 (p<0.001), IL-6 (p<0.01), TNFα (p<0.01), and MCP-1 (p<0.05) —in the rodent hypothalamus 6 to 24 hours after treatment during maximum fatigue/lethargy. CAF decreased orexin neuron activity as reflected by decreased nuclear cFos localization in orexin neurons 24 hours after treatment (p<0.05) and by decreased orexin-A in cerebrospinal fluid 16 hours after treatment (p<0.001). Most importantly, we found that central administration of1 μg orexin-A restored activity in CAF-treated rats (p<0.05). These results demonstrate that cytotoxic chemotherapy induces hypothalamic inflammation and that suppression of hypothalamic orexin neuron activity has a causal role in cytotoxic chemotherapy-induced fatigue in rodents.
Keywords: Orexin, Hypothalamus, Sickness behavior, Fatigue, Cytotoxic chemotherapy, Cancer treatment, Wakefulness, Activity, Inflammation
1. Introduction
Fatigue is the most common symptom with cytotoxic chemotherapeutic treatment for cancer (Reilly et al., 2013; Shi et al., 2011). Acute fatigue usually occurs after each dose of chemotherapy (de Jong et al., 2004; Schwartz et al., 2000) and it frequently persists weeks, months, or years after chemotherapy treatment is completed (Berger et al., 2012). Fatigue negatively impacts physical and social function, return to work, and quality of life (Curt et al., 2000; Shi et al., 2011; Spelten et al., 2003).
It is not known how cytotoxic chemotherapy causes fatigue. There is increasing evidence that chemotherapy-induced inflammation is an underlying causal mechanism (Bower and Lamkin, 2012; Cleeland et al., 2003; Dantzer et al., 2012; Ryan et al., 2007; Saligan and Kim, 2012; Wood and Weymann, 2013). Cytotoxic chemotherapy induces inflammatory cytokine production in macrophages in vitro and when administered to rodents (Elsea et al., 2008; Sauter et al., 2011; Wong et al., 2012; Wood et al., 2006). In clinical studies, elevated blood levels of IL-6 correlate with fatigue in cancer patients exposed to cytotoxic chemotherapy (Liu et al., 2012; Schubert et al., 2007). In addition, treatment for malignant melanoma with the cytokine interferon-α induces fatigue that is not responsive to antidepressant therapy (Capuron et al., 2002). The mechanisms by which chemotherapy-induced peripheral inflammation affects neural signaling to cause fatigue behavior are not understood. Our laboratory recently reported that inflammation-induced fatigue from lipopolysaccharide (LPS) administration was associated with reduced hypothalamic orexin neuron activity (Grossberg et al., 2011). Orexin neuron signaling promotes arousal and wakefulness (Anaclet et al., 2009; Carter et al., 2013; Kantor et al., 2013; Saper et al., 2005). Since cytotoxic chemotherapy is similar to LPS treatment in that they both induce peripheral inflammation and fatigue, the purpose of this study was to test our hypothesis that cytotoxic chemotherapy induces fatigue by disrupting orexin neuron activity. Since LPS treatment induces hypothalamic inflammatory cytokine expression, a second purpose of this study was to determine if cytotoxic chemotherapy induces inflammatory cytokine expression in the hypothalamus.
2. Materials and Methods
2.1. Animals
Mice and rats were used in experiments. Female C57BL/6J mice (strain #000664; 8-12 weeks of age) were purchased from Jackson Laboratories (Bar Harbor, ME). Male Sprague Dawley rats (250-350 g) were purchased from Charles River Laboratories (Wilmington, MA). Mice and rats show similar responses to both the absence of orexin signaling and the administration of orexin-A (Anaclet et al., 2009; Furutani et al., 2013; Gerashchenko et al., 2001; Gerashchenko et al., 2003). Mice were used in order to obtain voluntary wheel running activity after cytotoxic chemotherapy treatment. Rats were used for measurement of orexin-A in cerebrospinal fluid and to determine the effect of administration of orexin-A on ambulatory activity. Animals were maintained in pathogen-free rooms on a normal 12 hour light/dark period with lights on from 0600 to 1800 with ad libitum access to food (rodent diet 5001, Purina Mills) and water. In experiments where data was collected on animals more than 48 hours after receiving cytotoxic chemotherapy, both sham-treated and cytotoxic chemotherapy-treated animals were given water containing 150μg/mL amoxicillin (oral suspension, Sandoz) to reduce risk of infection secondary to neutropenia after cytotoxic chemotherapy. Animals were weighed daily during experiments. Experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Oregon Health & Science University Department of Comparative Medicine Institutional Animal Use and Care Committee.
2.2. Cytotoxic Chemotherapy Administration
Animals were administered a combination of cyclophosphamide (Cytoxan), doxorubicin (Adriamycin), and 5-fluorouracil (5-FU) (CAF) at concentrations of 167mg/kg, 4mg/kg, and 167mg/kg respectively in mice and 75 mg/kg, 4 mg/kg, and 75 mg/kg in rats. This drug regimen was chosen to reflect a clinically relevant adjuvant treatment regimen in breast cancer patients. The body surface area normalization method (Reagan-Shaw et al., 2007) was used to calculate the mouse dose in mg/kg based on the human dose of Cytoxan (500mg/m2), Adriamycin (50mg/m2) and 5-FU (500mg/m2) (Smalley et al., 1983). The tolerability of this dosing schema in mice has been examined extensively in our laboratory. Mice administered 4 separate doses of CAF at 3-week intervals did not meet any criteria for euthanasia during treatment (unpublished observations). Drug concentrations in rat were based on the following reports: the drug combination given at 65/6.5/65 mg/kg 21 days apart with euthanization required after the second dose when 6.5 mg/kg of adriamycin was in the cocktail but not with 6.5 mg/kg methotraxate (Little, 2007), cyclophosphamide at 75 mg/kg (Fardell et al., 2012), and 5-FU at 75 mg/kg in a cocktail given weekly for 4 weeks (Briones and Woods, 2011). In preliminary work we treated rats with 75/4/75 mg/kg of CAF for 2 doses given 8 days apart. Weight and activity in treated rats returned to baseline after 8 days. Cyclophosphamide was purchased from Baxter Healthcare Corporation. Doxorubicin-HCL was purchased from Bedford Labs (Bedford, OH, USA), dissolved in sterile, deionized water to attain a stock solution of 2 mg/ml, and stored at 4°C. 5-FU was purchased as a 50mg/ml solution from American Pharmaceutical Partners (Schaumburg, IL, USA).
Animals were administered the drugs in two separate intraperitoneal (ip) injections to prevent precipitation of Adriamycin by 5-fluorouracil. Cytoxan and Adriamycin were combined in a volume of 1 mL normal saline (NS) in mice and in 2 mL NS in rats and injected ip. Sixty minutes later, animals were injected ip with 1 mL (mice) or 2 mL (rats) NS containing 5-FU. Sham-treated mice were injected with the same volumes of NS without drug. This injection volume was used to reduce localized tissue inflammation at the injection site, to provide fluid, and to improve absorption of the drug. Animals were returned to their home cages after injection.
2.3. Experiment 1: The effect of cytotoxic chemotherapy on brain inflammatory gene expression
Mice housed 5 to a cage were injected with CAF or NS between 0800 and 1000 (for 3, 6, 24, and 48 hour samples) or between 1400 and 1600 (for 16 hour samples). At 3, 6, 16, 24, and 48 hours post injection, mice were deeply sedated with ketamine-xylazine (150 mg/kg-15 mg/kg ip) then perfused with 25 mL ice cold RNase-free 0.01 molar phosphate buffered solution (PBS). Whole brains were removed and hypothalamic blocks and brainstems excised. Hypothalamic blocks with median eminence attached were excised by making coronal cuts at the rostral extent of the optic chiasm caudal to the mammillary body and saggital cuts along the optic tracts. The cortex was removed at the corpus callosum. Brainstems were excised by cutting rostral to the cerebellum and gently removing the cerebellum. Tissue was placed in RNAlater® (Ambion) solution on ice, and stored at 4°C up to 48 hours prior to ribonucleic acid (RNA) extraction.
Total RNA was extracted from hypothalamic and brainstem tissue using QIAGEN RNeasy kits (QIAGEN, Inc., Valencia, CA) and included digestion of the homogenate with proteinase K (1% by volume) and removal of deoxyribonucleic acid (DNA) from total RNA using ribonuclease-free deoxyribonuclease (QIAGEN). Total RNA was quantified (Nanodrop) and stored at −80 C. Complementary DNA (cDNA) was generated using the TaqMan Reverse Transcription Kit (Applied Biosystems, Inc., Foster City, CA). Each cDNA synthesis reaction (10 μL) contained 100 ng RNA, 1 μL 10x RT buffer, 2 μL 25mM MgCl2, 1.6 μL 10mM deoxynucleotide triphosphates, 0.6 μL 50μM random hexamers, 0.4 μL RNAse inhibitor, 0.6 μL Multiscribe RTase, and nuclease-free water. Reverse transcriptase reactions were run on an Eppendorf Mastercycler (Eppendorf AG, Hamburg, Germany) programmed for 10 min at 25 C, 60 min at 37 C, and 5 min at 95 C. Samples were diluted with 40 μL nuclease-free water and stored at 4 C until RT-PCR was performed.
Relative levels of interleukin-1 beta (IL-1β), IL-1 receptor type I (IL-1RI), interleukin-6 (IL-6), tumor necrosis factor alpha (TNFα), and monocyte chemoattractant protein-1 (MCP-1) mRNAs were measured in brain tissue collected from CAF and sham-treated mice 3, 6, 16, 24, and 48 hours after injection (n≥5 per treatment and time). These cytokines and chemokine were tested because they are induced in serum in mice and in murine macrophages after treatment with cytotoxic chemotherapeutics (Elsea et al., 2008; Sauter et al., 2011). Quantitative RT-PCR was performed on an ABI 7300 Real-Time PCR System using prevalidated TaqMan master mix and commercial mouse-specific primer probes (IL-1 β,#Mn01336189_m1; IL-1RI, #Mn00434237_m1; IL-6, #Mn00446190_m1; TNFα, #Mn00443260_g1; MCP-1, #Mn00441242_m1; Applied Biosystems). Each RT-PCR reaction contained 5 μL of 2x Master Mix, 0.5 μL primer probe, and 5 μL of 2 ng/μL of cDNA. Reactions were run in duplicate or triplicate. Raw cycle threshold values from Eukaryotic 18S ribosomal subunit and GAPDH endogenous controls were compared between groups to validate that gene expression of internal controls was not affected by experimental treatment. Gene expression is expressed as fold change relative to sham treated group using the 2−ΔΔCT method (Livak and Schmittgen, 2001) (ABI 7300 relative quantity study software version 1.3). Statistical analyses were conducted on the ΔCT values for each gene since those values have a normal distribution.
2.4. Experiment 2. The effect of cytotoxic chemotherapy on fatigue and hypothalamic orexin neuronal activity
Voluntary wheel running was measured in 12 mice housed individually in shoe-box cages with an activity wheel. Wheel turns were collected automatically in 15 minute bins with a magnetic reed switch (MiniMitter) and the Vital View Data Acquisition System (Vital View, Bend, OR). After acclimation for 10 days, baseline wheel running activity was collected for 10 days. Running activity was calculated as the percent change in voluntary wheel running activity from baseline for each mouse.
To indirectly quantify orexin neural activity, cFos expression in orexin neurons was examined and quantified with immunohistochemistry (IHC) as described previously (Grossberg et al., 2011). Briefly, animals were deeply anesthetized using sodium pentobarbital (65 mg/kg) and killed by transcardial perfusion with PBS followed by fixation with 4% paraformaldehyde (PFA) between 1915 and 1945, 24 hours after sham or CAF injection (6 animals per treatment). The 24 hour treatment time coincides with acute reduction in locomotor activity after CAF injection. Animals were treated and tissue collected on sequential nights to allow for collection of tissue for all 12 animals between the desired times. Brains were post-fixed in PFA overnight and cryoprotected in 20% sucrose for 24 hours and frozen. Brains were sectioned at 30μm into a 1:4 series on a freezing microtome. Sections were blocked in 5% normal donkey serum then incubated for 72 hrs at 4C in primary antibody containing rabbit anti-cFos antibody (SC-52, Santa Cruz Biotechnology) diluted 1:5000 and goat anti-OrexinA antibody (SC-8070, Santa Cruz Biotechnology) diluted 1:1500. Sections were washed then incubated 2 hrs at room temperature in donkey anti-rabbit Alexa 594 and donkey anti-goat Alexa 488 (both 1:500, Invitrogen). Separate sections were incubated in the absence of primary antisera to ensure specificity of secondary antibodies. Sections were mounted and cover slipped in Aqua Polymount (Polysciences, Warrington, PA). Slides were viewed and photomicrographs obtained under a fluorescent microscope with appropriate filters (model 4000 DM, Leica Microsystems; or model LSM710, Carl Zeiss). Orexin-A positive neurons with and without nuclear localization of cFos immunoreactivity (IR) were counted (group assignment masked) using a dual red/green filter and counts confirmed with images at 488 and 594 taken separately with no significant differences in counts. Orexin neuron subpopulation locations in the dorsal medial hypothalamus (DMH), the perifornical hypothalamic area (PFA), and the lateral hypothalamic area (LHA) were based on mouse brain stereotactic measurements (Paxinos and Franklin, 2001). Images were photographed by an external investigator with group assignment masked.
2.5. Experiment 3. The effect of cytotoxic chemotherapy on cerebral spinal fluid (CSF) levels of orexin-A
Orexin-A neuropeptide levels were measured in CSF collected from rats at 16 hours after drug or sham treatment (n=9 per group) as an indicator of orexin neuron activity. Rats were used in order to obtain sufficient volume of cerebrospinal fluid. Individually housed rats were administered CAF or NS between 1500 and 1600. At 0630-0730 the next day, rats were anesthetized with 2% isofluorane and a needle inserted percutaneously into the cisterna magna to collect 50 uL CSF. Animals were returned to their home cage on a warming mat during recovery. Protease inhibitor (Roche) was added to the CSF samples according to manufacturer's instructions. Samples were frozen on dry ice and stored at −80C. Levels of orexin-A in each CSF sample was measured using a commercial radioimmunoassay (RIA) kit (Phoenix Pharmaceuticals). RIA was performed in duplicate using 25 uL of CSF according to the manufacturer's instructions. The interassay variability assessed by replicate analysis of a 250 pg/ml standard was 0.43%.
2.6. Experiment 4: To determine if central orexin-A injection restores ambulatory activity
Voluntary home-cage ambulatory activity and body temperature were measured in rats with telemetric transponders (MiniMitter) surgically implanted in the scapular region as described previously (Grossberg et al., 2011). A ventricular cannula was surgically implanted into rats (n=20) 3 to 4 days after transponder implantation as described previously (Grossberg et al., 2011). Briefly, a 22 gauge sterile guide cannula with obturator stylet (Plastics One, Roanoke, VA) was implanted into the lateral ventricle and fixed in place with multiple screws and dental cement. The coordinates used were 1.5 mm lateral to midline,1.0 mm posterior to bregma, and 4.1 mm below the skull surface (Paxinos and Watson, 1998). After 3 days of recovery, animals and the lateral ventricle cannula were handled daily for 3 days to minimize stress responses with intracerebroventricular (icv) administration of fluid. Animals were allowed to acclimate for at least 5 days following ventricular cannulation surgery. Placement of cannula in the lateral ventricle was confirmed with return of clear cerebral spinal fluid prior to icv administration of orexin-A/saline or injection of 1 μL dye and microscopic confirmation at the end of the experiment. Body temperature and movement on the x-, y-, and z- axes was recorded in 5 minute intervals throughout the experiment with Vital View Data Acquisition System (Vital View, Bend, OR). Baseline ambulatory activity was collected for 3 days. Rats were injected ip with CAF or NS between 1530 and 1730 (CAF n=12; NS n=8).
Orexin neuropeptide central injection was given 24 hours after CAF or sham injections. Rats were given 1 μL of either sterile 0.9% saline (NS) or I uL of saline with 1 μg of orexin-A (Ox-A) (California Peptide Research) into the lateral ventricle cannula between 1720 and 1740 (ip CAF-treated rats n=6 per icv group; ip NS-treated rats n=4 per icv group). The 1 μg dose of orexin-A was selected because it increased early dark phase activity in LPS-treated rats but not in sham-treated rats (Grossberg et al., 2011). Activity after ip CAF or sham treatment and after icv administration of NS or Ox-A was compared to baseline for each animal. Inactive bouts were assigned when a 5 minute interval had a recorded movement of 5 counts or less.
2.7. Experiment 5: Orexin neuronal activation after multiple doses of cytotoxic chemotherapy
Voluntary wheel running activity (VWRA) was measured in mice as described in Experiment 2 except wheel turns were summed over 60 min intervals. After establishing a 10-day baseline VWRA mice were separated into 2 groups. Mice in group 1 (n= 6) were injected with CAF while mice in group 2 were injected with NS. Mice were injected with an additional 3 doses of CAF or NS at 21-day intervals, mimicking a clinically relevant treatment regimen. All mice were provided with drinking water supplemented with antibiotics (150 μg/mL amoxicillin oral suspension, Sandoz) to prevent infection related to neutropenia, secondary to chemotherapy-induced bone marrow suppression.
Three weeks after the fourth dose of CAF or NS treatment, while CAF-treated animals still had fatigue, mice were terminally sedated between 1915 and 2000, perfused transcardially with 25 mL PBS followed by 25 mL 4% PFA as described in Experiment 2. Whole brains were removed, frozen, and sectioned for IHC as described in Experiment 2. Following IHC staining, sections (masked for treatment) were counted for nuclear visualization of c-Fos protein in neurons immunoreactive for orexin-A.
2.8. Statistical analysis
Data were graphed and analyzed using GraphPad Prism 5. Comparisons between two groups at a single time point were performed using two-tailed Student's t-test. Comparisons between two treatments at multiple time points were performed using two-way ANOVA with post hoc Bonferonni-corrected t-test. Comparisons among 3 or more groups were examined with a one-way ANOVA with post hoc Bonferonni-corrected t-test. Repeated measures ANOVA was used to examine change in repeated measures of activity between treatment groups. Differences between groups were considered significant when p<0.05.
3. Results
3.1. Cytotoxic chemotherapy induces brain inflammatory gene expression
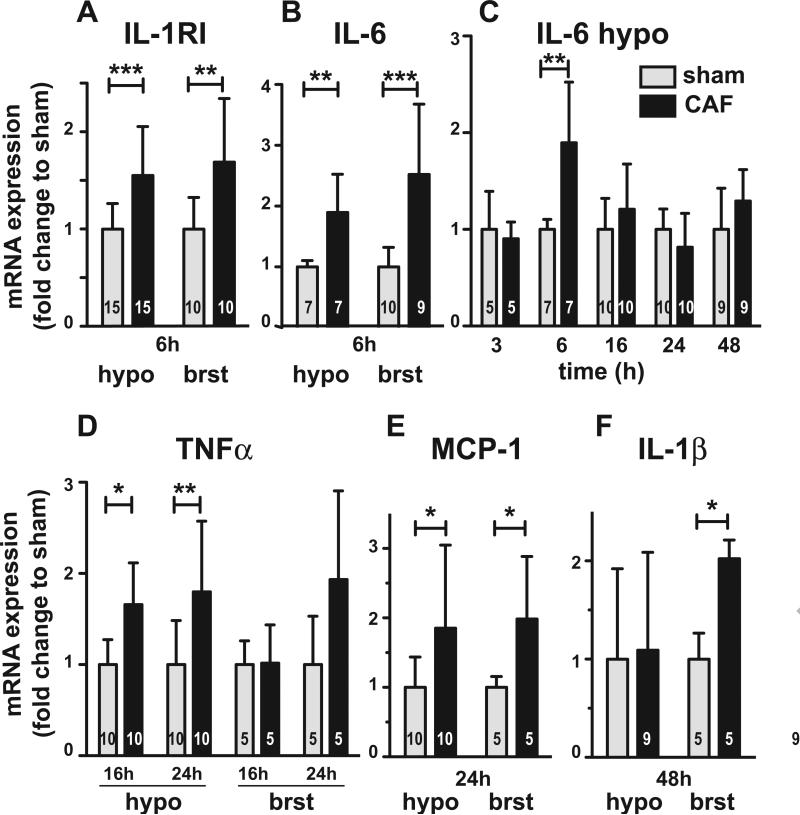
Since previous evidence suggests behavioral change such as fatigue/lethargy is mediated by neural signaling in the brainstem or hypothalamus, we tested if peripherally administered cytotoxic chemotherapy induces inflammatory gene expression in those brain regions. We examined gene expression of IL-1R1, IL-1β, IL-6, TNFα, and MCP-1 at 3, 6, 16, 24, and 48 hours after administration of NS or CAF. There was no increase in RNA expression of any of these inflammatory genes in either brain region at 3 hours after treatment. Il-1R1 gene expression was significantly increased in both the brainstem (1.7 fold increase±0.12, n=10) (p<.01) and hypothalamus (1.6 fold increase±0.20, n=15) (p<.001) at 6 hours after treatment (Fig 1A). IL-6 gene expression was also significantly increased in both the brainstem (2.5 fold increase±0.38, n=9) (p<0.001) and hypothalamus (1.9 fold increase±0.22, n=7) (p<0.01) at 6 hours after treatment (Fig 1B and C). This increase did not approach significance at any other time point tested (Fig 1C). TNFα gene expression was significantly induced in the hypothalamus at both 16 (1.7 fold increase±0.14, n=10) (p<0.05) and 24 hours (1.8 fold increase±0.24, n=10) (p<0.01) after treatment (Fig 1D). Although the average expression was elevated, it was not significantly increased in the brainstem at 24 hours after treatment likely due to variability (1.9 fold increase±0.43, n=5) (Fig. 1D). MCP-1 expression was significantly increased in both the brainstem (2.0 fold increase±0.40, n=5) (p<.05) and hypothalamus (1.8 fold increase±0.37, n=10) (p<0.05) at 24 hours after treatment (Fig. 1E), but not at other time points tested. Lastly, IL-1β gene expression was significantly increased in the brainstem, but not in the hypothalamus, at 48 hours after treatment (2.0 fold increase±0.08, n=5) (p<0.05) (Fig. 1F). Taken together, these results indicate that cytotoxic chemotherapy induces inflammatory gene expression in both the brainstem and hypothalamus within 48 hours of treatment.
Figure 1.
Hypothalamic (hypo) and brainstem (brst) mRNA levels of inflammatory genes at 3, 6, 16, 24, and 48 hours after ip sham or cyclophosphamide, adriamycin, and fluorouracil (CAF) administration in mice. Significant differences in gene expression are shown and presented as fold-change relative to sham treatment . The mean and standard error are graphed. The number in each group is given in bars. A, IL-1R1 gene expression is significantly induced in both brain regions 6h after ip CAF treatment. Expression after ip CAF administration does not differ from sham at other times after treatment. B, IL-6 gene expression is significantly induced 6 h after CAF treatment relative to sham in both brain regions. C, IL-6 gene expression in the hypothalamus after CAF treatment is not significantly different than sham at time points other than 6 h after treatment. D, TNFα gene expression at 16 h and 24h after CAF treatment is significant only in the hypothalamus. E, MCP-1 gene expression is significant in both brain regions at 24 h after treatment. F, IL-1β gene expression is significantly induced in the brain stem at 48 h after treatment. Interleukin 1 receptor type 1 (IL-1R1), Interleukin 1β (IL-1β), Interleukin 6 (IL-6), Tumor Necrosis Factor alpha (TNFα), Monocyte chemoattractant protein (MCP-1/CCL-2). *p<0.05, **p<0.01, ***p<0.001
3.2. Reduction in ambulatory activity after cytotoxic chemotherapy administration is associated with decreased dark-phase orexin neuron activity
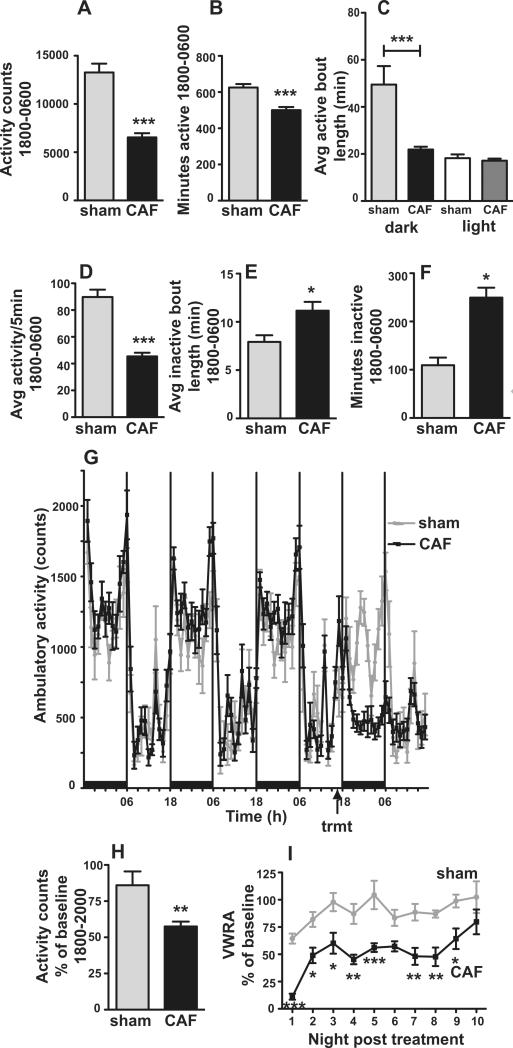
CAF administered intraperitoneally to rats 3 hours before the start of the dark phase caused a significant reduction in ambulatory activity during the dark phase (Fig. 2A-I). CAF-treatment caused a significant reduction in dark phase activity counts (t(18)=7.42, p<0.0001) (Fig 2A), total minutes of activity (t(18)=4.60, p<0.001) (Fig 2B), average duration of sustained activity between inactive bouts (t(18)=4.22, p<0.001) (Fig 2C), and average rate of activity throughout the dark phase (t(18)=7.93, p<0.0001) (Fig 2D). CAF-treatment caused a significant increase in average duration of inactive bouts (t(18)=2.59, p<0.05) (Fig 2E) and total minutes of inactivity (t(18)=4.96, p<0.001) (Fig 2F) during the dark phase.
Figure 2.
Cytotoxic chemotherapy reduces dark phase locomotor activity in rats and mice. A, Locomotor activity summed across the dark phase (1800-0600) is reduced following CAF treatment in rats. B, Total minutes of activity during the dark phase is reduced following CAF treatment. C, The average duration of bouts of activity are decreased during the dark phase but not during the light phase after CAF treatment. D, The average rate of activity (average of movement during 5 minute bins that had activity) is decreased during the dark phase. E, CAF increases the average duration of inactive periods during the dark phase. F, CAF treatment increases the total minutes of inactivity during the dark phase. G, Baseline activity in the dark and light phase prior to and after CAF or sham treatment. H, CAF treatment decreases ambulatory activity calculated as % of baseline activity in the first 2 hours of the dark phase, 1800-2000. Rats n=8 sham, n=12 CAF. I, Voluntary wheel running activity in mice (n=6 each treatment) slowly returns to baseline at 10 nights after treatment with CAF. *p<0.05, **p<0.01, ***p<0.001
Though the maximum decrease in activity occurred 6 to 12 hours after treatment (Fig 2G), activity was significantly decreased during the first 2 hours of darkness, 4 hours after CAF treatment (Fig 2H). During the first 2 hours of darkness (1800-2000), activity in CAF-treated animals was 57±3% of baseline activity (n=12) compared to sham (86±9%, n=8) (t(18)=3.28, p<0.01) (Fig.2H). Baseline activity between the groups prior to treatment was not significantly different at 1800-2000 (p=0.2) or throughout the dark phase, 1800-0600 (p=0.1) (Fig 2G). Locomotor activity, both ambulatory and voluntary wheel running, was initiated at the start of darkness in both treatment groups. Total counts of dark phase ambulatory activity in rats and voluntary wheel running activity in mice slowly returned to baseline 8 and 10 nights, respectively, after administration of a single dose of cytotoxic chemotherapy (Fig 2I). Irrespective of treatment, both rats and mice had limited activity during the light phase (Fig 2C and 2G). There was no difference in the counts of activity (p=0.6) or the average duration of bouts of activity (p=0.5) (Fig 2C) or inactivity (p=0.1) during the light phase between CAF and sham-treated animals.
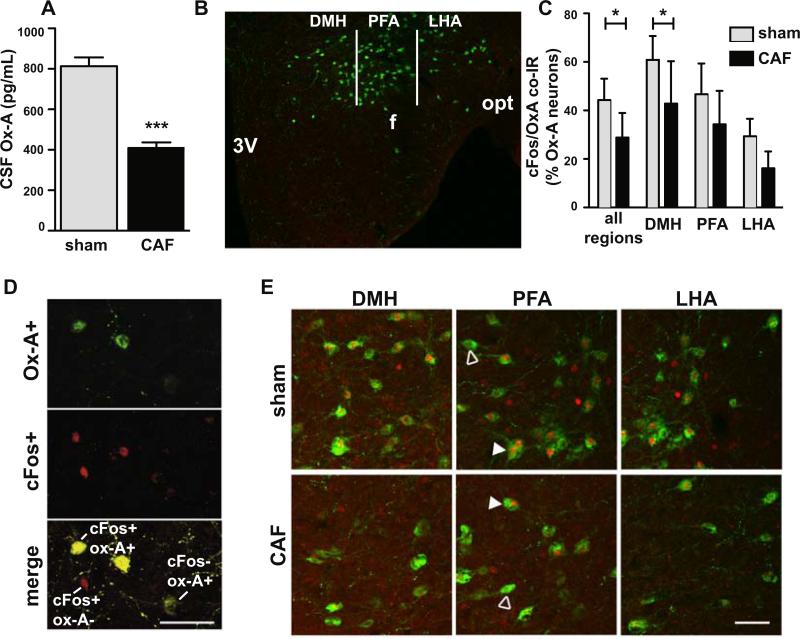
Since impaired orexin neuron activity is associated with decreased locomotor activity in rodents, we tested if CSF levels of orexin-A was decreased at the end of the dark phase after treatment with cytotoxic chemotherapy, during the period of acute fatigue. CAF-treated rats had a 49% reduction in CSF levels of orexin-A compared to sham-treated rats (Figure 3A) (mean-CAF=400 ± 27 pg/mL, n=9; mean-sham=813 ± 43 pg/mL, n=9; t(16)=8.04, p<0.0001).
Figure 3.
CAF treatment results in decreased dark phase orexin neuron activity. A, Rats treated with CAF at 1600 had decreased level of orexin-A (Ox-A) in cerebrospinal fluid (CSF) collected the following morning (n=9 each treatment). B, Image of the mouse hypothalamic area (near Bregma – 1.7 mm) illustrating orexin neurons in the dorsal medial hypothalamus (DMH) (width of 500 μm lateral from 3rd ventricle-3V), perifornical hypothalamic area (PFA) (width of 500 μm lateral from DMH and includes the fornix (f), and lateral hypothalamic area (LHA) (400 μm lateral from PFA to optic nerve (opt)) subpopulation areas counted. C, Percentage of orexin neurons with cFos immunoreactivity (IR) is less following treatment with CAF vs sham, indicating CAF treatment reduces the normal evening rise of cFos in orexin neurons in the hypothalamus in mice. Percentage of orexin neurons with cFos coexpression given for all orexin neurons in the hypothalamus and for separate anatomic subpopulations (n=6 each treatment). Data given as mean ± SEM. D, Photomicrographs of DMH orexin neurons after sham treatment showing immunoreactivity for orexin-A (green) in top panel, immunoreactivity for cFos (red) in middle panel, and the merged image showing 2 of the 3 orexin-A+ neurons co-stain for nuclear cFos (yellow cells), one orexin-A+ neuron is negative for cFos (green cell), and a cell negative for orexin-A that is positive for nuclear cFos (red nucleus). E, Hypothalamic orexin neurons subpopulations co-stained for cFos. Open arrowhead indicates cells expressing orexin-A but not nuclear cFos; closed arrowheads indicates cFos nuclear expression in orexin-A+ neuron. Scale bars=50 μm. *p<0.05, ***p<0.001.
To further examine the effect of chemotherapy on orexin neurons during the period of acute fatigue, we examined cFos IR in orexin neurons 24 hours after chemotherapy treatment. Hypothalamic orexin neurons in mice treated with CAF had significantly reduced nuclear cFos IR compared to sham treated controls (n=6 per group; p<0.05) (Fig. 3C and 3E). Colocalization of cFos in orexin neurons (depicted in Fig 3D) in the dorsomedial hypothalamus (DMH), perifornical area (PFA), and the lateral hypothalamic area (LHA) subpopulations (Fig 3B) revealed a significant effect of treatment (F(1,30)=13.32; p=0.001) and anatomic location (F(2,30)=18.15; p< 0.001) (Fig 3C and 3E). cFos IR in orexin neurons was significantly different between treatment groups in the DMH (Bonferroni-corrected t-test p<0.05) (Fig 3C). There was no difference in cFos IR in orexin neurons between treatment groups in the PFA and the LHA. The total number of orexin IR neurons counted was not different between treatment groups (p=0.9; data not shown).
3.3. Intracerebroventricular administration of orexin-A restores activity in rats administered cytotoxic chemotherapy
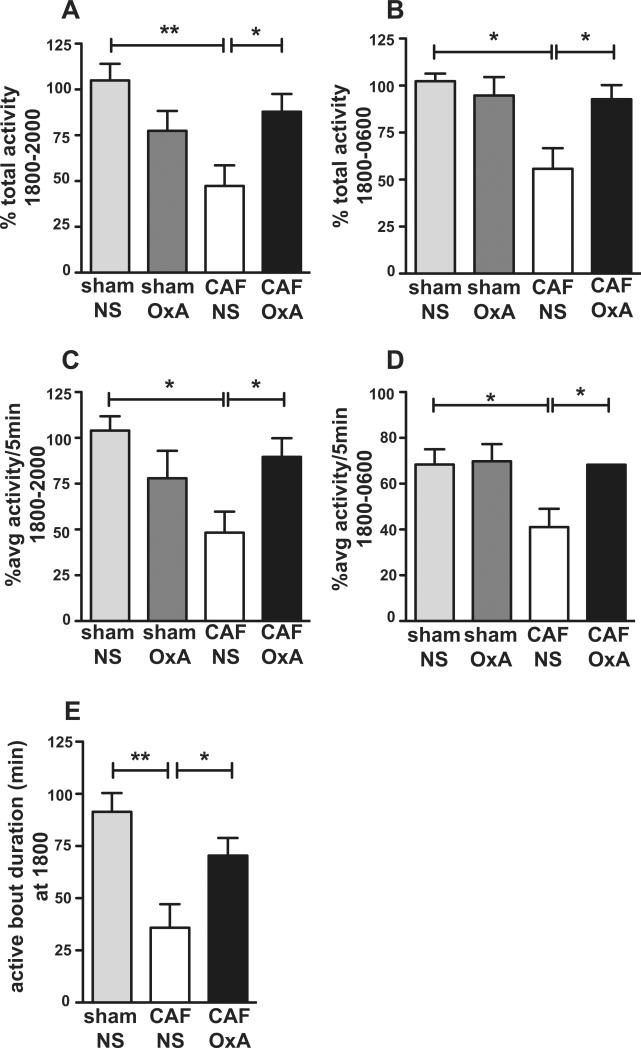
To determine if orexin has a role in the reduction in ambulatory activity following treatment with cytotoxic chemotherapy, we gave intracerebroventricular (icv) orexin-A neuropeptide or the NS diluent. We found that a1 ug bolus of orexin-A, given 30 minutes before the start of the dark phase, restored activity in CAF-treated animals. Among the chemotherapy treated rats, animals that received icv orexin-A had more activity (p<0.05) (Fig 4A) and a higher rate of activity (p<0.05) (Fig 4C) in the first 2 hours of the dark phase and throughout the 12 hour dark phase (p<.05) (Fig 4B and 4D) than animals that received icv NS. Orexin-A replacement in CAF-treated rats also increased the duration of initial activity at the beginning of the dark phase as compared to icv NS (Bonferroni t=2.61, p<0.05) (Fig 4E).
Figure 4.
Central orexin-A administration restores ambulatory activity counts (A, B) and the average activity rate per 5 minute bin (C, D) during the first 2 hours of the dark phase (A, C) and throughout the dark phase (B, D) as compared to baseline. Activity is calculated as percentage of baseline activity for each animal. E, Orexin-A administration increases duration of the first bout of activity at the beginning of dark phase. sham/NS=ip sham and icv NS (n-4), CAF/NS=ip CAF and icv NS (n=6), CAF/OxA= ip CAF and icv Orexin-A (n=6). Sham/OxA (n=4) was not significantly different than sham/NS in any of the measures.
Dark phase total activity (Fig 4B), rate of activity (Fig 4D), and duration of activity at the start of the dark phase (Fig 4E) in CAF-treated animals given icv orexin-A was not significantly different than activity in sham-treated control animals given icv NS. Animals treated with CAF that did not receive icv orexin-A showed the expected reduced activity (57% of baseline) at the beginning of the dark phase (p<.01) (Fig 4A) and throughout the dark phase (51% of baseline) (p<.05) (Fig 4B). Taken together, orexin administration in CAF-treated rats restored a pattern of dark phase activity--rate, duration, and total amount--similar to that in control animals. Activity of control animals given icv orexin-A was not statistically different than control animals given icv NS.
3.4. Orexin neuron activity suppressed after multiple cycles of cytotoxic chemotherapy
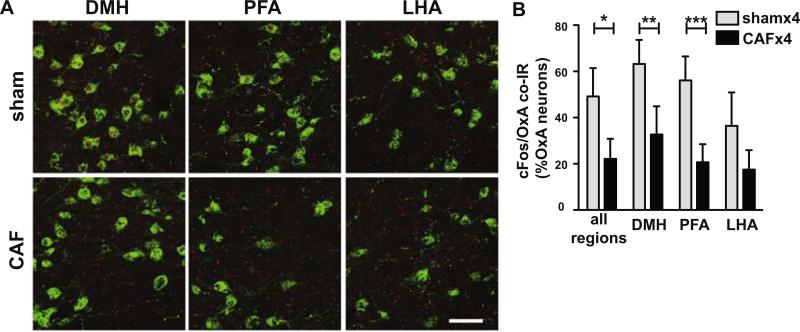
We also examined the effect of four cycles of CAF on hypothalamic orexin neuron cFos IR since prior work in our laboratory showed that fatigue can persist for weeks after multiple CAF cycles. CAF-treated mice had significantly reduced cFos IR in orexin-A-expressing neurons compared to sham treated controls (n=4 each treatment, t(6)=3.59, p=0.01) (Fig 5A and 5B). Across all regions, 22.1% of orexin-A-expressing neurons had cFos IR in CAF-treated mice vs 49.2% in sham. The 3 hypothalamic subpopulations of orexin neurons showed a significant effect of treatment (F(1,12)=14.99; 53% of variation, p<0.01) and anatomic location (F(2,12)=53.13; 20% of variation, p<0.0001). Post-hoc analysis showed a significant decrease in cFos IR in both the DMH (t=3.98, p<.01) and PFA (t=4.61, p<.001) orexin neurons (Fig 5B). No difference in cFos nuclear IR between treatment groups was observed in the LHA. Rostrocaudal location had no observed effect on cFos IR in orexin neurons. The number of orexin-A IR neurons counted across the hypothalamus and in each region did not differ with treatment (p=0.8; data not shown).
Figure 5.
The normal evening rise of cFos in orexin neurons in the hypothalamus is reduced in mice after multiple cycles of CAF treatment is given in doses 21 days apart. A, Representative photomicrographs of cFos (shown in red nuclei) in orexin neurons (shown in green) in DMH, PFA, and LHA in sham and CAF-treated mice. B, Percentage of orexin neurons with cFos IR following treatment with ip sham or CAF, given for all orexin neurons in the hypothalamus and for separate anatomic subpopulations. n=4 each treatment. Scale bar=50μM. *p<0.05, **p<0.01, ***p<0.001
3.5. Cytotoxic chemotherapy reduces body temperature
Though fever is often associated with infection and LPS-induced decreased activity, we found that rats treated with cytotoxic chemotherapy had significantly reduced dark phase body temperature (96.1% of dark phase baseline (range 34.1C to 36.8C); p<.001, n=12) compared to sham-treated controls (99.8 % of dark phase baseline (36.7C to 37.9C), n=8). Body temperature during the light phase was similar in both groups and did not differ from their light phase baseline temperature. Dark phase body temperature returned to baseline temperature the second night after cytotoxic chemotherapy treatment (data not shown), even though activity during the second night after drug treatment remained approximately 50% decreased from their dark phase baseline. Body temperature on the second night after sham or drug treatment, which had returned to baseline, was not further affected by icv central administration of the orexin-A neuropeptide or NS (data not shown).
4. Discussion
Fatigue is a common symptom associated with infection and cancer chemotherapy treatment. Infection induces peripheral inflammation that alters neural signaling in the brain, resulting in sickness behavior that conserves energy and promotes acute recovery (Dantzer et al., 2008; Kelley et al., 2003; Konsman et al., 2002). Since cytotoxic chemotherapy induces peripheral inflammation, fatigue associated with cytotoxic chemotherapy may have an underlying mechanism similar to infection-induced fatigue (Bower and Lamkin, 2012; Cleeland et al., 2003; Dantzer et al., 2012; Saligan and Kim, 2012). The neural mechanisms associated with cytotoxic chemotherapy induced fatigue are not known.
We report here three important results from this research. First, peripherally administered cytotoxic chemotherapy induces inflammatory gene expression in both the brainstem and hypothalamus within 48 hours after treatment. Second, cytotoxic chemotherapy suppresses hypothalamic orexin neuron activity. We demonstrate this by reduced orexin-A neuropeptide concentration in the cerebrospinal fluid and failure of normal physiological activation of orexin neurons (as measured by cFos nuclear localization). Third, central administration of the orexin-A neuropeptide restores ambulatory activity in cytotoxic chemotherapy-treated animals. Our results are consistent with studies demonstrating decreased orexin neuron activity in conditions involving central inflammation such as traumatic brain injury and treatment with bacterial endotoxin (Baumann et al., 2005; Gaykema and Goehler, 2009; Gerashchenko and Shiromani, 2004; Grossberg et al., 2011; Willie et al., 2012). Suppression of orexin neuron activity at a time of peak fatigue after cytotoxic chemotherapy along with restoration of activity with centrally administered orexin-A indicate that disrupted orexin neuron function contributes to fatigue/lethargy after cytotoxic chemotherapy. To our knowledge, this is the first report of altered neural activity associated with cytotoxic chemotherapy-induced fatigue.
4.1. Cytotoxic chemotherapy induces brain inflammatory signaling
The cytotoxic chemotherapeutic drugs used in this study, cyclophosphamide, adriamycin, and fluourouracil, acutely increase circulating inflammatory cytokine levels in mice (Sauter et al., 2011; Wong et al., 2012; Wood et al., 2006). Fluourouracil increases circulating MCP-1 levels at 5 and 15 days after multiple doses of drug (Mahoney et al., 2013). Yet it is not known if these cytotoxic chemotherapeutics induce hypothalamic inflammation. LPS induces peripheral and central inflammation in the hypothalamus and brainstem and anorexia and fatigue sickness behaviors (Capuron and Miller, 2011; Gayle et al., 1998). Chemotherapy induces similar sickness behaviors, suggesting a common mechanism with inflammatory stimuli such as LPS. On the other hand, we observed a decrease in core body temperature after CAF injection not followed by a febrile period. This is in contrast to the typical body temperature response after peripheral inflammatory insults, and suggests a different hypothalamic response may occur after cytotoxic chemotherapy.
We found that these cytotoxic chemotherapeutic drugs induce a similar pattern of inflammatory gene expression in both the brainstem and hypothalamus. This could result from peripheral inflammation communicated to both the brainstem and hypothalamus due to an attenuated blood brain barrier, vagal signaling (Bluthe et al., 1994), or signaling from the brainstem to the hypothalamus (Gaykema and Goehler, 2011). We were surprised to not detect elevated IL-1β gene expression at or prior to 6 hours after treatment since IL-1β is considered a key initiating cytokine for induction of inflammatory signaling. In vivo studies indicate that adriamycin induces formation of the NLRP3 inflammasome protein complex that cleaves pro-IL-1β to the active IL-1β (Sauter et al., 2011). This release of IL-1β could induce IL-1R1 and IL-6 gene expression at 6 hours after treatment without increased IL-1β gene expression. We found IL-1β gene expression was significantly induced in the brainstem at 48 hours after treatment, indicating a possible biphasic response. One limitation of this study is that we did not examine gene expression beyond 48 hours after treatment. It was not feasible to simultaneously measure the concentration of cytokines in these brain regions, so we do not know if the active form of IL-1β was increased in the hypothalamus or brainstem. In addition, we tested only the common intraperitoneal route of administration of drug. It is possible that an intravenous route could result in different central effects (Bluthe et al., 1996). We do not know how or if this central inflammation directly affects orexin neurons. Prostaglandin and central IL-1 signaling were not required in LPS-induced fatigue/lethargy (Grossberg et al., 2011).This area warrants additional study.
4.2. Physical activity to measure fatigue/lethargy induced by cytotoxic chemotherapy
The change in ambulatory activity in rodents after treatment with cytotoxic chemotherapy in this study captured the salient descriptions of this fatigue in people including decreased total activity, decreased duration of activity, slowed activity, and increased need for rest (Scott et al., 2011). Activity during the dark phase slowly returned to baseline in the subsequent 8-10 nights after treatment, consistent with reports of decreased activity with cytotoxic chemotherapy in rodents (Mahoney et al., 2013; Ray et al., 2011; Wood et al., 2006).
4.3. Decreased orexin neuron activity as a component of fatigue/lethargy
In nocturnal rodents, orexin neurons signal throughout the dark phase (Estabrooke et al., 2001; Lee et al., 2005), with discharge specific to wakefulness, exploration, and motivated behavior (Mileykovskiy et al., 2005; Takahashi et al., 2008). Orexin neurons project densely to neurons involved in arousal and wakefulness including noradrenergic neurons in the locus coeruleus (Carter et al., 2012) and monoaminergic, cholinergic, and histaminergic neurons (Date et al., 1999; Peyron et al., 1998). Orexin peptide administration in orexin knockout rodents restores locomotion and results in restored wakefulness (Anaclet et al., 2009; Kantor et al., 2013). We found that cytotoxic chemotherapy decreased orexin neuron activity during the dark phase.
Our results with acute and chronic administration of cytotoxic chemotherapy support a functional division of orexin neurons described previously with the DMH and PFA orexin subpopulations regulating arousal and stress responses (Harris and Aston-Jones, 2006; Nollet et al., 2011; Yoshida et al., 2006). We found a significant suppression of the DMH orexin neurons after a single dose and multiple doses of cytotoxic chemotherapy, and suppression of the PFA orexin neurons after multiple doses of chemotherapy. Our findings are similar, but not identical to those of Grossberg et al. (2011) who reported the PFA subpopulation is sensitive to LPS-induced inflammation and this suppression is associated with decreased locomotor activity.
It is not surprising that LPS and cytotoxic chemotherapy may have slightly different effects on orexin neurons. LPS causes decreased activity in rodents for less than one day, while cytotoxic chemotherapy reduces activity for many days following a single treatment. In our hands, LPS induces hypothalamic IL-1β gene expression within 2 hours after treatment, a result we did not observe with cytotoxic chemotherapy. LPS induces higher IL-6 gene expression after treatment as compared to our results with a single dose of cytotoxic chemotherapy (Grossberg et al., 2011). In addition, LPS often induces a febrile response, and we found cytotoxic chemotherapy induces hypothermia, indicating different effects between these agents on neuron populations in the hypothalamus. Differences in inflammatory gene expression induced by these treatments could be due to cytotoxic chemotherapy binding DNA and interfering with RNA and protein synthesis (Longley et al., 2003; Vacchelli et al., 2012). Differences in the timing and severity of hypothalamic inflammation could have a different impact on neurons and subsequent symptoms. In addition, the division of orexin neurons into three subpopulations is approximate and is difficult to discern on some hypothalamic sections. Since identification of subpopulations is subjective, it is important to note that we found a significant decrease in orexin neuron activity across all regions following both acute and chronic treatment with cytotoxic chemotherapy.
Our visual observation of decreased orexin neuron activity was confirmed with our finding that CSF concentration of orexin-A was decreased after administration of cytotoxic chemotherapy. Orexin peptides are released from orexin neurons throughout the active phase, which presumably drives a diurnal fluctuation of orexin-A in CSF (Desarnaud et al., 2004). CSF concentration of orexin-A was not measured at the nadir prior to the dark period since it was previously shown in our laboratory that the morning peak level of orexin-A and not the nadir level was altered in rodents given peripheral LPS (Grossberg et al., 2011). Orexin neurons were previously found to be sensitive to chronic administration of LPS (Gerashchenko and Shiromani, 2004). Although we did not find any difference in the number of hypothalamic orexin neurons, similar to results with a single dose of LPS or experimental traumatic brain injury (Grossberg et al., 2011; Willie et al., 2012), the acuity and duration of inflammation may influence the effect on orexin neurons.
4.4. Exogenous orexin-A restores activity following cytotoxic chemotherapy
If chemotherapy induces fatigue via a decrease in orexin neuronal function, then orexin administration should reverse this fatigue. We found central administration of orexin-A restored ambulatory activity movement and the rate of activity in the first 2 hours of the dark phase when healthy rodents have highest activity. Administration of orexin-A to CAF-treated rats also increased the duration of sustained activity at the start of the dark phase. Similar to our results, restoration of this normal pattern of arousal and sustained wakefulness was recently reported in rodents given orexin-A producing stem cells following orexin neuron ablation (Kantor et al., 2013). In our study, chemotherapy treated animals given icv orexin-A showed normal patterns of grooming, had restoration of movement in the cage, restoration of the rate of activity, and increased duration of activity upon wakening, indicators that orexin restored functional dark phase activity rather than inducing hyperactivity.
Centrally administered orexin-A has a limited physiological action (Kiwaki et al., 2004; Samson et al., 2010), so we were surprised to find it stimulated activity across the 12 hour dark phase. One explanation for this longer activity is that orexin-A stimulates orexin-A release (Yamanaka et al., 2010). Physical activity in animals may also stimulate increased CSF levels of orexin-A, resulting in a sustained active period (Wu et al., 2002).
We found that orexin-A administered at the start of the dark phase did not alter ambulatory activity during the subsequent light phase. This indicates the normal rodent diurnal pattern of light phase inactivity was not affected. We also found that administration of 1 μg orexin-A to control rats did not significantly affect dark phase activity, consistent with earlier reports (Anaclet et al., 2009; Grossberg et al., 2011). A dose of orexin-A four times greater than that used in our study was found to increase dark phase activity in control animals for two hours after orexin-A treatment (Kotz et al., 2002). Increased dose of orexin-A does increase activity (Kiwaki et al., 2004; Nakamura et al., 2000; Samson et al., 2010) perhaps explaining this difference in our findings. Administration of orexin-A during the light phase has repeatedly been shown to increase activity in control rodents for up to two hours during the normally inactive light phase (Fenzl et al., 2011; Kiwaki et al., 2004; Nakamura et al., 2000; Novak and Levine, 2009; Samson et al., 2010). Our treatment was aimed at restoring normal dark phase activity, not at producing a pharmacologic enhancement of activity during the normally quiescent light phase. The effects of exogenous orexin-A after multiple doses of chemotherapy and in female rats was not examined in this study.
4.5. Implications
Understanding the causal mechanisms of cytotoxic chemotherapy-induced fatigue can support development of interventions to prevent or treat this fatigue. This study supports earlier research indicating that suppression of the central inflammatory response and restoration of orexin signaling are both potential targets to prevent or treat cytotoxic chemotherapy induced fatigue (Grossberg et al., 2011; Wong et al., 2012; Wood and Weymann, 2013). We found that central administration of orexin-A neuropeptide restored functional activity during the active phase in rats. Central replacement of orexin-A is not clinically feasible. However, an oral agonist of the orexin receptor is in clinical trials to treat narcolepsy (NIH RePORT #1U01NS080400-01), a disorder in which orexin signaling is impaired (Chemelli et al., 1999; Lin et al., 1999).
Other drugs that stimulate arousal, such as methylphenidate and modafinil, have limited success in the treatment of persistent fatigue with cancer (Bruera et al., 2013; Jean-Pierre et al., 2010; Lower et al., 2009; Minton et al., 2011). An orexin agonist may be more suited to increase functional arousal as compared to altering dopamine or histamine signaling (Anaclet et al., 2009). Physical exercise is a non-pharmacologic intervention that has shown benefit in reducing cancer-related fatigue (Kummer et al., 2013; McNeely and Courneya, 2010; Mitchell et al., 2007). But, adherence to a regular schedule of physical activity is a barrier to this intervention (Wenzel et al., 2013). Based on results presented here and previous studies on orexin action, we propose that treatment with an orexin agonist may promote functional activity, daytime wakefulness, and increase success in maintaining physical activity in people treated with cytotoxic chemotherapy.
Research Highlight.
Cancer chemotherapy-induced fatigue is associated with suppressed hypothalamic orexin neuron activity and orexin administration reverses this fatigue.
Acknowledgments
This work was supported by NIH F31NR01329901 to KBW, NINR R01NR012479 to LJW, and NIH R01 DK70333 to DLM. The authors thank My Linh Nguyen, Caroline Anderson, Michael Harding, and the Advanced Light Microscopy Core-Jungers Center at Oregon Health & Science University for their expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anaclet C, Parmentier R, Ouk K, Guidon G, Buda C, Sastre JP, Akaoka H, Sergeeva OA, Yanagisawa M, Ohtsu H, Franco P, Haas HL, Lin JS. Orexin/hypocretin and histamine: distinct roles in the control of wakefulness demonstrated using knock-out mouse models. J Neurosci. 2009;29:14423–14438. doi: 10.1523/JNEUROSCI.2604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann CR, Stocker R, Imhof HG, Trentz O, Hersberger M, Mignot E, Bassetti CL. Hypocretin-1 (orexin A) deficiency in acute traumatic brain injury. Neurology. 2005;65:147–149. doi: 10.1212/01.wnl.0000167605.02541.f2. [DOI] [PubMed] [Google Scholar]
- Berger AM, Gerber LH, Mayer DK. Cancer-related fatigue: implications for breast cancer survivors. Cancer. 2012;118:2261–2269. doi: 10.1002/cncr.27475. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Michaud B, Kelley KW, Dantzer R. Vagotomy blocks behavioural effects of interleukin-1 injected via the intraperitoneal route but not via other systemic routes. Neuroreport. 1996;7:2823–2827. doi: 10.1097/00001756-199611040-00083. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Walter V, Parnet P, Laye S, Lestage J, Verrier D, Poole S, Stenning BE, Kelley KW, Dantzer R. Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. Comptes rendus de l'Academie des sciences. 1994;317:499–503. [PubMed] [Google Scholar]
- Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: Mechanisms, contributing factors, and treatment implications. Brain, behavior, and immunity. 2012 doi: 10.1016/j.bbi.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones TL, Woods J. Chemotherapy-induced cognitive impairment is associated with decreases in cell proliferation and histone modifications. BMC Neurosci. 2011;12:124. doi: 10.1186/1471-2202-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruera E, Yennurajalingam S, Palmer JL, Perez-Cruz PE, Frisbee-Hume S, Allo JA, Williams JL, Cohen MZ. Methylphenidate and/or a nursing telephone intervention for fatigue in patients with advanced cancer: a randomized, placebo-controlled, phase II trial. J Clin Oncol. 2013;31:2421–2427. doi: 10.1200/JCO.2012.45.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Brill J, Bonnavion P, Huguenard JR, Huerta R, de Lecea L. Mechanism for Hypocretin-mediated sleep-to-wake transitions. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2635–2644. doi: 10.1073/pnas.1202526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, de Lecea L, Adamantidis A. Functional wiring of hypocretin and LC-NE neurons: implications for arousal. Frontiers in behavioral neuroscience. 2013;7:43. doi: 10.3389/fnbeh.2013.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Bennett GJ, Dantzer R, Dougherty PM, Dunn AJ, Meyers CA, Miller AH, Payne R, Reuben JM, Wang XS, Lee BN. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97:2919–2925. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, Johnson DH, Miaskowski C, Scherr SL, Portenoy RK, Vogelzang NJ. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. The oncologist. 2000;5:353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Capuron L, Irwin MR, Miller AH, Ollat H, Perry VH, Rousey S, Yirmiya R. Identification and treatment of symptoms associated with inflammation in medically ill patients. Psychoneuroendocrinology. 2008;33:18–29. doi: 10.1016/j.psyneuen.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Meagher MW, Cleeland CS. Translational approaches to treatment-induced symptoms in cancer patients. Nat Rev Clin Oncol. 2012;9:414–426. doi: 10.1038/nrclinonc.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong N, Candel MJ, Schouten HC, Abu-Saad HH, Courtens AM. Prevalence and course of fatigue in breast cancer patients receiving adjuvant chemotherapy. Ann Oncol. 2004;15:896–905. doi: 10.1093/annonc/mdh229. [DOI] [PubMed] [Google Scholar]
- Desarnaud F, Murillo-Rodriguez E, Lin L, Xu M, Gerashchenko D, Shiromani SN, Nishino S, Mignot E, Shiromani PJ. The diurnal rhythm of hypocretin in young and old F344 rats. Sleep. 2004;27:851–856. doi: 10.1093/sleep/27.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsea CR, Roberts DA, Druker BJ, Wood LJ. Inhibition of p38 MAPK suppresses inflammatory cytokine induction by etoposide, 5-fluorouracil, and doxorubicin without affecting tumoricidal activity. PloS one. 2008;3:e2355. doi: 10.1371/journal.pone.0002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardell JE, Vardy J, Shah JD, Johnston IN. Cognitive impairments caused by oxaliplatin and 5-fluorouracil chemotherapy are ameliorated by physical activity. Psychopharmacology (Berl) 2012;220:183–193. doi: 10.1007/s00213-011-2466-2. [DOI] [PubMed] [Google Scholar]
- Fenzl T, Romanowski CP, Flachskamm C, Deussing JM, Kimura M. Wake-promoting effects of orexin: Its independent actions against the background of an impaired corticotropine-releasing hormone receptor system. Behavioural brain research. 2011;222:43–50. doi: 10.1016/j.bbr.2011.03.026. [DOI] [PubMed] [Google Scholar]
- Furutani N, Hondo M, Kageyama H, Tsujino N, Mieda M, Yanagisawa M, Shioda S, Sakurai T. Neurotensin co-expressed in orexin-producing neurons in the lateral hypothalamus plays an important role in regulation of sleep/wakefulness States. PloS one. 2013;8:e62391. doi: 10.1371/journal.pone.0062391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaykema RP, Goehler LE. Lipopolysaccharide challenge-induced suppression of Fos in hypothalamic orexin neurons: their potential role in sickness behavior. Brain, behavior, and immunity. 2009;23:926–930. doi: 10.1016/j.bbi.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaykema RP, Goehler LE. Ascending caudal medullary catecholamine pathways drive sickness-induced deficits in exploratory behavior: brain substrates for fatigue? Brain, behavior, and immunity. 2011;25:443–460. doi: 10.1016/j.bbi.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayle D, Ilyin SE, Flynn MC, Plata-Salaman CR. Lipopolysaccharide (LPS)- and muramyl dipeptide (MDP)-induced anorexia during refeeding following acute fasting: characterization of brain cytokine and neuropeptide systems mRNAs. Brain research. 1998;795:77–86. doi: 10.1016/s0006-8993(98)00280-7. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Kohls MD, Greco M, Waleh NS, Salin-Pascual R, Kilduff TS, Lappi DA, Shiromani PJ. Hypocretin-2-saporin lesions of the lateral hypothalamus produce narcoleptic-like sleep behavior in the rat. J Neurosci. 2001;21:7273–7283. doi: 10.1523/JNEUROSCI.21-18-07273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerashchenko D, Murillo-Rodriguez E, Lin L, Xu M, Hallett L, Nishino S, Mignot E, Shiromani PJ. Relationship between CSF hypocretin levels and hypocretin neuronal loss. Experimental neurology. 2003;184:1010–1016. doi: 10.1016/S0014-4886(03)00388-1. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Shiromani PJ. Effects of inflammation produced by chronic lipopolysaccharide administration on the survival of hypocretin neurons and sleep. Brain research. 2004;1019:162–169. doi: 10.1016/j.brainres.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Grossberg AJ, Zhu X, Leinninger GM, Levasseur PR, Braun TP, Myers MG, Jr., Marks DL. Inflammation-induced lethargy is mediated by suppression of orexin neuron activity. J Neurosci. 2011;31:11376–11386. doi: 10.1523/JNEUROSCI.2311-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends in neurosciences. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Jean-Pierre P, Morrow GR, Roscoe JA, Heckler C, Mohile S, Janelsins M, Peppone L, Hemstad A, Esparaz BT, Hopkins JO. A phase 3 randomized, placebo-controlled, double-blind, clinical trial of the effect of modafinil on cancer-related fatigue among 631 patients receiving chemotherapy: a University of Rochester Cancer Center Community Clinical Oncology Program Research base study. Cancer. 2010;116:3513–3520. doi: 10.1002/cncr.25083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor S, Mochizuki T, Lops SN, Ko B, Clain E, Clark E, Yamamoto M, Scammell TE. Orexin gene therapy restores the timing and maintenance of wakefulness in narcoleptic mice. Sleep. 2013;36:1129–1138. doi: 10.5665/sleep.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain, behavior, and immunity. 2003;17(Suppl 1):S112–118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Kiwaki K, Kotz CM, Wang C, Lanningham-Foster L, Levine JA. Orexin A (hypocretin 1) injected into hypothalamic paraventricular nucleus and spontaneous physical activity in rats. Am J Physiol Endocrinol Metab. 2004;286:E551–559. doi: 10.1152/ajpendo.00126.2003. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends in neurosciences. 2002;25:154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- Kotz CM, Teske JA, Levine JA, Wang C. Feeding and activity induced by orexin A in the lateral hypothalamus in rats. Regulatory peptides. 2002;104:27–32. doi: 10.1016/s0167-0115(01)00346-9. [DOI] [PubMed] [Google Scholar]
- Kummer F, Catuogno S, Perseus JM, Bloch W, Baumann FT. Relationship between Cancer-related Fatigue and Physical Activity in Inpatient Cancer Rehabilitation. Anticancer research. 2013;33:3415–3422. [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Little K. A Rat Model of Sytemic Chemotherapy for Breast Cancer to Evaluate and Treat Chemobrain. 2007 http://www.researchgate.net/publication/235098916.
- Liu L, Mills PJ, Rissling M, Fiorentino L, Natarajan L, Dimsdale JE, Sadler GR, Parker BA, Ancoli-Israel S. Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain, behavior, and immunity. 2012;26:706–713. doi: 10.1016/j.bbi.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nature reviews. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- Lower EE, Fleishman S, Cooper A, Zeldis J, Faleck H, Yu Z, Manning D. Efficacy of dexmethylphenidate for the treatment of fatigue after cancer chemotherapy: a randomized clinical trial. Journal of pain and symptom management. 2009;38:650–662. doi: 10.1016/j.jpainsymman.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Mahoney SE, Davis JM, Murphy EA, McClellan JL, Gordon B, Pena MM. Effects of 5-fluorouracil chemotherapy on fatigue: role of MCP-1. Brain, behavior, and immunity. 2013;27:155–161. doi: 10.1016/j.bbi.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeely ML, Courneya KS. Exercise programs for cancer-related fatigue: evidence and clinical guidelines. J Natl Compr Canc Netw. 2010;8:945–953. doi: 10.6004/jnccn.2010.0069. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton O, Richardson A, Sharpe M, Hotopf M, Stone PC. Psychostimulants for the management of cancer-related fatigue: a systematic review and meta-analysis. Journal of pain and symptom management. 2011;41:761–767. doi: 10.1016/j.jpainsymman.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Mitchell SA, Beck SL, Hood LE, Moore K, Tanner ER. Putting evidence into practice: evidence-based interventions for fatigue during and following cancer and its treatment. Clinical journal of oncology nursing. 2007;11:99–113. doi: 10.1188/07.CJON.99-113. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Uramura K, Nambu T, Yada T, Goto K, Yanagisawa M, Sakurai T. Orexin-induced hyperlocomotion and stereotypy are mediated by the dopaminergic system. Brain research. 2000;873:181–187. doi: 10.1016/s0006-8993(00)02555-5. [DOI] [PubMed] [Google Scholar]
- Nollet M, Gaillard P, Minier F, Tanti A, Belzung C, Leman S. Activation of orexin neurons in dorsomedial/perifornical hypothalamus and antidepressant reversal in a rodent model of depression. Neuropharmacology. 2011;61:336–346. doi: 10.1016/j.neuropharm.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Novak CM, Levine JA. Daily intraparaventricular orexin-A treatment induces weight loss in rats. Obesity (Silver Spring, Md. 2009;17:1493–1498. doi: 10.1038/oby.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. Academic Press; New York: 2001. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1998. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray MA, Trammell RA, Verhulst S, Ran S, Toth LA. Development of a mouse model for assessing fatigue during chemotherapy. Comparative medicine. 2011;61:119–130. [PMC free article] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. Faseb J. 2007;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Reilly CM, Bruner DW, Mitchell SA, Minasian LM, Basch E, Dueck AC, Cella D, Reeve BB. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer. 2013;21:1525–1550. doi: 10.1007/s00520-012-1688-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. Mechanisms of cancer-related fatigue. The oncologist. 2007;12(Suppl 1):22–34. doi: 10.1634/theoncologist.12-S1-22. [DOI] [PubMed] [Google Scholar]
- Saligan LN, Kim HS. A systematic review of the association between immunogenomic markers and cancer-related fatigue. Brain, behavior, and immunity. 2012;26:830–848. doi: 10.1016/j.bbi.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson WK, Bagley SL, Ferguson AV, White MM. Orexin receptor subtype activation and locomotor behaviour in the rat. Acta physiologica (Oxford, England) 2010;198:313–324. doi: 10.1111/j.1748-1716.2009.02056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Sauter KA, Wood LJ, Wong J, Iordanov M, Magun BE. Doxorubicin and daunorubicin induce processing and release of interleukin-1beta through activation of the NLRP3 inflammasome. Cancer biology & therapy. 2011;11:1008–1016. doi: 10.4161/cbt.11.12.15540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain, behavior, and immunity. 2007;21:413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Schwartz AL, Nail LM, Chen S, Meek P, Barsevick AM, King ME, Jones LS. Fatigue patterns observed in patients receiving chemotherapy and radiotherapy. Cancer investigation. 2000;18:11–19. doi: 10.3109/07357900009023057. [DOI] [PubMed] [Google Scholar]
- Scott JA, Lasch KE, Barsevick AM, Piault-Louis E. Patients' experiences with cancer-related fatigue: a review and synthesis of qualitative research. Oncology nursing forum. 2011;38:E191–203. doi: 10.1188/11.ONF.E191-E203. [DOI] [PubMed] [Google Scholar]
- Shi Q, Smith TG, Michonski JD, Stein KD, Kaw C, Cleeland CS. Symptom burden in cancer survivors 1 year after diagnosis: a report from the American Cancer Society's Studies of Cancer Survivors. Cancer. 2011;117:2779–2790. doi: 10.1002/cncr.26146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley RV, Lefante J, Bartolucci A, Carpenter J, Vogel C, Krauss S. A comparison of cyclophosphamide, adriamycin, and 5-fluorouracil (CAF) and cyclophosphamide, methotrexate, 5-fluorouracil, vincristine, and prednisone (CMFVP) in patients with advanced breast cancer. Breast cancer research and treatment. 1983;3:209–220. doi: 10.1007/BF01803563. [DOI] [PubMed] [Google Scholar]
- Spelten ER, Verbeek JH, Uitterhoeve AL, Ansink AC, van der Lelie J, de Reijke TM, Kammeijer M, de Haes JC, Sprangers MA. Cancer, fatigue and the return of patients to work-a prospective cohort study. Eur J Cancer. 2003;39:1562–1567. doi: 10.1016/s0959-8049(03)00364-2. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Lin JS, Sakai K. Neuronal activity of orexin and non-orexin waking-active neurons during wake-sleep states in the mouse. Neuroscience. 2008;153:860–870. doi: 10.1016/j.neuroscience.2008.02.058. [DOI] [PubMed] [Google Scholar]
- Vacchelli E, Galluzzi L, Fridman WH, Galon J, Sautes-Fridman C, Tartour E, Kroemer G. Trial watch: Chemotherapy with immunogenic cell death inducers. Oncoimmunology. 2012;1:179–188. doi: 10.4161/onci.1.2.19026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel JA, Griffith KA, Shang J, Thompson CB, Hedlin H, Stewart KJ, DeWeese T, Mock V. Impact of a home-based walking intervention on outcomes of sleep quality, emotional distress, and fatigue in patients undergoing treatment for solid tumors. The oncologist. 2013;18:476–484. doi: 10.1634/theoncologist.2012-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie JT, Lim MM, Bennett RE, Azarion AA, Schwetye KE, Brody DL. Controlled cortical impact traumatic brain injury acutely disrupts wakefulness and extracellular orexin dynamics as determined by intracerebral microdialysis in mice. Journal of neurotrauma. 2012;29:1908–1921. doi: 10.1089/neu.2012.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Smith LB, Magun EA, Engstrom T, Kelley-Howard K, Jandhyala DM, Thorpe CM, Magun BE, Wood LJ. Small molecule kinase inhibitors block the ZAK-dependent inflammatory effects of doxorubicin. Cancer biology & therapy. 2012;14:56–63. doi: 10.4161/cbt.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LJ, Nail LM, Perrin NA, Elsea CR, Fischer A, Druker BJ. The cancer chemotherapy drug etoposide (VP-16) induces proinflammatory cytokine production and sickness behavior-like symptoms in a mouse model of cancer chemotherapy-related symptoms. Biological research for nursing. 2006;8:157–169. doi: 10.1177/1099800406290932. [DOI] [PubMed] [Google Scholar]
- Wood LJ, Weymann K. Inflammation and neural signaling: etiologic mechanisms of the cancer treatment-related symptom cluster. Current opinion in supportive and palliative care. 2013;7:54–59. doi: 10.1097/SPC.0b013e32835dabe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MF, John J, Maidment N, Lam HA, Siegel JM. Hypocretin release in normal and narcoleptic dogs after food and sleep deprivation, eating, and movement. American journal of physiology. 2002;283:R1079–1086. doi: 10.1152/ajpregu.00207.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Tabuchi S, Tsunematsu T, Fukazawa Y, Tominaga M. Orexin directly excites orexin neurons through orexin 2 receptor. J Neurosci. 2010;30:12642–12652. doi: 10.1523/JNEUROSCI.2120-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. The Journal of comparative neurology. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]