Abstract
Introduction
Phosgene is a rare exposure with strong clinical implications. We report a phosgene exposure that resulted in the patient's death.
Case Report
A 58 year-old man arrived to the emergency department 1 hour after exposure to phosgene with complaints of a sore throat. Initial vital signs were blood pressure 175/118 mmHg, heart rate 98/min, respirations 12/min, and oxygen saturation of 93% on room air. Physical exam revealed few scattered rhonchi, without signs of distress. Initial arterial blood gases (ABG's) revealed pH 7.42, pCO2 43 mmHg, pO2 68 mmHg, HCO3 27 meq/L, and oxygen saturation of 93% on room air. Initial chest x-ray 2 hours after the exposure demonstrated clear lung fields. Approximately 2.5 hours after the exposure, he began complaining of dyspnea, restlessness and his oxygen saturation dropped below 90%. He received nebulized albuterol, 1 gram intravenous methylprednisolone, and 100 % oxygen via face mask. Minimal improvement was noted and he was intubated. The post intubation chest x-ray, 3.5 hours after the exposure, revealed diffuse alveolar infiltrates. Acetylcysteine, terbutaline, and IV steroids were administered without improvement. The patient died 30 hours after exposure.
Discussion
There are many misunderstandings concerning phosgene due to its rare presentation. Traditional treatment modalities are often unproven in human trials and were unsuccessful in this case.
Conclusion
This case highlights the significant toxicity that results from phosgene exposure and the challenges of the limited treatment modalities. There is concern for the use of this agent in chemical terrorism.
Keywords: Phosgene, ARDS, Chemical warfare, Pulmonary edema
Introduction
Phosgene (carbonyl chloride, CAS 75-44-5) is a clear, colorless, irritant gas commonly used in the production of pharmaceuticals, polycarbonates, dyestuffs, isocyanates, carbamates, and other chemicals. It was used historically as a chemical weapon, along with chlorine and mustard gas, causing an estimated 80 % of the 100,000 poison gas deaths occurring in World War I [1]. Today, it remains a chemical of industrial significance with worldwide production in excess of 5 billion pounds [2]. Also, it may be a by-product of welding and is produced during the combustion of substances such as polyvinyl chloride and isocyanates [3]. We report on a case of significant phosgene exposure in a 58-year-old man resulting in rapidly progressive pulmonary edema and death.
Case
A 58-year-old man presents to the emergency department 45 min after he was exposed to a liquid/gaseous mixture at a local chemical plant. Housed in an open-air shed, containers of phosgene were kept and transferred to larger tanks via stainless steel hoses, as part of a production process for a local chemical plant. While checking the tanks, a stainless steel hose carrying liquid phosgene, having not been purged appropriately, burst, and a liquid/gaseous mixture was sprayed across the face and chest of the patient. He immediately called for help and removed himself from the area. After changing his clothes and washing his face, he was transported to the nearest emergency department for evaluation complaining of only minor throat irritation. En route to the hospital, his vital signs were as follows: blood pressure 190/100 mmHg, pulse 105/min, respirations 19/min, and oxygen saturations were 98 % on room air.
Upon arrival to the emergency department, he complained of minor throat irritation and was mildly anxious in appearance. On physical exam, his vital signs were the following: blood pressure 175/108 mmHg, pulse 98/min, respirations 12/min, and oxygen saturation of 93 % on room air. He was placed on supplemental oxygen via a nasal cannula. Chest auscultation revealed a few scattered rhonchi, equal breath sounds with good air movement, and no costal retractions. The remainder of the exam revealed no other acute findings of erythema, edema, or caustic injury about the eyes, face, oropharynx, or chest. There were no signs of pharyngeal irritation or lesions. The initial chest radiograph revealed no evidence of acute pulmonary infiltrates. Past medical history was significant for mild hypertension. There was no history of chronic obstructive pulmonary disease or other cardiorespiratory disorders. There is no history of ethanol, tobacco, or other illicit drug use. He had no known drug allergies.
Serum chemistries were performed revealing serum sodium of 143 mmol/L, potassium 4.1 mmol/L, chloride 106 mmol/L, bicarbonate 32 mmol/L, blood urea nitrogen 19 mg/dL, and creatinine 0.8 mg/dL. A complete blood count revealed WBC 8.8 k/μL, hemoglobin 14.3 g/dL, hematocrit 42.8 %, and platelets 188 k/μL. An arterial blood gas showed pH 7.42, pCO2 43 mmHg, pO2 68 mmHg, bicarbonate 27 mmol/L, oxygen saturation 93 % on 2 L of supplemental oxygen via a nasal cannula. Lactic acid was 1.3 mmol/L. Electrocardiogram showed a sinus tachycardia at 99 beats per minute with QRS duration of 94 ms and QTc of 324 ms. Q waves were noted in lead V1, and inverted T waves in V3–V6, which were unchanged from a previous EKG.
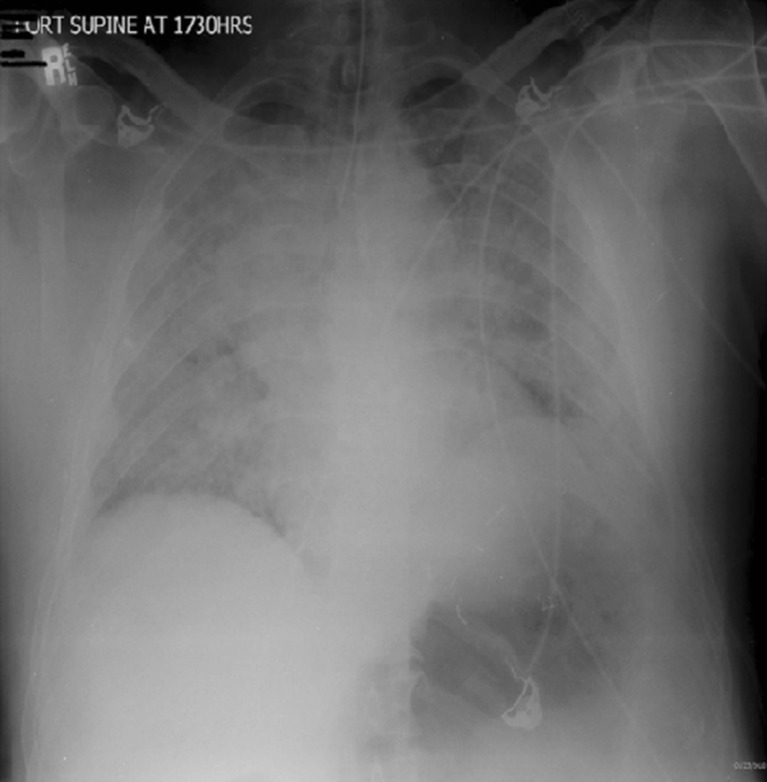
During the emergency department evaluation, his condition continued to decline. At 2 h and 45 min post-exposure, he began having increased dyspnea and restlessness for which he was treated intravenously with three 1 mg of lorazepam doses over a 30-min time period to decrease anxiety. At 3 h and 15 min post-exposure, his symptoms had worsened with the development of a cough, increased restlessness, and audible wheezing. He was then treated with nebulized albuterol and 1 g of methylprednisolone given intravenously. The patient remained awake and alert, but his oxygen saturations continued to decrease into the 80s despite high flow of oxygen via a non-rebreather facemask. At that point, the decision was made, with the patient’s consent, to proceed with endotracheal intubation and mechanical ventilation. After intubation, his oxygen saturations improved to 93 % on 100 % FiO2. A post-intubation chest radiograph approximately 4 h post-exposure revealed diffuse pulmonary edema (see Fig. 1).
Fig. 1.
Diffuse pulmonary infiltrates at 4 h post-exposure
Treatment recommendations were discussed and initiated per recommendations from the local Poison Control Center and representatives from the patient’s employer. Nebulized N-acetylcysteine (10 mL of 20 % solution every 6 h), nebulized albuterol (every 4 h), oral administration of ibuprofen (800 mg every 8 h), and terbutaline (0.5 mg given intravenously times one dose) were initiated. He was admitted to the Medical Intensive Care Unit where intravenously administered methylprednisolone was continued at 125 mg four times daily. Ventilator support was transitioned from bilevel to pressure control ventilation with transient improvement in oxygenation. Norepinephrine and vasopressin were started for hypotension. Extracorporeal membrane oxygenation (ECMO) was considered but was not available at this facility. The patient was not stable for transfer to another facility, so prone positioning was attempted with successful removal of excretions and temporary improvement in oxygenation.
Despite the early identification of exposure and initiation of multiple therapeutic interventions, the patient remained profoundly hypoxemic with respiratory acidosis and fulminant pulmonary edema consistent with overwhelming acute respiratory distress syndrome (ARDS). Ultimately, this patient succumbed to his injuries and was pronounced dead approximately 31.5 h post-exposure. Later reports based on a dosimetry badge worn by the patient and calculations of the amount of liquid phosgene contained in the hose revealed that this patient received a potentially lethal dose of phosgene 300 ppm/min in less than a tenth of a second [4].
Discussion
Phosgene is a volatile acid chloride that exists as a colorless, fuming liquid at standard temperature and pressure. It has a boiling point of 8.2 °C (47 °F). At room temperature, phosgene exists as a gas. It is commonly stored under pressure as a liquid that quickly vaporizes into a gas upon release. Phosgene gas is heavier than air, so it stays close to the ground and spreads rapidly. Phosgene lacks any irritating warning signs to prevent harmful exposures. Its characteristic odor is commonly described as moldy or freshly cut hay. Poisoning is dependent upon several variables including concentration, duration of exposure, and route of exposure. The lethal concentration for 50 % of the population (LC50) in humans is approximately 300 ppm/min [3].
Mechanistically, phosgene is postulated to cause toxicity by two different mechanisms, hydrolysis, and acylation [5]. In the first mechanism, phosgene hydrolyses into hydrogen chloride and carbon dioxide when it comes into contact with water in the respiratory tract. At normal physiologic conditions, only small amounts of hydrochloric acid are produced [5, 6]. The second and likely more harmful mechanisms by which phosgene causes its effects is through acylation. By this mechanism, phosgene reacts with hydroxyl, thiol, amine, and sulfhydryl groups on proteins, carbohydrates, and lipids [5, 6]. This mechanism causes extreme oxidative damage and quickly depletes glutathione stores, worsening the free radical damage. Multiple animal studies and human case reports have shown an increase in vascular permeability, alveolar leakage, and development of pulmonary edema [5–7]. Animal models have demonstrated that the pulmonary edema is the result of increased permeability of the alveolar membrane [8]. Increasing evidence suggest that exposure initiates a cascade of inflammatory cytokines and other mediators, leading to increased pulmonary capillary permeability and edema formation [9, 10].
Symptoms are traditionally divided into immediate and delayed. Immediate effects are determined by concentration and are mostly reported as mucous membrane irritation via the hydrolysis pathway [5]. The delayed effects are dependent upon the total inhaled dose rather than on exposure concentration. Individuals may be symptom-free for up to 48 h following an acute exposure before developing a cough, shortness of breath, tachypnea, and respiratory distress [6].
Moreover, clinical effects are often divided into three phases: reflex, latency, and pulmonary edema. The reflex phase is that time period immediately following the inhalation of phosgene and often occurs when the exposure concentration is greater than 3 ppm [5]. In this phase, the local irritant effects on the sensory receptors in the bronchial tree cause a self-protective vagal reflex. This often results in rapid, shallow breathing, causing decreased vital capacity, elevated partial pressure of carbon dioxide, mild hypoxemia, and mild respiratory acidosis. These symptoms vary in intensity among individuals and are likely influenced by emotional factors [5]. Individuals may persist in this initial phase for several hours before returning to baseline. This phase may not occur if it is a prolonged exposure to concentrations of <3 ppm. In the latency phase, individuals are generally asymptomatic. The biochemical effects of phosgene are immediate, but the clinical effects can be delayed for several hours [5]. The duration of this latency phase is inversely related to the inhaled dose [5, 10–12]. There are currently no biologic markers capable of predicting the onset or severity of pulmonary symptoms. The earliest pulmonary effects can usually be seen midway through the latency phase with enlargement of the hila and ill-defined shadows predominately in the central portions of the lungs on x-ray. The end of this phase is characterized by the development of cough, shortness of breath, tachypnea, and early signs of respiratory distress. The third and final phase is characterized as frank pulmonary edema. Copious amounts of frothy, protein-rich pulmonary secretions may progress over the next 24–30 h at which time death may result from suffocation [5].
Treatment recommendations for acute phosgene exposure are varied. Basic measures such as the removal from the site of exposure and decontamination seem to be the most helpful. There are numerous therapies that have been recommended based on animal models, often with little to no human experience. Grainge and Rice divide therapeutic strategies into the following pathways: the antioxidant pathway, the cyclic 3,5-adenosine monophosphate pathway, and the anti-inflammatory pathway. In addition to these pathways, supportive therapy strategies such as protective ventilation and reduced oxygen concentration therapy are recommended. However, there is no evidence strongly supporting the use of these therapies in humans.
Therapies aimed at the antioxidant pathway include acetylcysteine, nonsteroidal anti-inflammatory drugs, eicosatetraynoic acid, and furosemide. A portion of the pathologic effect of phosgene appears to be mediated by oxidant damage to the respiratory epithelium [13, 14]. Dietary supplementation with n-propyl gallate (nPG), vitamin E, and butylated hydroxyanisole have been studied in mouse models [15–17]. These therapies are thought to offset the oxidant damage to the respiratory epithelium; however, increasing antioxidant defenses with nPG were only of benefit if initiated prior to exposure. Vitamin E at doses greater than 450 mg/kg in mouse models was not shown to cause any protective effect from phosgene exposure and led to higher mortality rates than control groups. There are currently no data suggesting benefit in human exposures. Furthermore, except for vitamin E, these medications are not easily accessible for emergency use.
Acetylcysteine is an antioxidant that was shown in an isolated rabbit lung study to reduce lung weight gain, reduce leukotriene concentrations, and lipid peroxidation in dosage equivalents of 3,200 mg in 80-kg humans [17]. A more recent animal study also demonstrated improvement in lung wet-weight-to-dry-weight ratio when rats were given an intraperitoneal dose after exposure [18]. This study further postulated that glutathione reconstitution was fundamental to acetylcysteine’s therapeutic action. The human equivalent dose in this study was similar to those commonly used in acetaminophen toxicity. Case reports on anecdotal improvements with nebulized acetylcysteine in human exposures have been reported, although the dose of phosgene in these anecdotal reports is often unknown. Early treatment with nebulized acetylcysteine, 1–10 mL of the 20 % solution or 2–20 mL of the 10 % solution every 2–6 h may be beneficial after significant exposure [19].
Ibuprofen is a nonsteroidal anti-inflammatory agent that is thought to act as an iron and hydroxyl scavenger. Animal studies measuring lung wet weights after phosgene exposure have shown some benefit [20]. These studies, however, used large doses (2–9 g in human equivalents) that may cause renal damage and gastrointestinal bleeding.
Furosemide is thought to act through multiple mechanisms. These include the prevention of bronchoconstriction, inhibition of mast cell degranulation, and decreased mucosal permeability. However, in a pig model, no improvement in survival has been reported in using 24 h of survival as the primary outcome measure [21].
Cyclic 3,5-adenosine monophosphate (cAMP) concentrations are decreased in lung tissue following phosgene exposure [9]. It is thought that the use of beta agonists like isoprenaline, terbutaline, and albuterol, as well as phosphodiesterase inhibitors like aminophylline will cause an up-regulation on intracellular cAMP. Numerous isolated lung studies have been performed showing improved lung weight gain in treatment groups. Beta 1 and beta 2 agonists like isoprenaline and albuterol have shown benefit in treating acute lung injury (ALI)/ARDS. Studies involving large animals have shown significant reduction in lung inflammation with decreasing amounts of neutrophils in bronchoalveolar lavage [22]. However, no improvement in survival was found.
The anti-inflammatory pathway encompasses the use of colchicine and corticosteroids. Colchicine was found to improve survival in mouse models 30 min after phosgene exposure when colchicine doses of 1 mg/kg were given [10]. This dosage range is well beyond the known toxic human dose and would be expected to cause significant systemic toxicity and death. It is unknown if lower doses would be beneficial in human exposures. Corticosteroids were shown in one pig model to have no effect on 24 h of survival following phosgene exposure [23].
Other measures of therapy to consider include the use of ARDS ventilation strategies and supplemental oxygen use. ARDSNet-recommended ventilation strategies have shown improvement in 24 h of survival in one large pig model [24]. Oxygen therapy has been widely advocated since World War I; however, early high-dose oxygen could be potentially harmful through the production of reactive oxygen species. Improved survival has been demonstrated with the use of low-oxygen concentration (<40 %) and delayed oxygen therapy until signs and symptoms of phosgene inhalation have become apparent [25].
Future cases of phosgene exposure will require a unique approach to therapy, as many of the current recommended therapies are based solely on anecdotal case reports and limited animal models. Based on the available data and our experiences with this case, we recommend the following treatments as “first-tier” actions to perform in the absence of clear contraindications.
Increase suspicion for possible phosgene exposure in patients working with fluorinated hydrocarbons and industrial chemical processes.
Limit the formation of reactive oxygen species by delaying supplemental oxygen until signs of toxicity from phosgene inhalation are present and restrict oxygen use to only low concentrations (<40 %).
Early treatment with corticosteroids (methylprednisolone of 1 g administered intravenously every 4–6 h)
Use nebulized treatments of N-acetylcysteine, 1–10 mL of the 20 % solution or 2–20 mL of the 10 % solution every 2–6 h, as both are a scavenger of oxygen free radicals and replete glutathione stores. Intravenous N-acetylcysteine may also be used in doses used for acetaminophen toxicity.
Terbutaline 2.5–5 mg per os (PO) tid should be considered as well as inhaled albuterol 2.5 mg/3 mL solution every few minutes in attempt to up-regulate intracellular cAMP.
Administer nonsteroidal anti-inflammatory agents such as ibuprofen 800 mg PO tid to reduce tissue injury through hydroxyl scavenger effects.
Should the exposure and resultant injury require endotracheal intubation, then use of ARDS ventilation strategies such as lower tidal volumes and adjusted positive end-expiratory pressure for alveoli recruitment and lung protection strategies is recommended.
Frequent suctioning for copious airway secretions and pulmonary edema
Balance the use of fluid administration with vasopressor agents such as norepinephrine to reduce excess pulmonary edema formation.
We recommend the following as “second-tier” actions to perform in the absence of clear contraindications.
The use of leukotriene antagonists such as zafirlukast (10–20 mg PO bid)
In extreme cases that do not respond to the above, the use of ECMO may be considered. Further studies are needed to show efficacy.
Individuals that survive phosgene inhalation generally have a good long-term prognosis [26–29]. However, survivors may have persistent exertional dyspnea, reduced pulmonary function, and decreased levels of physical fitness for months or even years after exposure [30].
Conclusions
This is a case report on a single patient exposed to phosgene. This case highlights the unique clinical presentation of a massive phosgene exposure, but few conclusions can be made concerning the treatment of phosgene-exposed patients since this patient died so rapidly. Potentially fatal phosgene exposures remain a concern in our industrialized society, and physicians should familiarize themselves with this chemical exposure. Phosgene is still used as a chemical manufacturing agent and may be used as an agent of terrorism. This case highlights the clinical presentation and treatment strategies to an extremely rare, yet life-threatening, exposure to phosgene. The mechanisms underlying the acute lung injury associated with phosgene exposures are not well understood. Available treatment modalities are limited with little to no evidence to support their use in human exposures. Potentially fatal phosgene exposures may present with little to no symptoms that may progress many hours after exposure into fulminant pulmonary edema. Further studies are needed to better understand the mechanisms through which phosgene causes its toxicity and to improve treatment modalities for life-threatening exposures. Although industrial standards regarding personal protective equipment use and handling of phosgene in processes posing potential exposure risks have been revised since this incident, the appropriate treatment of potentially fatal phosgene exposure remains uncertain.
Acknowledgments
Conflict of Interest
None
Footnotes
This article has been previously presented in abstract form in the 2011 NACCT Conference, Washington, DC, USA, and was based on the study of Pizon, AF, Hardison L, Wright E, Turner JM, Phosgene Exposure: A Case Report, Clinical Toxicology (2011) 49: 586.
References
- 1.Winternitz MC. Pathology of war gas poisoning. New Haven: Yale University Press; 1920. [Google Scholar]
- 2.Environmental health criteria 193: phosgene. Geneva: World Health Organization; 1997. [Google Scholar]
- 3.Collins, et al. Results from the US industry-wide phosgene surveillance: the Diller registry. J. Occup. Env. Med. 2011;53:239–244. doi: 10.1097/JOM.0b013e31820c90cf. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Chemical Safety and Hazard Investigation Board (2011) Investigation report. Draft report for public comment. Report no. 2010-6-I-WV. U.S. Chemical Safety and Hazard Investigation Board, Washington, DC
- 5.Borak J, Diller WF. Phosgene exposure: mechanisms of injury and treatment strategies. Joccup Environ Med. 2000;43:110–119. doi: 10.1097/00043764-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Grainge C, Rice P. Management of phosgene-induced acute lung injury. Clin Toxicol. 2010;48:497–508. doi: 10.3109/15563650.2010.506877. [DOI] [PubMed] [Google Scholar]
- 7.Brown RF, Jugg BJ, Harban FM, Asley Z, Kenward CE, Paltt J, Hill A, Rice P, Watkins PE. Pathophysiological responses following phosgene exposure in the anesthetized pig. J APll Toxicol. 2002;22:263–269. doi: 10.1002/jat.857. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy TP, Michael JR, Hoidal JR, Hasty D, Sciuto AM, Hopkins C, Lazar R, Bysani GK, Tolly E, Gurtner GH. Dibutyryl cAMP, aminophylline, and beta-adrenergic agonists protect against pulmonary edema caused by phosgene. J Appl Physiol. 1989;67:2542–2552. doi: 10.1152/jappl.1989.67.6.2542. [DOI] [PubMed] [Google Scholar]
- 9.Guo YL, Kennedy TP, Michael JR, et al. Mechanism of phosgene-induced lung toxicity: role of arachidonate mediators. J Appl Physiol. 1990;69:1615–1622. doi: 10.1152/jappl.1990.69.5.1615. [DOI] [PubMed] [Google Scholar]
- 10.Ghio AJ, Kennedy TP, Hatch GE, Tepper JS. Reduction of neutrophil influx diminishes lung injury and mortality following phosgene inhalation. J Appl Physiol. 1991;71:657–665. doi: 10.1152/jappl.1991.71.2.657. [DOI] [PubMed] [Google Scholar]
- 11.Diller WF. Pathogenesis of phosgene poisoning. Toxicol Ind Health. 1985;1:7–15. doi: 10.1177/074823378500100202. [DOI] [PubMed] [Google Scholar]
- 12.Currie WD, Hatch GE, Frosolono MF. Pulmonary alterations in rats due to acute phosgene inhalation. Fundam Appl Toxicol. 1987;8:107–114. doi: 10.1016/0272-0590(87)90106-0. [DOI] [PubMed] [Google Scholar]
- 13.Sciuto AM, Cascio MB, Moran TS, Forster JS. The fate of antioxidant enzymes in bronchoalveolar lavage fluid over 7 days in mice with acute lung injury. Inhal Toxicol. 2003;15:675–685. doi: 10.1080/713857424. [DOI] [PubMed] [Google Scholar]
- 14.Scuito AM, Phillips CS, Orzolek LD, Hege AI, Moran TS, DIlman JF. Genomic analysis of murine pulmonary tissue following carbonyl chloride inhalation. Chen Res Toxicol. 2005;18:1654–1660. doi: 10.1021/tx050126f. [DOI] [PubMed] [Google Scholar]
- 15.Scuito AM, Moran TS. Effect of dietary treatment with n-propyl galate or vitamin E on the survival of mice exposed to phosgene. J Appl Toxicol. 2001;21:33–39. doi: 10.1002/jat.729. [DOI] [PubMed] [Google Scholar]
- 16.Scuito AM, Moran TS. BHA diet enhances the survival of mice exposed to phosgene: the effect of BHA on glutathione levels in the lung. Inhal Toxicol. 1999;11:855–871. doi: 10.1080/089583799196772. [DOI] [PubMed] [Google Scholar]
- 17.Sciuto AM, Strickland PT, Kennedy TP, Gurtner GH. Protective effects of N-acetylcysteine treatment after phosgene exposure in rabbits. Am J Respir Crit Care Med. 1995;151:768–772. doi: 10.1164/ajrccm/151.3_Pt_1.768. [DOI] [PubMed] [Google Scholar]
- 18.Ji L, Liu R, Zhang XD, Chen HL, Bai H, Wang X, Zhao HL, Liang X, Hai CX. N-acetylcysteine attenuates phosgene-induced acute lung injury via up-regulation of Nrf2 expression. Inhal Toxicol. 2010;22:535–542. doi: 10.3109/08958370903525183. [DOI] [PubMed] [Google Scholar]
- 19.Gutch M, Jain N, Agrawal A, Consul S (2012) Acute accidental phosgene poisoning. BMJ Case Reports doi:10.1136/bcr.%2011.2011.5233 [DOI] [PMC free article] [PubMed]
- 20.Kennedy TP, Rao NV, Noah W, Michael JR, Jafri MH, Gurtner GH, Hoidal JR. Ibuprofen prevents oxidant lung injury and in vitro lipid peroxidation by chelating iron. J Clin Invest. 1990;86:1565–1573. doi: 10.1172/JCI114876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grainge C, Smith AJ, Jugg B, Jenner J, Rice P. Furosemide therapy in the treatment of phosgene induced acute lung injury. J R Army Med Corps. 156(4):245–250. [PubMed]
- 22.Grainge C, Brown R, Jugg BJ, Smith AJ, Mann TM, Jenner J, Rice P, Parkahouse DA. Early treatment with nebulised salbutamol worsens physiologic measures and does not improve survival following phosgene induced acute lung injury. J R Army Med Corps. 2009;155:105–109. doi: 10.1136/jramc-155-02-05. [DOI] [PubMed] [Google Scholar]
- 23.Smith A, Brown R, Jugg B, Platt J, Mann T, Masey C, Jenner J, Ice P. The effect of steroid treatment with inhaled budesonide or intravenous methylprednisolone on phosgene-induced acute lung injury in a porcine model. Mil Med. 2009;174:1287–1294. doi: 10.7205/MILMED-D-09-00050. [DOI] [PubMed] [Google Scholar]
- 24.Parkhouse DA, Brown RF, Jugg BJ, Harban FM, Platt J, Kenward CE, Jenner J, Rice P, Smith AJ. Protective ventilation strategies in the management of phosgene-induced acute lung injury. Mil Med. 2007;172:295–300. doi: 10.7205/milmed.172.3.295. [DOI] [PubMed] [Google Scholar]
- 25.Grainge C, Jugg BJ, Smith AJ, Brown RFR, Jenner J, Parkhouse DA, Rice P. Delayed low-dose supplemental oxygen improves survival following phosgene-induced acute ling injury. Inhal Toxicol. 2010;22:552–560. doi: 10.3109/08958370903571831. [DOI] [PubMed] [Google Scholar]
- 26.Rossman H. Phosgene (1985) In: ParmeggianiL (ed.) Encyclopedia of occupational health and safety. International Labour Office, Geneva. 1678–1679
- 27.Diller WF. Late sequelae after phosgene poisoning: a literature review. Toxicol Ind Health. 1985;1:129–136. doi: 10.1177/074823378500100213. [DOI] [PubMed] [Google Scholar]
- 28.Polednak AP. Mortality among men occupationally exposed to phosgene in 1943–1945. Environ Res. 1980;22:357–367. doi: 10.1016/0013-9351(80)90147-4. [DOI] [PubMed] [Google Scholar]
- 29.Waldron HA. Non-neoplastic disorders due to metallic, chemical and physical agents. In: Parkes WR, editor. Occupational lung disorders. Oxford: Butterworth-Heinemann; 1994. pp. 593–643. [Google Scholar]
- 30.Gladston M, Leutscher JA, Longcope WT, et al. A study of the residual effects of phosgene poisoning in human subjects. I. After chronic exposure. J Clin Invest. 1947;26:169–181. doi: 10.1172/JCI101796. [DOI] [PMC free article] [PubMed] [Google Scholar]