Abstract
The subcellular compartmentalization of kinase activity allows for regulation of distinct cellular processes involved in cell differentiation or survival. The PTEN-induced kinase-1 (PINK1) is a neuroprotective kinase localized to cytosolic and mitochondrial compartments. While mitochondrial targeting of PINK1 is important for its activities regulating mitochondrial homeostasis, the physiological role of the cytosolic pool of PINK1 remains unknown. Here, we demonstrate a novel role for cytosolic PINK1 in neuronal differentiation/neurite maintenance. Overexpression of wild-type PINK1, but not a catalytically inactive form of PINK1(K219M), promoted neurite outgrowth in SH-SY5Y cells and increased dendritic lengths in primary cortical and midbrain dopaminergic neurons. To identify the subcellular pools of PINK1 involved in promoting neurite outgrowth, we transiently transfected cells with PINK1 constructs designed to target PINK1 to the outer mitochondrial membrane (OMM-PINK1), or restrict PINK1 to the cytosol (ΔN111-PINK1). Both constructs blocked cell death associated with loss of endogenous PINK1. However, transient expression of ΔN111-PINK1, but not of OMM-PINK1 or ΔN111-PINK1(K219M), promoted dendrite outgrowth in primary neurons, and rescued the decreased dendritic arborization of PINK1-deficient neurons. Mechanistically, the cytosolic pool of PINK1 regulated neurite morphology through enhanced anterograde transport of dendritic mitochondria and amplification of PKA-related signaling pathways. Our data supports a novel role for PINK1 in regulating dendritic morphogenesis.
Keywords: PINK1, PKA, dendrite, cytosol, differentiation, mitochondria, Parkinson disease
Introduction
Neuronal differentiation requires the coordinated activation of pathways that mediate changes in cell shape, metabolism and suppression of apoptosis. Often a single kinase is involved in regulating distinct cellular pathways through compartmentalized activity (Feliciello et al. 2001, Sim & Scott 1999). The PTEN-induced kinase 1 (PINK1) is a ser/thr kinase with dual mitochondrial and cytosolic localization (Matsuda et al. 2010, Lin & Kang 2008). Mutations in PINK1 cause autosomal recessive Parkinson Disease (PD) (Valente et al. 2004) associated with neuropsychiatric symptoms (Steinlechner et al. 2007). A large body of evidence suggests that PINK1 is a cytoprotective ser/thr kinase, conferring resistance against staurosporine, proteasome inhibitors and the PD toxins MPP+ and 6-hydroxydopamine (6-OHDA) (Dagda & Chu 2009, Valente et al. 2004, Petit et al. 2005, Haque et al. 2008). While PINK1 may inhibit cell death through multiple mechanisms (Petit et al. 2005, Pridgeon et al. 2007, Wang et al. 2007) including activation of the cytosolic Akt/mTOR pathway (Murata et al.2011), the possible involvement of mitochondrially-anchored or cytosolic pools of PINK1 remain undefined.
Structural domains of PINK1 include an N-terminal mitochondrial import sequence, a transmembrane domain that can anchor the protein to the outer mitochondrial membrane (OMM), a catalytic domain, and a C-terminal regulatory domain that affects its localization (Cookson 2010, Cookson et al. 2007, Becker et al.2012). Under physiological conditions, a large fraction of PINK1 is imported into mitochondria for processing by up to four mitochondrial proteases (Deas et al.2011), with export of lower molecular weight forms of PINK1 to the cytosol where they may be degraded or stabilized by association with heat shock protein 90 (Hsp90) (Lin & Kang 2008).
PINK1 regulates mitochondrial morphology (Dagda et al. 2009, Exner et al. 2007, Yang et al. 2008), mitochondrial calcium buffering, mitochondrial transport and mitochondrial autophagy (mitophagy) through interactions with the ubiquitin ligase Parkin (Dagda et al. 2009, Gandhi et al. 2009, Wood-Kaczmar et al. 2008, Poole et al. 2008, Marongiu et al. 2009, Matsuda et al.2010, Narendra et al. 2010). However, the mitochondrial import sequence is not always necessary for neuroprotection in vitro or in vivo (Haque et al. 2008).
Less is known about functions regulated by cytosolic pools of PINK1. At the postsynaptic compartment, PINK1 positively upregulates the neuroprotective activity of NR2A-containing NMDA receptors in cortical neurons (Chang et al.2010), regulating mitochondrial transport by associating with or phosphorylating Milton or Miro (Liu et al. 2012, Matenia et al.2012). PINK1 also regulates vesicular release of dopamine, regulates long-term potentiation, and modulates calcium activated potassium channels (Shan et al. 2009, Kitada et al. 2007), implicating PINK1 in neuron-specific functions in addition to its more widely studied role at the OMM of depolarized mitochondria.
Here, we elucidate a novel physiological role for the cytosolic pool of PINK1. Either transient or stable overexpression of full-length PINK1 (PINK1-FL) promotes neurite outgrowth in naïve SH-SY5Y cells and enhances dendritic complexity in primary cortical or midbrain dopaminergic neurons. Conversely, PINK1-deficient SH-SY5Y cells and Pink1−/− cortical or midbrain neurons show decreased neurite lengths compared to controls. Cytosolic PINK1 not only blocks cell death induced by chronic loss of PINK1, but exhibits through functional interactions with PKA a novel neurite-regulatory function distinct from that of OMM-tethered PINK1.
Materials and Methods
Plasmids
PINK1-GFP and PINK1-3xFlag were purchased from Genecopoeia (Rockville, MD). OMM-GFP, D-AKAP1-GFP and Mito-PKI-GFP were provided by Dr. Stefan Strack (University of Iowa) (Cribbs and Strack, 2007). Human PINK1 cDNA lacking the residues 1–111 was amplified from the full length sequence (Haque et al., 2008) and ligated downstream of the TOM20 presequence (residues 1–32) in the pEGFP-N1 vector. A catalytically inactive construct of ΔN111-PINK1(K219M) was generated from ΔN111-PINK1 in pReceiver M03 using the QuickChange Site Directed Mutagenesis kit (Stratagene). Wild type and K219M versions of PINK1-V5 were provided by Anurag Tandon (University of Toronto).
Cell culture
Parental SH-SY5Y cells, stable vector control (pReceiver), PINK1shRNA line D14 and shControl line V17 (Dagda et al., 2009) were grown in DMEM (Invitrogen), 2mM glutamine, HEPES, 10% FBS). In some experiments, cells were differentiated with dibutyryl-cAMP (250µM, SIGMA, St. Louis, MO) for 24h before analysis as described below. Due to unique features of post-mitotic neurons, primary neuron cultures from mice are necessary to fully study the effects of PINK1 on neuron-specific processes. All experiments involving mice were performed in accord with ARRIVE guidelines. Primary cortical and midbrain neurons were prepared from wild type C57BL/6 or PINK1 knockout mice as previously described (Dagda et al., 2011), using procedures to minimize distress that have been approved by the University of Pittsburgh Institutional Animal Care and Use Committee (IACUC). The University of Pittsburgh is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, International (AAALAC). Primary cortical and midbrain neurons were prepared from 14-day C57BL/6 mouse embryos (Hilltop Laboratory Animals,Scottdale, PA) or from PINK1 knockout mouse and control littermates. Approximately 16 wells of 200,000 cells/well were obtained from 6–8 embryos (male and female) per timed pregnant female for midbrain cultures or roughly 10× as many total cortical cells from 8 embryos plated at 300,000 cells per well for multiple transfection experiments. After 3 days, two-thirds of the media was exchanged with fresh Neurobasal (Gibco/Invitrogen, Carlsbad, CA) containing B27 and 0.75mM L-glutamine. Primary cortical and midbrain neurons were transiently transfected with wild-type or mutant PINK1 at 5 DIV as described (Chu, 2008). The PINK1 genotype of each individual pup was confirmed by PCR.
Immunofluorescence and live-cell imaging
For studies of neurite length and mitochondrial morphology, cells previously transfected as indicated were formaldehyde-fixed, immunolabeled using rabbit anti-GFP (1:5000; Invitrogen, Carlsbad, CA), mouse anti-cytochrome c (1:400;BD Pharmingen, San Jose, CA), and/or mouse anti-human mitochondrial p60 (Biogenex, 1:1000, Fremont, CA), mouse anti-neurofilament heavy chain (200kDa) (1:400; Millipore, Billerica, MA), mouse anti-MAP2B (1:400; Millipore, Billerica, MA), mouse anti-human phospho-CREB (ser 133; 1:1000, Cell Signaling, Billerica, MA) or rabbit anti-human CREB (1:1000, Cell Signaling, Billerica, MA), mouse anti-human tyrosine hydroxylase (TH; 1:1000, Millipore, Billerica, MA), rabbit anti-human tyrosine hydroxylase (TH: 1:1000, Life Technologies, Grand Island, NY), goat anti-GFP (1:1000,Rockland Immunochemicals, Gibertsville,PA) using Alexa 488 (Molecular Probes, Eugene, CA) or Cy3-conjugated (1:400; Jackson Immuno-Research Laboratories, West Grove, PA) secondary antibodies, and counterstained with 1.25 µg/ml DAPI (Molecular Probes, Eugene, CA). Cells were imaged at 25°C using an IDX71 fluorescence microscope with a DP70 camera (Olympus America Inc., Melville, NY) at a magnification of 20× (0.45NA) or 40× (0.60NA) (excitation/emission filter: 490 nm/520 nm; 541 nm/572 nm).
Primary cortical neurons or cAMP-differentiated SH-SY5Y cells were co-transfected with mito-RFP and GFP-tagged wild-type, ΔN111-PINK1 or ΔN111-PINK1(K219M). Mitochondrial movement was analyzed by time-lapse fluorescence imaging every 5 seconds for five minutes at 60×. Kymographs were assembled and analyzed using using Image J version 1.44 (Bethesda, MD) with “Multiple Kymograph” plug-in (J. Rietdorf,A. Seitz, EMBL, Heidelberg), tracking up to 40 mitochondria per cell.
Neurite lengths and complexities were analyzed in cortical and midbrain neurons using the NIH Image J plug-in programs “Neuron J” (Erik Meijering, Biomedical Imaging Group Rotterdam, Netherlands) and “Scholl Analysis” respectively. All neurite length and complexity studies were scored blindly by an independent participant who was not directly involved with the experiment.
Western blot analysis and densitometry
Proteins were resolved on precast 5–15% Tris-HCl polyacrylamide gels (Biorad, Hercules, CA) as previously described (Dagda et al., 2009), immunoblotting for endogenous PINK1 using C8830 rabbit anti-PINK1 (1:2000)(Dagda et al., 2009) or Novus CB100 494 (1:1000), mouse anti-Flag (1:1000) (Sigma, St. Louis, MO), rabbit anti-GFP (1:1000) (Invitrogen, Carlsbad, CA), mouse anti-neurofilament heavy chain (200kDa)(1:400; Millipore, Billerica, MA), mouse anti-MAP2B antibody (1:400; Millipore, Billerica, MA), mouse anti-PSD95 (BD transduction labs, San Jose, CA) respectively, mouse anti-human mitochondrial p60 (1:1000, Biogenex, Fremont, CA), rabbit anti-human TFAM (1:1000, serum, gift of Dr. Emine Koc, Marshall University, Huntington, WV), rabbit-antihuman TOM20 (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-GADPH (1:4000), rabbit anti-β-tubulin (1:10,000; Abcam, Cambridge, MA) or mouse anti-β-actin (1:1000) (Santa Cruz Biotechnology, Santa Cruz, CA). Following overnight incubation, PVDF membranes were incubated with the appropriate secondary antibodies conjugated to horseradish peroxidase (1:5,000; Amersham/GE Healthcare) in 5% milk in PBST, and analyzed by film detection of chemiluminescence.
Mitochondrial isolation and trypsin sensitivity
Mitochondria were isolated from 3 × 106 cells per 10-cm tissue culture dish 3d after transfection as previously described (Dagda et al., 2009), using the Subcell Focus mitochondrial isolation kit (G-Biosciences, St. Lois, MO). Approximately four equal parts of mitochondria (20µl of an 80µl total material at 0.50µg/ul) solubilized in working storage buffer (G-Biosciences, St. Lois, MO) were exposed to increasing concentrations of trypsin in the presence or absence of 1% Triton-X-100 to lyse mitochondrial pellets. Mitochondrial pellets were incubated for 30 minutes on ice and the protease reaction was terminated using ovomucoid trypsin inhibitor (final concentration of 50µg/ml). Mitochondria lysed in 5mM CaCl2, 10mM Tris acetate at pH 8.0, 0.1mM PMSF, 2µg/ml of leupeptin, 2µg/ml aprotonin and 0.5% NP40 were analyzed by immunoblot for PINK1 (BC100-494), TOM20, TFAM and GAPDH.
Cell survival-death, ROS and transmembrane potential assays
Cells seeded on 24 well plates and transfected with GFP tagged forms of PINK1 were fixed in 3.9% paraformaldehyde 2 days later, permeabilized in 0.1% Triton X-100 for 10 minutes and counterstained with DAPI (1.25 µg/ml; Molecular Probes, Eugene, CA) for five minutes prior to washing. 20–30 transfected cells per epifluorescence micrograph were scored for nuclear fragmentation/pyknosis by an observer blinded to the experimental conditions.
Two days after transfection with PINK1 fusion constructs, the cells were stained with MitoSOX, (2.5µM, Molecular Probes, Eugene, CA) as previously described (Dagda et al., 2009). The average intensity of MitoSOX fluorescence per cell in GFP transfected cells was quantified using NIH Image J as the ratio of the mean intensity of MitoSOX fluorescence to the mitochondrial area per transfected cell.
Stable control (V17) and PINK1shRNA cells (clones A4 and A14) were loaded with 100nM of tetra-methyl rhodamine methyl-esther (TMRM, SIGMA, St. Lois, MO) two days after transfection, and incubated for 40 min at 37C in a 5% CO2 cell culture incubator, then washed with pre-warmed complete DMEM media. TMRM fluorescence was visualized in live cells using an inverted FluoView 1000 laser scanning confocal microscope at a 60× magnification (1.42 numerical aperture) using the linear, sequential scan mode function (Olympus America Inc., Melville, NY; excitation/emission filter: 488 nm/510 nm; 561 nm/592 nm). Mitochondrial membrane potential was quantified by dividing the average pixel intensity of the mitochondria by the background cytosolic/nuclear fluorescence intensity as previously described (Dagda et al., 2011).
Statistics
Results are expressed as compiled mean±s.e.m from at least three independent experiments. Multiple group comparisons were performed using one-way analysis of variance (ANOVA) followed by Tukey’s test or a Fisher’s LSD t-test to identify paired differences. Values of p<0.05 were considered significant.
Results
PINK1 modulates neurite morphology in SH-SY5Y cells and primary cortical neurons
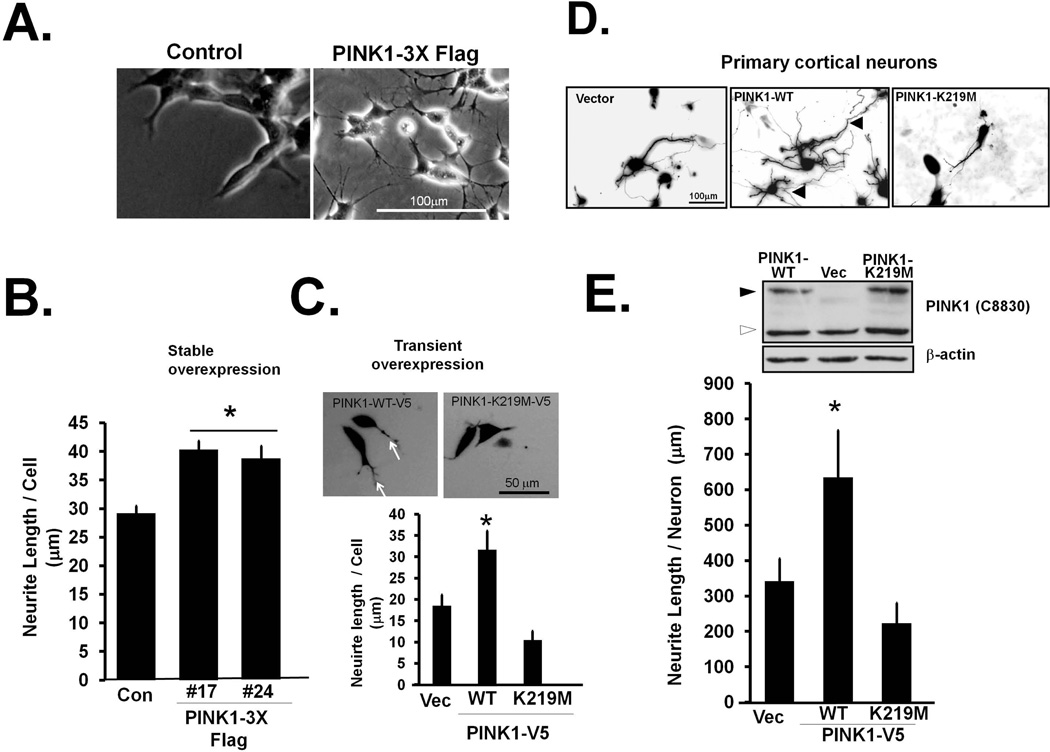
Endogenous PINK1 is localized to both cytosolic and mitochondrial compartments (Supplementary Fig. S1AB) (Dagda et al. 2009, Beilina et al. 2005, Gandhi et al. 2006), yet few studies have examined the potential role of cytosolic PINK1 in regulating neuronal health. We previously reported that an amino-truncated version of PINK1 that lacks the mitochondrial targeting sequence suppresses macroautophagy and blocks neuronal cell death induced by chronic loss of endogenous PINK1 (Dagda et al. 2009). Immunofluorescence studies of endogenous PINK1 reveal that it is diffusely present within neurites as well as colocalizing with mitochondria (Supplementary Fig. S1B). To investigate the possible role of PINK1 in neurite outgrowth, we studied the effects of PINK1 overexpression in SH-SY5Y cells, in the absence of neurotrophic factors or other differentiating stimuli, using a set of previously characterized stable cell lines (Dagda et al. 2009). Whereas the stable vector control line (pReceiver M14) exhibited stubby-ended pseudopodia that are characteristic of parental SH-SY5Y cells, increased PINK1 expression elicited elongated, slender cytoplasmic extensions that resembled neurites (Fig. 1A–B). Moreover, transient expression of a V5-tagged form of PINK1-FL not only increased the percentage of SH-SY5Y cells containing elongated cytoplasmic extensions, but also increased the average length of neurites in primary cortical neurons (Fig. 1C–E). In contrast, an engineered kinase deficient version of PINK1(K219M) had no such effect (Fig. 1C–E), despite achieving approximately similar levels of recombinant PINK1 expression compared to wild-type PINK1 (Fig. 1E, inset), suggesting that intact kinase activity is required for the neurite elongating effect of PINK1 in both SH-SY5Y cells and primary neurons.
Figure 1. Overexpression of PINK1 promotes neurite outgrowth.
(A–C). (A) previously characterized SH-SY5Y stable cell line (#24) that overexpresses a C-terminally 3xFlag-tagged version of PINK1 (Dagda et al. 2009), was analyzed by bright field microscopy for morphological differences (B) Compiled quantification of the average neurite length per cell shows that stable expression of PINK1 in two different clones induces a 33% increase in neurite length (Means ± S.EM, three independent experiments, *:p<0.05 vs. control cell line). (C) SH-SY5Y cells were transiently transfected with GFP and co-transfected with full-length or catalytically inactive PINK1(K219M) and analyzed for the average neurite length per cell. Representative epifluorescence images show an increase in elongated cytoplasmic extensions (white arrows) not observed with PINK1(K219M). (Means ± SEM, n=20–40 cells from two independent experiments, *:p<0.05 vs. vector transfected cells; **:p<0.05 vs. PINK1-WT-V5). (D–E) Primary cortical neurons were co-transfected with GFP and the indicated plasmids for three days. Dendritic arbors were visualized by epifluorescence microscopy of GFP-expressing neurons, revealing more complex dendritic arbors with PINK1-WT, with a 33% increase in summated neurite lengths €, Means ± SEM, n= 5–7 independent experiments. *:p<0.05 vs. vector; **:p<0.05 vs. PINK1-WT-V5). Inset shows a representative Western blot of the indicated V5 tagged PINK1 constructs of PINK1 showing similar recombinant PINK1 expression levels (full-length PINK1; black arrows) as determined by immunoblotting for PINK1 (C8830; Dagda et al., 2009) in SH-SY5Y cells transiently expressing the indicated constructs. Black arrowhead points to full-length PINK1 (66kDa) while the white arrowhead points to processed PINK1).
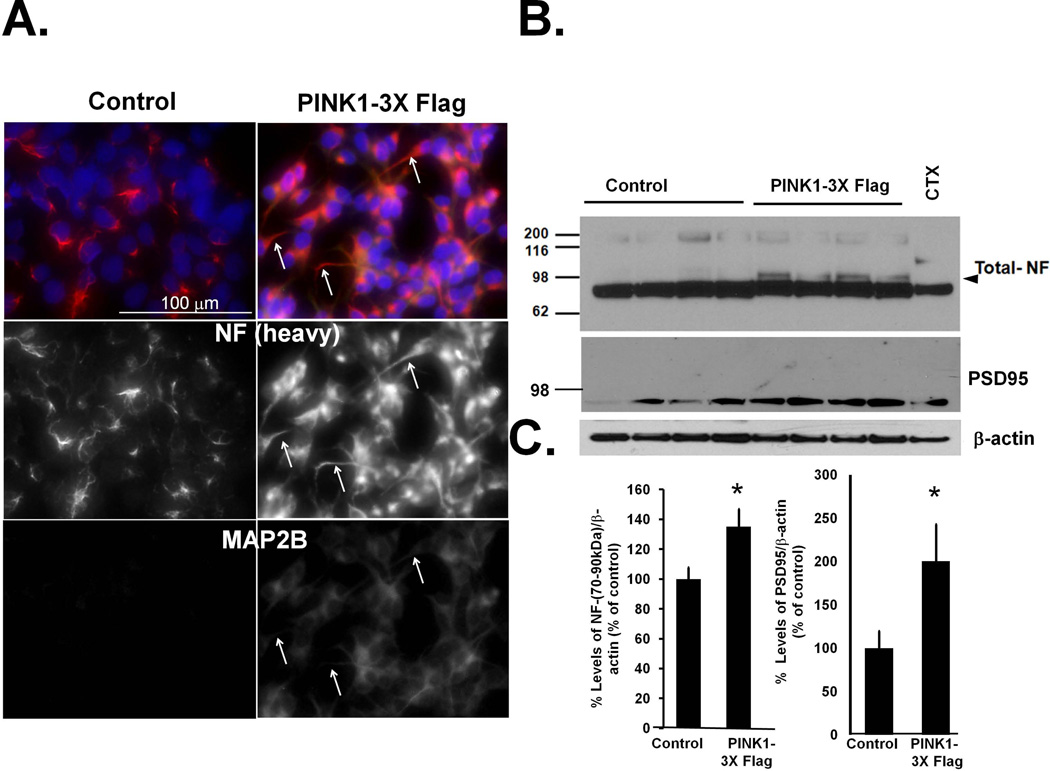
By indirect immunofluorescence, we observed that stable overexpression of PINK1-3xFlag elicited formation of cytoplasmic extensions containing high levels of the neurofilament heavy chain (200kDa) and the dendritic marker microtubule associated protein 2B (MAP2B), further supporting the interpretation that PINK1 promotes neuritogenesis in naïve SH-SY5Y cells in the absence of differentiation factors (Fig. 2A). Western blot analysis showed that PINK1 overexpression elevated levels of the postsynaptic marker PSD95. In addition, cell lysates from PINK1-3xFlag cells exhibited a higher molecular weight form of neurofilament medium chain (70–90kDa), consistent with higher phosphorylation status of neurofilaments in differentiated axons (Grant & Pant 2000), which was not seen in cell lysates from the vector control (Fig. 2B–C).
Figure 2. Stable overexpression of PINK1 upregulates proteins associated with neuronal differentiation.
(A) The stable PINK1-3xFlag cell line (#24) was fixed in 4% paraformaldehyde and immunolabeled for the heavy chain of neurofilament (200kDa) and MAP2B to label axons and dendrites respectively and analyzed for morphology by epifluorescence microscopy. Representative epifluorescence micrographs demonstrates that stable expression of PINK1 increases the expression levels and induces a redistribution of neurofilament heavy chain subunit and MAP2B from the soma to the neurites compared to a stable control cell line (pReceiverM14). White arrows point to the presence of elongated neurites in PINK1-3X Flag cells. (B) Cell lysates derived from control (pReceiver M14) and PINK1 overexpressing cell lines were immunoprobed for total levels of neurofilament proteins, and the post-synaptic marker PSD95. Arrowhead indicates a higher molecular weight form of neurofilament medium chain, consistent with phosphorylation observed in differentiated axons (Grant & Pant 2000). (C). The integrated intensities of immunoreactive bands for the medium chain of neurofilament and PSD95 were quantified using the Image J software and normalized to β-actin (Means ± S.EM, n=10 plates compiled from three independent experiments, *:p<0.05 vs. control cell line).
The cytosolic pool of PINK1 enhances neurite/dendrite lengths
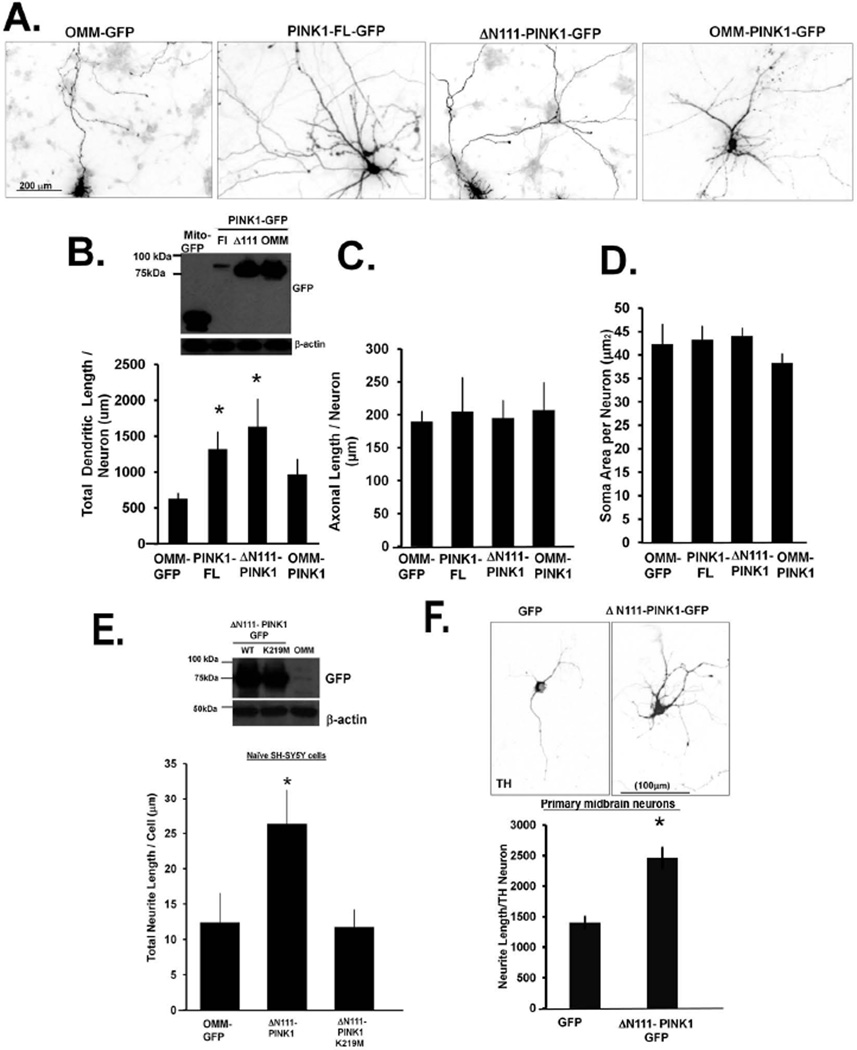
To investigate whether the action of PINK1 on neurite lengths was related to its activity at mitochondrial or cytosolic subcompartments, we generated OMM-targeted PINK1-GFP by swapping the first 111 amino acids of PINK1 with a TOM20 mitochondrial targeting sequence (OMM-PINK1-GFP) for comparison with PINK1 lacking its mitochondrial targeting sequence (Δ111N-PINK1-GFP), which was previously shown to exhibit cytosolic localization (Haque et al. 2008). Two days after transfection, the subcellular localization of the recombinant proteins was verified for appropriate targeting of the construct. In contrast to wild type PINK1-GFP, which has a dual cytoplasmic and mitochondrial distribution (Supplementary Fig. S2A; left panel), OMM-PINK1-GFP was exclusively localized to mitochondria (Supplementary Fig. S2A; middle panel), while Δ111N-PINK1-GFP was localized to the cytosol (Supplementary Fig. 2A, right panel). Analysis of mitochondrial fractions isolated from transfected cells confirmed that full-length PINK1 showed a mixed cytosolic and mitochondrial distribution with the mitochondrial pool accessible to trypsin (Supplementary Fig. 2B), in agreement with the OMM distribution reported by others (Zhou et al. 2008, Lin & Kang 2010). The OMM-PINK1 was similarly accessible to trypsin (Supplementary. Fig. 2C), while ΔN111-PINK1-3XFlag, a construct that lacks both mitochondrial targeting and transmembrane domains, is almost exclusively localized to the cytosol (Supplementary. Fig. 2D), as previously reported (Haque et al., 2008).
Chronic loss of endogenous PINK1 leads to a significant increase in basal apoptosis compared to a control cell line (3% cell death in control cell line vs. 22% in PINK1shRNA A14 clone). Transient expression of either OMM-PINK1 or ΔN111-PINK1-GFP was able to significantly suppress cell death induced by loss of endogenous PINK1, suggesting that both subcellular pools of PINK1 function in pro-survival pathways (Supplementary Fig. 3)
In primary cortical neurons, either PINK1-FL-GFP or ΔN111-PINK1-GFP, but not OMM-PINK1-GFP, increased neurite lengths (Fig. 3A). Likewise, transient expression of ΔN111-PINK1-GFP was sufficient to increase neurite lengths in dopamine midbrain neurons compared to OMM-GFP transfected neurons (Fig. 3F). Using the dendritic marker MAP2B in transfected primary cortical neurons, we observed that ΔN111-PINK1-GFP overexpression is sufficient for enhancing dendritic lengths (Figure. 3B), while a similar degree of overexpression of OMM-PINK1-GFP failed to do so (Fig. 3B, inset).
Figure 3. Cytosolic localized PINK1 increases dendritic complexity/length in primary cortical neurons and midbrain neurons.
(A) Primary cortical neurons (DIV14) were transiently transfected with the indicated PINK1 fusion constructs for three days, fixed in paraformaldehyde and immunolabeled for GFP and MAP2B to label dendrites. Representative epifluorescence micrographs show that transient expression of ΔN111-PINK1-GFP qualitatively increases the complexity and length of neurites while OMM-PINK1transient expression has no effect. (B) Compiled quantification derived from five to seven experiments show that transient expression of ΔN111-PINK1-GFP enhances neurite length in primary cortical neurons. (Means ± SEM, n= five to seven independent experiments *:p<0.05 vs. OMM-GFP). (C) Quantification of axonal lengths as analyzed by immunolabeling for the neurofilament heavy chain subunit in transiently transfected primary cortical neurons. (Means± S.EM n=20 transfected neurons from two experiments, no significant changes found across groups). (D) Quantification of the soma area of primary cortical neurons transfected with the indicated plasmids showing no differences (Means ± S.EM, n=20 transfected neurons from two experiments, no significant changes found across groups). (E) Quantification of neurite lengths in transfected SH-SY5Y cells not exposed to any other differentiating stimuli (naïve). (F) Representative epifluorescence micrographs of DIV9 midbrain dopamine neurons transfected with GFP or ΔN111-PINK1-GFP for two days prior to immunolabeling for tyrosine hydroxylase to identify dopamine neurons (the TH channel is shown). Quantification of neurite lengths derived from three experiments show that transient expression of ΔN111-PINK1-GFP enhances neurite length in dopamine neurons (For E, means ± S.EM, n=20 transfected SH-SY5Y cells from two experiments, *:p<0.05 vs. OMM-GFP. For F, compiled means ± S.EM, N=3 experiments, n=15–22 neurons per condition, *p<0.05 vs. GFP). Insets show expression levels of GFP-tagged PINK1 in transiently transfected cells.
To examine the possible effects of PINK1 on axons, primary cortical neurons transiently expressing different PINK1 constructs were immunolabeled for the heavy chain of neurofilament (200kDa) and analyzed for axonal length in GFP transfected primary cortical neurons using Neuron J. We found no significant effects of PINK1-GFP on the average axon length per transfected cell (Fig. 3C). It is unlikely that the increased dendritic lengths observed in primary neurons expressing PINK1-GFP is a consequence of selectively transfecting a subpopulation of primary cortical neurons, as analyzed cells showed no significant differences in the average soma size (Fig. 3D). Our data further indicates that the kinase deficient mutant of ΔN111-PINK1(K219M)-GFP failed to influence neurite lengths in SH-SY5Y cells (Fig. 3E) or primary cortical neurons (data not shown). Taken together, these data indicate that the cytosolic pool of PINK1 is sufficient for enhancing dendritic arbors in neurons.
Primary cortical and midbrain neurons from Pink1 knockout mice have reduced dendrite lengths
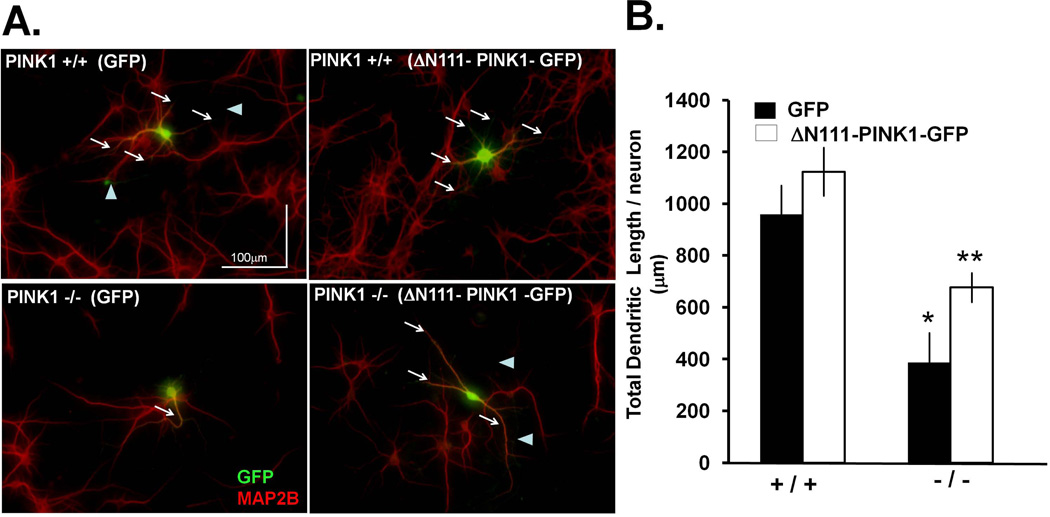
To further investigate the possible role of endogenous PINK1 in regulating dendrite lengths, we studied primary cortical neurons derived from Pink1 knockout mice. By measuring the lengths of MAP2B-immunoreactive dendrites in paraformaldehyde-fixed primary cortical neurons, we found that neurons derived from Pink1 knockout mice showed a 60% decrease in summated dendritic length per neuron compared to primary cortical neurons derived from wild-type mice (Fig. 4A). Furthermore, transfecting Pink1 knockout neurons with ΔN111-PINK1-GFP significantly restored dendrite lengths (Fig. 4A–B). In addition, dopamine neurons in dissociated primary midbrain cultures derived from Pink1 knockout mice also showed significantly reduced complexity and total length of dendrites compared to wild-type neurons, with no significant effect on axon lengths (Supplementary Fig. 4). These results indicate that loss of endogenous PINK1 elicits a similar attenuation of the dendritic arbor in both midbrain dopaminergic neurons and cortical neurons.
Figure 4. PINK1 knockout primary cortical neurons showed decreased dendritic lengths.
(A) DIV5 primary cortical neurons from PINK1 +/+ and PINK1 −/− littermates were transfected with GFP or ΔN111-PINK1-GFP, fixed three days post-transfection and immunolabeled for MAP2B to visualize dendrites. Representative images demonstrate that PINK1 deficient (−/−) primary cortical neurons exhibited reduced dendritic lengths as visualized by immunolabeling for MAP2B (white arrows in bottom left panel) compared to wild-type neurons (top left panel). Dendritic lengths are restored in PINK1 −/− neurons by transiently expressing ΔN111-PINK1-GFP. White arrowheads points to GFP positive axons that were MAP2B negative and that were excluded from dendritic length quantification. (B) Quantification of total dendritic lengths in primary cortical neurons transiently expressing the indicated plasmids (Means ± S.EM., n=30–50 primary cortical neurons pooled from 3–5 prenatal mouse pups per genotype, *:p<0.05 vs. PINK1 (+/+)/ GFP; **:p<0.05 vs. PINK1 (−/−) / GFP).
PINK1 enhances mitochondrial anterograde transport and density in dendrites
Achieving the proper density of mitochondria in dendrites is essential for synaptogenesis and dendritic maintenance in neurons (Li et al. 2004). By quantifying the percentage of the dendrite length occupied by mitochondria, we found that PINK-FL-GFP increased mitochondrial density in the dendrites compared to primary cortical neurons expressing OMM-GFP. This effect could be recapitulated by transfection with ΔN111-PINK1-GFP, but not OMM-PINK1-GFP (Supplementary Fig. 5).
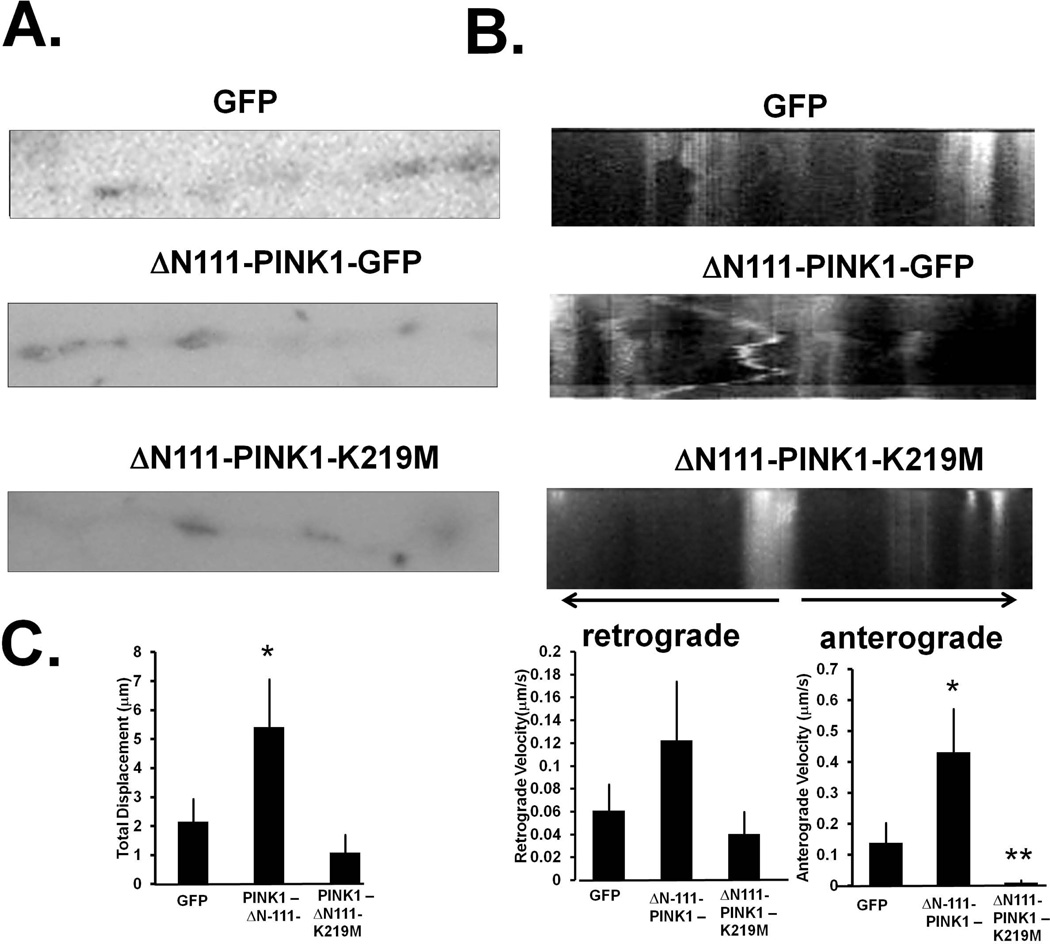
One mechanism by which either PINK1-FL-GFP or ΔN111-PINK1-GFP could act to regulate mitochondrial density within dendrites is via mitochondrial transport. Live cell imaging of mitochondria in cells co-transfected with mito-RFP confirmed previously reports (Cherra et al.) that only a small fraction of mitochondria is mobile in dendrites of control DIV8 primary neurons transfected with OMM-GFP (Fig. 5B). Expression of ΔN111-PINK1-GFP significantly enhanced mitochondrial movement, particularly anterograde velocities, while expression of the kinase deficient ΔN111-PINK1(K219M)-GFP resulted in cessation of movement (Fig. 5B–C).
Figure 5. The cytosolic pool of PINK1 enhances anterograde movement of mitochondria to neurites.
(A) Representative epifluorescence micrographs of dendrites from DIV9 primary cortical neurons transiently expressing either GFP, ΔN111-PINK1-GFP or kinase deficient ΔN111-PINK1-K219M-GFP along with mito-RFP to analyze for mitochondrial movement and distribution in dendrites. (B) DIV9 primary cortical neurons transiently expressing the indicated PINK1 fusion constructs and mito-RFP were visualized for mitochondrial movement by time lapse epifluorescence microscopy in live cells for 5 minutes with 5 second intervals per image capture. Image sequences were assembled into kymographs to visualize mitochondrial movement. The representative kymographs show bidirectional movement of mitochondria at the dendrites across time for the indicated transfection conditions. (C) Total displacement (left graph), average anterograde velocity (right graph) and retrograde velocity (middle graph) were quantified by using the line scan tool of Image J for up to eight kymographs per condition. Note that transient expression of the kinase deficient mutant K219M stalls mitochondria movement while ΔN111-PINK1-GFP enhances anterograde movement towards the distal end of the dendrites (right graph) compared to GFP control cells. (Means ± S.EM, n=120 mitochondria from 5–8 transfected primary cortical neurons *:p<0.05 vs. GFP **:p<0.05 vs. ΔN111-PINK1).
We conducted similar studies in the neurites of cyclic AMP-differentiated SH-SY5Y cells (Supplementary Fig. 6). In contrast to primary cortical neurons, mitochondria of SH-SY5Y cells predominantly moved in a retrograde manner. Like primary cortical neurons, however, differentiated SH-SY5Y cells exhibited enhanced anterograde velocities when transfected with ΔN111-PINK1-GFP, while mobility was stalled by expression of ΔN111-PINK1(K219M)-GFP (Supplementary Fig. 6B–C).
PINK1shRNA cells are impaired for cyclic AMP-mediated neurite outgrowth
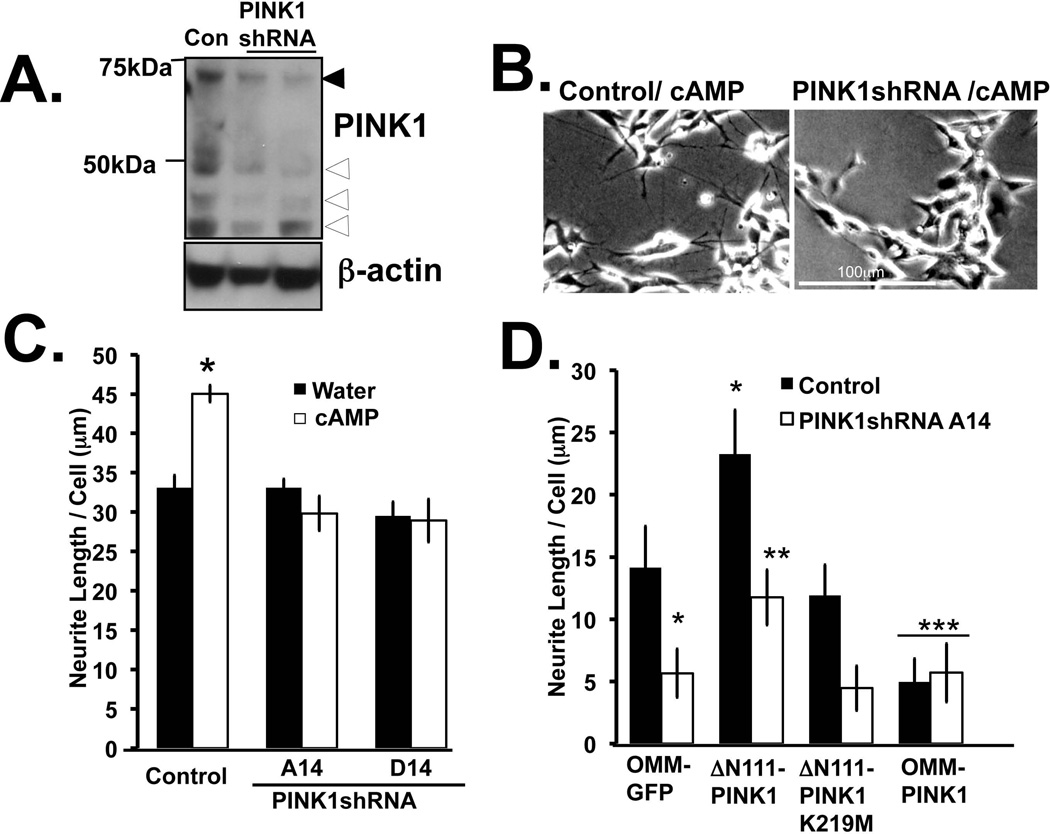
To elucidate mechanism(s) underlying the regulation of neurite outgrowth by PINK1, we utilized two previously characterized PINK1shRNA cell lines that stably reduce full length (51% and 81% for PINK1shRNA A14 and clone D14 respectively) and lower molecular weight forms of endogenous PINK1 compared to a stable vector control line (for 45kDa band: 58% and 60% for PINK1shRNA A14 and clone D14 respectively and more than 60% for PINK1 bands less than 36kDa) (Fig. 6A), with resultant mitochondrial dysfunction (Dagda et al. 2009). Neither of these lines responded well to cAMP-induced neuronal differentiation (Fig. 6B–C). Complementation studies show that transient expression of ΔN111-PINK1-GFP but not ΔN111-PINK1(K219M)-GFP was sufficient to stimulate neurite outgrowth in both control and PINK1-deficient SH-SY5Y cells, while OMM-PINK1 failed to do so (Fig. 6D). These data indicate divergent roles for cytosolic and OMM-tethered forms of PINK1.
Figure 6. PINK1 deficient cells are impaired for cyclic AMP mediated neurite outgrowth.
(A) PINK1 knockdown SH-SY5Y clonal cell lines (clone A14 and D14) exhibit reduced expression of full length (black arrow) and processed forms of PINK1 (white arrows), relative to the stable shRNA control (clone V17). (B) Representative bright field images of control cells show that a single dose of cAMP (24 hr) elicits neurite outgrowth in control cells, while PINK1shRNA cells remain unresponsive. (C) Bar graph showing that PINK1shRNA cells are unresponsive to cyclic-AMP-mediated neurite extension (Means ± S.EM, n=30–40 cells analyzed from two experiments, *:p<0.05 vs. control cell line/water). (D) Control and PINK1shRNA SH-SY5Y cells were transiently transfected with the indicated PINK1 fusion constructs and neurite length was quantified using Image J. (Means ± S.EM, n=30–40 cells analyzed from two experiments, *:p<0.05 vs. control cell line/OMM-GFP, **:p<0.05 vs. PINK1shRNA/OMM-GFP, ***:p<0.05 vs. control/ΔN111- PINK1 -GFP or PINK1shRNA / ΔN111- PINK1 -GFP).
Mitochondrially localized PINK1 modulates mitochondrial homeostasis
We next wanted to verify that the OMM-PINK1 construct was functional by examining its ability to complement known phenotypes induced by loss of endogenous PINK1 (Dagda et al.2011, Lazarou et al. 2012, Greene et al.2012). As indicated above, both OMM- and cytosolic PINK1 retained the ability to protect against apoptosis (Supplementary Fig. 3). Moreover, OMM-PINK1, but not ΔN111-PINK1, reversed previously described mitochondrial effects of PINK1-deficiency on transmembrane potential, mitochondrial superoxide levels, and mitochondrial morphology (Supplementary Fig. 7). Thus, the failure of OMM-PINK1 to affect neurite morphology was not due to loss of biological activity induced by addition of the TOM20 targeting sequence. In addition to confirming a role for OMM-tethered PINK1 in regulating mitochondrial homeostasis, these data further support the concept of distinct biological roles for OMM- versus cytosolic pools of PINK1.
PINK1 enhances neurite length through activation of PKA-regulated signaling pathways
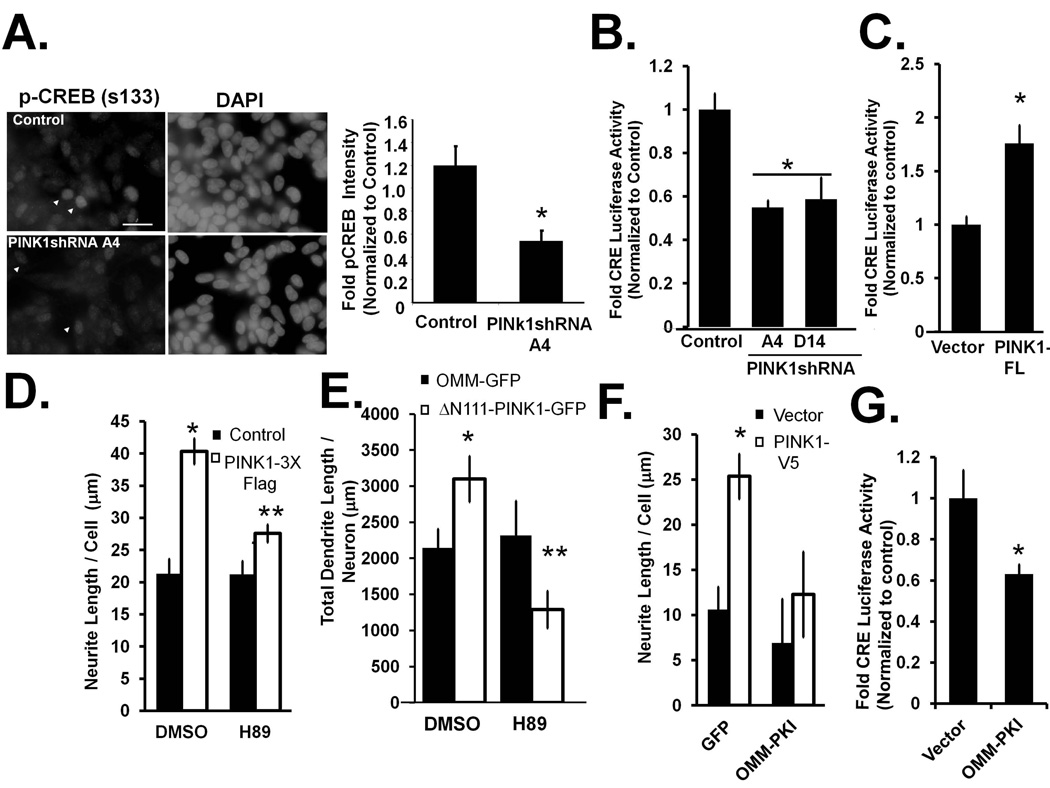
The selective effects of PINK1 knockdown on neuronal differentiation elicited by cAMP, but not by retinoic acid (data not shown), implicated possible involvement of PKA, which regulates synaptogenesis, long term potentiation and survival through activation of CREB (Grewal et al. 2000, Merrill et al. 2011, Tominaga-Yoshino et al. 2002, Dickey & Strack 2011, Xu et al.2012). PINK1shRNA lines showed significant decreases in phosphorylated nuclear CREB (ser 133) compared to the stable control line, suggesting decreased activation or translocation of phospho-CREB (Fig. 8A). Transfection of PINK1shRNA cells with a CRE-luciferase reporter plasmid also supported a decrease in intracellular PKA activity (Fig. 7B). Conversely, transient overexpression of PINK1-FL significantly enhanced CRE-luciferase activity (Fig. 7C), indicating that PINK1 modulates cAMP-dependent signaling pathways in SH-SY5Y cells.
Figure 7. Mitochondrially localized PKA is required for PINK1’s ability to promote neurite outgrowth.
(A)Representative epifluorescence micrographs of control and PINK1shRNA SH-SY5Y cells showing that stable knock down of endogenous PINK1 leads to decreased nuclear localization of p-CREB (ser 133), as quantified by the mean intensity of nuclear p-CREB immunofluorescence normalized to total CREB immunoreactivity. (Means ± S.EM, n=30 cells per cell line from two experiments, *:p<0.05 vs. control).
(B–C) Knockdown of endogenous PINK1 decreases the CRE-luciferase activity/protein level ratios compared to control cells (clone V17), an indirect measure of intracellular PKA activity in two PINK1shRNA cell lines (A14 and D14), while PKA activity is increased in cells stably expressing PINK1-3X Flag (C) (Means ± S.EM, n= 3–4 experiments, *:p<0.05 vs. vector or control).
(D) Total neurite length per cell was measured in PINK1 overexpressing cells (clones #24) in the presence or absence of H89 (means ± SEM, n = 30 cells/cell line from one a representative experiment of three *:p<0.05 vs. control/DMSO, **:p<0.05 vs. PINK1-3X Flag/DMSO). (E) Primary cortical neurons were transiently transfected with OMM-GFP and ΔN111-PINK1-GFP constructs in the presence or absence of H89 (0.5 µM) and analyzed for neurite length via image analysis. (Means ± S.E.M, n = 30 cells/cell line from one representative experiment of two, *:p<0.05 vs. control/DMSO, **:p<0.05 vs. ΔN111-PINK1-GFP /DMSO) (F) SH-SY5Y cells were transiently transfected cells with V5 tagged PINK1 Flag and co-transfected with GFP-tagged mitochondrially targeted PKA inhibitor (mito-PKI) or GFP as a control and analyzed for neurite length via image analysis (Means ± S.E.M, n = 30 cells/line from two experiments, *:p<0.05 vs. vector/GFP, **:p<0.05 vs. PINK1-V5/GFP). (G) Representative quantification showing that transient expression of mito-PKI decreases CRE-luciferase activity by 40% in parental SH-SY5Y cells. (Means ± S.E.M, n=6 transfected wells per condition, *:p<0.05 vs. vector/GFP).
To determine the role of PKA signaling in PINK1-mediated neurite outgrowth, we treated the PINK1-3xFlag line with the PKA inhibitor H89, using conditions that do not elicit mitochondrial fragmentation or cell injury (Dagda et al. 2011). H89 significantly inhibited neurite outgrowth induced by PINK1 overexpression in SH-SY5Y cells (Fig. 7D) and in primary neurons transfected with Δ111-PINK1-GFP (Fig. 7E). In control experiments, we found that H89 had no effect on PINK1 autophosphorylation (Cherra & Chu, unpublished data). Taken together, these data suggest a functional interaction of cytosolic PINK1 and PKA signaling.
PKA activities are compartmentalized to specific subcellular compartments by binding to A-kinase anchoring proteins (AKAP). Transient expression of D-AKAP1 increases dendritic length and complexity in hippocampal neurons while suppressing premature synaptogenesis, suggesting that PKA exerts its effects on dendrite morphogenesis at the mitochondrion in neurons (Dickey & Strack, 2011). To determine whether mitochondrial PKA was similarly implicated in PINK1-dependent neurite outgrowth, cells were co-transfected with PINK1 and a GFP-tagged protein kinase A inhibitor peptide (PKI) targeted to the OMM (mito-PKI). Transient expression of mito-PKI for two days resulted in a 40% decrease in intracellular PKA activity (Fig. 7G). Mito-PKI blocked the ability of overexpressed PINK1 to promote neurite outgrowth (Fig.7F), while expression of a constitutively active mitochondrial PKA further amplified the effects of PINK1 (data not shown).
Overall, the requirement for PINK1 in cAMP-mediated differentiation combined with the ability of PINK1 to positively regulate PKA/CREB activities indicates functional interaction between cytosolic PINK1 and PKA in neuritic/dendritic formation and maintenance.
Discussion
While the role of PINK1 in regulating mitochondrial structure and function have been heavily studied over the past decade, there is less knowledge with regards to the effects of PINK1 in other subcellular compartments, particularly in neurons. At the mitochondrial level, PINK1 regulates mitochondrial transport, morphology, biogenesis, function, calcium buffering capacity and mitophagy (Wood-Kaczmar et al. 2008, Gegg et al. 2009, Dagda & Chu 2009, Van Laar & Berman 2009, Gandhi et al. 2009, Petit et al. 2005, Narendra et al. 2010, Matsuda et al. 2010, Sun et al. 2012). However, an N-terminal truncation mutant of PINK1 that lacks the transmembrane and mitochondrial targeting domain is sufficient for neuroprotection against MPTP toxicity in vivo (Haque et al. 2008), suggesting that mitochondrial targeting is not essential for all PINK1 activities. Here, we present data for a novel role for cytosolic PINK1 in regulating neurite morphogenesis, enhancing anterograde mitochondrial transport and density of mitochondria in dendrites and upregulating expression of neuronal differentiation proteins (Fig. 2, 3, and Supplementary Fig. 5 and 6). Furthermore, loss of PINK1 function is not only associated with impaired mitochondrial function, but also shortening and simplification of dendritic arbors and decreased PKA signaling (Fig. 4 and 7). Given that synaptic dysfunction and dendritic pathology may contribute to the earliest stages of neurodegeneration (Bywood & Johnson 2000, Cherra et al. 2010, Bendiske et al. 2002), these findings may offer additional insight for mechanisms contributing to the development of PD.
Mechanistically, we found that PINK1 is required for cAMP-induced neurite extension and promotes PKA activation, implicating an amplifying interaction between these pathways in dendrite maintenance. (Fig. 3–5, 7). PKA is critical for several neuronal functions including dendrite extension, spine formation and modulation of mitochondrial fission (Dickey & Strack, 2011 Merrill et al.2011, Dagda et al.2011). The effects of PKA are regulated by AKAPs, which confer selectivity to its actions at distinct subcellular sites (Feliciello et al. 2001). Although the mechanism by which PINK1 is targeted to the mitochondria is distinct from that of PKA, (Becker et al.2012, Lin & Kang 2010, Zhou et al. 2008), emerging data suggest functional interactions of PINK1 and PKA at multiple subcellular compartments. Like mitochondrial PKA, expression of PINK1 promotes mitochondrial fusion in mammalian cells (Sandebring et al. 2009, Exner et al. 2007, Dagda et al. 2009). Moreover, mitochondrial PKA can reverse multiple parameters of mitochondrial dysfunction induced by chronic deficiency in endogenous PINK1 (Dagda et al.2011). In the cytosolic compartment, PKA phosphorylates the autophagy protein LC3 to protect against MPP+ and G2019S LRRK2-mediated dendrite retraction (Cherra et al. 2010). Likewise, cytosolic Δ111-PINK1 is sufficient to inhibit autophagy induced by loss of endogenous PINK1 (Dagda et al. 2009). Interestingly, PINK1 promotes PKA-mediated phosphorylation of both Drp1 (Sandebring et al. 2009) and LC3 (Cherra & Chu, unpublished data). The current data showing that cytosolic PINK1 affects dendritogenesis through effects on PKA add further support to an important functional relationship between these pathways.
Future studies are required to elucidate the mechanisms by which PINK1 activates downstream PKA signaling for promoting dendritic outgrowth. It is conceivable that more than one signaling mechanism may contribute to the ability of PINK1 to elicit dendritic outgrowth in neurons. Given that MAP2 is a neuronal-specific AKAP (Harada et al. 2002), and PINK1 elicited increased levels of MAP2 (Fig. 2 and 3), it is possible that MAP2-tethered PKA holoenzymes are also increased at the dendrites to promote dendritic outgrowth. (Huang et al. 2013). Alternatively, cytosolic PINK1 may promote phosphorylation of the RIIβ subunit, a substrate of many ser/thr kinases including PKA (Braun et al. 1991), to release the catalytic subunits of PKA holoenzymes. This may occur either directly or indirectly, as the increased mitochondrial density in observed in dendrites may lead to increased levels of OMM-localized PKA, setting up an amplifying mechanism by which phosphorylation of cytoskeletal-associated MAP2A/B results in destabilization of MAP2-tethered RIIβ subunits to liberate active PKA.
Prior studies have also suggested that PINK1 may play a role in regulating cytoskeletal dynamics. PINK1 promotes lamellopodia extension and cellular motility in non-neuronal cells (Murata et al.2011). Transient expression of PINK1 opposes the effects of LRRK2 in modulating axonal morphology in C. elegans. (Samann et al. 2009). Furthermore, PINK1 may play a developmental role for the proper patterning, migration and contact of dopaminergic axons in zebrafish (Samann et al.2009). In human hippocampal neurons, overexpression of full length PINK1 is correlated with mitochondrial fragmentation, increased mitochondrial number, and decreased density at dendritic spines (Yu et al.2011), raising the possibility of differences based on neuron type. The current data showing that transient or stable overexpression of PINK1 enhanced neurite outgrowth in SH-SY5Y cells in the absence of added neurotrophic/differentiation factors, combined with the effects on dendrite morphology observed in cortical and dopaminergic midbrain neurons, suggest that cytosolic PINK1 is a bona fide regulator of dendritogenesis in neurons (Fig. 1, 3).
Interestingly, the regulation of dendritic versus axonal outgrowth exhibits distinct as well as shared mechanisms (Dickey & Strack 2011, Xu et al.2012, Goshima et al. 1993). PD-associated mutations in LRRK2 engage different mechanisms to regulate presynaptic/axonal versus postsynaptic/dendritic compartments (Lin et al. 2010, Lee et al. 2010, and Cherra et al. 2013) Transient overexpression of PINK1 in Drosophila motor neurons or mouse hippocampal neurons reduces axonal mitochondrial velocities, leading to decreased mitochondrial occupancy in presynaptic terminals (Liu et al. 2012, Wang et al. 2011, Yu et al.2011). The mechanism by which PINK1 reduces mitochondrial delivery to axon terminals is through to involve phosphorylation of microtubule adaptor proteins Milton and Miro (Weihofen et al. 2009), and the subsequent Parkin-mediated autophagic clearance of mitochondria (Liu et al.2012). In contrast, our study suggests cytosolic PINK1 promotes anterograde mitochondrial transport into dendrites. These findings may relate to the ability of different TRAK family adaptor proteins to engage different transport machineries to steer mitochondria into axons versus dendrites (van Spronsen et al.2013).
In summary, we found that cytosolic PINK1 exhibited distinct activities from OMM-tethered PINK1. In particular, cytosolic PINK1 was involved in regulating neurite outgrowth and dendritic extensions in SH-SY5Y cells and primary cortical neurons through mechanisms involving dendritic mitochondrial transport and amplification of PKA signaling. PINK1 is widely expressed throughout the body, and has been implicated in mitochondrial dysfunction in a growing number of tissues (Billia et al. 2011, Rakovic et al. 2010). Despite this, patients with homozygous or compound heterozygous mutations in PINK1 exhibit neurodegeneration as the primary manifestation of disease. Thus, the effects of PINK1 in promoting or maintaining neuronal differentiation may be of particular relevance to Parkinson’s disease.
Supplementary Material
Acknowledgements
We thank Simon Watkins and Jason Shields of the Center for Biological Imaging for their technical support, training and access to specialized imaging equipment, and the scientists listed in Methods for their gifts of reagents.
Funding
This study was supported by the National Institutes of Health R01 NS065789 to CTC. RKD was supported in part by F32 AG030821 and GM103554.
Abbreviations
- OMM
Outer mitochondrial membrane
- PINK1
PTEN-induced kinase-1
- PKA
Protein kinase A
- PKI
Inhibitor of protein kinase A
- MAP2B
Microtubule associate protein 2 B
Footnotes
Competing interests.
All authors that contributed to this study declare no conflict of interest.
References
- Becker D, Richter J, Tocilescu MA, Przedborski S, Voos W. Pink1 kinase and its membrane potential (Deltapsi)-dependent cleavage product both localize to outer mitochondrial membrane by unique targeting mode. The Journal of biological chemistry. 2012;287:22969–22987. doi: 10.1074/jbc.M112.365700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilina A, Van Der Brug M, Ahmad R, Kesavapany S, Miller DW, Petsko GA, Cookson MR. Mutations in PTEN-induced putative kinase 1 associated with recessive parkinsonism have differential effects on protein stability. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5703–5708. doi: 10.1073/pnas.0500617102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendiske J, Caba E, Brown QB, Bahr BA. Intracellular deposition, microtubule destabilization, and transport failure: an "early" pathogenic cascade leading to synaptic decline. Journal of neuropathology and experimental neurology. 2002;61:640–650. doi: 10.1093/jnen/61.7.640. [DOI] [PubMed] [Google Scholar]
- Billia F, Hauck L, Konecny F, Rao V, Shen J, Mak TW. PTEN-inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9572–9577. doi: 10.1073/pnas.1106291108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun RK, Vulliet PR, Carbonaro-Hall DA, Hall FL. Phosphorylation of RII subunit and attenuation of cAMP-dependent protein kinase activity by proline-directed protein kinase. Archives of biochemistry and biophysics. 1991;289:187–191. doi: 10.1016/0003-9861(91)90460-z. [DOI] [PubMed] [Google Scholar]
- Bywood PT, Johnson SM. Dendrite loss is a characteristic early indicator of toxin-induced neurodegeneration in rat midbrain slices. Experimental neurology. 2000;161:306–316. doi: 10.1006/exnr.1999.7259. [DOI] [PubMed] [Google Scholar]
- Chang N, Li L, Hu R, et al. Differential regulation of NMDA receptor function by DJ-1 and PINK1. Aging cell. 2010;9:837–850. doi: 10.1111/j.1474-9726.2010.00615.x. [DOI] [PubMed] [Google Scholar]
- Cherra SJ, 3rd, Kulich SM, Uechi G, Balasubramani M, Mountzouris J, Day BW, Chu CT. Regulation of the autophagy protein LC3 by phosphorylation. The Journal of cell biology. 2010;190:533–539. doi: 10.1083/jcb.201002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherra SJ, 3rd, Steer E, Gusdon AM, Kiselyov K, Chu CT. Mutant LRRK2 elicits calcium imbalance and depletion of dendritic mitochondria in neurons. The American journal of pathology. 2013;182:474–484. doi: 10.1016/j.ajpath.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson MR. DJ-1, PINK1, and their effects on mitochondrial pathways. Mov Disord. 2010;25(Suppl 1):S44–S48. doi: 10.1002/mds.22713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson MR, Dauer W, Dawson T, Fon EA, Guo M, Shen J. The roles of kinases in familial Parkinson's disease. J Neurosci. 2007;27:11865–11868. doi: 10.1523/JNEUROSCI.3695-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagda RK, Cherra SJ, 3rd, Kulich SM, Tandon A, Park D, Chu CT. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. The Journal of biological chemistry. 2009;284:13843–13855. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagda RK, Chu CT. Mitochondrial quality control: insights on how Parkinson's disease related genes PINK1, parkin, and Omi/HtrA2 interact to maintain mitochondrial homeostasis. Journal of bioenergetics and biomembranes. 2009;41:473–479. doi: 10.1007/s10863-009-9255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagda RK, Gusdon AM, Pien I, Strack S, Green S, Li C, Van Houten B, Cherra SJ, 3rd, Chu CT. Mitochondrially localized PKA reverses mitochondrial pathology and dysfunction in a cellular model of Parkinson's disease. Cell death and differentiation. 2011;18:1914–1923. doi: 10.1038/cdd.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deas E, Plun-Favreau H, Gandhi S, et al. PINK1 cleavage at position A103 by the mitochondrial protease PARL. Human molecular genetics. 2011;20:867–879. doi: 10.1093/hmg/ddq526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey AS, Strack S. PKA/AKAP1 and PP2A/Bbeta2 regulate neuronal morphogenesis via Drp1 phosphorylation and mitochondrial bioenergetics. J Neurosci. 2011;31:15716–15726. doi: 10.1523/JNEUROSCI.3159-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner N, Treske B, Paquet D, et al. Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J Neurosci. 2007;27:12413–12418. doi: 10.1523/JNEUROSCI.0719-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliciello A, Gottesman ME, Avvedimento EV. The biological functions of A-kinase anchor proteins. Journal of molecular biology. 2001;308:99–114. doi: 10.1006/jmbi.2001.4585. [DOI] [PubMed] [Google Scholar]
- Gandhi S, Muqit MM, Stanyer L, et al. PINK1 protein in normal human brain and Parkinson's disease. Brain. 2006;129:1720–1731. doi: 10.1093/brain/awl114. [DOI] [PubMed] [Google Scholar]
- Gandhi S, Wood-Kaczmar A, Yao Z, et al. PINK1-associated Parkinson's disease is caused by neuronal vulnerability to calcium-induced cell death. Molecular cell. 2009;33:627–638. doi: 10.1016/j.molcel.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegg ME, Cooper JM, Schapira AH, Taanman JW. Silencing of PINK1 expression affects mitochondrial DNA and oxidative phosphorylation in dopaminergic cells. PLoS ONE. 2009;Vol. 4:e4756. doi: 10.1371/journal.pone.0004756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima Y, Ohsako S, Yamauchi T. Overexpression of Ca2+/calmodulin-dependent protein kinase II in Neuro2a and NG108-15 neuroblastoma cell lines promotes neurite outgrowth and growth cone motility. J Neurosci. 1993;13:559–567. doi: 10.1523/JNEUROSCI.13-02-00559.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant P, Pant HC. Neurofilament protein synthesis and phosphorylation. Journal of neurocytology. 2000;29:843–872. doi: 10.1023/a:1010999509251. [DOI] [PubMed] [Google Scholar]
- Greene AW, Grenier K, Aguileta MA, Muise S, Farazifard R, Haque ME, McBride HM, Park DS, Fon EA. Mitochondrial processing peptidase regulates PINK1 processing, import and Parkin recruitment. EMBO reports. 2012;13:378–385. doi: 10.1038/embor.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal SS, Fass DM, Yao H, Ellig CL, Goodman RH, Stork PJ. Calcium and cAMP signals differentially regulate cAMP-responsive element-binding protein function via a Rap1-extracellular signal-regulated kinase pathway. The Journal of biological chemistry. 2000;275:34433–34441. doi: 10.1074/jbc.M004728200. [DOI] [PubMed] [Google Scholar]
- Haque ME, Thomas KJ, D'Souza C, et al. Cytoplasmic Pink1 activity protects neurons from dopaminergic neurotoxin MPTP. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1716–1721. doi: 10.1073/pnas.0705363105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada A, Teng J, Takei Y, Oguchi K, Hirokawa N. MAP2 is required for dendrite elongation, PKA anchoring in dendrites, and proper PKA signal transduction. The Journal of cell biology. 2002;158:541–549. doi: 10.1083/jcb.200110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Kao JW, Tseng DT, Chen WS, Chiang MH, Hwang E. Microtubule-Associated Type II Protein Kinase A Is Important for Neurite Elongation. PLoS ONE. 2013;8:e73890. doi: 10.1371/journal.pone.0073890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Pisani A, Porter DR, et al. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarou M, Jin SM, Kane LA, Youle RJ. Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Developmental cell. 2012;22:320–333. doi: 10.1016/j.devcel.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Liu HP, Lin WY, Guo H, Lu B. LRRK2 kinase regulates synaptic morphology through distinct substrates at the presynaptic and postsynaptic compartments of the Drosophila neuromuscular junction. J Neurosci. 2010;30:16959–16969. doi: 10.1523/JNEUROSCI.1807-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Lin CH, Tsai PI, Wu RM, Chien C. T. LRRK2 G2019S mutation induces dendrite degeneration through mislocalization and phosphorylation of tau by recruiting autoactivated GSK3ss. J Neurosci. 2010;30:13138–13149. doi: 10.1523/JNEUROSCI.1737-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Kang UJ. Characterization of PINK1 processing, stability, and subcellular localization. Journal of neurochemistry. 2008;106:464–474. doi: 10.1111/j.1471-4159.2008.05398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Kang UJ. Structural determinants of PINK1 topology and dual subcellular distribution. BMC cell biology. 2010;11:90. doi: 10.1186/1471-2121-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Sawada T, Lee S, et al. Parkinson's disease-associated kinase PINK1 regulates Miro protein level and axonal transport of mitochondria. PLoS genetics. 2012;8:e1002537. doi: 10.1371/journal.pgen.1002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Sawada T, Lee S, et al. Parkinson's disease-associated kinase PINK1 regulates Miro protein level and axonal transport of mitochondria. PLoS genetics. 2012;8:e1002537. doi: 10.1371/journal.pgen.1002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marongiu R, Spencer B, Crews L, Adame A, Patrick C, Trejo M, Dallapiccola B, Valente EM, Masliah E. Mutant Pink1 induces mitochondrial dysfunction in a neuronal cell model of Parkinson's disease by disturbing calcium flux. Journal of neurochemistry. 2009;108:1561–1574. doi: 10.1111/j.1471-4159.2009.05932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matenia D, Hempp C, Timm T, Eikhof A, Mandelkow EM. Microtubule affinity-regulating kinase 2 (MARK2) turns on phosphatase and tensin homolog (PTEN)-induced kinase 1 (PINK1) at Thr-313, a mutation site in Parkinson disease: effects on mitochondrial transport. The Journal of biological chemistry. 2012;287:8174–8186. doi: 10.1074/jbc.M111.262287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N, Sato S, Shiba K, et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. The Journal of cell biology. 2010;189:211–221. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill RA, Dagda RK, Cribbs JT, Dickey A, H GS, Usachev YM, Strack S. Mechanism of neuroprotective mitochondrial remodeling by PKA/AKAP1. PLoS biology. 2010;9(4):e1000612. doi: 10.1371/journal.pbio.1000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata H, Sakaguchi M, Jin Y, Sakaguchi Y, Futami J, Yamada H, Kataoka K, Huh NH. A new cytosolic pathway from a Parkinson disease-associated kinase, BRPK/PINK1: activation of AKT via mTORC2. The Journal of biological chemistry. 2011;286:7182–7189. doi: 10.1074/jbc.M110.179390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS biology. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit A, Kawarai T, Paitel E, et al. Wild-type PINK1 prevents basal and induced neuronal apoptosis, a protective effect abrogated by Parkinson disease-related mutations. The Journal of biological chemistry. 2005;280:34025–34032. doi: 10.1074/jbc.M505143200. [DOI] [PubMed] [Google Scholar]
- Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pridgeon JW, Olzmann JA, Chin LS, Li L. PINK1 Protects against Oxidative Stress by Phosphorylating Mitochondrial Chaperone TRAP1. PLoS biology. 2007;5:e172. doi: 10.1371/journal.pbio.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakovic A, Grunewald A, Seibler P, Ramirez A, Kock N, Orolicki S, Lohmann K, Klein C. Effect of endogenous mutant and wild-type PINK1 on Parkin in fibroblasts from Parkinson disease patients. Human molecular genetics. 2013;19:3124–3137. doi: 10.1093/hmg/ddq215. [DOI] [PubMed] [Google Scholar]
- Samann J, Hegermann J, von Gromoff E, Eimer S, Baumeister R, Schmidt E. Caenorhabditits elegans LRK-1 and PINK-1 act antagonistically in stress response and neurite outgrowth. The Journal of biological chemistry. 2009;284:16482–16491. doi: 10.1074/jbc.M808255200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandebring A, Thomas KJ, Beilina A, et al. Mitochondrial alterations in PINK1 deficient cells are influenced by calcineurin-dependent dephosphorylation of dynamin-related protein 1. PLoS ONE. 2009;4:e5701. doi: 10.1371/journal.pone.0005701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Y, Liu B, Li L, et al. Regulation of PINK1 by NR2B-containing NMDA receptors in ischemic neuronal injury. Journal of neurochemistry. 2009;111:1149–1160. doi: 10.1111/j.1471-4159.2009.06398.x. [DOI] [PubMed] [Google Scholar]
- Sim AT, Scott JD. Targeting of PKA, PKC and protein phosphatases to cellular microdomains. Cell calcium. 1999;26:209–217. doi: 10.1054/ceca.1999.0072. [DOI] [PubMed] [Google Scholar]
- Steinlechner S, Stahlberg J, Volkel B, et al. Co-occurrence of affective and schizophrenia spectrum disorders with PINK1 mutations. Journal of neurology, neurosurgery, and psychiatry. 2007;78:532–535. doi: 10.1136/jnnp.2006.105676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Vashisht AA, Tchieu J, Wohlschlegel JA, Dreier L. VDACs recruit Parkin to defective mitochondria to promote mitochondrial autophagy. The Journal of biological chemistry. 2012;287:40652–40660. doi: 10.1074/jbc.M112.419721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga-Yoshino K, Kondo S, Tamotsu S, Ogura A. Repetitive activation of protein kinase A induces slow and persistent potentiation associated with synaptogenesis in cultured hippocampus. Neuroscience research. 2002;44:357–367. doi: 10.1016/s0168-0102(02)00155-4. [DOI] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science (New York, N.Y. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- Van Laar VS, Berman SB. Mitochondrial dynamics in Parkinson's disease. Experimental neurology. 2009;218:247–256. doi: 10.1016/j.expneurol.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Spronsen M, Mikhaylova M, Lipka J, et al. TRAK/Milton motor-adaptor proteins steer mitochondrial trafficking to axons and dendrites. Neuron. 2013;77:485–502. doi: 10.1016/j.neuron.2012.11.027. [DOI] [PubMed] [Google Scholar]
- Wang HL, Chou AH, Yeh TH, Li AH, Chen YL, Kuo YL, Tsai SR, Yu ST. PINK1 mutants associated with recessive Parkinson's disease are defective in inhibiting mitochondrial release of cytochrome c. Neurobiology of disease. 2007;28:216–226. doi: 10.1016/j.nbd.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Wang X, Winter D, Ashrafi G, et al. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell. 2011;147:893–906. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihofen A, Thomas KJ, Ostaszewski BL, Cookson MR, Selkoe DJ. Pink1 forms a multiprotein complex with Miro and Milton, linking Pink1 function to mitochondrial trafficking. Biochemistry. 2009;48:2045–2052. doi: 10.1021/bi8019178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood-Kaczmar A, Gandhi S, Yao Z, et al. PINK1 is necessary for long term survival and mitochondrial function in human dopaminergic neurons. PLoS ONE. 2008;3:e2455. doi: 10.1371/journal.pone.0002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N, Engbers J, Khaja S, Xu L, Clark JJ, Hansen MR. Influence of cAMP and protein kinase A on neurite length from spiral ganglion neurons. Hearing research. 2012;283:33–44. doi: 10.1016/j.heares.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ouyang Y, Yang L, Beal MF, McQuibban A, Vogel H, Lu B. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7070–7075. doi: 10.1073/pnas.0711845105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Sun Y, Guo S, Lu B. The PINK1/Parkin pathway regulates mitochondrial dynamics and function in mammalian hippocampal and dopaminergic neurons. Human molecular genetics. 2011;20:3227–3240. doi: 10.1093/hmg/ddr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Huang Y, Shao Y, May J, Prou D, Perier C, Dauer W, Schon EA, Przedborski S. The kinase domain of mitochondrial PINK1 faces the cytoplasm. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12022–12027. doi: 10.1073/pnas.0802814105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.