Abstract
Adrenomedullin is a neuropeptide known for its cardiovascular activities and anti-inflammatory effects. Here, we investigated the effect of adrenomedullin in a model of experimental autoimmune encephalomyelitis (EAE) that mirrors chronic progressive multiple sclerosis. A short-term systemic treatment with adrenomedullin reduced clinical severity and incidence of EAE, the appearance of inflammatory infiltrates in spinal cord and the subsequent demyelination and axonal damage. This effect was exerted at multiple levels affecting both early and late events of the disease. Adrenomedullin decreased the presence/activation of encephalitogenic Th1 and Th17 cells and down-regulated several inflammatory mediators in peripheral lymphoid organs and central nervous system. Noteworthy, adrenomedullin inhibited the production by encephalitogenic cells of osteopontin and of Granulocyte Macrophage Colony-Stimulating Factor (GM-CSF), two critical cytokines in the development of EAE. At the same time, adrenomedullin increased the number of IL-10-producing regulatory T cells with suppressive effects on the progression of EAE. Furthermore, adrenomedullin generated dendritic cells with a semi-mature phenotype that impaired encephalitogenic responses in vitro and in vivo. Finally, adrenomedullin regulated glial activity and favored an active program of neuroprotection/regeneration. Therefore, the use of adrenomedullin emerges as a novel multimodal therapeutic approach to treat chronic progressive multiple sclerosis.
Keywords: Autoimmunity, Regulatory T cells, Dendritic cells, Glial cells, Neuropeptide, Adrenomedullin, Multiple Sclerosis
1. Introduction
Multiple sclerosis (MS) is a disabling demyelinating disease of the central nervous system (CNS) affecting more frequently young people. Although the mechanisms of disease pathogenesis remain unclear, MS is considered as an archetypal neuroinflammatory and autoimmune disease in which Th1 and Th17 CD4 cells reactive to components of the myelin sheath infiltrate the CNS parenchyma, release proinflammatory cytokines and chemokines, and further promote inflammatory cell infiltration and activation (Goverman, 2009; Steinman, 2006). The production of inflammatory mediators such as cytokines and free radicals by infiltrating immune cells and resident glial cells plays a critical destructive role in the process of demyelination and contributes to oligodendrocyte loss and axonal degeneration. Moreover, evidence indicates that a deregulation in the mechanisms involved in the maintenance of immune tolerance, especially those affecting regulatory T (Treg) cells, critically contributes to the establishment and progression of the autoimmune response (Viglietta et al., 2004). Although available therapies based on immunosuppressive agents reduce the relapse rate or delay disease onset, they do not suppress progressive clinical disability. Therefore, we need novel multistep therapeutic approaches that prevent the inflammatory and autoimmune components of the disease, restore immune tolerance and promote and/or allow mechanisms of neuroprotection and neuroregeneration.
Adrenomedullin is a 52-aa neuropeptide belonging to the family of calcitonin-gene related peptide (CGRP) that was initially discovered through its vasodilatory activity and cardiovascular protective actions (Kitamura et al., 1993). Recent evidence indicates that adrenomedullin is a major regulator of the immune response. Besides its presence in the CNS and cardiovascular system (Hinson et al., 2000), adrenomedullin and its receptors are expressed by macrophages, monocytes, T cells and dendritic cells (DCs), mainly in response to inflammatory and immune stimulation (Elsasser and Kahl, 2002; Ishimitsu et al., 1998; Kubo et al., 1998a, 1998b; Rulle et al., 2012; Yang et al., 2001). Moreover, adrenomedullin shows potent anti-inflammatory activity by downregulating the production of a wide panel of inflammatory mediators by activated macrophages, microglia and DCs (Consonni et al., 2011; Gonzalez-Rey et al., 2006a, 2007a; Rulle et al., 2012; Wong et al., 2005; Wu et al., 2003). In addition, adrenomedullin is able to inhibit antigen-specific Th1 responses and increase the number of Treg cells in vivo (Gonzalez-Rey et al., 2006b, 2007a). Several studies demonstrated that treatment with adrenomedullin protected from experimental sepsis, inflammatory bowel disease and arthritis (Cui et al., 2005; Gonzalez-Rey et al., 2006a, 2006b, 2007a; Koo et al., 2001; Okura et al., 2008; Zudaire et al., 2006). In view of these findings, the aim of this study was to investigate the potential therapeutic effect of adrenomedullin in an animal model of experimental autoimmune encephalomyelitis (EAE) that mimics chronic progressive MS, characterized by the worst clinical prognosis and lack of effective treatment (Steinman, 1999).
2. Methods
2.1. Induction and treatment of experimental autoimmune encephalomyelitis (EAE)
To induce chronic EAE, female C57BL/6 mice (8 weeks old, Charles River) were immunized subcutaneously (s.c). with 200 µg of myelin oligodendrocyte protein (MOG35–55, MEVGWYRSPFSRVVHLYRNGK, GeneScript) emulsified in complete Freund´s adjuvant (CFA) containing 400 µg of Mycobacterium tuberculosis H37 RA (Difco). Mice also received intraperitoneal (i.p). injections of 200 ng of pertussis toxin (Sigma) on days 0 and 2. Treatment consisted in the i.p. injection of adrenomedullin (1 nmol/day, American Peptides) or Phosphate buffered saline (PBS, controls) for 5 consecutive days after disease onset in animals with a clinical score of 0.5–1 (onset) or with a clinical score of 1–1.5 or >2 (acute phase). Mice were scored daily for signs of EAE according to the following clinical scoring system (Miller et al, 2010): 0, no clinical signs; 0.5, partial loss of tail tonicity; 1, complete loss of tail tonicity; 2, flaccid tail and abnormal gait; 3, hind leg paralysis; 4, hind leg paralysis with hind body paresis; 5, hind and fore leg paralysis; and 6, death. All experiments with animals were performed in accordance the European ethical guidelines and approved by the Animal Care Unit Committee IPBLN-CSIC (# protocol SAF2010-16923).
2.2. Tissue collection and cell isolation
Spleen, draining lymph nodes (DLNs: cervicals, inguinals and axillaries), brain and spinal cord were removed at various time-points from mice with EAE that were treated with PBS or with adrenomedullin for 5 consecutive days after the onset of disease (with a clinical score between 1 and 2). Single-cell suspensions were obtained from spleen or pooled DLNs and used for flow cytometry analysis, determination of autoreactive responses and adoptive transfer of EAE as described below. Brain and spinal segments of the cervical and lumbar regions were prepared separately and used for RNA isolation, protein extraction and histopathological analysis as described below. Brain and spinal cord mononuclear cells were isolated by enzymatic tissue digestion and gradient centrifugation as previously described (Kong et al., 2011) and used for flow cytometry analysis and determination of autoreactive responses as described below. Proteins were extracted from cervical and lumbar segments of spinal cord and brain by homogenization (50 mg tissue/ml) in lysis buffer (50 mM Tris-HCl, pH 7.4, 0.5 mM Dithiothreitol and 10 µg/ml of protease inhibitors phenylmethylsulfonyl fluoride, pepstatin and leupeptin). Samples were centrifuged (20,000g, 15 min, 4°C) and the supernatants were assayed for cytokine contents using sandwich ELISA following manufacturer’s recommendations (BD Bioscience and Peprotech), and for adrenomedullin levels using a competitive ELISA (Phoenix Pharmaceuticals).
2.3. Histopathological analysis of EAE
For light microscopy, cervical and lumbar spinal cord segments were fixed in buffered 10% formalin for 48 h and processed for paraffin inclusion and sectioning. Transversal sections (4-µm thickness) were stained with luxol fast blue, cresyl violet and hematoxylin following the technique described by Kluver and Barrera (1953) and analyzed for the presence of areas of demyelination and cell infiltration using a light microscope (Olympus).
For immunofluorescence staining, cervical and lumbar spinal cord segments were fixed in 4% paraformaldehyde pH 7.4 for 4–8 h at 4°C, equilibrated in 30% sucrose for 24h, and embedded in OCT. Transversal cryosections (10-µm thickness) were blocked with 10% goat serum in PBS-T (PBS+0.2% Triton X-100) for 30 min at 22°C, incubated with FITC-labeled anti-CD4 mAb (2.5 µg/ml, BD Bioscience), PE-labeled anti-CD45 mAb (1 µg/ml, BD Bioscience) or anti-Iba1 Ab (1 µg/ml, Wako) for 18 h at 4°C, followed by incubation with Alexa Fluor 546-labeled anti-rabbit Ab (2 µg/ml, Invitrogen). Nuclear staining was performed with Hoechst (Sigma). Between steps, samples were extensively washed with PBS-T. Samples were observed in a fluorescence microscope (Olympus IX81).
For immunohistochemistry, spinal cord sections were obtained as described for paraffin processing followed by incubation steps with peroxidase blocking reagents, heat-treated in 1 mM EDTA pH 8.0 at 95°C during 20 min for antigenic unmasking, and incubated for 30 min at room temperature with polyclonal anti-Myelin Basic Protein Ab (Ab980, Millipore). The immunohistochemical study was done on an Autostainer480 (Thermo Fisher Scientific Inc) using the polymer-peroxidase-based method and developed with diaminobenzidine. We used a non-specific peroxidase-conjugated IgG anti-rabbit serum as a negative control of isotype. Nuclei were hematoxylin-counterstained.
2.4. Flow cytometry analysis
For FoxP3 staining, spleen cells, DLN cells and brain/spinal cord mononuclear cells were isolated from C57Bl/6 mice with EAE at the peak of the disease and incubated with FITC-labeled anti-CD25 and APC-labeled anti-CD4 mAbs (4–5 µg/ml, BD Bioscience) for 8 h at 4°C. After extensive washing, cells were fixed/permeabilized (eBioscience), stained with PE-labeled anti-FoxP3 Abs (4–5 µg/ml, eBioscience) for 30 min at 4°C and analyzed in a FACScalibur flow cytometer (BD Bioscience). We used isotype-matched Abs as controls, and Mouse BD Fc Block (BD Bioscience) to avoid nonspecific binding to Fc-receptors.
For intracellular analysis of cytokines, brain/spinal cord mononuclear cells, spleen cells and DLN cells were isolated at the peak of the disease and stimulated at 106 cells/ml with Phorbol-12-myristate-13-acetate (PMA, 25 ng/ml) plus ionomycin (500 ng/ml) for 8–12 h, in the presence of 3 µM monensin for the last 6 h. Cells were stained with APC-anti-CD4 mAb (4 µg/ml) for 1 h at 4°C, fixed/saponin permeabilized with Cytofix/Cytoperm (BD Bioscience), stained with FITC- and PE-conjugated anti-cytokine specific mAbs (4 µg/ml, BD Pharmingen) for 30 min at 4°C, and analyzed in a FACScalibur flow cytometer.
2.5. Determination of autoreactive response
Brain/spinal cord mononuclear cells and spleen and DLN cells (106/ml) recovered from the C57Bl/6 mice at peak of clinical EAE (18–20 days postimmunization) were stimulated in complete medium (RPMI 1640 containing 10% Fetal bovine serum (FBS), 50 µM 2-mercaptoethanol, 2 mM L-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin) with 15 µM MOG35–55. In brain/spinal cord cell cultures, plastic-adherent C57Bl/6 splenocytes (2 × 105/ml) were added to cultures as antigen presenting cells. Cell proliferation was evaluated after 72 h by adding 2.5 µCi/ml [3H]-TdR during the last 8 h of culture and determining cpm incorporation in a Microbeta counter 1450. After 48 h, cytokine and chemokine contents in culture supernatants were determined by sandwich ELISAs. Concanavalin A (Con A, 2.5 µg/ml) was employed as control for non-specific polyclonal stimulation. To investigate the direct effect of adrenomedullin on autoreactive responses, the peptide (100 nM) was added to cultures of brain/spinal cord mononuclear and spleen/DLN cells isolated from untreated EAE mice and stimulated with 15 µM MOG35–55.
To assay the capacity to transfer immune tolerance, spleen and DLN cells recovered from untreated and adrenomedullin-treated C57Bl/6 mice at the peak of disease (18 days postimmunization) were T-cell enriched by plastic adherence (2 h, 37°C) and the non-adherent cells were injected i.p. (107 cells/mouse) into EAE mice (clinical score 0.5–1). Where indicated, isolated CD4 cells were depleted of CD25 population (>99% depletion) before transfer, by using immunomagnetic beads (Miltenyi Biotech) following the manufacturer’s recommendations and injected i.p. (15 × 106 cells/mouse) into EAE mice at disease onset.
2.6. Determination of autoantibodies
We used ELISA to determine the specific anti-MOG Ab responses. Maxisorb plates (Millipore) were coated overnight at 4°C with MOG35–55 (10 µg/ml) in 0.1 M biphosphate buffer (pH 9.6), blocked with PBS/10% FBS and incubated for 2 h at 37°C with serial dilutions of sera obtained at the disease peak by cardiac puncture. Biotinylated anti-IgG1 or anti-IgG2a Abs (2.5 µg/ml) (Serotec) were added for 1 h at 37°C. After washing, the plates were incubated with Streptavidin-Horseradish Peroxidase (HRP), developed with ABTS [2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)] and the absorbance was determined using a spectrophotometer.
2.7. DC isolation and treatments
DCs were differentiated from bone marrow cells obtained from femurs and tibiae of C57Bl/6 mice. Bone marrow cells (2 × 106) were incubated in Petri dishes in complete medium containing 20 ng/ml of rmGM-CSF (PreproTech). At day 6 of culture, non-adherent cells were collected (routinely containing 80–90% CD11c+ cells) and stimulated with bacterial lipopolysaccharide (LPS, 1 µg/ml) and pulsed with MOG35–55 (20 µg/ml) in the absence (DCs-control) or presence (DCs-adrenomedullin) of adrenomedullin (100 nM). After 48 h of culture, flow cytometry analysis for CD40, CD80 and CD86 was performed as described previously (Chorny et al., 2005) and the content of cytokines in the culture supernatants was determined by ELISA. After 24 h of culture, MOG-pulsed DCs-control or DCs-adrenomedullin (2 × 105/ml) were added to DLN cells (106/ml) isolated from EAE mice at peak of disease and restimulated with MOG35–55 (15 µM) or Concanavalin A (2.5 µg/ml) to determine the proliferative response and production of cytokines as above. Alternatively, MOG-pulsed DCs-control or DCs-adrenomedullin (2 × 106/mouse) were injected i.p. in mice with EAE at disease onset and the progression of the disease was evaluated as described above.
2.8. Neuron and glial cell isolation and culture
Primary mixed neuron-glia, microglia, astrocytes and oligodendrocytes were obtained from brains of newborns (postnatal days 1–3, P1–P3) of naïve C57Bl/6 mice as previously described (Souza-Moreira et al., 2013; see also Supplementary data). Cell cultures were used 10–14 days after plating. When indicated, cytokine contents were determined by ELISA in supernatants and nitric oxide production was determined by measuring oxidized nitrite amounts in culture supernatants by using the Griess reagent (Gonzalez-Rey et al., 2006a). Oligodendrocyte cell death caused by oxidative stress was assayed by the MTT (3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide) method after 24 h of culture.
2.9. Determination of gene expression of neurotrophic factors by real-time PCR
Total RNA was isolated using Tripure (Roche) from spinal cord segments. After DNase I treatment, RNA (1 µg/sample) was reverse transcribed using RevertAid First Strand cDNA Synthesis kit (Fermentas) and random hexamer primers. cDNA was used for quantifying gene expression of activity-dependent neuroprotective protein (ADNP) and brain-derived neurotrophic factor (BDNF) by real-time quantitative RT-PCR (60°C as annealing temperature) by using iQ SYBR Green Supermix (Bio-Rad) according to the manufacturer’s instructions. We used beta-actin for normalization and estimated fold change expression with Delta-Delta Ct method.
2.10. Statistical analysis
All data are expressed as the mean±SEM. In all in vivo animal studies, we calculated that we required a minimum of 8–12 mice per group in order to have 80% power of detecting approximately a 30% change, assuming a standard deviation of 30% at a significance level of α = 0.05. It has been our experience that this is sufficient to detect clinically and statistically significant differences with repetitive studies. As clinical scores to assess EAE are non-linear we used non-parametric statistical analysis. These include Mann-Whitney U test or the Kruskal–Wallis test when comparing more than two groups. We use these tests for detecting statistical differences between the individual EAE scores on a specific day for the adrenomedullin-treated and untreated EAE mice. In addition, their scores over each day of the experiment were compared by two-way ANOVA or one-way ANOVA (for more than two groups) with appropriate post tests. The rest of the experiments were statistically analyzed by unpaired t-test and by the non parametric Mann-Whitney test and we evaluate if the normalizing transformations were effective by comparing results between parametric and nonparametric analysis. We used Mann-Whitney test when we found different results. We considered significance at p<0.05. All statistical analyses were performed using the Graph-Pad Prism software.
3. Results
3.1. Treatment with adrenomedullin reduces severity and incidence of chronic EAE
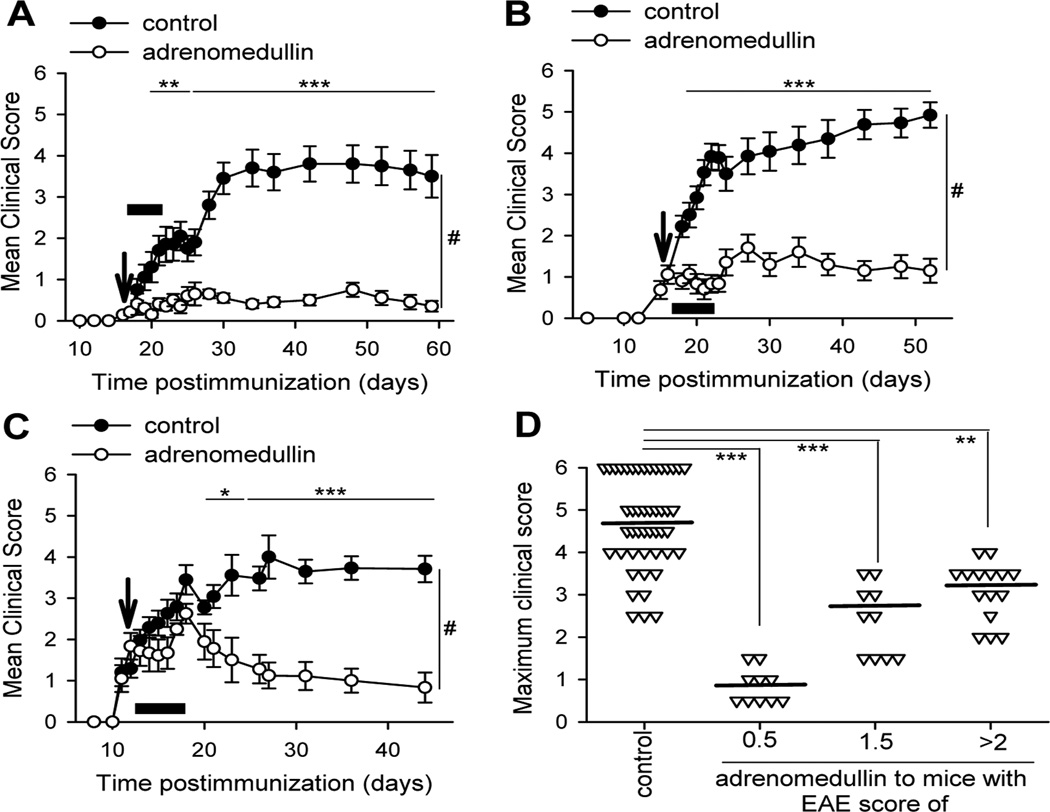
We investigated the effect of the treatment with adrenomedullin in a model of chronic progressive EAE induced by MOG35–55 in C57BL/6 mice (Baxter, 2007). Without treatment, these mice developed moderate to severe clinical symptoms without subsequent recovery (Fig. 1). Systemic administration of adrenomedullin after the onset (Fig. 1A) or during the effector phase of the disease (Fig. 1B and 1C), greatly reduced disease incidence and severity (Fig. 1; Table 1). It is remarkable that most of the adrenomedullin-treated EAE mice displayed mild symptoms and a significant number recovered completely and were entirely asymptomatic 30–40 days after disease onset (Table 1). Interestingly, a short treatment with adrenomedullin was enough to generate a long lasting protective effect (Fig. 1).
Figure 1. Treatment with adrenomedullin reduces severity of chronic EAE.
Chronic progressive EAE was induced in C57BL/6 mice by immunization with MOG35–55. Mice were treated i.p. with PBS (control) or adrenomedullin (1 nmol/day, black bars) for 5 consecutive days at disease onset (A, arrow indicated adrenomedullin administration started at clinical score <1) or at two points during the acute phase in the therapeutic regimen (B, arrow indicated adrenomedullin administration started at clinical score 1–2; C, at clinical score >2). Data represent the mean clinical score ± SEM (A–C) or maximum peak of disease per animal (D, mean: horizontal line). n=10 mice/group in A; n=13 mice/control group and 10 mice/adrenomedullin group in B; n=24 mice/control group and 15 mice/adrenomedullin group in C; all performed in three independent experiments. *p<0.05; **p<0.01; ***p<0.001 vs. control with Mann-Whitney test (A–C) and Kruskal Wallis Test (D). #p<0.001 with ANOVA t-test.
Table 1.
Effect of adrenomedullin in chronic progressive EAE.
| Timing of Adrenomedullin administration in EAE |
Incidencea | Mortality | CDIb | |||
|---|---|---|---|---|---|---|
| None | Mild | Moderate | Severe | |||
| 1. Disease Onset | ||||||
| Control (n=10) | 0% | 0% | 70% | 30% | 20% | 76.2±9.6 |
| adrenomedullin (n=10) | 30% | 70% | 0% | 0% | 0% | 17.6±1.7*** |
| 2. Early acute phase | ||||||
| Control (n=13) | 0% | 0% | 54% | 46% | 46% | 103.6±7.8 |
| adrenomedullin (n=10) | 10% | 80% | 10% | 0% | 0% | 33.1±4.5*** |
| 3. Advanced acute phase | ||||||
| Control (n=24) | 0% | 0% | 54% | 46% | 29% | 82.5±7.1 |
| adrenomedullin (n=15) | 13% | 86% | 0% | 0% | 0% | 41.2±4.1** |
Chronic progressive EAE was induced in C57BL/6 mice by immunization with MOG35–55. Immunized mice were treated i.p. for 5 days with PBS (control) or with adrenomedullin (1 nmol/day) starting at the onset of clinical signs (with a clinical score of 0.5) or at two points during the acute phase of the disease (early acute phase with a clinical score of 1–2; or advanced acute phase with a clinical score >2). The number of animals per group used in each condition is shown in parenthesis. Data represent results from three independent experiments.
Disease incidence (expressed as percentage) is graded as severe (clinical score: >4), moderate (clinical score: 2–4), mild (clinical score: <2) or none (no clinical signs) at day 36.
Cumulative Disease Index (CDI) is the mean of the sum of the daily disease scores for each animal between days 20 and 44 postimmunization.
p<0.005,
p<0.0001 vs. control.
3.2. Adrenomedullin reduces the inflammatory infiltration and demyelination in the CNS of mice with EAE
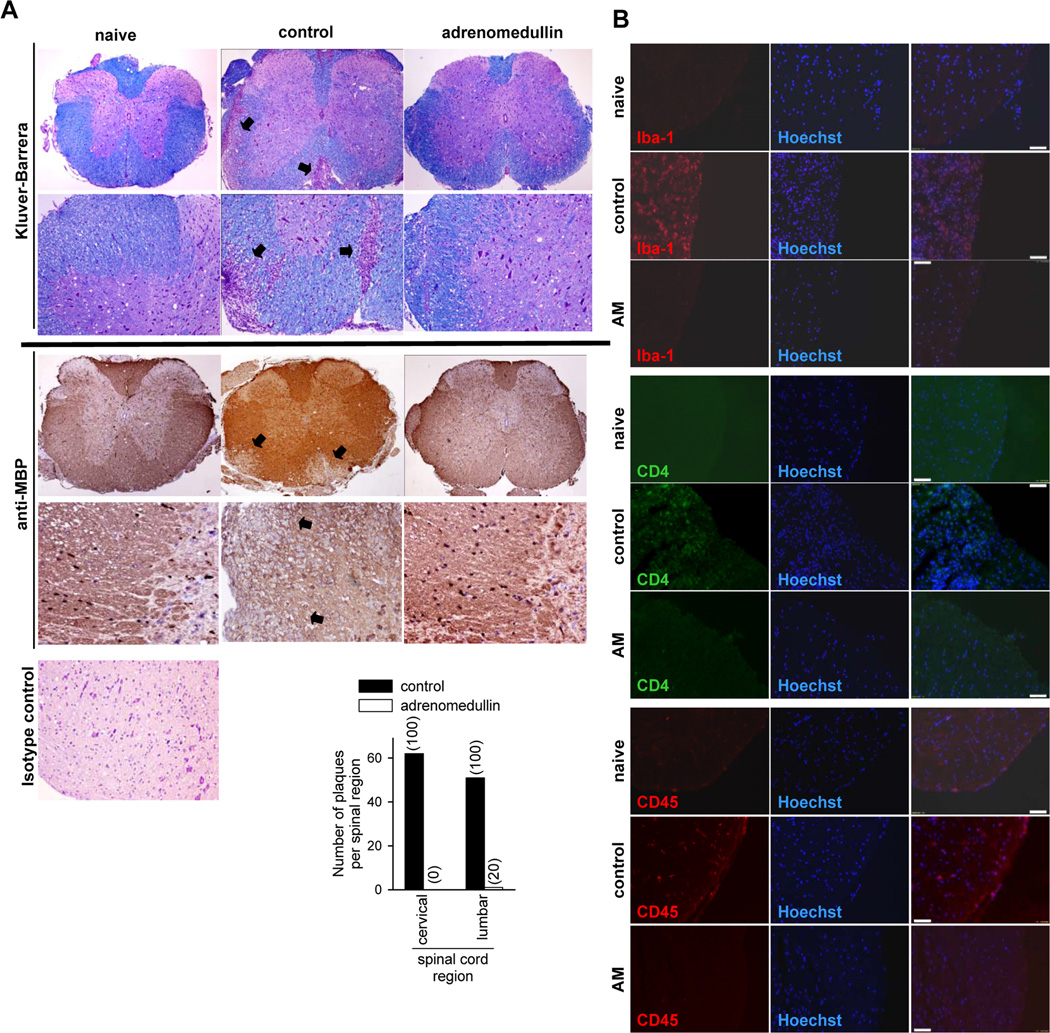
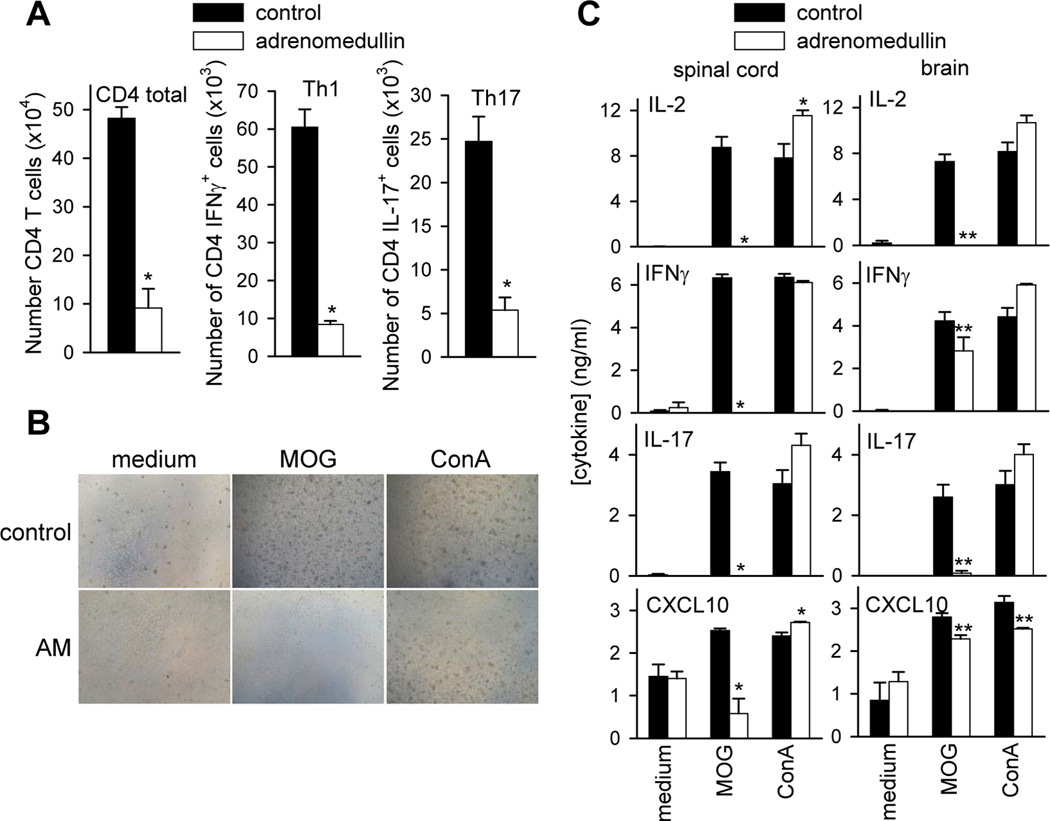
We next investigated the mechanisms underlying the beneficial effects of adrenomedullin during the effector phase of EAE in mice with established clinical signs (scores between 1 and 2). The pathology of MS and EAE is characterized by focal areas of inflammatory infiltration and demyelination with oligodendrocyte depletion (Baxter, 2007; Steinman, 1996). Histopathologic analysis of spinal cords in both lumbar and cervical regions showed that treatment with adrenomedullin drastically decreased the number of plaques with inflammatory infiltrates and areas of demyelination (Fig. 2A). Immunofluorescence evaluation of CNS infiltrates in animals with EAE revealed that the inflammatory cells (CD45+) close to the perivascular area were mostly CD4+ cells and Iba1+ macrophages (Fig. 2B). Adrenomedullin decreased the numbers of CD45+, CD4+, and Iba1+ cells (Fig. 2B).
Figure 2. Adrenomedullin reduces inflammatory infiltration and demyelination in CNS of mice with EAE.
Mice with MOG35–55-induced chronic EAE were treated with PBS (control) or with adrenomedullin for 5 days starting at disease onset (6/group, two independent experiments). Naïve animals were used as negative controls. (A) Transverse sections of spinal cord randomly selected at the peak of clinical disease were stained with Kluver-Barrera or immunostained for myelin (anti-MBP). Original magnification for upper images, ×40; lower, ×100 for Kluver-Barrera images and ×200 for anti-MBP images. Arrows point to areas of demyelination and inflammatory infiltration. A section of spinal cord of naïve mice stained with isotype control antibody was included to assess non-specific immunostaining (magnification, × 200). The mean number of plaques of demyelination in the lumbar and cervical regions and the incidence of occurrence of plaques (in parenthesis) was determined. (B) The phenotype of the infiltrating cells in the lumbar region of spinal cord was assayed by immunofluorescence for CD45, CD4 and Iba1. AM: adrenomedullin. Scale bars: 50 µm.
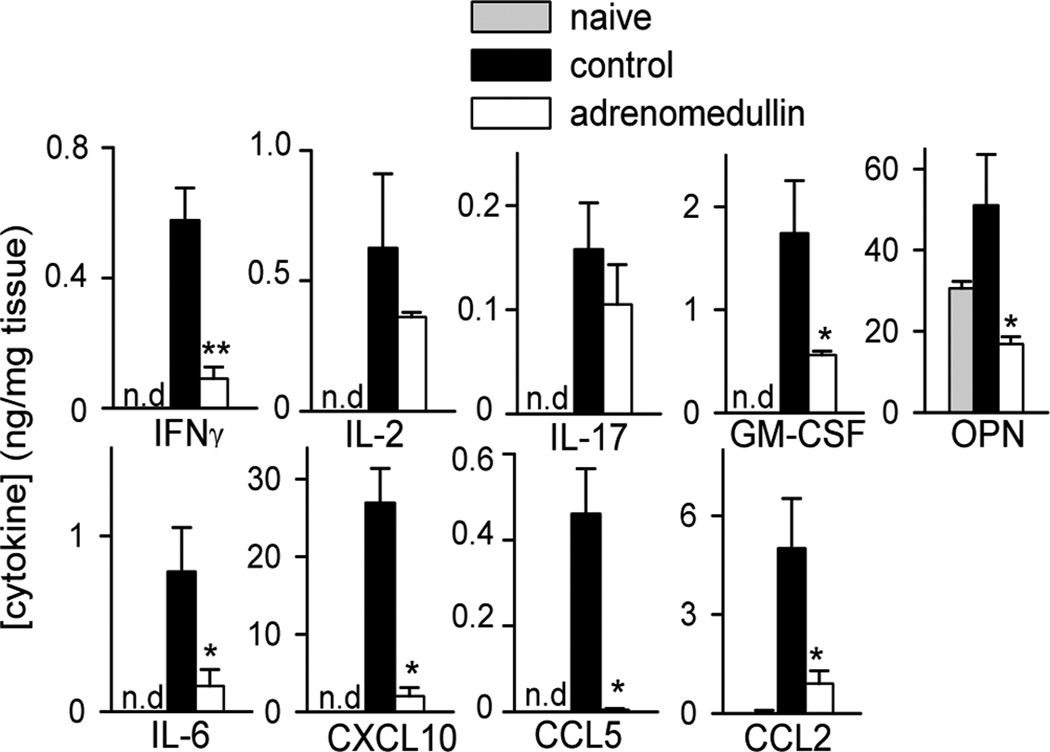
The reduction of CNS infiltrating cells in the adrenomedullin-treated EAE mice correlated with the decrease in the amounts of inflammation-related cytokines and chemokines, including IL-6, IL-17, IFNγ, CCL5, CCL2 and CXCL10 in spinal cords (Fig. 3). Moreover, treatment with adrenomedullin reduced the contents of GM-CSF and osteopontin in the CNS of EAE mice (Fig. 3), two inflammatory markers that play a major role in the induction and progression of EAE and MS (Codarri et al., 2011; Chabas et al., 2001; Jansson et al., 2002; Murugaiyan et al., 2008, 2010).
Figure 3. Adrenomedullin reduces the production of inflammatory markers in the CNS of EAE mice.
Mice with MOG35–55-induced chronic EAE were treated with PBS (control) or adrenomedullin for 5 days at the disease onset (clinical score 1–2). Levels of inflammatory cytokines, chemokines, GM-CSF, and osteopontin (OPN) were determined by ELISA in protein extracts purified from spinal cord at peak of the disease. Samples from naïve mice were used as reference (n.d.: under detection limit). n=6 mice/group, two independent experiments. *p<0.05; **p<0.01, vs. control with Mann-Whitney test.
3.3. Adrenomedullin modulates the autoimmune component of EAE in the periphery
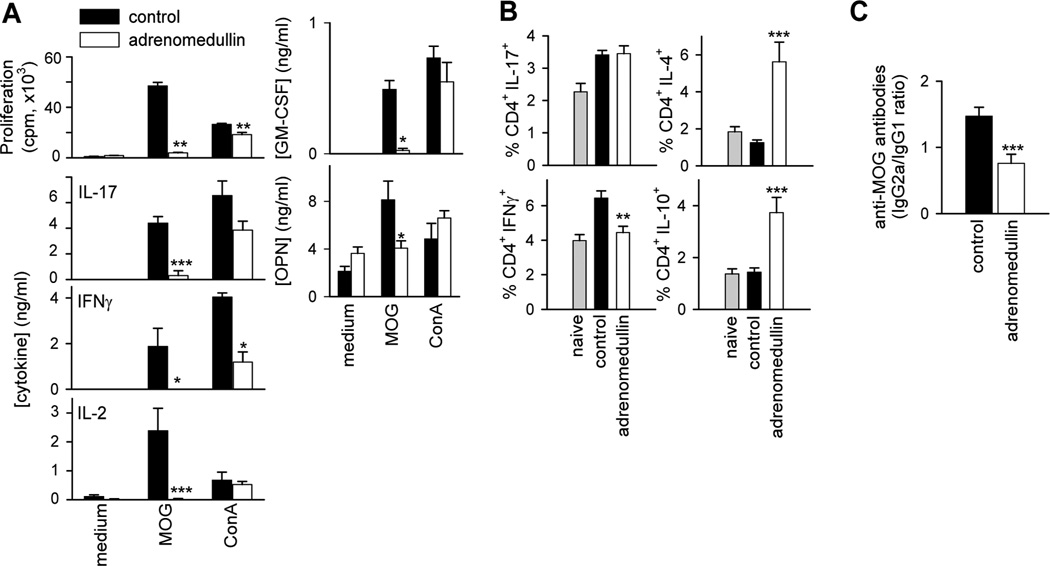
In EAE, autoreactive IFNγ-producing Th1 cells and IL-17-secreting Th17 cells infiltrate CNS and promote disease progression (Hu and Ivashki et al, 2009; Jager et al., 2009; Stromnes et al., 2008). Therefore, we investigated whether adrenomedullin could ameliorate EAE by reducing encephalitogenic T-cell responses and/or their migration to CNS. Lymphocytes from DLNs and spleen of EAE mice showed marked MOG-dependent proliferation and production of IFNγ, IL-2 and IL-17 (Fig. 4A). However, T cells from adrenomedullin-treated mice proliferated much less and did not produce Th1 and Th17 cytokines in the MOG-specific recall response (Fig. 4A). Interestingly, adrenomedullin treatment also reduced the capacity of spleen cells to produce GM-CSF and osteopontin upon MOG-restimulation (Fig. 4A). The effect of adrenomedullin was mostly antigen specific since polyclonal T cell activation with Concanavalin A resulted in similar proliferation and cytokine secretion (except for IFNγ) in both groups (Fig. 4A). Moreover, adrenomedullin treatment reduced the number of effector T cells secreting IFNγ, but not IL-17 in DLNs and spleen, and at the same time elevated the percentage of IL-4- and IL-10-expressing CD4 cells (Fig. 4B). These results indicate that adrenomedullin administration during the effector phase of EAE partially inhibits autoreactive Th1- and Th17-cell activation and clonal expansion in the periphery. In order to evaluate whether this action is exerted directly on lymphoid cells, we assayed the in vitro effect of adrenomedullin on MOG-induced proliferation and cytokine production by DLNs isolated from EAE mice. Adrenomedullin deactivated MOG-specific recall responses in vitro (Supplementary Fig. 1). Finally, because high levels of circulating antibodies directed against myelin antigens invariably accompany the development of MS and EAE (Owens, 2003), and are major factors in determining susceptibility to EAE (Steinman, 1999), we next examined the effect of adrenomedullin treatment on the serum levels of MOG-specific IgG autoantibodies. Adrenomedullin reduced the ratio between MOG-specific IgG2a and IgG1 isotypes, generally reflective of Th1 and Th2 activities, respectively (Fig. 4C).
Figure 4. Adrenomedullin ameliorates EAE severity by downregulating the peripheral encephalitogenic Th1/Th17 response.
Mice with MOG35–55-induced chronic EAE were treated with PBS (control) or adrenomedullin for 5 days at disease onset (clinical score 1–2). (A) Adrenomedullin-treated EAE mice showed reduced encephalitogenic Th1/Th17-mediated responses in lymphoid organs. Proliferation and cytokine production by DLN cells isolated at peak of the disease and stimulated with medium, the encephalitogenic antigen (MOG35–55) or a polyclonal stimulus (Con A). We obtained similar results with spleen cells (data not shown). n=6 mice/group, two independent experiments. (B) Adrenomedullin treatment regulated the number of Th1, Th17 and Th2 cells in EAE mice. Spleen cells isolated at peak of the disease were assayed for intracellular cytokine expression by flow cytometry in the CD4 gated population. We used naïve mice as basal controls. We obtained similar results with DLNs (data not shown). n=6–8 mice/group, three independent experiments. (C) MOG-specific IgG1 and IgG2a serum levels (peak of disease). n=14 mice/group, three independent experiments. *p<0.05; **p<0.01; ***p<0.001 vs control with Mann-Whitney test (A) and t-test (B and C).
3.4. Adrenomedullin impairs the encephalitogenic response in the CNS
We next investigated whether, beside its effect in the periphery, the treatment with adrenomedullin also impaired the encephalitogenic response in the CNS. In agreement with data obtained from histopathology (Fig. 2B), flow cytometry analysis of infiltrating mononuclear cells isolated from the brains of EAE mice showed that adrenomedullin administration resulted in significantly lower numbers of infiltrating CD4 T cells, particularly of IFNγ- and IL-17-producing Th1 and Th17 cells (Fig. 5A). We next examined the encephalitogenic capacity of the few infiltrating T cells found in the CNS parenchyma of adrenomedullin-treated EAE mice. As expected, infiltrating mononuclear cells isolated from brain and spinal cord of untreated EAE mice showed robust proliferation (Fig. 5B) and a Th1/Th17 cytokine profile upon ex vivo restimulation with MOG (Fig. 5C). In contrast, CNS mononuclear cells from adrenomedullin-treated EAE mice neither proliferated nor produced Th1/Th17 cytokines in the MOG-specific recall response, although they did respond to polyclonal activation (Fig. 5B and C). Interestingly, addition of adrenomedullin to cultures of MOG-stimulated CNS mononuclear cells isolated from EAE mice reduced proliferation (not shown) and the production of IL-2 and IL-17, but not of IFNγ (Supplementary Fig. 2). These results support that adrenomedullin could regulate the encephalitogenic response of infiltrating immune cells in the CNS parenchyma during the progression of EAE.
Figure 5. Treatment with adrenomedullin impairs the encephalitogenic response in the CNS of mice with EAE.
Mice with MOG35–55-induced chronic EAE were treated with PBS (control) or adrenomedullin for 5 days at disease onset (clinical score 1–2). (A) Phenotypic analysis by flow cytometry of brain infiltrating mononuclear cells isolated at the peak of disease. Results show total numbers of CD4 T cells, CD4+IFNγ+ cells or CD4+IL-17+ cells per brain. n=6 mice/group, two independent experiments. (B) Proliferation (depicted by the formation of cell clusters of activation; original magnification, ×100) and (C) cytokine production by mononuclear cells isolated from brains and spinal cords at peak of the disease and restimulated with MOG35–55 or with a polyclonal stimulus (ConA). n=6–8 mice/group, two independent experiments. AM: adrenomedullin. *p<0.05; **p<0.01 vs control with Mann-Whitney test.
3.5. Adrenomedullin increases the number of functional Treg cells in EAE
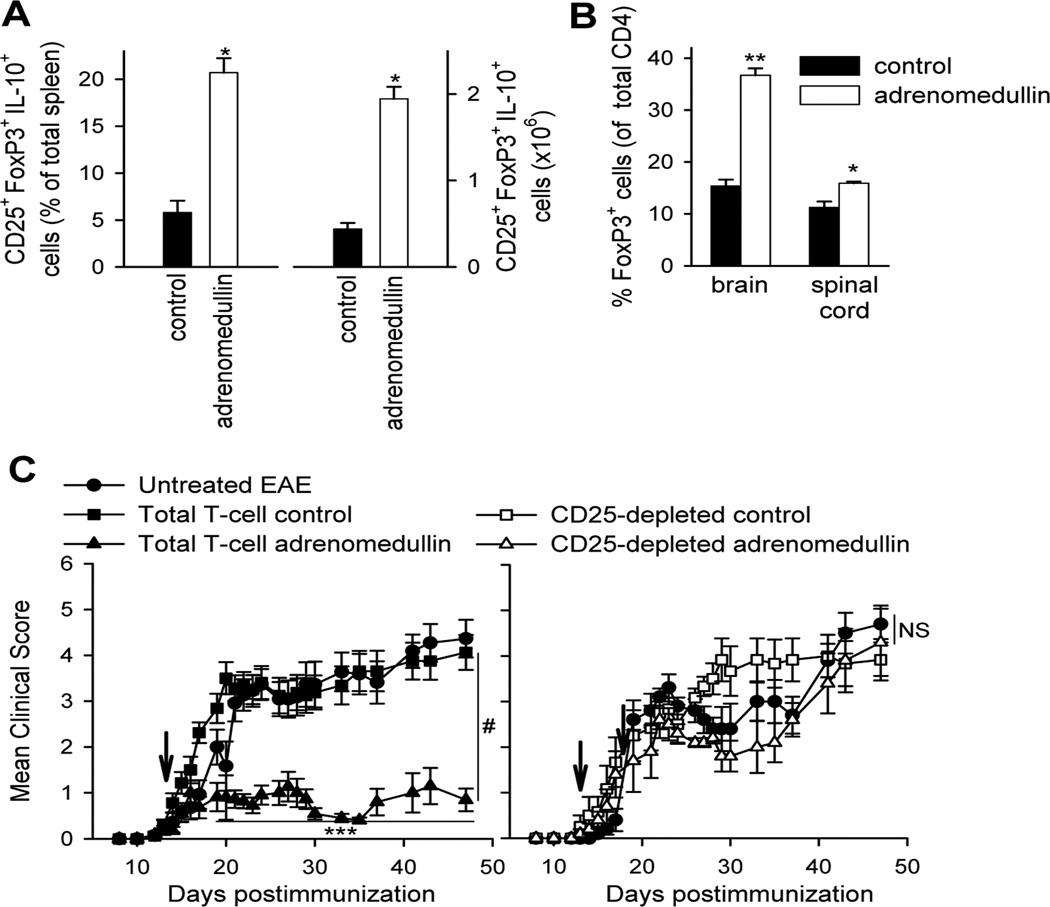
Evidence supports that Treg cells confer significant protection against EAE by deactivating autoreactive T cells and their homing to the CNS (Bynoe et al., 2003; Kohm et al., 2002). Therefore, we next evaluated the capacity of adrenomedullin to generate Treg cells during EAE. We found that adrenomedullin injection increased the number and percentage of IL-10-secreting CD4+CD25+FoxP3+ Treg cells in DLNs and spleens of EAE mice (Fig. 6A). In the CNS, despite lower numbers of CD4 T cells, adrenomedullin increased significantly the percentage of FoxP3+ cells (Fig. 6B).
Figure 6. Treatment with adrenomedullin increases the number of Treg cells which suppress EAE progression.
Mice with MOG35–55-induced chronic EAE were treated with PBS (control) or adrenomedullin for 5 days at disease onset (clinical score 1–2). (A) Adrenomedullin induces the emergence of peripheral Treg cells in EAE mice. Percentage and number of CD4+CD25+FoxP3+IL-10+ Treg cells in spleens isolated at peak of the disease. n=18 mice/group, three independent experiments. (B) Adrenomedullin increases the percentages of Treg cells in the CNS inflammatory infiltrates. Spinal cord and brain mononuclear cells isolated at peak of the disease were analyzed for the presence of CD4+CD25+FoxP3+ Treg cells by flow cytometry and expressed as percentage of FoxP3+ cells in the CD4 population. n=10 mice/group, two independent experiments. (C) Treatment (arrow) of EAE mice with CD4 T cells or CD25-depleted CD4 T cells isolated from spleen/DLNs of EAE mice that were previously treated with PBS (control) or adrenomedullin. Untreated EAE mice were used as reference. n=7–9 mice/group, two independent experiments. *p<0.05; **p<0.01; ***p<0.001 vs control with Mann-Whitney test (A and B) and Kruskal-Wallis test (C). #p<0.01; NS: not significant vs control with Kruskal-Wallis test.
To evaluate the suppressive capacity of Treg cells generated by the treatment with adrenomedullin in vivo, we examined their potential protective effect in the progression of EAE. Thus, injection of T cells from spleen/DLNs of adrenomedullin-treated EAE mice, but not of untreated EAE mice, into EAE mice ameliorated clinical symptoms (Fig. 6C, left panel). Moreover, depletion of CD25+ T cells prior to transfer abrogated the protective effect in vivo, suggesting that the capacity to generate tolerance resides in the CD4+CD25+ Treg population (Fig. 6C, right panel).
3.6. Adrenomedullin generates dendritic cells that suppress the encephalitogenic response in vitro and in vivo
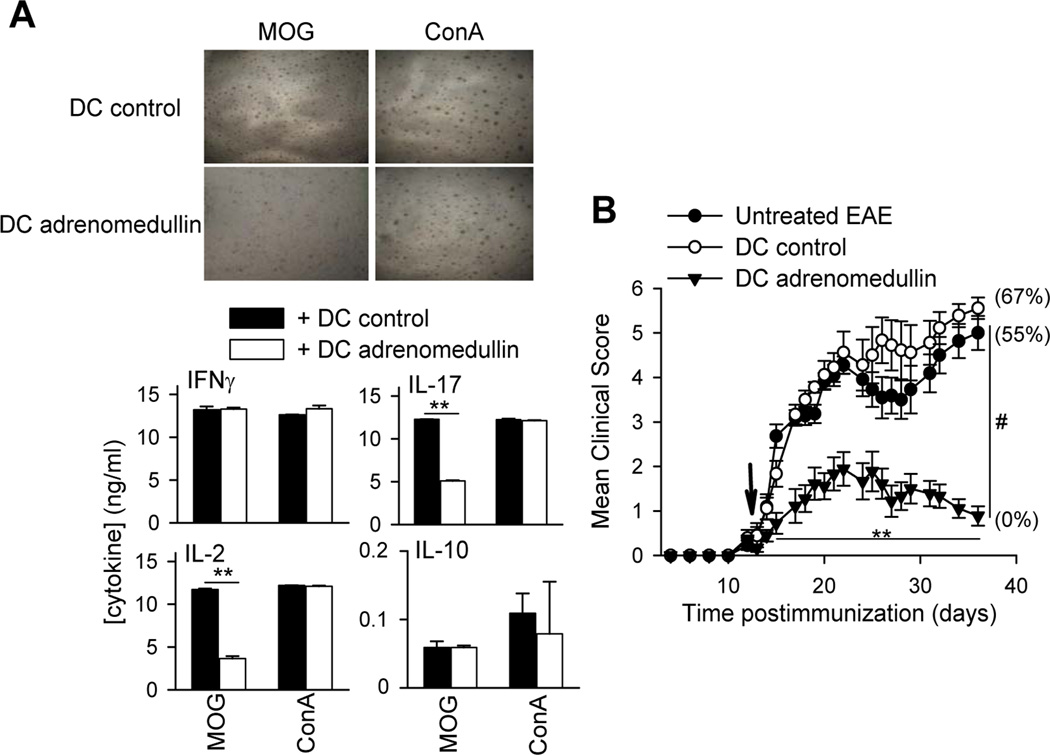
A recent report demonstrated the capacity of adrenomedullin to regulate DC function by inducing a semi-mature phenotype (Rulle et al., 2012). Because DCs play a major role in antigen presentation during autoimmune responses, we investigated how adrenomedullin may modulate DCs in the scenario of EAE. We first confirmed that addition of adrenomedullin during the process of activation/maturation of DCs with LPS reduced the expression of the costimulatory molecules CD40 and CD80, and the production of inflammatory cytokines such as TNFα, IL-12 and IL-6, inducing IL-10 secretion (Supplementary Fig. 3). MOG-pulsed DCs activated in the presence of adrenomedullin also impaired the proliferative response and the production of IL-2 and IL-17, but not of IFNγ, by MOG-activated DLN cells isolated from EAE mice (Fig. 7A). Interestingly, they did not affect the polyclonal activation of T cells (Fig. 7A). Noteworthy, MOG-pulsed DCs exposed to adrenomedullin kept the suppressive activity in vivo, because they reduced disease severity and incidence following administration to EAE mice (Fig. 7B). These data suggest that adrenomedullin could generate antigen-specific responses during EAE progression by modulating DC function.
Figure 7. Adrenomedullin generates dendritic cells (DCs) with the capacity to deactivate the encephalitogenic response in vitro and in vivo.
(A) DCs matured with LPS and pulsed with MOG35–55 in the absence (DC-control) or presence of adrenomedullin (DC-adrenomedullin) were co-incubated with DLN cells isolated from mice suffering EAE and stimulated with MOG35–55 or Concanavalin A. Cell proliferation (evidenced by the presence of clusters of activation in the culture; original magnification, ×100) and the production of cytokines were determined 48 h later. n=5, in duplicate. (B) DCs matured with LPS and pulsed with MOG35–55 in the absence (DC-control) or presence of adrenomedullin (DC-adrenomedullin) were administered i.p. to EAE mice at disease onset (arrow). Untreated EAE mice were used as reference. Numbers in parenthesis represent the mortality rate in each group. n=6–8 mice/group, two independent experiments. **p<0.01 vs control with Mann-Whitney test; #p<0.001 vs control with Kruskal-Wallis test.
3.7. Adrenomedullin down-regulates inflammatory mediators by glial cells and promotes neuroprotective responses
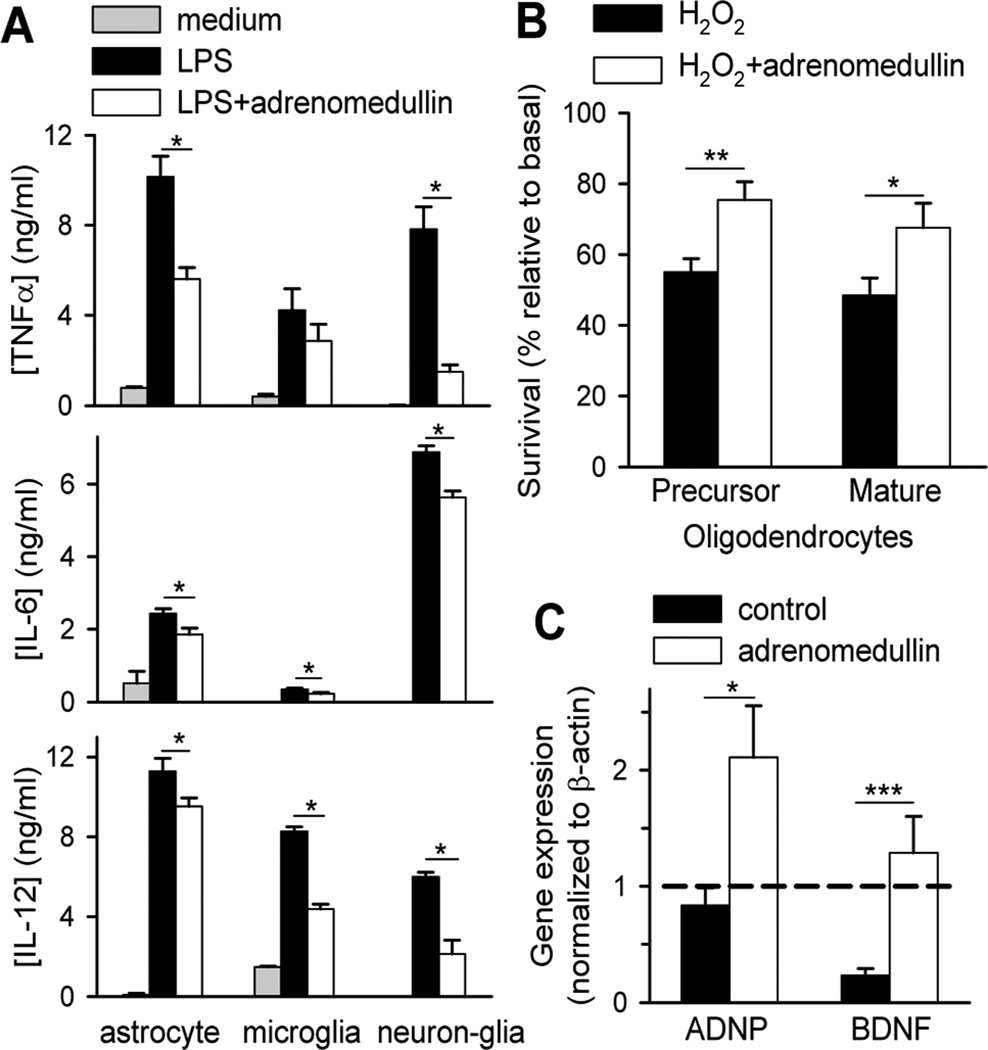
We next investigated whether, besides its immunoregulatory activity, adrenomedullin exerts an active protective effect in CNS by directly acting on resident cells. Because local production of cytotoxic factors by activated microglia and astrocytes in an inflammatory milieu critically contributes to MS/EAE pathology, by inducing demyelination, oligodendrocyte loss and axonal degeneration (Goverman, 2009; Owens, 2003; Steinman, 1996), we investigated the effect of adrenomedullin on the production of inflammatory mediators by activated glial cells. This neuropeptide reduced the production of inflammatory cytokines like TNFα, IL-12 and IL-6 from LPS-activated astrocytes, microglia and neuron-glia cultures (Fig. 8A) and of nitric oxide by LPS/IFNγ-activated microglia (nitrite: 1.4±0.1 µM by untreated cells vs. 0.5±0.2 µM by adrenomedullin-treated cells, p<0.005). Notably, adrenomedullin decreased cell death induced by oxidative stress in precursor and mature oligodendrocytes (Fig. 8B). In agreement with this protective response, we observed that treatment with adrenomedullin significantly increased the expression of neuroprotective factors, like brain-derived neurotrophic factor (BDNF) and activity-dependent neuroprotector protein (ADNP) in the CNS of EAE mice (Fig. 8C). The neurotrophic factors are involved in processes of remyelination, axonal growth and neuroregeneration (Braitch et al., 2010; Lewin et al., 1996; Linker et al., 2010). All together these data suggest that adrenomedullin could promote protective responses in EAE by inducing CNS neurotrophic factors, by downregulating the destructive inflammatory response mediated by resident glial cells and by directly preventing oligodendrocyte cell loss.
Figure 8. Adrenomedullin promotes neuroprotective responses in EAE mice.
(A) Adrenomedullin downregulates the inflammatory response of CNS resident cells. Microglia, astrocytes and mixed neuron-glia isolated from newborn mice were cultured in medium or stimulated with LPS in the absence or presence of adrenomedullin (100 nM) and cytokine contents in culture supernatants were determined 24 h later. n=4, in duplicates. (B) Adrenomedullin protects oligodendrocytes from oxidative-induced cell death. Cell survival of precursor and mature oligodendrocytes cultured for 24 h with 200 µM H2O2 in the absence or presence of 100 nM adrenomedullin. n=3, in duplicates. (C) Adrenomedullin increases neurotrophic factors. BDNF and ADNP gene expression (relative to β-actin) in spinal cords isolated from EAE mice treated with PBS (control) or adrenomedullin at disease onset. Dashed line corresponds to expression in naïve mice normalized to 1. n=8 mice/group, three independent experiments. *p<0.05; **p<0.01; ***p<0.001vs control with Mann-Whitney test.
3.8. EAE mice have reduced levels of adrenomedullin in the CNS
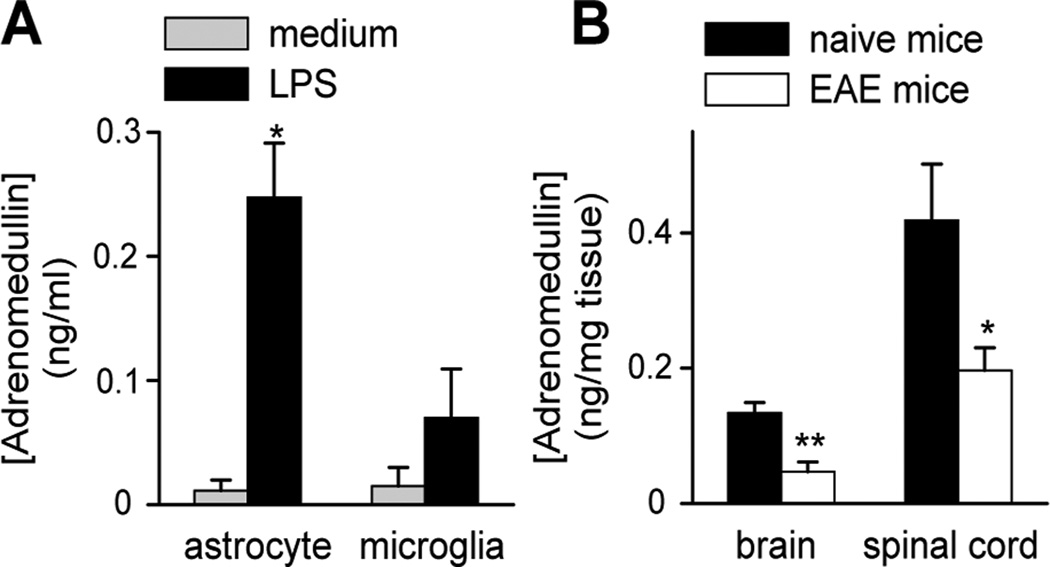
Several studies report that immune cells including lymphocytes, macrophages and DCs secrete adrenomedullin following activation (Elsasser and Kahl, 2002; Ishimitsu et al., 1998; Kubo et al., 1998a, 1998b; Rulle et al., 2012; Yang et al., 2001). Similarly, microglia and astrocytes increase adrenomedullin production upon LPS stimulation (Fig. 9A). We assessed whether the levels of adrenomedullin changed during EAE progression. Surprisingly, we found that the content of adrenomedullin in the CNS decreased in EAE mice (Fig. 9B), supporting an inverse correlation between EAE progression and endogenous adrenomedullin production.
Figure 9. EAE mice show decreased CNS adrenomedullin content.
Levels of adrenomedullin in culture supernatants of LPS-activated astrocytes and microglia (24 h) (A, n=5, in duplicates) and in the brains and spinal cords of naïve and EAE mice (B, n=5–8 mice/group, in duplicates). *p<0.05 vs medium with Mann-Whitney test; *p<0.05; **p<0.01 vs naïve with t-test.
4. Discussion
In this study, we report that the neuropeptide adrenomedullin provides a highly effective therapy for chronic EAE. Treatment with adrenomedullin reduced the level of inflammatory infiltrates in the CNS and subsequent demyelination and axonal damage typical of EAE. This therapeutic effect is associated with a striking reduction of the two deleterious components of the disease, namely the autoimmune and neuroinflammatory responses.
Our data indicate that treatment with adrenomedullin decreased the presence of encephalitogenic Th1 and Th17 cells in the periphery and CNS. This effect is mostly exerted by regulating the encephalitogenic sensitization in the peripheral immune compartment. Adrenomedullin reduced the number of Th1 and Th17 cells in lymphoid organs of EAE mice and specifically impaired MOG-specific Th1 and Th17 recall responses, while at the same time, it increased the number of Th2 cells, as reflected by a class switch in autoantibodies. Importantly, this treatment was antigen-specific and it did not result in general immunosuppression, since the response to a polyclonal T cell stimulation remained mostly unaffected in the adrenomedullin-treated EAE mice. Our data indicate that adrenomedullin could regulate the self-reactive T cell responses directly in the peripheral lymphoid organs. Whether this effect is exerted directly on T cells, affecting their activation, clonal expansion or differentiation, or indirectly through the modulation of antigen presenting cells, remains to be determined. Our data could support either possibility. We have previously found that adrenomedullin inhibited the activation of T cells in the absence of antigen presenting cells (unpublished data). Here we demonstrate that adrenomedullin regulates DC function by generating a semi-mature phenotype with the capacity to impair the encephalitogenic response. The effect on DCs could partially explain the fact that adrenomedullin inhibited the T cell response in an antigen-specific manner.
Beside its effect in the peripheral immune compartment, our in vitro experiments suggest that adrenomedullin could exert direct actions on infiltrating encephalitogenic T cells in the CNS parenchyma following traffic through the compromised blood-brain-barrier. In this case, whereas adrenomedullin fully suppressed MOG-specific Th17 responses, it failed to inhibit the production of IFNγ. For over a decade, Th1 cells were thought to be the driving force behind MS neuroinflammation (Hu and Ivashkiv, 2009). However, this traditional view has been challenged by studies describing exacerbated EAE development in animals deficient in IFNγ, which supports a protective role for endogenous IFNγ (Chu et al., 2000; Ferber et al., 1996). While this remains the subject of some debate, evidence now indicates that IL-17 producing T cells are critical for EAE (Komiyama et al., 2006; Park et al., 2005; Reboldi et al., 2009; Stromnes et al., 2008), and that T cell infiltration and inflammation in the CNS in EAE occur only when Th17 cells outnumber Th1 cells (Stromnes et al., 2008). Therefore, the potent suppressive effect of adrenomedullin on the activation of peripheral and central encephalitogenic Th17 cells might be an important component in its protective effect in EAE. Although we previously reported the inhibitory effect of adrenomedullin on Th1 responses in experimental colitis and arthritis (Gonzalez-Rey et al., 2006b, 2007a), the present study is the first demonstrating the impairment of self-reactive Th17 responses by this neuropeptide. Of relevance is also the fact that adrenomedullin downregulated the production of GM-CSF and osteopontin by encephalitogenic T cells, since both cytokines serve a nonredundant function in the initiation of autoimmune inflammation and in the generation of Th1 and Th17 cells in EAE and MS (Codarri et al., 2011; Chabas et al., 2001; Jansson et al., 2002; Murugaiyan et al., 2008, 2010).
On the other hand, the increase in the number of IL-10-producing CD4+CD25+FoxP3+ Treg cells in lymphoid organs and CNS could partially explain the antigen specificity of the long lasting protective response generated by adrenomedullin, and the fact that adrenomedullin administration subsequent to the activation/differentiation of antigen-specific effector Th1/Th17 cells still inhibited the inflammatory phase of EAE. In agreement with the present study, we previously described the generation of CD4+CD25+ Treg cells by adrenomedullin in animal models of rheumatoid arthritis and inflammatory bowel disease (Gonzalez-Rey et al., 2006b, 2007a). Further investigations are necessary to determine whether adrenomedullin affects the expansion of already existing Treg cells or their de novo generation. Other neuropeptides, such as vasoactive intestinal peptide and urocortin, increased Treg cells by promoting their generation from the non-Treg compartment through direct actions on T cells and indirectly on tolerogenic DCs (Gonzalez-Rey et al., 2007b). A recent study demonstrates that stimulation of DCs with adrenomedullin induce the expression of indoleamine 2,3-dioxygenase and the expansion of FoxP3+ Treg cells in vitro (Rulle et al., 2012). Here, we found that DCs stimulated and pulsed with MOG in the presence of adrenomedullin are capable to transfer tolerance to mice with EAE, although it remains to be determined whether they induce antigen-specific Treg cells in vivo. The IL-10-secreting CD4 Treg cells increased by adrenomedullin in EAE mice could represent a subtype of Tr1-like cells generated by tolerogenic DCs (Maldonado and von Adrian, 2010).
Regarding the inflammatory response in EAE, it is evident that the regulation of a wide spectrum of inflammatory mediators by adrenomedullin has an advantage over therapies directed against single mediators. The reduction of inflammation in CNS in adrenomedullin-treated EAE mice is associated with a decrease in the levels of chemokines in CNS parenchyma. This is especially relevant for chemokines such as CXCL10 (chemotactic for Th1 cells), CCL5 (for T cells) and CCL2 (for macrophages and T cells) that contribute to MS neuropathology (Goverman, 2009; Owens, 2003). Whether the effect on chemokines is exerted at local level or as a consequence of the peripheral action of adrenomedullin on IL-17 or GM-CSF (which play major roles in the initial recruitment of inflammatory cells to the CNS parenchyma in EAE), remains to be determined. A recent study reporting the capacity of adrenomedullin to inhibit the secretion of CCL2 and CCL3 by activated microglia in vitro (Consonni et al., 2011) supports a local effect of adrenomedullin. Moreover, the present study and others (Consonni et al., 2011) demonstrate that adrenomedullin might exert its anti-inflammatory action locally by downregulating the production of cytokines and nitric oxide by astrocytes and microglia. The effect on resident inflammatory cells, together with the inhibitory action of adrenomedullin on infiltrating macrophages (Gonzalez-Rey et al., 2006a, 2006b), probably contributes to the protection against oligodendrocyte/neuronal cell loss and axonal damage in this inflammatory milieu.
Attention has recently focused on regenerative mechanisms as targets for MS therapy, especially in the secondary progressive phase of the disease. Notably, the delayed treatment with adrenomedullin induced complete recovery in a significant number of animals, suggesting a role of adrenomedullin in repair and/or neuroregeneration. Supporting this, we found that adrenomedullin protected oligodendrocytes from cell death in an oxidative milieu. Moreover, adrenomedullin increased the local levels of BDNF and ADNP, which induce axonal outgrowth, remyelination, and rescue of degenerating neurons (Braitch et al., 2010; Lewin et al., 1996; Linker et al., 2010).
How does adrenomedullin regulate such a wide spectrum of immune mediators? The answer to this question could lie in the fact that adrenomedullin is the endogenous ligand of the calcitonin-related-like receptor (CRLR). CRLR forms complexes with various receptor activity-modifying proteins (RAMP1, RAMP2 and RAMP3) and the binding specificity for the ligand and the activity of the receptor depend on the RAMP subtype associated to CRLR (Gibbons et al., 2007). In contrast to CGRP which specifically binds to the CRLR-RAMP1 complex, adrenomedullin binds preferentially to CRLR associated to RAMP2 and RAMP3 (Hinson et al., 2000). Because CRLR-RAMP2/3 complexes are coupled to Gαs proteins, adrenomedullin signals through the elevation of cAMP and activation of protein kinase A, an intracellular pathway generally involved in the downregulation of inflammatory mediators, inhibition of Th1 responses, generation of Treg cells and induction of tolerogenic DCs (Torgersen et al., 2002; Tasken and Shokka., 2006; Klein et al., 2012). Noteworthy, macrophages, lymphocytes and DCs specifically express CRLR-RAMP2/3 complexes, which are differentially up-regulated during inflammatory processes (Ono et al. 2000), or during the differentiation and maturation of DCs (Rulle et al., 2012; EG-R, unpublished data).
What is the role of endogenous adrenomedullin in EAE? Evidence suggests that adrenomedullin is an endogenous immunomodulatory factor since both immune and glial cells produce adrenomedullin in response to inflammatory-related substances, cytokines and oxidative stress (Elsasser and Kahl, 2002; Ishimitsu et al., 1998; Kubo et al., 1998a, 1998b; Rulle et al., 2012; Yang et al., 2001; see also Fig. 9A). Adrenomedullin is increased in several inflammatory conditions, including rheumatoid arthritis, systemic lupus erythematosus and systemic sclerosis (Chosa et al., 2003; Mak et al., 2006; Mok et al., 2007; Yudoh et al., 1999). However, we found an inverse correlation between EAE severity and adrenomedullin levels in the CNS. Because adrenomedullin deficiency is embryonic lethal due to severe prenatal vascular failures (Shindo et al., 2001), cell- and tissue-conditional knockout mice are required to study the role of endogenous adrenomedullin in EAE. A recently generated brain conditional knockout mouse demonstrated the role of adrenomedullin in the differentiation of adult neural stem/progenitor cells and the development of oligodendrocytes (Vergaño-Vera et al., 2010). Finally, it was demonstrated that carriers of a single nucleotide polymorphism in the proximity of the adrenomedullin gene have lower levels of circulating peptide (Cheung et al., 2011; Martinez-Herrero and Martinez, 2013). Therefore, it should be interesting to investigate whether this single nucleotide polymorphism and low circulating adrenomedullin correlates with the susceptibility to suffer the disease or with its severity in patients with MS or other autoimmune encephalomyelitis.
5. Summary and conclusions
Our findings suggest that adrenomedullin may be a therapeutic option in MS. This novel therapeutic strategy is targeted to the inhibition of various neuropathological components of the disease, while promoting long-lasting antigen-specific suppressive responses through the induction of Treg cells and tolerogenic DCs and mounting an active program of neuroprotection. This multimodal action represents a therapeutic advantage over current treatments. The ability of adrenomedullin upon delayed administration to ameliorate ongoing disease also fulfils an essential prerequisite for its development as a therapeutic agent for MS. Translation of our findings to clinical practice could be imminent since adrenomedullin-based treatments have been proven safe and effective in several human pathologies, including inflammatory disorders (Nagaya et al., 2000) and EAE is an established preclinical model for MS therapies. Finally, our study supports that adrenomedullin is a key player in the bidirectional communication between the neuroendocrine and immune systems.
Supplementary Material
Highlight.
Adrenomedullin is a neuropeptide with therapeutic effects in EAE which acts at multiple levels on the autoimmune, inflammatory and degenerative components of the disease.
Acknowledgments
This work was supported by grant SAF2010-16923 from Spanish Ministry of Science and Innovation (to EGR) and the NIH grant 2RO1AI47325 (to DG). Support was also provided through Fondo Social Europeo (to MM, program JAE-DOC) and FPU grant from Spanish Ministry of Education (to MP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Financial Interest: Authors declare no competing financial interest.
REFERENCES
- Baxter AG. The origin and application of experimental autoimmune encephalomyelitis. Nat. Rev. Immunol. 2007;7:904–912. doi: 10.1038/nri2190. [DOI] [PubMed] [Google Scholar]
- Braitch M, Kawabe K, Nyirenda M, Gilles LJ, Robins RA, Gran B, Murphy S, Showe L, Constantinescu CS. Expression of activity-dependent neuroprotective protein in the immune system: possible functions and relevance to multiple sclerosis. Neuroimmunomodulation. 2010;17:120–125. doi: 10.1159/000258695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bynoe MS, Evans JT, Viret C, Janeway CA., Jr Epicutaneous immunization with autoantigenic peptides induces T suppressor cells that prevent experimental allergic encephalomyelitis. Immunity. 2003;19:317–328. doi: 10.1016/s1074-7613(03)00239-5. [DOI] [PubMed] [Google Scholar]
- Chabas D, Baranzini SE, Mitchell D, Bernard CC, Rittling SR, Denhardt DT, Sobel RA, Lock C, Karpuj M, Pedotti R, Heller R. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294:1731–1735. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- Cheung BM, Ong KL, Tso AW, Leung RY, Cherny SS, Sham PC, Lam TH, Lam KS. Plasma adrenomedullin level is related to a single nucleotide polymorphism in the adrenomedullin gene. Eur. J. Endocrinol. 2011;165:571–577. doi: 10.1530/EJE-11-0513. [DOI] [PubMed] [Google Scholar]
- Chorny A, Gonzalez-Rey E, Fernandez-Martin A, Pozo D, Ganea D, Delgado M. Vasoactive intestinal peptide induces regulatory dendritic cells with therapeutic effects on autoimmune disorders. Proc. Natl. Acad. Sci. U S A. 2005;102:13562–13567. doi: 10.1073/pnas.0504484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chosa E, Hamada H, Kitamura K, Eto T, Tajima N. Increased plasma and joint tissue adrenomedullin concentrations in patients with rheumatoid arthritis compared to those with osteoarthritis. J. Rheumatol. 2003;30:2553–2556. [PubMed] [Google Scholar]
- Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated CD4 T cell population in interferon γ-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J. Exp. Med. 2000;192:123–128. doi: 10.1084/jem.192.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codarri L, Gyülvészi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- Consonni A, Morara S, Codazzi F, Grohovaz F, Zacchetti D. Inhibition of lipopolysaccharide-induced microglia activation by calcitonin gene related peptide and adrenomedullin. Mol. Cell. Neurosci. 2011;48:151–160. doi: 10.1016/j.mcn.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Wu R, Zhou M, Dong W, Ulloa L, Yang H, Wang H, Tracey KJ, Simms HH, Wang P. Adrenomedullin and its binding protein attenuate the proinflammatory response after hemorrhage. Crit. Care. Med. 2005;33:391–398. doi: 10.1097/01.ccm.0000153416.41398.a9. [DOI] [PubMed] [Google Scholar]
- Elsasser TH, Kahl S. Adrenomedullin has multiple roles in disease stress: development and remission of the inflammatory response. Microsc. Res. Tech. 2002;57:120–129. doi: 10.1002/jemt.10058. [DOI] [PubMed] [Google Scholar]
- Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J. Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- Gibbons C, Dackor R, Dunworth W, Fritz-Six K, Caron KM. Receptor activity-modifying proteins: RAMPing up adrenomedullin signaling. Mol. Endocrinol. 2007;21:783–796. doi: 10.1210/me.2006-0156. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Chorny A, Robledo G, Delgado M. Urocortin and adrenomedullin prevent lethal endotoxemia by downregulating the inflammatory response. Am. J. Pathol. 2006a;168:1921–1930. doi: 10.2353/ajpath.2006.051104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Fernandez-Martin A, Chorny A, Delgado M. Therapeutic effect of urocortin and adrenomedullin in a murine model of Crohn’s disease. Gut. 2006b;55:824–832. doi: 10.1136/gut.2005.084525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Chorny A, O'Valle F, Delgado M. Adrenomedullin protects from experimental arthritis by down-regulating inflammation and Th1 response and inducing regulatory T cells. Am. J. Pathol. 2007a;170:263–271. doi: 10.2353/ajpath.2007.060596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Chorny A, Delgado M. Regulation of immune tolerance by anti- inflammatory neuropeptides. Nat. Rev. Immunol. 2007b;7:52–63. doi: 10.1038/nri1984. [DOI] [PubMed] [Google Scholar]
- Goverman J. Autoimmune T cell responses in the central nervous system. Nat. Rev. Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr. Rev. 2000;21(2):138–167. doi: 10.1210/edrv.21.2.0396. [DOI] [PubMed] [Google Scholar]
- Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity. 2009;31:539–550. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimitsu T, Miyata A, Matsuoka H, Kangawa K. Transcriptional regulation of human adrenomedullin gene in vascular endothelial cells. Biochem. Biophys. Res. Commun. 1998;243:463–470. doi: 10.1006/bbrc.1998.8110. [DOI] [PubMed] [Google Scholar]
- Jager A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J. Immunol. 2009;183:7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson M, Panoutsakopoulou V, Baker J, Klein L, Cantor H. Cutting edge: Attenuated experimental autoimmune encephalomyelitis in eta-1/osteopontindeficient mice. J. Immunol. 2002;168:2096–2099. doi: 10.4049/jimmunol.168.5.2096. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem. Biophys. Res. Comm. 1993;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- Klein M, Vaeth M, Scheel T, Grabbe S, Baumgrass R, Berberich-Siebelt F, Bopp T, Schmitt E, Becker C. Repression of cyclic adenosine monophosphate upregulation disarms and expands human regulatory T cells. J. Immunol. 2012;188(3):1091–1097. doi: 10.4049/jimmunol.1102045. [DOI] [PubMed] [Google Scholar]
- Kluver H, Barrera E. A method for the combined staining of cells and fibers in the nervous system. J. Neuropathol. Exp. Neurol. 1953;12:400–403. doi: 10.1097/00005072-195312040-00008. [DOI] [PubMed] [Google Scholar]
- Kohm AP, Carpentier PA, Anger HA, Miller SD. CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J. Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- Kong W, Yen JH, Ganea D. Docosahexaenoic acid prevents dendritic cell maturation, inhibits antigen-specific Th1/Th17 differentiation and suppresses experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2011;25:872–882. doi: 10.1016/j.bbi.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo DJ, Zhou M, Chaudry IH, Wang P. The role of adrenomedullin in producing differential hemodynamic responses during sepsis. J. Surg. Res. 2001;95:207–218. doi: 10.1006/jsre.2000.6013. [DOI] [PubMed] [Google Scholar]
- Kubo A, Minamino N, Isumi Y, Kangawa K, Dohi K, Matsuo H. Adrenomedullin production is correlated with differentiation in human leukemia cell lines and peripheral blood monocytes. FEBS Lett. 1998;426:233–237. doi: 10.1016/s0014-5793(98)00349-4. [DOI] [PubMed] [Google Scholar]
- Kubo A, Minamino N, Isumi Y, Katafuchi T, Kangawa K, Dohi K, Matsuo H. Production of adrenomedullin in macrophage cell line and peritoneal macrophage. J. Biol. Chem. 1998;273:16730–16738. doi: 10.1074/jbc.273.27.16730. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Barde YA. Physiology of the neurotrophins. Annu. Rev. Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- Linker RA, Lee DH, Demir S, Wiese S, Kruse N, Siglienti I, Gerhardt E, Neumann H, Sendtner M, Lühder F, Gold R. Functional role of brain-derived neurotrophic factor in neuroprotective autoimmunity: therapeutic implications in a model of multiple sclerosis. Brain. 2010;133:2248–2263. doi: 10.1093/brain/awq179. [DOI] [PubMed] [Google Scholar]
- Mak A, Cheung BMY, Mok CC, Leung R, Lau CS. Adrenomedullina potential disease activity marker and suppressor of nephritis activity in systemic lupus erythematosus. Rheumatology. 2006;45:1266–1272. doi: 10.1093/rheumatology/kel105. [DOI] [PubMed] [Google Scholar]
- Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv. Immunol. 2010;108:111–165. doi: 10.1016/B978-0-12-380995-7.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Herrero S, Martinez A. Cancer protection elicited by a single nucleotide polymorphism close to the adrenomedullin gene. J. Clin. Endocrinol. Metab. 2013;98:E807–E810. doi: 10.1210/jc.2012-4193. [DOI] [PubMed] [Google Scholar]
- Miller SD, Karpus WJ, Davidson TS. Experimental autoimmune encephalomyelitis in the mouse. In: Wiley Interscience, editor. Current Protocols in Immunology. Supplement 88. John Wiley & Sons, Inc.; 2010. pp. 15.1.1–15.1.20. [DOI] [PubMed] [Google Scholar]
- Mok MY, Cheung BM, Lo Y, Leung RY, Wong WS, Lau CS. Elevated plasma adrenomedullin and vascular manifestations in patients with systemic sclerosis. J. Rheumatol. 2007;34:2224–2229. [PubMed] [Google Scholar]
- Murugaiyan G, Mittal A, Weiner HL. Increased osteopontin expression in dendritic cells amplifies IL-17 production by CD4+ T cells in experimental autoimmune encephalomyelitis and in multiple sclerosis. J. Immunol. 2008;181:7480–7488. doi: 10.4049/jimmunol.181.11.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugaiyan G, Mittal A, Weiner HL. Identification of an IL-27/osteopontin axis in dendritic cells and its modulation by IFN-gamma limits IL-17-mediated autoimmune inflammation. Proc. Natl. Acad. Sci U S A. 2010;107:11495–11500. doi: 10.1073/pnas.1002099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaya N, Satoh T, Nishikimi T, Uematsu M, Furuichi S, Sakamaki F, Oya H, Kyotani S, Nakanishi N, Goto Y, Masuda Y, Miyatake K, Kangawa K. Hemodynamic, renal, and hormonal effects of adrenomedullin infusion in patients with congestive heart failure. Circulation. 2000;101:498–503. doi: 10.1161/01.cir.101.5.498. [DOI] [PubMed] [Google Scholar]
- Okura T, Marutsuka K, Hamada H, Sekimoto T, Fukushima T, Asada Y, Kitamura K, Chosa E. Therapeutic efficacy of intra-articular adrenomedullin injection in antigen induced arthritis in rabbits. Arthritis. Res. Ther. 2008;10:R133. doi: 10.1186/ar2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y, Okano I, Kojima M, Okada K, Kangawa K. Decreased gene expression of adrenomedullin receptor in mouse lungs during sepsis. Biochem. Biophys. Res. Commun. 2000;271:197–202. doi: 10.1006/bbrc.2000.2606. [DOI] [PubMed] [Google Scholar]
- Owens T. The enigma of multiple sclerosis: inflammation and neurodegeneration cause heterogeneous dysfunction and damage. Curr. Opin. Neurol. 2003;16:259–265. doi: 10.1097/01.wco.0000073925.19076.f2. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Donq C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, Sallusto F. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- Rullé S, Ah Kioon MD, Asensio C, Mussard J, Ea HK, Boissier MC, Lioté F, Falgarone G. Adrenomedullin, a neuropeptide with immunoregulatory properties induces semi-mature tolerogenic dendritic cells. Immunology. 2012;136:252–264. doi: 10.1111/j.1365-2567.2012.03577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo T, Kurihara Y, Nishimatsu H, Moriyama N, Kakoki M, Wang Y, Imai Y, Ebihara A, Kuwaki T, Ju KH, Minamino N, Kangawa K, Ishikawa T, Fukuda M, Akimoto Y, Kawakami H, Imai T, Morita H, Yazaki Y, Nagai R, Hirata Y, Kurihara H. Vascular abnormalities and elevated blood pressure in mice laking adrenomedullin gene. Circulation. 2001;104:1964–1971. doi: 10.1161/hc4101.097111. [DOI] [PubMed] [Google Scholar]
- Souza-Moreira L, Morell M, Delgado-Maroto V, Pedreño M, Martinez-Escudero L, Caro M, O'Valle F, Luque R, Gallo M, de Lecea L, Castaño JP, Gonzalez-Rey E. Paradoxical effect of cortistatin treatment and its deficiency on experimental autoimmune encephalomyelitis. J. Immunol. 2013;191:2144–2154. doi: 10.4049/jimmunol.1300384. [DOI] [PubMed] [Google Scholar]
- Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- Steinman L. Assessment of animal models for MS and demyelinating disease in the design of rational therapy. Neuron. 1999;24:511–514. doi: 10.1016/s0896-6273(00)81107-1. [DOI] [PubMed] [Google Scholar]
- Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat. Med. 2008;14:337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgersen KM, Vang T, Abrahamsen H, Yaqub S, Taskén K. Molecular mechanisms for protein kinase A-mediated modulation of immune function. Cell. Signal. 2002;14:1–9. doi: 10.1016/s0898-6568(01)00214-5. [DOI] [PubMed] [Google Scholar]
- Taskén K, Stokka AJ. The molecular machinery for cAMP-dependent immunomodulation in T-cells. Biochem. Soc. Trans. 2006;34:476–479. doi: 10.1042/BST0340476. [DOI] [PubMed] [Google Scholar]
- Vergaño-Vera E, Fernández AP, Hurtado-Chong A, Vicario-Abejón C, Martínez A. Lack of adrenomedullin affects growth and differentiation of adult neural stem/progenitor cells. Cell. Tissue. Res. 2010;340:1–11. doi: 10.1007/s00441-010-0934-3. [DOI] [PubMed] [Google Scholar]
- Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J. Exp. Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong LY, Cheung BM, Li YY, Tang F. Adrenomedullin is both proinflammatory and antiinflammatory: its effects on gene expression and secretion of cytokines and macrophage migration inhibitory factor in NR8383 macrophage cell line. Endocrinology. 2005;146:1321–1327. doi: 10.1210/en.2004-1080. [DOI] [PubMed] [Google Scholar]
- Wu R, Zhou M, Wang P. Adrenomedullin and adrenomedullin binding protein-1 downregulate TNF-alpha in macrophage cell line and rat Kupffer cells. Regul. Pept. 2003;112:19–26. doi: 10.1016/s0167-0115(03)00018-1. [DOI] [PubMed] [Google Scholar]
- Yang S, Zhou M, Chaudry IH, Wang P. The role of lipopolysaccharide in stimulating adrenomedullin production during polymicrobial sepsis. Biochem. Biophys. Res. Acta. 2001;1537:167–174. doi: 10.1016/s0925-4439(01)00069-2. [DOI] [PubMed] [Google Scholar]
- Yudoh K, Matsuno H, Kimura T. Plasma adrenomedullin in rheumatoid arthritis compared with other rheumatic diseases. Arthritis. Rheum. 1999;42:1297–1298. doi: 10.1002/1529-0131(199906)42:6<1297::AID-ANR30>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Zudaire E, Portal-Núñez S, Cuttitta F. The central role of adrenomedullin in host defense. J. Leukoc. Biol. 2006;80:237–244. doi: 10.1189/jlb.0206123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.