Abstract
Background and aims
Initial subjective reactions to cannabis and tobacco, broadly classified as positive or negative, have previously been explored for their associations with onset and maintenance of subsequent abuse/dependence. We examine (a) the factorial architecture of self-reported initial reactions to cannabis and tobacco; (b) whether these factors associate with concurrently reported age at onset of DSM-IV diagnosis of nicotine dependence and cannabis abuse/dependence; and (c) estimate heritable variation in and covariation between the factors.
Design
Factorial and exploratory structural equation modeling was conducted to examine the factor structure of initial reactions. Cox proportional hazards modeling was employed to examine their association with time to onset of diagnosis of DSM-IV nicotine dependence and cannabis abuse/dependence. Classical twin modeling, using univariate and multivariate models, was used to parse variance in each factor (and the covariance between factors) to their additive genetic, shared environmental and non-shared environmental sources.
Setting and Participants
General population sample of Caucasian female twins aged 18–32 years, with a lifetime history of tobacco [N=2393] and cannabis [N=1445] use.
Measurement
Self-report of initial subjective reactions to tobacco (cigarettes) and cannabis the first time they were used and time to onset of lifetime history of DSM-IV diagnosis of abuse (cannabis) and dependence (cannabis or nicotine).
Findings
Factors representing putatively positive and negative reactions to cannabis and tobacco emerged. Initial reactions to tobacco were associated with onset of DSM-IV diagnosis of nicotine dependence and cannabis abuse/dependence while initial reactions to cannabis were associated with onset of DSM-IV diagnosis of cannabis abuse/dependence alone. Genetic factors played a moderate role in each factor (heritability of 27–35%, p < 0.05) with the remaining variance attributed to individual-specific environment. Covariation across the factors indexing positive and negative initial reactions was attributable to genetic sources (0.18–0.58, p < 0.05), and to overlapping individual-specific environmental factors (−0.16–0.36, p < 0.05).
Conclusions
Initial subjective reactions to tobacco are associated with later onset of DSM-IV diagnosis of nicotine dependence and cannabis abuse/dependence while initial subjective reactions to cannabis are only associated with onset of diagnosis of DSM-IV cannabis abuse/dependence. Genetic and environmental factors underpin the overlap across the factors representing initial reactions, both positive and negative.
Keywords: cannabis, tobacco, reactions, genetics, twin
INTRODUCTION
Numerous studies, including those with genetically informed designs, have explored the role of subjective reactions (e.g. liking the taste, feeling dizzy, feeling nauseous, heart racing) to tobacco (1–9) and cannabis smoking (10–14). In general, these studies have had three primary objectives – to categorize subjective reactions as positive or negative, to examine their association and predictive utility (e.g. whether the experience of positive subjective reactions correlates with or prospectively predicts dependence or persistent use) and, more recently, whether heritable variation contributes to the etiology of these subjective responses (10;15;16). To these ends, first, studies have generally classified reactions such as relaxation and a pleasurable rush or buzz as positive and nausea and coughing as negative while items assessing dizziness remain ambiguously categorized (15;17;18). Second, while positive subjective reactions have been consistently implicated as a risk factor for nicotine and cannabis dependence, the role of negative reactions in this regard remains unclear. Finally, a few studies have explored the extent to which genetic and environmental factors influence the variance in and covariation between these facets of subjective reactions. Overall, the role of heritable variation is modest (ranging between 0.16 and 0.32) (10;16). Furthermore, these genetic factors explain a significant degree of correlation across cannabis and tobacco within the positive and negative domain of subjective reactions, however, the role of drug-specific genetic influences is also apparent (16).
In reviewing the extant literature, an important distinction should be made between typical [e.g. shortly after you last used cannabis, did it make you feel dizzy] and initial [e.g. when using cannabis for the first time or two, did you experience dizziness] subjective reactions. A majority of studies have focused on typical reactions and some differences in the association and predictive utility of typical versus initial reactions have been noted. For instance, for cannabis, Grant and colleagues (2005) found that greater endorsement of both typical adverse (i.e. greater endorsement of paranoia, confusion) and positive reactions (i.e. greater endorsement of sociability and feeling good) were associated with an increased risk for cannabis use disorders. In contrast, examining initial reactions to cannabis, Fergusson and colleagues (2003) reported that only positive symptoms predicted later cannabis use disorders. For tobacco, while positive reactions, regardless of whether they referenced the earliest or the most typical experiences with tobacco, have been found to positively correlate with nicotine dependence (5;19) and relapse after cessation (20). In contrast, negative reactions (both early and typical) have been found to exert negligible impact on nicotine dependence (3;21).
In this study, we use a sample of young adult twin women to examine (a) the phenotypic factorial architecture underlying retrospectively reported initial reactions to cannabis and tobacco; (b) the association between scores on these factors and onset of (abuse)/dependence on cannabis and tobacco; and (c) the extent to which heritable factors influence variance in, and covariance between, factors representing initial reactions to cannabis and tobacco.
METHODS
Sample
Data for these analyses are drawn from a longitudinal study of adult twin women born in a Midwestern state of the United States. The Missouri Adolescent Female Twin Study (MOAFTS) represents a cohort of same-sex female twin pairs identified from birth records who were born between July 1st 1975 and June 30th 1985 (22;23). Further details regarding sample recruitment, and characteristics of the baseline (adolescent) interview data (not used in this study), are given elsewhere (23). As the baseline assessment was targeted at behaviors specific to childhood and adolescence, sensitive questions regarding illicit drug use were not administered. During 2002–2005, all eligible twins who were then adults, regardless of whether they had participated in the baseline assessments or not (and as long as they had not declined to participate in future interviews) were invited to participate in the first full-length adult follow-up interview (Interview 1) which included individuals previously interviewed at baseline and an additional 1,431 eligible twins aged 18–29 years. The final sample for Interview 1 (N=3,787, 14.6% African-American with the remaining 3,233 of Caucasian ancestry) represented 80% of live born female twins identified via state birth records. The individual twins could be classified as 964 monozygotic (MZ) and 809 dizygotic (DZ) pairs with an additional 97 MZ and 145 DZ twins whose co-twin did not participate. A second follow-up interview (Interview 2) was conducted two years later, in 2005–2007, and included 3,427 women (2928 of Caucasian descent), an overwhelming majority of whom had been previously interviewed during Interview 1.
The flow of data collection for the initial subjective reactions is illustrated in Figure 1. As shown, initial subjective reactions to tobacco were queried at both Interview 1 and 2 (for interview 2, only if the respondent had initiated smoking in the past 24 months, N=32) while subjective reactions to cannabis (for use across the lifetime) were queried only at Interview 2. As norms surrounding cannabis and tobacco use can markedly differ in African-American populations, and as only a modest proportion of our sample is African-American, we elected to focus the current analyses on the Caucasian subset of data. Of these Caucasian women, 72.7% [N=2393], and 43.9% [N=1445] reported lifetime tobacco (ever smoked even one cigarette) and cannabis (ever used cannabis even once) smoking respectively - data on initial reactions for these individuals are utilized in these analyses. Thus, with missing data for either tobacco or cannabis-related initial reactions, 606 MZ and 455 DZ pairs and an additional 163 MZ and 223 DZ twins whose co-twin did not participate [N=2508] were included in the study.
Figure 1.
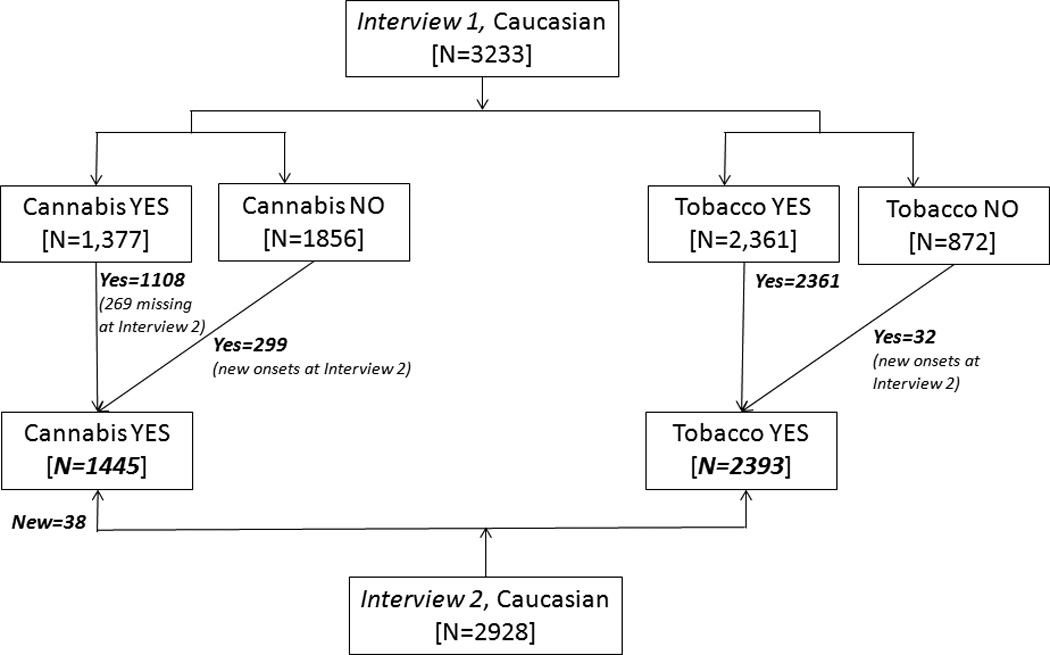
Flow of data collection for initial reactions to tobacco and cannabis from Interview 1 and 2 of the Missouri Adolescent Female Twin Study (MOAFTS). Baseline (adolescent) interview that preceded Interview 1 for a subset of subjects is not shown as data from that assessment were not used (except in recall bias analyses).
Measures
Respondents who reported ever having smoked cigarettes or cannabis, even once in their lifetime, were asked to recall whether they experienced certain sensations when smoking their very first cigarette or using cannabis for the first time or two, respectively. Ten items (liking the taste, coughing, dizziness, feeling relaxed, feeling a pleasurable rush or buzz, headaches, heart racing, nausea, trembling muscles and a burning sensation in the throat) were identically queried for both tobacco and cannabis while “feeling confused” was only queried as an initial reaction to cannabis. Responses were coded dichotomously. The items used to assess initial reactions are presented in Table 1. In addition to the initial reactions, nicotine dependence and cannabis abuse/dependence were coded according to DSM-IV criteria (24) using the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA)(25) and the age at diagnosis used in survival modeling.
Table 1.
Standardized factor loadings from an exploratory structural equation model of positive and negative subjective initial reactions to cannabis and tobacco in European-American female twins.
| % | Factor 1 | Factor 2 | Factor 3 | Factor 4 | |
|---|---|---|---|---|---|
| Tobacco initial reactions (N=2393) | |||||
| T: Like the taste | 12.7 | 0.50* | −0.44* | 0.02 | −0.006 |
| T: Cough | 76.5 | −0.07 | 0.60* | 0.06 | −0.005 |
| T: Dizzy | 47.2 | 0.73* | 0.52* | 0.007 | −0.02 |
| T: Relax | 15.5 | 0.70* | −0.29* | 0.003 | 0.07 |
| T: Headache | 26.5 | 0.09* | 0.72* | −0.05 | 0.02 |
| T: Rush/Buzz | 22.5 | 0.76* | 0.07 | 0.12* | −0.02 |
| T: Heart race | 19.2 | 0.51* | 0.50* | 0.03 | 0.03 |
| T: Nausea | 19.2 | 0.01 | 0.76* | −0.02 | 0.02 |
| T: Tremble | 4.8 | 0.47* | 0.56* | −0.11 | 0.02 |
| T: Burn in throat | 34.2 | −0.04 | 0.52* | 0.08 | −0.002 |
| Cannabis initial reactions (N=1445) | |||||
| C: Like the taste | 17.8 | 0.26* | −0.07 | 0.54* | −0.07 |
| C: Cough | 79.0 | −0.13* | 0.02 | 0.28* | 0.80* |
| C: Dizzy | 51.1 | −0.02 | 0.06 | 0.16* | 0.77* |
| C: Relax | 50.5 | 0.05 | 0.03 | 0.88* | 0.06 |
| C: Headache | 14.9 | 0.06 | −0.007 | −0.42* | 0.62* |
| C: Rush/Buzz | 44.6 | 0.03 | −0.02 | 0.74* | 0.31* |
| C: Heart race | 26.5 | 0.07 | 0.007 | 0.02 | 0.65* |
| C: Nausea | 13.8 | −0.03 | 0.04 | −0.40* | 0.63* |
| C: Tremble | 8.1 | 0.11 | −0.15* | −0.19* | 0.70* |
| C: Burn in throat | 37.1 | −0.10* | 0.06 | 0.04 | 0.56* |
| C: Feel confused | 20.9 | 0.04 | −0.04 | −0.005 | 0.69* |
| Factor label | Tobacco positive |
Tobacco negative |
Cannabis positive |
Cannabis negative |
|
indicates significant at p < 0.05.
Statistical analysis
Exploratory structural equation modeling (ESEM; (26)) was conducted in MPlus (version 7; (27)). In the absence of covariates, ESEM approximates an exploratory factor analysis. The best-fitting factor model was selected based on the comparative fit index (CFI), root mean square error of approximation (RMSEA) and standard root mean square residual (SRMR) (28). Factors were extracted and used in STATA (29) to conduct Cox proportional hazards survival modeling (30). Finally, univariate and multivariate twin models were fitted to the factor scores by decomposing the total variance in (and covariance between) the factors into their additive genetic, shared environmental (or non-additive genetic, if applicable) and non-shared environmental sources. The software package Mx (31) was used to model the classical twin design.
RESULTS
Prevalence of initial reactions to cannabis and tobacco
Mean age at first tobacco use was 14.3 years [SD=3.0] while first cannabis use, on average occurred at age 17 [SD 2.7]. Overwhelmingly, first use of tobacco preceded first use of cannabis with only 6.5% [of those reporting lifetime use of both substances] reporting use of cannabis prior to first tobacco use and 13.6% reporting onsets at the same age. As shown in Table 1, coughing was the most commonly reported initial reaction to both cannabis and tobacco, followed by dizziness. For cannabis, 45–51% of the participants also reported feeling relaxed and a pleasurable rush or buzz although the endorsement of these subjective initial reactions to tobacco were lower (16–23%). About 34–37% of the respondents endorsed experiencing a burning sensation in their throat the first time they smoked tobacco or cannabis. Headaches were more commonly reported as an initial reaction to smoking tobacco.
Exploratory Factor Analysis
From a series of 1 to 5 factor models, the 4 factor model appeared to fit the data well (3 factor: CFI=0.88, RMSEA=0.05, SRMR=0.09; 4 factor: CFI=0.96, RMSEA=0.03, SRMR=0.06; 5 factor: CFI=0.97, RMSEA=0.03, SRMR=0.04). Standardized factor loadings from the 4 factor model, which was reanalyzed in an ESEM framework, are presented in Table 1. Broadly, the factors represent positive and negative reactions to tobacco and cannabis. For both drugs, liking the taste, feeling relaxed and experiencing a pleasurable rush or buzz were unequivocally classified as positive reactions while coughing, headaches, nausea and feeling a burning sensation in the throat constituted negative experiences (as was ‘feeling confused’ for cannabis). For tobacco, dizziness, heart racing and trembling loaded positively on both the positive and negative reactions factors while for cannabis, these appeared to aggregate with items assessing negative reactions. Inter-factor correlations (Table 2) ranged from −0.12 (between negative reactions to tobacco and positive reactions to cannabis) and 0.28 (between negative reactions to cannabis and tobacco).
Table 2.
Phenotypic correlations across positive and negative subjective initial reactions to cannabis and tobacco in European-American female twins.
| Tobacco + | Tobacco − | Cannabis + | Cannabis − | |
|---|---|---|---|---|
| Tobacco + | 1.0 | - | - | - |
| Tobacco − | 0.08 [0.05–0.12] |
1.0 | - | - |
| Cannabis + | 0.17 [0.15–0.20] |
−0.12 [-0.16- −0.09] |
1.0 | - |
| Cannabis − | 0.23 [0.19–0.25] |
0.28 [0.24–0.30] |
0.11 [0.07–0.13] |
1.0 |
Associations with onset of nicotine dependence and cannabis use disorders
In the sample, 25.8% of those who had ever smoked a cigarette met criteria for DSM-IV nicotine dependence and 10.3% of those who had ever used cannabis met criteria for DSM-IV cannabis abuse or dependence. Nicotine dependence and cannabis abuse/dependence were highly correlated (Odds Ratio 4.15, 95% Confidence Interval 2.90–5.90).
We examined whether the factors representing positive and negative initial reactions (entered into the Cox proportional hazards model simultaneously) were significantly associated with age at onset (time to onset) of nicotine dependence or cannabis abuse/dependence. As shown in Table 3, positive and negative initial reactions to tobacco were associated with increased hazards of onset of DSM-IV nicotine dependence, but initial reactions to cannabis were not. Positive and negative reactions to both cannabis and tobacco were associated with increased hazards of onset of cannabis abuse/dependence. There was no evidence across the analyses for violations of the proportional hazards assumption.
Table 3.
Hazards ratios [95% Confidence limits] reflecting the association between factor scores representing positive and negative initial reactions to cannabis and tobacco and time to onset of nicotine dependence and cannabis abuse/dependence in European-American female twins.
| Nicotine Dependence |
Cannabis Abuse/Dependence |
|
|---|---|---|
| Tobacco + | 1.42 [1.29–1.56] |
1.29 [1.10–1.51] |
| Tobacco − | 1.21 [1.10–1.34] |
1.23 [1.02–1.47] |
| Cannabis + | 1.08 [1.00–1.17] |
1.46 [1.28–1.67] |
| Cannabis − | 1.09 [1.00–1.19] |
1.22 [1.06–1.40] |
Heritability and covariation across initial subjective reactions
An omnibus test revealed that all shared environmental influences (10 degrees of freedom) could be constrained to zero without a significant deterioration in fit (Δχ2=9.72, p=0.47). Subsequently, additive genetic and non-shared environmental factors explained the variance in initial subjective reactions. Genetic factors played a moderate role in positive and negative reactions to tobacco and cannabis (heritability of 27–35%, Table 4).
Table 4.
Proportion of variance positive and negative subjective initial reactions to cannabis and tobacco in European-American female twins attributable to additive genetic and non-shared environmental factors.
| Tobacco | Cannabis | |||
|---|---|---|---|---|
| Additive genetic | Positive | Negative | Positive | Negative |
| 0.34 [0.27–0.40] | 0.33 [0.26–0.39] | 0.35 [0.29–0.40] | 0.27 [0.20–0.36] | |
| Non-shared environment |
0.66 [0.60–0.73] | 0.67[0.61–0.74] | 0.65[0.60–0.71] | 0.72 [0.64–0.80] |
The net phenotypic covariance between the initial subjective responses could also be attributed to overlapping additive genetic and non-shared environmental influences as shown in Table 5.
Table 5.
Extent of additive genetic (below diagonal) and non-shared environmental (above diagonal) overlap across positive and negative subjective initial reactions to cannabis and tobacco in European-American female twins.
| Tobacco + | Tobacco − | Cannabis + | Cannabis − | |
|---|---|---|---|---|
| Tobacco + | 1.0 | 0.00 | 0.08 [-0.01 to 0.17] | 0.21 [0.12–0.28] |
| Tobacco − | 0.36 [0.23–0.49] | 1.0 | −0.16 [-0.25 to −0.07] | 0.36 [0.30–0.42] |
| Cannabis + | 0.58 [0.35–0.85] | −0.07 [-0.32 to 0.20] | 1.0 | 0.12 [0.05–0.19] |
| Cannabis − | 0.52 [0.32–0.72] | 0.18 [0.09–0.30] | 0.30 [0.15–0.50] | 1.0 |
Positive and negative reactions to tobacco
The modest correlation (r=0.08, Table 2) between these initial reactions was solely attributable to genetic factors. About 13% (Table 5, rg=0.36; (0.36)2=0.13) of the genetic variance in negative initial reactions to tobacco was due to genetic factors influencing positive initial reactions to tobacco.
Positive and negative reactions to cannabis
Both genetic and environmental factors were responsible for this modest correlation (r=0.11).
Positive reactions to cannabis and tobacco
Primarily shared genetic influences contributed to the correlation between positive reactions to cannabis and tobacco but there was also substantial evidence for substance-specific genetic effects. For instance, only 34% of the genetic variance in positive reactions to cannabis overlapped with those influencing positive reactions to tobacco.
Negative reactions to cannabis and tobacco
Both genetic and environmental factors contributed to this covariance. However, unlike positive reactions to both drugs, non-shared environmental factors exerted a stronger influence than genetic factors on the covariation (re=0.36).
Positive reactions to tobacco and negative reactions to cannabis
Both genetic and non-shared environmental factors played a role (Table 5) with additive genetic factors influencing positive reactions to tobacco explaining 27% of the genetic variance in negative reactions to cannabis.
Negative reactions to tobacco and positive reactions to cannabis
A modest but significant degree of non-shared environmental overlap (re=−0.16) was responsible for this negative correlation with no evidence for significant overlapping genetic contributions.
Discussion
Consistent with the literature, factors that broadly represented domains of subjectively positive and negative initial reactions emerged. Nonetheless, we noted that certain reactions tended to load well on putatively positive and negative factors. Evidence for such cross-loadings have been previously reported for dizziness from initial experiences with tobacco supporting its affective neutrality (15;17;18). Furthermore, Sartor et al., (2010) reported that endorsement of dizziness was common in those reporting exclusively negative reactions (e.g. coughing, headache, nausea) as well as those reporting pleasurable reactions (e.g. liking the taste). Interestingly, for cannabis, even though cross-factor loadings were statistically apparent, dizziness appeared to load more clearly on a negative reactions factor – this is consistent with other studies as well (10;14).
While the variable valence of dizziness to tobacco is well recognized, the high cross-loadings of putatively physiological reactions such as heart racing or trembling muscles were unexpected. Sartor and colleagues (2010) have previously identified subgroups of tobacco smokers who retrospectively report both pleasurable and putatively negative physiological initial reactions (e.g. heart racing). Why these reactions cohere should be further investigated. As posited by de Wit and Phillips (32), expectancies from substances and personality can play a pivotal role in the reporting of initial reactions, particularly when retrospectively recalled. It is plausible that certain individuals perceive these physiological responses as less aversive because they expect to experience them and interpret them as the desired psychoactive effect of nicotine. Relatedly, disinhibited individuals may find an elevated heart rate to be stimulating. There is mixed evidence, for instance, linking higher impulsivity to increased heart rate reactivity (33) such that highly impulsive individuals tend to have lower resting/baseline heart rates (i.e. under-aroused) that tend to increase more dramatically when challenged (34). Once again, results for cannabis were less ambiguous with these putatively physiological items loading more clearly on a negative experiences factor. It is also worth noting that ours is amongst few large-scale epidemiological studies that assess these physiological initial reactions to cannabis, and thus, future studies might consider their inclusion as well.
Next, we explored the relationship between these factor scores and onset of cannabis and nicotine (abuse)/dependence. Previous literature strongly supports the role of positive initial reactions to tobacco and cannabis in being associated with, even predicting, subsequent nicotine dependence and cannabis use disorders respectively (see (31) for a review). While we cannot be certain about temporality (i.e. whether greater positive reactions predicted dependence or whether those with dependence were more likely to recall greater positive experiences), we did find similar evidence for elevated hazards of onset of (abuse)/dependence in those with higher scores on the positive reaction factors. However, in contrast to the extant literature, we found that negative reactions to tobacco and cannabis were also associated with an increased likelihood of onset of nicotine dependence and cannabis abuse/dependence respectively. We speculate that this either reflects the enhanced set of negative reactions assessed in this study, especially for cannabis or, alternatively can be attributed to dependent individuals being more likely to be (or, to recall in a biased or unbiased manner) more sensitive to the physiological effects of tobacco and cannabis, regardless of its valence. Indeed, two studies of typical reactions to cannabis (12;13) as well as a study of initial reactions to tobacco (5) have identified subgroups of such “high responders” who also exhibit heightened vulnerability to problematic cannabis and tobacco use respectively.
An additional feature of the present study is that we explored cross-drug relationships. In our study, time to onset of cannabis use disorders was associated with positive and negative initial reactions to tobacco. A similar association between positive (but not negative) reactions to tobacco and cannabis abuse/dependence has been noted elsewhere (21) as have other cross-drug associations with other psychoactive substances (12;13;35).
Leveraging the twin design, we also explored the sources of variation in, and covariation between positive and negative reactions to cannabis and tobacco. Overall, the factors were moderately heritable, as has been previously reported (10;16). Prior research has noted similar heritability estimates (ranging from 0.15–0.30) for these factors. However, we are only aware of one other study by Haberstick and colleagues (16) that explored the sources of covariation across reactions (albeit, typical) to cannabis and tobacco, as well as alcohol. That study made several key observations. First, the authors noted minimal overlap across positive and negative reactions (and hence, unlike the present analysis, studied them separately). For positive and negative reactions, individually, a common, heritable underlying genetic vulnerability to substance sensitivity contributed to covariation, although substance-specific genetic factors were also evident. Even though we examined initial (not typical) reactions, we found similar evidence for genetic overlap, even when looking across substances. However, in contrast to Haberstick et al., (16) we found evidence for a significant, albeit modest, phenotypic correlation between positive and negative reactions to cannabis and tobacco. Possibly, this significant correlation between the positive and negative domains was due to several items cross-loading on both factors.
The present study has some limitations. Most importantly, we used retrospective reports from young adult women who, on average had started smoking tobacco and cannabis 7.6 and 5.6 years respectively prior to the interviews. While short term test-retest reliabilities were not available, a subset of the tobacco initial reactions questions were also assessed during the baseline interview administered to 1163 lifetime tobacco smokers who were also part of these analyses. As there was considerable variation in years lapsed (3–9 years, median 6 years) between the baseline and Interview 1 data used here, we restricted analysis of test-retest to those who had participated in Interview 1 within 5 years of the baseline interview. Kappas for 6 individual tobacco initial reactions (like the taste, cough, dizzy, headache, heart race and nausea) in these 203 women ranged from 0.20 (like the taste) to 0.40 (dizzy). Kappas were fairly similar to those from a one-year follow-up reported by Riedel et al (9) as were test-retest correlations (ranging from 0.39–0.61) to those reported by Urbán et al (36) for a 6-month follow-up. Reliability declined with increasing time elapsed between interviews indicating that recall bias may be a concern.
Another possible caveat is that those with more involved nicotine and cannabis use may have preferentially recalled a greater preponderance of initial reactions. For dizziness, headache and heart racing those who endorsed the initial reaction at baseline, but not at Interview 1, were less likely to be nicotine dependent and meet criteria for cannabis abuse/dependence than those who endorsed it at Interview 1 (regardless of their endorsement at baseline). However, no significant differences in age at onset were observed.
Another potential limitation is that as alcohol-related initial reactions were only collected from a subset of the sample during baseline, we were unable to study them here, as done by Haberstick et al (16). Lastly, we restricted the present analyses to Caucasian women only.
Similar to prior work using latent class modeling (37) and Mokken Scaling Analyses (38), we find that initial reactions to cannabis and tobacco tend to aggregate together, resembling a heritable and general sensitivity to psychoactive substances. Those who are high responders (i.e. more sensitive to the initial or typical effects) of drugs, in general, may be at greater risk for developing substance use disorders. Thus, endorsement of a large number of initial reactions, regardless of valence, may portend future substance misuse across a range of drugs. In fact, in this study, cross-drug cross-valence genetic correlations were noted, supporting the idea of general sensitivity to drugs. Furthermore, it is well documented that common heritable variation underlies a variety of substance use disorders (39). If the genetic influences on substance use disorders are partly in common with those influencing general sensitivity to substance use, then this may provide further avenues for gene identification and facilitate our understanding of yet another aspect of the etiology of addiction.
It is also possible that some of our results are unique to the study of cannabis and tobacco. These drugs typically share a route of administration (i.e. smoked) and are frequently co-used, therefore the likelihood that initial reactions to one drug (say, cannabis) are shaped by experiences with the other (such as tobacco), is highly likely (40). For instance, we have previously found that regular tobacco smokers are more likely to endorse positive initial reactions to cannabis (41). In this study as well, items indicating respiratory adaptations to inhaled smoke (e.g. negative loadings for coughing, burning throat) as well as subjective liking loaded on the factor representing positive reactions to tobacco. From a public health standpoint, research into this co-use is at a watershed. There has been a documented decline in rates of tobacco smoking in youth while, conversely, rates of cannabis use appear to have stabilized (42). This study adds to a growing body of literature that indicates that the association between these substances begins fairly early in the substance use trajectory, even as early as the first time they are used. Therefore, initial reactions may serve as the first stage of substance use at which intervention efforts may be targeted.
Acknowledgements
This work is supported by K02DA32573 & R01DA23668 to AA, as well as AA11998, AA07728, AA09022 & K05AA17688 (ACH), AA12640 & DA14363 (KKB), DA12854 (PAFM) and DA18660 (MTL).
Declaration of interest
Dr. Agrawal has received peer-reviewed grant funding, travel reimbursements and an honorarium from ABMRF/Foundation for Alcohol Research, which receives some of its funds from brewers.
Reference List
- 1.DiFranza JR, Savageau JA, Fletcher K, Ockene JK, Rigotti NA, McNeill AD, et al. Recollections and repercussions of the first inhaled cigarette. Addict Behav. 2004 Feb;29(2):261–272. doi: 10.1016/j.addbeh.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Kandel DB, Hu MC, Griesler PC, Schaffran C. On the development of nicotine dependence in adolescence. Drug Alcohol Depend. 2007 Nov 2;91(1):26–39. doi: 10.1016/j.drugalcdep.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pomerleau OF, Pomerleau CS, Namenek RJ. Early experiences with tobacco among women smokers, ex-smokers, and never-smokers. Addiction. 1998 Apr;93(4):595–599. doi: 10.1046/j.1360-0443.1998.93459515.x. [DOI] [PubMed] [Google Scholar]
- 4.Pomerleau CS, Pomerleau OF, Namenek RJ, Marks JL. Initial exposure to nicotine in college-age women smokers and never-smokers: a replication and extension. J Addict Dis. 1999;18(3):13–19. doi: 10.1300/J069v18n03_02. [DOI] [PubMed] [Google Scholar]
- 5.Sartor CE, Lessov-Schlaggar CN, Scherrer JF, Bucholz KK, Madden PA, Pergadia ML, et al. Initial response to cigarettes predicts rate of progression to regular smoking: findings from an offspring-of-twins design. Addict Behav. 2010 Aug;35(8):771–778. doi: 10.1016/j.addbeh.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eissenberg T, Balster RL. Initial tobacco use episodes in children and adolescents: current knowledge, future directions. Drug Alcohol Depend. 2000 May 1;59(Suppl 1):S41–S60. doi: 10.1016/s0376-8716(99)00164-7. [DOI] [PubMed] [Google Scholar]
- 7.DiFranza JR, Savageau JA, Fletcher K, O'Loughlin J, Pbert L, Ockene JK, et al. Symptoms of tobacco dependence after brief intermittent use: the Development and Assessment of Nicotine Dependence in Youth-2 study. Arch Pediatr Adolesc Med. 2007 Jul;161(7):704–710. doi: 10.1001/archpedi.161.7.704. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Stacy A, Zheng H, Shan J, Spruijt-Metz D, Unger J, et al. Sensations from initial exposure to nicotine predicting adolescent smoking in China: a potential measure of vulnerability to nicotine. Nicotine Tob Res. 2003 Aug;5(4):455–463. doi: 10.1080/14622200307239. [DOI] [PubMed] [Google Scholar]
- 9.Riedel BW, Blitstein JL, Robinson LA, Murray DM, Klesges RC. The reliability and predictive value of adolescents' reports of initial reactions to smoking. Nicotine Tob Res. 2003 Aug;5(4):553–559. doi: 10.1080/1462220031000118658. [DOI] [PubMed] [Google Scholar]
- 10.Lyons MJ, Toomey R, Meyer JM, Green AI, Eisen SA, Goldberg J, et al. How do genes influence marijuana use? The role of subjective effects. Addiction. 1997 Apr;92(4):409–417. [PubMed] [Google Scholar]
- 11.Fergusson DM, Horwood LJ, Lynskey MT, Madden PA. Early reactions to cannabis predict later dependence. Arch Gen Psychiatry. 2003 Oct;60(10):1033–1039. doi: 10.1001/archpsyc.60.10.1033. [DOI] [PubMed] [Google Scholar]
- 12.Scherrer JF, Grant JD, Duncan AE, Sartor CE, Haber JR, Jacob T, et al. Subjective effects to cannabis are associated with use, abuse and dependence after adjusting for genetic and environmental influences. Drug Alcohol Depend. 2009 Nov 1;105(1–2):76–82. doi: 10.1016/j.drugalcdep.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant JD, Scherrer JF, Lyons MJ, Tsuang M, True WR, Bucholz KK. Subjective reactions to cocaine and marijuana are associated with abuse and dependence. Addict Behav. 2005 Sep;30(8):1574–1586. doi: 10.1016/j.addbeh.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Zeiger JS, Haberstick BC, Corley RP, Ehringer MA, Crowley TJ, Hewitt JK, et al. Subjective effects to marijuana associated with marijuana use in community and clinical subjects. Drug Alcohol Depend. 2010 Jun 1;109(1–3):161–166. doi: 10.1016/j.drugalcdep.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haberstick BC, Ehringer MA, Lessem JM, Hopfer CJ, Hewitt JK. Dizziness and the genetic influences on subjective experiences to initial cigarette use. Addiction. 2011 Feb;106(2):391–399. doi: 10.1111/j.1360-0443.2010.03133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haberstick BC, Zeiger JS, Corley RP, Hopfer CJ, Stallings MC, Rhee SH, et al. Common and drug-specific genetic influences on subjective effects to alcohol, tobacco and marijuana use. Addiction. 2011 Jan;106(1):215–224. doi: 10.1111/j.1360-0443.2010.03129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pomerleau OF, Pomerleau CS. Commentary on Haberstick et al (2011): Dizziness upon initial experimentation with cigarettes - implications for smoking persistence. Addiction. 2011 Feb;106(2):400–401. doi: 10.1111/j.1360-0443.2010.03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rios-Bedoya CF, Pomerleau CS, Neuman RJ, Pomerleau OF. Using MIMIC models to examine the relationship between current smoking and early smoking experiences. Nicotine Tob Res. 2009 Sep;11(9):1035–1041. doi: 10.1093/ntr/ntp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu MC, Davies M, Kandel DB. Epidemiology and correlates of daily smoking and nicotine dependence among young adults in the United States. Am J Public Health. 2006 Feb;96(2):299–308. doi: 10.2105/AJPH.2004.057232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strong DR, Leventhal AM, Evatt DP, Haber S, Greenberg BD, Abrams D, et al. Positive reactions to tobacco predict relapse after cessation. J Abnorm Psychol. 2011 Nov;120(4):999–1005. doi: 10.1037/a0023666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeiger JS, Haberstick BC, Corley RP, Ehringer MA, Crowley TJ, Hewitt JK, et al. Subjective effects for alcohol, tobacco, and marijuana association with cross-drug outcomes. Drug Alcohol Depend. 2012 Mar 22;123(Suppl 1):S52–S58. doi: 10.1016/j.drugalcdep.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heath AC, Howells W, Bucholz KK, Glowinski AL, Nelson EC, Madden PA. Ascertainment of a mid-western US female adolescent twin cohort for alcohol studies: assessment of sample representativeness using birth record data. Twin Res. 2002 Apr;5(2):107–112. doi: 10.1375/1369052022974. [DOI] [PubMed] [Google Scholar]
- 23.Knopik VS, Sparrow EP, Madden PA, Bucholz KK, Hudziak JJ, Reich W, et al. Contributions of parental alcoholism, prenatal substance exposure, and genetic transmission to child ADHD risk: a female twin study. Psychol Med. 2005 May;35(5):625–635. doi: 10.1017/s0033291704004155. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edition. Washington, DC: American Psychiatric Association; 1994. Revised ed. [Google Scholar]
- 25.Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994 Mar;55(2):149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- 26.Asparouhov T, Muthen BO. Exploratory structural equation modeling. Structural Equation Modeling. 2010;16:397–438. [Google Scholar]
- 27.Muthen B, Muthen LK. Mplus User's Guide. Seventh Edition. 7 ed. Los Angeles, CA: Muthen & Muthen; 2012. [Google Scholar]
- 28.Hu L, Bentler PM. Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychological Methods. 1998;3:424–453. [Google Scholar]
- 29.Stata Corp. STATA version 9. College Station, TX: 2012. [Google Scholar]
- 30.Cleves MA, Gould WW, Gutierrez RG. The Cox Model: Diagnostics. An Introduction to Survival Analysis Using STATA. College Station, TX: Stata Corp; 2004. [Google Scholar]
- 31.Neale MC. Statistical Modeling with Mx. Box # 980710, Richmond VA 23298: Dept of Psychiatry; 2004. [Google Scholar]
- 32.de Wit H, Phillips TJ. Do initial responses to drugs predict future use or abuse? Neurosci Biobehav Rev. 2012 Jul;36(6):1565–1576. doi: 10.1016/j.neubiorev.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen MT, Hogan AM, Laird LK. The relationships of impulsivity and cardiovascular responses: the role of gender and task type. Int J Psychophysiol. 2009 Sep;73(3):369–376. doi: 10.1016/j.ijpsycho.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Mathias CW, Standford MS. Impulsiveness and arousal: heart rate under conditions of rest and challenge in healthy males. Pers Indiv Dif. 2003;35:355–371. [Google Scholar]
- 35.Perkins KA, Coddington SB, Karelitz JL, Jetton C, Scott JA, Wilson AS, et al. Variability in initial nicotine sensitivity due to sex, history of other drug use, and parental smoking. Drug Alcohol Depend. 2009 Jan 1;99(1–3):47–57. doi: 10.1016/j.drugalcdep.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urban R, Sutfin E. Do early smoking experiences count in development of smoking?: temporal stability and predictive validity of an early smoking experience questionnaire in adolescents. Nicotine Tob Res. 2010 Dec;12(12):1265–1269. doi: 10.1093/ntr/ntq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCutcheon AL. Latent Class Analysis. Newbury Park, CA: Sage; 1987. [Google Scholar]
- 38.Mokken RJ, Lewis C. A nonparametric approach to the analysis of dichotomous item responses. Applied Psychological Measurement. 1982;6:417–430. [Google Scholar]
- 39.Kendler KS, Myers J, Prescott CA. Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Arch Gen Psychiatry. 2007 Nov;64(11):1313–1320. doi: 10.1001/archpsyc.64.11.1313. [DOI] [PubMed] [Google Scholar]
- 40.Agrawal A, Budney AJ, Lynskey MT. Cannabis and Tobacco Co-Involvement: A Review. Addiction. 2012;107(7):1221–1233. doi: 10.1111/j.1360-0443.2012.03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agrawal A, Madden PA, Martin NG, Lynskey MT. Do early experiences with cannabis vary in cigarette smokers? Drug Alcohol Depend. 2013 Mar 1;128(3):255–259. doi: 10.1016/j.drugalcdep.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2012. Ann Arbor: Institute for Social Research: The University of Michigan; 2013. [Google Scholar]


