Abstract
Background
Deaths during induction chemotherapy for pediatric acute lymphoblastic leukemia (ALL) account for one-tenth of ALL-associated mortality and half of ALL treatment-related mortality. We sought to ascertain patient- and hospital-level factors associated with induction mortality.
Procedure
We performed a retrospective cohort analysis of 8,516 children ages 0 to <19 years with newly diagnosed ALL admitted to freestanding US children’s hospitals from 1999–2009 using the Pediatric Health Information System database. Induction mortality risk was modeled accounting for demographics, intensive care unit-level interventions, and socioeconomic status (SES) using Cox regression. The association of ALL induction mortality with hospital-level factors including volume, hospital-wide mortality and payer mix was analyzed with multiple linear regression.
Results
ALL induction mortality was 1.12%. Race and patient-level SES factors were not associated with induction mortality. Patients receiving both mechanical ventilation and vasoactive infusions experienced nearly 50% mortality (hazard ratio 122.30, 95% CI 66.56–224.80). Institutions in the highest induction mortality quartile contributed 27% of all patients but nearly half of all deaths (47 of 95). Hospital payer mix was associated with ALL induction mortality after adjustment for other hospital-level factors (p=0.046).
Conclusions
The overall risk of induction death is low but substantially increased in patients with cardio-respiratory and other organ failures. Induction mortality varies up to three-fold across hospitals and is correlated with hospital payer mix. Further work is needed to improve induction outcomes in hospitals with higher mortality. These data suggest an induction mortality rate of less than 1% may be an attainable national benchmark.
Keywords: acute lymphoblastic leukemia, mortality, Medicaid, insurance
INTRODUCTION
Acute lymphoblastic leukemia (ALL) is the most common pediatric malignancy, with approximately 2,400 children diagnosed annually in the US.1 While most ALL patients are cured with standard chemotherapy, 10–15% die of disease or treatment-related complications. The initial month of treatment, or “induction”, has a reported mortality rate of 1–2%.2–6 Induction mortality accounts for about one-tenth of all ALL-associated deaths and up to 50% of all treatment-related mortality.7,8
Much of the available data on ALL induction mortality stems from cooperative group chemotherapy trials. These studies have led to improvements in ALL survival from single-digit percentages in the 1960s to 90.4% 5-year overall survival among patients treated from 2000–2005.7 Treatment-related mortality in patients enrolled on clinical trials has declined from 2.16% (1990–1994) to 1.57% (2000–2005).7 However, cooperative group trials do not capture important data on socioeconomic status (SES), hospital resources used (i.e., non-chemotherapeutic medication exposures or procedures performed), or hospital-level factors, such as payer mix. These data could be used to develop precise estimates of induction mortality in particular clinical scenarios and analyses of hospital-level variation in induction mortality.
Administrative/billing data have been used to study multiple pediatric clinical outcomes,9–13 to evaluate effectiveness of medical and clinical pathway interventions,11,14 and to show improved mortality rates in centers with high procedure-specific volume.15,16 Such analyses aim to improve the quality of medical care for specific conditions but have not been performed for pediatric ALL.
We used an established cohort of children with ALL from the Pediatric Health Information System (PHIS) database17 to address the following aims: 1) to estimate the induction mortality rate in a national sample of free-standing children’s hospitals; 2) to identify patient-level factors associated with increased mortality risk; 3) to estimate mortality for patients with organ system failure requiring intensive care unit (ICU)-level care; and 4) to identify hospital-level factors associated with increased ALL induction mortality. With these analyses, we aim to stimulate a discussion to establish a national benchmark for ALL induction mortality and to identify processes for improvement so that all institutions can reach such a benchmark.
METHODS
Data source
The PHIS database was used to establish a cohort of patients diagnosed with new-onset ALL from 1999–2009. Detailed descriptions of this database are previously published.9,10,12 Briefly, PHIS contains inpatient, emergency department, ambulatory surgery, and observation data from 43 not-for-profit, tertiary care pediatric hospitals in the US that can be linked over time by unique PHIS-generated identifiers. These hospitals are affiliated with the Children’s Hospital Association, a business alliance of children’s hospitals. PHIS data represent 85% of all freestanding children’s hospitals registered with the National Association of Children’s Hospitals and Related Institutions (NACHRI) and have been used in over 160 peer-reviewed publications in a wide range of pediatric conditions, including work by our group evaluating induction mortality, acute renal failure, and cefepime-associated mortality in pediatric acute myeloid leukemia.18–20
Establishment of de novo ALL cohort
The methodology for the assembly and validation of the de novo ALL cohort used in this study has been described previously.17 Briefly, this cohort includes 8,733 children ages 0 to <19 years admitted to PHIS-participating hospitals between 1999–2009 who were assigned an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) discharge diagnosis code consistent with ALL (204.xx, excluding 204.02 — relapsed ALL). Patients were included in the cohort only if they had chemotherapy exposures matching standard ALL induction regimens on review of their daily pharmaceutical billing codes.
Definition of induction periods
Each patient’s induction period was defined as starting on the first inpatient day chemotherapy was administered and lasting until the first of three events: start of consolidation chemotherapy, death, or 60 days from first chemotherapy (Supplemental Figure 1). Patients who died before day 29 were counted as cases of induction mortality and assigned the time from first chemotherapy to day of death as their observation period. Children who were no longer inpatient on day 29 were assumed to have received consolidation chemotherapy as an outpatient and were assigned a 29-day induction period. A child with an admission including day 29 was observed until discharge, start of consolidation chemotherapy or death and assigned the length of time from first chemotherapy exposure to the date of the first of these endpoints as the induction period. Some regimens gave certain high-risk patients additional doses of induction-like chemotherapy 4 weeks from start of therapy (“extended induction”); children who received extended induction chemotherapy were expected to begin consolidation chemotherapy by day 43 and were observed until discharge death or observed inpatient consolidation. Finally patients whose admissions lasted beyond an expected induction period but were not observed to receive consolidation chemotherapy were assigned a 60-day maximum observation period; deaths occurring beyond this point were not included in our analysis.
Outcome
The primary outcome for each analysis was inpatient mortality. Each hospital admission in PHIS is associated with a discharge disposition variable, which includes a code for death. This variable was used to identify patients with inpatient mortality, and the discharge date for that admission was used to assign date of death.
Patient-level factors
Age at first chemotherapy exposure was categorized according to National Cancer Institute age-based ALL risk groups: <1 year, 1 to <10 years, and ≥10 years. Other demographic variables included gender and race, dichotomized into white or non-white (black, Asian/Pacific islander, native American, other, and unknown). Hispanic ethnicity data are frequently missing in PHIS and thus were not included in this study. A patient’s primary insurance status was determined by data available for his/her first ALL admission and was dichotomized into public or private/self-pay/other (Supplemental Table I). Income was estimated using median household income for each patient’s ZIP code.21 Each inpatient day was dichotomized as containing ICU-level care based on the presence of specific pharmaceutical and clinical billing codes, as well as ICD-9-CM procedure codes consistent with ICU-level resources (Supplemental Table II).
Hospital-level factors
The following hospital-level summary variables were created: percent of patients in each of the three age groups; percent male; percent non-white; percentages with anthracycline and dexamethasone exposure; percent with public insurance at discharge; percent with Down syndrome; median of median household income by ZIP code; mean proportion of in-hospital days per induction period; percentages of patients receiving mechanical ventilation, vasopressors, extra-corporeal membrane oxygenation (ECMO), and hemodialysis; and total number of ALL diagnoses per year. Hospital-wide (i.e. not limited to ALL patients) summaries of variables hypothesized a priori to have a potential association with ALL induction mortality rates were created: annual hospital mortality rate (number of hospital admissions ending in death per all hospital admissions); annual patient volume (total admissions per year); percentage of admissions at the hospital with public insurance as the primary carrier; and race and gender frequencies for all hospital admissions.
Human subjects protection
In accordance with the Common Rule (45 CFR 46.102(f)) and the policies of The Children’s Hospital of Philadelphia Institutional Review Board this study using a de-identified dataset was not considered human subjects research.
Statistical methods
Patient-level analyses
Descriptive statistics were used to summarize patient-level demographic variables. Frequencies and percentages are reported for categorical variables, while mean with standard deviation (SD) or median with range are reported for continuous variables. Cox proportional hazard models were used to evaluate associations of patient-level factors with induction mortality. Patients were followed until completion of their induction periods, as defined above. The proportional hazard assumption was assessed for each factor graphically using log-log plots. Results from each Cox model are presented as hazard ratios (HRs) with corresponding 95% confidence intervals (CIs). Robust variance estimates were used to account for hospital-level clustering. First, univariate Cox models were used to screen for significant baseline factors. A multivariate Cox model was constructed next, including age, sex, race, anthracycline and dexamethasone exposures a priori and additional baseline factors with p-value <0.2 in the univariate Cox model. The effects of need for post-baseline ICU level resource utilization were evaluated using a multivariate Cox model adjusting for all the baseline factors included in the previous model.
Hospital-level analyses
Pearson correlations were used to identify associations among hospital summary variables and hospital ALL mortality rates. Multiple linear regression was performed using ALL patient and hospital-wide summary variables as the explanatory variables and hospital-level ALL induction mortality as the outcome. Finally, multiple linear regression was performed with hospital-wide summary variables as the covariates and hospital-wide mortality as the outcome.
RESULTS
Demographic characteristics
Review of the initial cohort of 8,733 patients17 for initiation of consolidation-like chemotherapy to ascertain the end of induction identified 123 (1.44%) patients with upfront exposures to chemotherapy typically only given after induction or in relapse who were then excluded. Additional exclusion of patients over age 19 (n= 94) resulted in a final cohort of 8,516 patients. This refined cohort retained similar demographic characteristics (Table I) to the previously published cohort, which was comparable with Surveillance Epidemiology and End Results (SEER) data.17,22
Table I.
Demographic, SES, and length-of-stay characteristics of the PHIS ALL cohort, 1999–2009
| Patient characteristic | N (%) |
|---|---|
| Age at diagnosis, median 5.7 years, range 0 – 19 years* | |
| <1 year | 243 (2.9) |
| 1 – <10 years | 6,029 (70.8) |
| 10 – <19 years | 2,244 (26.4) |
| Female | 3,739 (43.9) |
| Race | |
| White | 6,506 (76.4) |
| Black | 634 (7.4) |
| Asian/Pacific Islander | 264 (3.1) |
| Native American | 63 (0.7) |
| Other | 625 (7.3) |
| Unknown | 424 (5.0) |
| Down syndrome | 200 (2.4) |
| Insurance | |
| Private | 3,334 (39.2) |
| Government | 3,130 (36.8) |
| Self-pay | 244 (2.9) |
| Other | 1,775 (20.8) |
| Unknown | 33 (0.4) |
| Anthracycline and dexamethasone exposure | |
| Both | 1,045 (12.3) |
| Dexamethasone only | 3,886 (45.6) |
| Anthracycline only | 2,958 (34.7) |
| Neither | 627 (7.4) |
| Income estimate | Median (Range) |
| Median household income by patients’ home ZIP codes** | $42,224 (10,395 –192,641) |
| Hospitalization data | Median (Range) |
| Number of hospital days | 9 (1–60)*** |
| Proportion of study days in the hospital | 0.31 (0.03–1) |
| Number of admissions | 1 (1–6) |
Only patients up to age 19 years were included.
ZIP code data were missing for 1428 (16.8%) patients.
Patients who remained inpatient beyond day 29 without evidence of consolidation chemotherapy were censored at day 60.
Patient-level factors and induction mortality
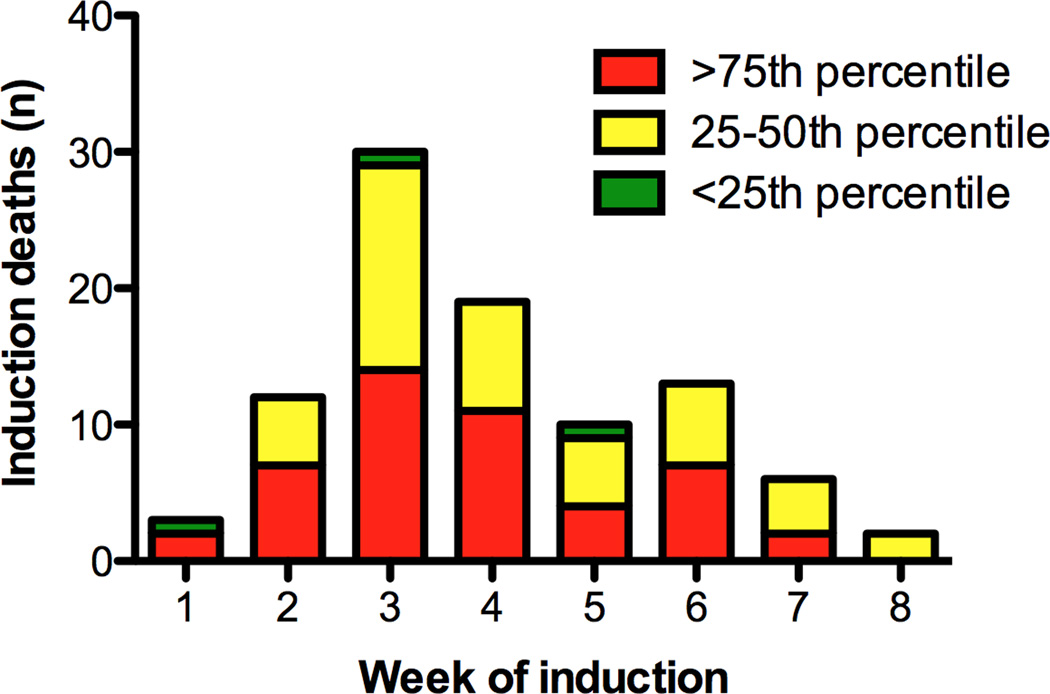
Overall inpatient induction mortality was 1.12%, with a median time to death of 21 days (range 3–50 days; Figure 1). There were no significant differences in mortality by gender, race, income, or insurance type in either unadjusted (Supplemental Table III) or adjusted models (Table II). Additionally, there were no significant differences in mortality risk by geographic region or year of diagnosis (Supplemental Tables IV and V). Compared to children aged 1 to <10 years, infants and older patients had a significantly increased adjusted risk of induction mortality (HR 3.34; 95% CI 1.22–9.13, p=0.02, and HR 2.89; 95% CI 1.55–5.41, p=0.001). Children with Down syndrome did not have a significantly increased risk of death in induction. While anthracycline exposure was associated with an increased risk of induction mortality in an unadjusted analysis, neither anthracycline nor dexamethasone exposure was associated with mortality in the multivariate analysis.
Figure 1.
Table II.
Adjusted mortality hazard by patient demographics and insurance status
| Risk factor | Raw mortality, N (%) | Adjusted HR (95% CI) | P |
|---|---|---|---|
| Overall | 95 (1.1) | — | — |
| Age at diagnosis | |||
| <1 year | 13 (5.4) | 3.34 (1.22–9.13) | 0.02 |
| 1 – <10 years | 35 (0.6) | 1.0 | — |
| 10 – <19 years | 47 (2.1) | 2.89 (1.55–5.41) | 0.001 |
| Sex | |||
| Female | 44 (1.2) | 1.0 | — |
| Male | 51 (1.0) | 0.92 (0.66–1.26) | 0.59 |
| Race | |||
| White | 73 (1.1) | 1.0 | — |
| Non-white | 22 (1.1) | 0.91 (0.59–1.42) | 0.69 |
| Down syndrome | |||
| Yes | 3 (1.5) | 1.27 (0.51–3.16) | 0.61 |
| No | 92 (1.1) | 1.0 | — |
| Anthracycline | |||
| Yes | 69 (1.7) | 1.23 (0.64–2.4) | 0.53 |
| No | 26 (0.6) | 1.0 | — |
| Dexamethasone | |||
| Yes | 44 (0.9) | 1.13 (0.72–1.78) | 0.58 |
| No | 51 (1.4) | 1.0 | — |
| Insurance | |||
| Private/other | 51 (1.0) | 1.0 | — |
| Public | 44 (1.4) | 1.41 (0.89–2.25) | 0.15 |
Race was dichotomized into white or non-white. Insurance was dichotomized to public and private/other. Age, sex, race, Down syndrome, anthracycline and dexamethasone exposure were included in the model based on a priori clinical hypotheses. Additional variables were included only if p <0.2 in univariate Cox models.
Clinical scenarios in which patients required ICU-level of care often progressed to death. Specifically, 20% of children with respiratory failure requiring mechanical ventilation, 5% of patients with cardiovascular failure resulting in vasopressor administration and nearly 50% of patients with cardiorespiratory failure requiring both mechanical ventilation and vasopressor support died. Each of these scenarios resulted in a significantly higher hazard for death in a multivariate model (Table III).
Table III.
Adjusted mortality hazard by organ failure requiring ICU-level resource utilization
| Resource used | Raw mortality, N (%) | Adjusted HR (95% CI) | P |
|---|---|---|---|
| Cardiorespiratory failure requiring mechanical ventilation or vasopressors | |||
| Both | 44 (47.8) | 122.30 (66.56–224.80) | <0.001 |
| Pressors only | 12 (4.8) | 17.47 (8.38–36.41) | <0.001 |
| Ventilation only | 22 (20.4) | 74.12 (37.80–145.40) | <0.001 |
| Neither | 17 (0.2) | 1.0 | — |
| Respiratory failure requiring ECMO | |||
| Yes | 2 (50.0) | 5.66 (1.68–19.11) | 0.005 |
| No | 93 (1.1) | 1.0 | — |
| Renal failure requiring hemodialysis | |||
| Yes | 21 (20.0) | 2.59 (1.81–3.71) | <0.001 |
| No | 74 (0.9) | 1.0 | — |
Raw mortality indicates the raw number and the percent of patients receiving each intervention who died. Hazard ratio adjusted for patient age, sex, race, Down syndrome, anthracycline exposure, dexamethasone exposure, and insurance type.
Hospital-level factors and induction mortality
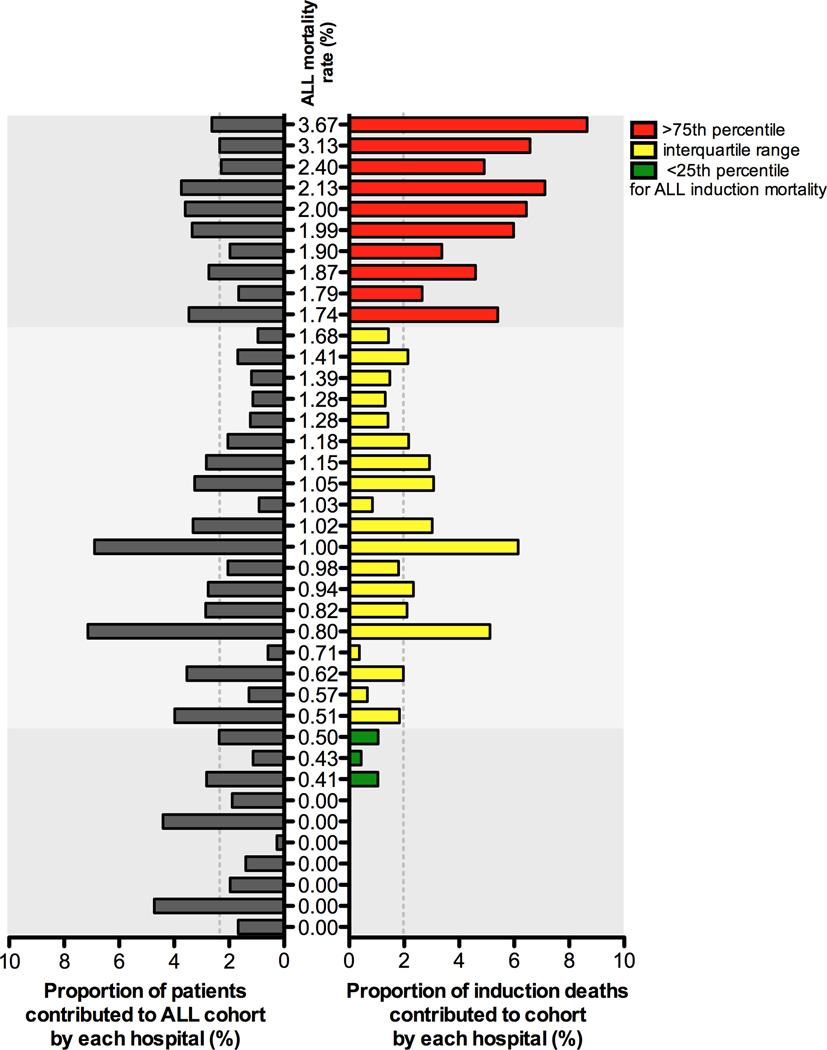
The median annual induction mortality rate across all hospitals was 1.02% (range: 0–3.74%; interquartile range: 0.50–1.74%). Hospitals in the highest induction mortality quartile contributed 27% of patients (n=2267) to the cohort but accounted for nearly half the mortality (47 of 95 deaths; Figure 2). Conversely, hospitals in the lowest induction mortality quartile comprised 21% of the cohort (1,793 patients) but contributed only 3 deaths.
Figure 2.
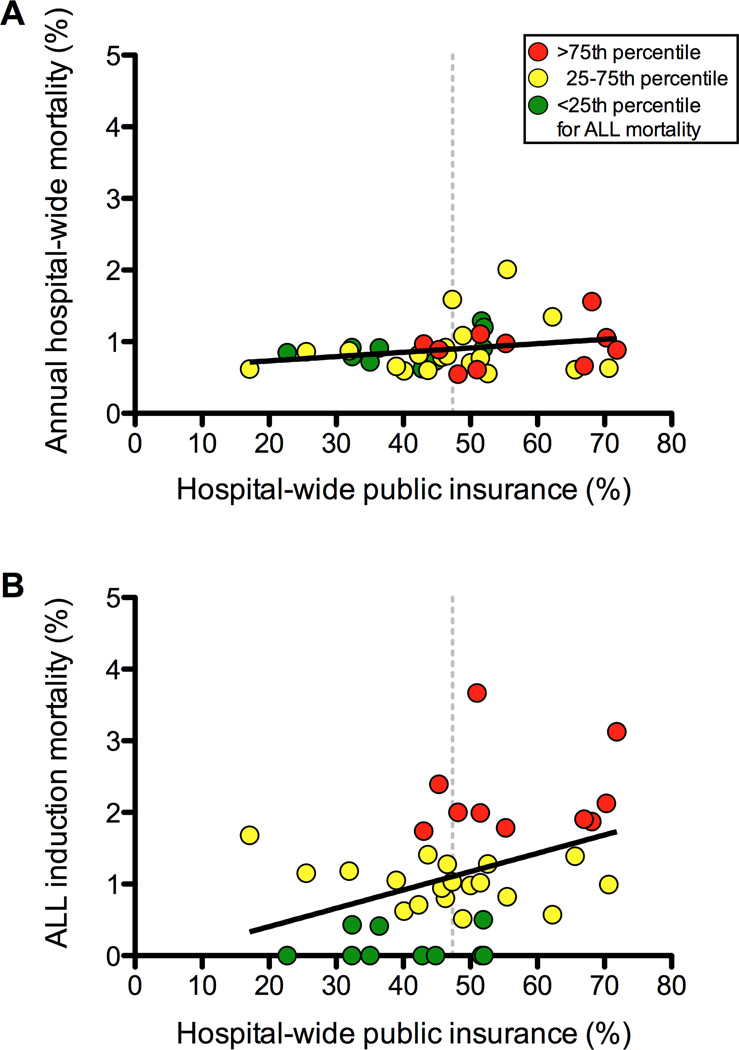
In unadjusted analyses, higher hospital-level ALL induction mortality rates correlated with higher hospital percentages of ALL patients with Down syndrome (ρ = 0.36; p=0.03), ALL patients requiring mechanical ventilation (ρ = 0.42; p <0.001), and ALL patients on ECMO (ρ = 0.39; p =0.01). A hospital’s ALL mortality rate also correlated with the proportion of publicly-insured ALL patients at that hospital (ρ = 0.36; p=0.03). Except for percentage of patients requiring mechanical ventilation, each of these hospital-level factors remained positively correlated with increasing mortality rates in a multiple linear regression (Supplemental Table VI). Hospital induction mortality rates were not associated with total number of ALL patients per year.
The median hospital public insurance composition was 47.33% (range: 17.10 – 71.84%). In regression analyses of hospital-level covariates, only hospital-wide proportion of publicly insured patients was significantly associated with ALL mortality (p=0.046; Figure 3). Linear regression analysis of these covariates on annual hospital-wide mortality showed no significant associations.
Figure 3.
DISCUSSION
These analyses of induction mortality in children receiving chemotherapy for newly diagnosed ALL in freestanding pediatric hospitals support several clinically relevant conclusions. First, induction mortality is approximately 1% and varies up to three-fold among treating centers. Second, induction mortality peaks in the third week of induction, potentially offering a window for medical intervention. Third, this analysis shows that induction mortality is highest among children requiring mechanical ventilation and vasoactive infusions and uses daily billing data to estimate specific mortality risks for children with organ failures needing ICU-level support. Fourth, hospitals with higher ALL induction mortality had higher proportions of hospital-wide inpatient admissions with public insurance. This association provides evidence that a hospital’s payer mix may account for some of the variability in ALL outcomes noted between hospitals with the highest and lowest mortality rates. Finally, these data suggest induction mortality rates of less than 1% could serve as a national benchmark.
Although a pediatric ALL patient with organ failure requiring ICU-level resources is intuitively at higher risk for mortality, specific probabilities of surviving induction therapy for children requiring ICU-level interventions are not available in the literature. The significant increase in mortality probability for patients requiring both mechanical ventilation and vasopressor support is relevant to ICU and oncology clinicians caring for patients receiving such interventions. Further work is ongoing to refine these estimates and to develop models incorporating duration of resource utilization and to predict time to mortality. Once validated, such models may provide quantitative guidance for clinicians counseling families whose children are critically ill during ALL induction.
These data show no correlation between a hospital’s overall annual mortality rate and its proportion of publicly insured patients. However, the association between a hospital’s ALL induction mortality and payer mix suggests that the effect of payer mix may become evident among patient populations at high risk of mortality. Medicaid-heavy hospitals have been shown to have worse outcomes for adult colon cancer patients and children with non-oncologic diagnoses, although this has not previously been described in pediatric cancer diagnoses.23,24 The etiology of the association between ALL induction mortality and payer mix is currently unknown. One hypothesis is that hospitals with a high proportion of admissions reimbursed by Medicaid may be under-resourced relative to hospitals with more favorable payer mixes. A relative lack of resources may manifest in a number of ways, such as higher patient-to-nurse ratios, which have been associated with increased mortality and failure to rescue in adult surgical patients and poor weight gain in preterm neonates.25–27 Conversely, legislation of increased staffing in California has led to improved outcomes in pediatric heart surgeries.28 Additional hospital-level factors potentially related to resource availability or efficiency of use of such resources, such as use of early warning systems29 or checklists,30 presence of rapid response teams,31,32 nursing Magnet® status,33 specific expertise in oncology, and hospital financial performance,34 may contribute to variability in mortality.
The observed differences for outcomes in high-risk illnesses are likely multifactorial, perhaps including a stressed hospital system and unknown pre-diagnosis SES-related health factors. Indeed, hospitals may face a double effect: those serving low-SES populations have a greater chance of caring for patients at higher risk due to pre-diagnosis factors, while simultaneously facing lower reimbursements for that care. Conversely, analysis of care patterns at the institutions with high Medicaid penetration and low induction mortality may be very informative. Work is ongoing to determine the role potential resource-related factors play in predicting ALL induction mortality in this cohort.
These data obtained from PHIS hospitals include approximately 35% of new ALL cases diagnosed in the US, assuming a national annual incidence of 2,400 cases.35 This cohort has gender, age and race distributions similar to published SEER data,22 and a Down syndrome prevalence consistent with previous ALL trials.36,37 The 1.12% overall induction mortality rate is consistent with prior studies.2,3,5–7,38–41 as is the increased mortality risk in infants and children older than 10 years of age.2,7 Although increased induction mortality in children with Down syndrome has been reported, this association was not observed in the present study, perhaps due to therapy modifications and improved supportive care practices resulting from the reports that largely predate the study cohort.
Despite these strengths, PHIS data have limitations, many of which have been previously described.17 PHIS only captures inpatient data, while most ALL therapy is delivered on an outpatient basis; however, ALL diagnosis, initiation of induction therapy, and induction mortality typically occur in the inpatient setting. Since cohort inclusion requires evaluation of billed chemotherapy, children who died before receiving chemotherapy were not captured. These patients represent 0.2–0.3% of new-onset ALL cases,6,8 and by excluding them, these results may reflect preventable treatment-related mortality, rather than rare fulminant presentations of ALL. Finally, the primary outcome was all-cause inpatient mortality and did not identify specific etiologies for induction death. A subset of PHIS hospitals will contribute laboratory test results in the near future;42 such daily laboratory data will enable a more nuanced analysis of factors associated with mortality, particularly when combined with data from cooperative group ALL trials.43
We have identified an association between ALL induction mortality and hospital payer mix. Such analyses have particular importance if payer mix and related availability of hospital-level resources are associated with mortality in other high-risk pediatric conditions, such in children with as cystic fibrosis or in the neonatal ICU, since further Medicaid cuts may lead to an increase in potentially avoidable pediatric deaths.24 Additional investigations are needed to identify modifiable factors that may further improve pediatric outcomes. Finally, we hope these data will stimulate discussion about defining a national benchmark for ALL induction mortality. Since one quarter of hospitals achieved an ALL induction mortality rate of less than 0.5%, a benchmark of 1% induction mortality may be an appropriate target for all institutions. Further studies are needed both to validate this goal and to identify pathways to achieve it.
Supplementary Material
ACKNOWLEDGMENTS
Funding sources: National Cancer Institute 1 R01 CA133881-01 (Aplenc); Canuso Foundation Innovation Award (Seif); Alex’s Lemonade Stand Foundation/Center for Childhood Cancer Research Seed Grant (Seif); and American Cancer Society Mentored Research Scholar Award in Applied and Clinical Research, MRSG-12-215-01-LIB (Seif).
Footnotes
DISCLOSURES
Conflict of Interest Statement: Dr. Seif received an honorarium and travel expenses from OptumHealth for giving a continuing education lecture on stem cell transplantation for pediatric solid tumors. Dr. Fisher receives research funding from Pfizer. Dr. Walker is employed at Bristol-Myers Squibb. The remaining authors have no financial disclosures. The authors have no conflicts of interest to declare.
REFERENCES
- 1.Smith M, Ries L, Gurney J, Ross J. Leukemia. In: Ries L, Smith M, Gurney J, et al., editors. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. Bethesda, MD: National Cancer Institute, SEER Program; 1999. pp. 17–34. NIH Pub. No. 99-4649. [Google Scholar]
- 2.Gaynon PS, Angiolillo AL, Carroll WL, et al. Long-term results of the children's cancer group studies for childhood acute lymphoblastic leukemia 1983–2002: a Children's Oncology Group Report. Leukemia. 2010 Feb;24(2):285–297. doi: 10.1038/leu.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prucker C, Attarbaschi A, Peters C, et al. Induction death and treatment-related mortality in first remission of children with acute lymphoblastic leukemia: a population-based analysis of the Austrian Berlin-Frankfurt-Munster study group. Leukemia. 2009 Jul;23(7):1264–1269. doi: 10.1038/leu.2009.12. [DOI] [PubMed] [Google Scholar]
- 4.Schmiegelow K, Forestier E, Hellebostad M, et al. Long-term results of NOPHO ALL-92 and ALL-2000 studies of childhood acute lymphoblastic leukemia. Leukemia. 2010 Feb;24(2):345–354. doi: 10.1038/leu.2009.251. [DOI] [PubMed] [Google Scholar]
- 5.Silverman LB, Stevenson KE, O'Brien JE, et al. Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985–2000) Leukemia. 2010 Feb;24(2):320–334. doi: 10.1038/leu.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slats AM, Egeler RM, van der Does-van den Berg A, et al. Causes of death--other than progressive leukemia--in childhood acute lymphoblastic (ALL) and myeloid leukemia (AML): the Dutch Childhood Oncology Group experience. Leukemia. 2005 Apr;19(4):537–544. doi: 10.1038/sj.leu.2403665. [DOI] [PubMed] [Google Scholar]
- 7.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children's oncology group. J Clin Oncol. 2012 May 10;30(14):1663–1669. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lund B, Asberg A, Heyman M, et al. Risk factors for treatment related mortality in childhood acute lymphoblastic leukaemia. Pediatr Blood Cancer. 2011 Apr;56(4):551–559. doi: 10.1002/pbc.22719. [DOI] [PubMed] [Google Scholar]
- 9.Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children's hospitals. JAMA. 2011 Feb 16;305(7):682–690. doi: 10.1001/jama.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008 May 7;299(17):2048–2055. doi: 10.1001/jama.299.17.2048. [DOI] [PubMed] [Google Scholar]
- 11.Morse RB, Hall M, Fieldston ES, et al. Hospital-level compliance with asthma care quality measures at children's hospitals and subsequent asthma-related outcomes. JAMA. 2011 Oct 5;306(13):1454–1460. doi: 10.1001/jama.2011.1385. [DOI] [PubMed] [Google Scholar]
- 12.Ponsky TA, Huang ZJ, Kittle K, et al. Hospital- and patient-level characteristics and the risk of appendiceal rupture and negative appendectomy in children. JAMA. 2004 Oct 27;292(16):1977–1982. doi: 10.1001/jama.292.16.1977. [DOI] [PubMed] [Google Scholar]
- 13.Weiss PF, Klink AJ, Localio R, et al. Corticosteroids may improve clinical outcomes during hospitalization for Henoch-Schonlein purpura. Pediatrics. 2010 Oct;126(4):674–681. doi: 10.1542/peds.2009-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fieldston ES, Hall M, Sills MR, et al. Children's hospitals do not acutely respond to high occupancy. Pediatrics. 2010 May;125(5):974–981. doi: 10.1542/peds.2009-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allareddy V, Ward MM, Konety BR. Effect of meeting Leapfrog volume thresholds on complication rates following complex surgical procedures. Ann Surg. 2010 Feb;251(2):377–383. doi: 10.1097/SLA.0b013e3181cb853f. [DOI] [PubMed] [Google Scholar]
- 16.Schilling PL, Davis MM, Albanese CT, Dutta S, Morton J. National trends in adolescent bariatric surgical procedures and implications for surgical centers of excellence. J Am Coll Surg. 2008 Jan;206(1):1–12. doi: 10.1016/j.jamcollsurg.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 17.Fisher BT, Harris T, Torp K, et al. Establishment of an 11-Year Cohort of 8733 Pediatric Patients Hospitalized at United States Free-standing Children's Hospitals With De Novo Acute Lymphoblastic Leukemia From Health Care Administrative Data. Med Care. 2012 Mar 8; doi: 10.1097/MLR.0b013e31824deff9. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher BT, Aplenc R, Localio R, Leckerman KH, Zaoutis TE. Cefepime and mortality in pediatric acute myelogenous leukemia: a retrospective cohort study. Pediatr Infect Dis J. 2009 Nov;28(11):971–975. doi: 10.1097/INF.0b013e3181a75939. [DOI] [PubMed] [Google Scholar]
- 19.Fisher BT, Zaoutis TE, Leckerman KH, Localio R, Aplenc R. Risk factors for renal failure in pediatric patients with acute myeloid leukemia: a retrospective cohort study. Pediatr Blood Cancer. 2010 Oct;55(4):655–661. doi: 10.1002/pbc.22601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kavcic M, Fisher BT, Li Y, et al. Induction mortality and resource utilization in children treated for acute myeloid leukemia at free-standing pediatric hospitals in the United States. Cancer. 2013 May 15;119(10):1916–1923. doi: 10.1002/cncr.27957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colvin JD, Zaniletti I, Fieldston ES, et al. Socioeconomic status and in-hospital pediatric mortality. Pediatrics. 2013 Jan;131(1):e182–e190. doi: 10.1542/peds.2012-1215. [DOI] [PubMed] [Google Scholar]
- 22.Linabery AM, Ross JA. Childhood and adolescent cancer survival in the US by race and ethnicity for the diagnostic period 1975–1999. Cancer. 2008 Nov 1;113(9):2575–2596. doi: 10.1002/cncr.23866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhoads KF, Ackerson LK, Jha AK, Dudley RA. Quality of colon cancer outcomes in hospitals with a high percentage of Medicaid patients. J Am Coll Surg. 2008 Aug;207(2):197–204. doi: 10.1016/j.jamcollsurg.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Smith RB, Dynan L, Fairbrother G, Chabi G, Simpson L. Medicaid, hospital financial stress, and the incidence of adverse medical events for children. Health Serv Res. 2012 Aug;47(4):1621–1641. doi: 10.1111/j.1475-6773.2012.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friese CR, Lake ET, Aiken LH, Silber JH, Sochalski J. Hospital nurse practice environments and outcomes for surgical oncology patients. Health Serv Res. 2008 Aug;43(4):1145–1163. doi: 10.1111/j.1475-6773.2007.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aiken LH, Clarke SP, Sloane DM, Sochalski J, Silber JH. Hospital nurse staffing and patient mortality, nurse burnout, and job dissatisfaction. JAMA. 2002 Oct 23–30;288(16):1987–1993. doi: 10.1001/jama.288.16.1987. [DOI] [PubMed] [Google Scholar]
- 27.Profit J, Petersen LA, McCormick MC, et al. Patient-to-nurse ratios and outcomes of moderately preterm infants. Pediatrics. 2010 Feb;125(2):320–326. doi: 10.1542/peds.2008-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hickey PA, Gauvreau K, Jenkins K, Fawcett J, Hayman L. Statewide and national impact of California's Staffing Law on pediatric cardiac surgery outcomes. J Nurs Adm. 2011 May;41(5):218–225. doi: 10.1097/NNA.0b013e3182171b2e. [DOI] [PubMed] [Google Scholar]
- 29.Demmel KM, Williams L, Flesch L. Implementation of the pediatric early warning scoring system on a pediatric hematology/oncology unit. J Pediatr Oncol Nurs. 2010 Jul-Aug;27(4):229–240. doi: 10.1177/1043454209358410. [DOI] [PubMed] [Google Scholar]
- 30.Schulman J, Stricof R, Stevens TP, et al. Statewide NICU central-line-associated bloodstream infection rates decline after bundles and checklists. Pediatrics. 2011 Mar;127(3):436–444. doi: 10.1542/peds.2010-2873. [DOI] [PubMed] [Google Scholar]
- 31.Kotsakis A, Lobos AT, Parshuram C, et al. Implementation of a multicenter rapid response system in pediatric academic hospitals is effective. Pediatrics. 2011 Jul;128(1):72–78. doi: 10.1542/peds.2010-0756. [DOI] [PubMed] [Google Scholar]
- 32.VandenBerg SD, Hutchison JS, Parshuram CS. A cross-sectional survey of levels of care and response mechanisms for evolving critical illness in hospitalized children. Pediatrics. 2007 Apr;119(4):e940–e946. doi: 10.1542/peds.2006-0852. [DOI] [PubMed] [Google Scholar]
- 33.Hickey P, Gauvreau K, Connor J, Sporing E, Jenkins K. The relationship of nurse staffing, skill mix, and Magnet recognition to institutional volume and mortality for congenital heart surgery. J Nurs Adm. 2010 May;40(5):226–232. doi: 10.1097/NNA.0b013e3181da3f71. [DOI] [PubMed] [Google Scholar]
- 34.Bazzoli GJ, Chen HF, Zhao M, Lindrooth RC. Hospital financial condition and the quality of patient care. Health Econ. 2008 Aug;17(8):977–995. doi: 10.1002/hec.1311. [DOI] [PubMed] [Google Scholar]
- 35.Children's Hospital Association. Hospital Directory. [Accessed 2/25/2013];2013 http://www.childrenshospitals.net/AM/Template.cfm?Section=Hospital_Directory1&Template=/CustomSource/HospitalProfiles/HospitalProfileResultNew.cfm&ShowAll=1. [Google Scholar]
- 36.Bassal M, La MK, Whitlock JA, et al. Lymphoblast biology and outcome among children with Down syndrome and ALL treated on CCG-1952. Pediatr Blood Cancer. 2005 Jan;44(1):21–28. doi: 10.1002/pbc.20193. [DOI] [PubMed] [Google Scholar]
- 37.Whitlock JA, Sather HN, Gaynon P, et al. Clinical characteristics and outcome of children with Down syndrome and acute lymphoblastic leukemia: a Children's Cancer Group study. Blood. 2005 Dec 15;106(13):4043–4049. doi: 10.1182/blood-2003-10-3446. [DOI] [PubMed] [Google Scholar]
- 38.Arico M, Ziino O, Valsecchi MG, et al. Acute lymphoblastic leukemia and Down syndrome: presenting features and treatment outcome in the experience of the Italian Association of Pediatric Hematology and Oncology (AIEOP) Cancer. 2008 Aug 1;113(3):515–521. doi: 10.1002/cncr.23587. [DOI] [PubMed] [Google Scholar]
- 39.Christensen MS, Heyman M, Mottonen M, Zeller B, Jonmundsson G, Hasle H. Treatment-related death in childhood acute lymphoblastic leukaemia in the Nordic countries: 1992–2001. Br J Haematol. 2005 Oct;131(1):50–58. doi: 10.1111/j.1365-2141.2005.05736.x. [DOI] [PubMed] [Google Scholar]
- 40.Rubnitz JE, Lensing S, Zhou Y, et al. Death during induction therapy and first remission of acute leukemia in childhood: the St. Jude experience. Cancer. 2004 Oct 1;101(7):1677–1684. doi: 10.1002/cncr.20532. [DOI] [PubMed] [Google Scholar]
- 41.van der Linden MH, Valsecchi MG, De Lorenzo P, et al. Outcome of congenital acute lymphoblastic leukemia treated on the Interfant-99 protocol. Blood. 2009 Oct 29;114(18):3764–3768. doi: 10.1182/blood-2009-02-204214. [DOI] [PubMed] [Google Scholar]
- 42.Narus SP, Srivastava R, Gouripeddi R, et al. Federating clinical data from six pediatric hospitals: process and initial results from the PHIS+ Consortium. AMIA Annu Symp Proc. 2011;2011:994–1003. [PMC free article] [PubMed] [Google Scholar]
- 43.Aplenc R, Fisher BT, Huang YS, et al. Merging of the National Cancer Institute-funded cooperative oncology group data with an administrative data source to develop a more effective platform for clinical trial analysis and comparative effectiveness research: a report from the Children's Oncology Group. Pharmacoepidemiol Drug Saf. 2012 May;21(Suppl 2):37–43. doi: 10.1002/pds.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.