Abstract
Background
The objective of this study is to investigate the association between childhood trauma and lipid profiles in adults from a highly traumatized population at-risk for cardiovascular disease.
Method
We recruited 452 participants, primarily African American, low socioeconomic status from general medical clinics in a large urban hospital. We performed direct comparisons, univariate ANOVA and regression analyses together and separated by sex, examining the associations of child abuse, BMI, lipid lowering drug use, blood pressure, age, and substance use to HDL levels and HDL/LDL ratios.
Results
A history of moderate to severe levels of childhood trauma and abuse was associated with a significant decrease in HDL levels (p≤0.01) and HDL/LDL ratios (p≤0.001) relative to males with low levels of abuse. This relationship held when the status of lipid-lowering drugs was considered. When controlling for age, substance abuse, tobacco use, and adult trauma, the effects of childhood trauma remained significant. We found a significant child abuse by sex interaction on HDL/LDL ratios (F(1, 369)=13.0, p≤0.0005) consistent with a differential effect of trauma on dyslipidemia in male but not female subjects
Conclusions
Our data suggest that childhood trauma exposure, obtained with self-report measures, may contribute to increased risk of cardiovascular disease by way of stress-mediated alterations of lipid concentration and composition in male, but not female, subjects.
Keywords: Trauma, African-American, Genetic, Cardiovascular, Lipids
Introduction
Exposure to chronic stress and/or traumatic experience during development has been shown to result in dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis during adult life in both animal (1) and human (2) models. The consequences of developmental stress exposure appear to be, in part, through altered sensitivity of the neuroendocrine response to environmental demands resulting in dysregulation of cortisol release. Evidence of HPA axis disturbances has been demonstrated via altered cortisol secretion among maltreated and neglected children (3) and maltreated children with symptoms of post-traumatic disorder have also been shown to have elevated cortisol levels throughout the day (4). Dysregulated HPA function is also associated with many components of metabolic and cardiovascular disease.
The experience of abusive or traumatic experiences during childhood is also associated with increased rates of obesity, ischemic heart disease, and type 2 diabetes mellitus during adulthood (5–10). Although the high prevalence of various forms of cardiometabolic disease in the African American population is well established, the reasons for this important health disparity are unclear (11–15). Some investigators have found that socioeconomic status (SES) plays an important role in health disparity (16).
Previous studies have found that many urban, low-SES minority populations are exposed to extremely high rates of trauma during the course of their lifetime, putting them at an increased risk for post-traumatic stress disorder (PTSD) (18–21). Furthermore, exposure to childhood trauma is one of the largest risk factors for the later development of PTSD and other stress-related disorders (20, 22–31, 34, 51). Exposure to trauma and diagnosis of PTSD are associated with greater rates of physical morbidity and mortality, as well as increased health care utilization and expenses (22–25). In addition, trauma exposure and/or a diagnosis of PTSD are associated with a variety of metabolic abnormalities (26–31) which may contribute to the adverse health outcomes repeatedly observed in low-SES minority populations. Consistent with these findings, elevated rates of diabetes and cardiovascular disease have been reported in civilians and combat veterans with PTSD (32, 33).
One possible mechanism whereby trauma exposure and/or the presence of stress-related psychiatric disorders that are observed in low-SES, urban African American populations (18, 20) may contribute to cardiometabolic disease is through pathological alteration of lipid metabolism. Catecholamines, such as epinephrine and other counter-regulatory hormones released during acute stress, function to increase circulating levels of glucose and fatty acids to provide fuel for an adaptive response to threat. However, frequent stimulation of the stress response as a result of ongoing exposure to environmental stress may result in persistent metabolic dysregulation in the form of hyperglycemia and dyslipidemia.
Because exposure to childhood abuse has been associated with an exaggerated biological response to stress as well as increased risk for cardiometabolic disease, we hypothesized that exposure to high rates of child abuse would be associated with lipid profiles predictive of cardiometabolic risk.
Methods
2.1. Recruitment and general study procedures
The data for this study was collected to investigate the effects of childhood trauma on circulating HDL levels and HDL/LDL ratios. The data were drawn from a larger sample of participants enrolled in a study that examines the risk factors for PTSD in an urban, low-income, predominantly AfricanAmerican population (20, 34). A total of 452 were examined for whom we have childhood trauma and other medical data, although fewer subjects were examined for some analyses when not all data variables were available, as noted in results. Individual subjects were recruited from waiting rooms in multiple primary care clinics at Grady Memorial Hospital, a publicly funded hospital primarily serving individuals of low SES in Atlanta, GA. Prospective subjects were invited to complete a screening interview, and a subset of subjects returned for additional study visits to participate in more in-depth psychiatric interviews and physiological assessments. Demographic data were ascertained through subject self-report. As part of the multiday study, subjects underwent psychological assessments, a physical examination, a fasting morning blood draw and anthropometric measurements. To be eligible for participation, subjects had to be at least 18 years or older, provide informed consent and be able to speak English. Subjects were excluded if they had active/unstable psychotic symptoms or prominent suicidal ideation at the time of the study, had dementia, were pregnant, or took oral corticosteroids within the past month. All other subjects from whom we collected metabolic data were included from the larger study. A detailed account of the general Grady Trauma Project study methods has been previously published and can be found in Gillespie et al. (20). The final sample size included 452 subjects with self-report data and HDL/LDL measures, and a lesser number of subjects for some specialized analyses (including medication history and specific substance abuse measures).
2.2. Measures of Demographic and Health Characteristics, Substance Abuse, Trauma Exposure and Psychiatric Symptoms and Diagnoses
2.2.1. Demographics Form
The Demographics Form is locally developed and assesses subject age, self-identified race, marital status, education, income, employment, and disability status.
2.2.2. Kreek-McHugh-Schluger-Kellogg Scale
The Kreek-McHugh-Schluger-Kellogg scale (KMSK) is designed to quantify self-exposure to opiates, cocaine, alcohol, and/or tobacco. Each section of the KMSK scale assesses the frequency, amount, and duration of use of a particular substance during the individual’s period of greatest consumption. The scale also assesses the mode of use, whether the substance use is current or past, and whether each substance is the substance of choice (40). The KMSK provided a continuous measure for lifetime history of substance abuse. This measure also complemented our self-report inventory of presence or absence current and past substance abuse.
2.2.3. Traumatic Events Inventory
The Traumatic Events Inventory (TEI) (20,34) is a 14- item instrument for assessing exposure to traumatic events in childhood and adulthood. For each traumatic life event, the TEI assesses experiencing and witnessing of events separately. In addition to assessing the presence and absence of each type of trauma exposure, the TEI also assesses frequency of exposure within each type of trauma. The TEI, which was developed for use with our specific study population, is similar in number of questions and format to other self-report assessments of trauma exposure (see Cusack et al. (35) and Norris et al. (36) for a review of self-report instruments of civilian trauma exposure).
2.2.4. Childhood Trauma Questionnaire
The CTQ (47–49) is a 28-item, self-report inventory assessing three types of child abuse: sexual, physical, and emotional. Cutoff scores for each category have shown excellent sensitivity and specificity in correctly classifying cases of abuse (47,50). The CTQ yields a total score and subscale scores for each of the 3 types of child abuse. Bernstein and Fink (48) established scores for mild, moderate and severe for each type of abuse. To summarize the level of exposure to child abuse trauma, we summed the total number of different types of child abuse trauma to create a 3-level categorical variable (0, 1, ≥2) reflecting number of types of child abuse trauma because in our prior work (20, 24, 51) it relates in a predictable and consistent manner with a number of measures of adaptive functioning and trauma-related functioning.
2.2.5. Clinician Administered PTSD Scale
PTSD symptoms and PTSD diagnosis were assessed using the Clinician Administered PTSD Scale (CAPS) (37,38). The CAPS is a structured interview with excellent psychometric properties (39) that provides a diagnosis of PTSD and can also be used as a continuous measure of PTSD symptomatology. A current (last 30 days) or lifetime diagnosis of PTSD was based on the presence/absence of each of the Diagnostic and Statistical Manual of MentalDisorders, Fourth Edition (DSM-IV) diagnostic criteria using a frequency rating of 1 or higher paired with an intensity score of 2 or higher as the scoring rule.
2.2.6. Modified PTSD Symptom Scale
The Modified PTSD Symptom Scale (MPSS) 18-item psychometrically valid self-report scale assessing PTSD symptoms over two weeks prior to the rating, according to DSM-IV criteria (41, 42). Within our cohort it was cross-validated with the CAPS for a subset of subjects.
2.2.7. Beck Depression Inventory
The Beck Depression inventory is a widely used self-report measure of depressive symptoms in clinical and non-clinical populations (43, 44). It is a 21- item psychometrically valid self-report scale which aims to assess depression symptom severity during the past month.
2.3. Anthropometric, Physiological, and Laboratory Measures and Prescription Medication History
A study physician determined each subject’s eligibility based on a medical examination at the beginning of the study visit or based on review of records from a medical exam by a Grady Hospital physician completed within 2 months of study participation. Blood pressure, weight (kg), and height (cm) were obtained by trained nursing staff at the Grady Hospital Clinical Research Center. Fasting blood samples were obtained by venipuncture between 0800 and 0900 h during the baseline study assessment for each subject. Serum samples were obtained, processed, and then stored at −80°C for later processing in batches. Triglyceride (TG) and glucose were measured by enzymatic methods using reagent kits from Beckman Coulter Diagnostics (Fullerton, CA, USA). High-density lipoprotein (HDL) was ascertained by homogenous enzymatic methods using kits from Equal Diagnostics (Exton, PA, USA). Prescription medications were recorded for use in data analyses.
2.4. Statistical Methods
Subjects were stratified into low and high levels of childhood trauma exposure using data from the CTQ. To summarize the level of exposure to child abuse trauma, we summed the total number of different types of child abuse trauma to create a 3-level categorical variable (0, 1, ≥2) reflecting number of types of child abuse trauma as in our prior work, (20, 34, 51) where it relates in a predictable and consistent manner with a number of measures of adaptive functioning and trauma-related functioning. For our analyses, subjects with 0 or 1 type of child abuse trauma exposure were classified as having “low” childhood trauma exposure whereas subjects with ≥2 types of child abuse trauma exposure were classified as having “high” child abuse trauma exposure, based on these and other prior studies.
We performed all analyses using SPSS (v. 20). One-way analysis of variance (ANOVA)was performed to assess differences in continuous demographic (age), post-traumatic stress symptom (PSS total score and PSS subscale scores), depressive symptom (BDI total score), body mass index (BMI), and systolic and diastolic blood pressure variables between study samples. For categorical variables (sex, monthly income, education, lifetime smoking history, receipt of prescription for lipid-lowering medication), we used chi-squared tests to determine statistical differences between samples with low or high childhood trauma exposure. We examined the effect of childhood trauma exposure level with respect to these variables using chi square tests, t-tests, and ANOVA. The relationships between child abuse exposure as a continuous variable (CTQ total score and subscale scores) and continuous cholesterol (serum HDL and HDL/LDL ratio) variables were examined using bivariate correlation.
Linear regression was used to examine the effects of potential confounders in study analyses. Several regressions were performed, and tables 2–3 list two sets of potential confounders that were based on two different potential hypotheses: 1) that the phenomenon was due to differential levels of lipid-lowering medications as a function of childhood trauma (for example, those with a history of abuse may be less likely to seek medical care and thus less likely to receive lipid lowering medications); and 2) Other demographic and trauma-related risk factors that are known to be associated with cardiometabolic disease (age, race, alcohol use, tobacco and adult trauma exposure) may be accounting for the primary effect of childhood trauma exposure (which may also be associated with these separate risk variables).
Table 2.
The effect of the Childhood Trauma on HDL/LDL Ratio when controlling for prescribed lipid lowering agents
| Sex | Model | Beta | t | P-value | |
|---|---|---|---|---|---|
| Male | 1 | Statins (% Prescribed) | .114 | 1.092 | .278 |
| NonStatin Lipid Medication (% Prescribed) | −.094 | −.905 | .368 | ||
| 2 | Statins (% Prescribed) | .083 | .805 | .423 | |
| NonStatin Lipid Medication (% Prescribed) | −.091 | −.889 | .376 | ||
| Childhood Trauma (CTQ total score) | −.234 | −2.345 | .021 | ||
|
| |||||
| Female | 1 | Statins (% Prescribed) | .027 | .350 | .727 |
| NonStatin Lipid Medication (% Prescribed) | −.069 | −.905 | .367 | ||
| 2 | Statins (% Prescribed) | .027 | .356 | .722 | |
| NonStatin Lipid Medication (% Prescribed) | −.069 | −.899 | .370 | ||
| Childhood Trauma (CTQ total score) | .007 | .097 | .923 | ||
Table 3.
The effect of the Childhood Trauma on HDL/LDL Ratio when controlling for additional variables previously associated with cardiovascular risk
| Sex | Model | Predictor Variables | Beta | t | P-Value |
|---|---|---|---|---|---|
| Male | 1 | Age | .132 | 1.626 | .106 |
| Race | −.167 | −2.061 | .041 | ||
|
| |||||
| 2 | Age | .137 | 1.679 | .095 | |
| Race | −.163 | −1.996 | .048 | ||
| Alcohol Lifetime | .103 | 1.154 | .250 | ||
| Tobacco Lifetime | −.084 | −.934 | .352 | ||
|
| |||||
| 3 | Age | .151 | 1.861 | .065 | |
| Race | −.133 | −1.612 | .109 | ||
| Alcohol Lifetime | .126 | 1.407 | .162 | ||
| Tobacco Lifetime | −.055 | −.603 | .547 | ||
| Total Adult Trauma Exposure (TEI) | −.167 | −1.940 | .054 | ||
|
| |||||
| 4 | Age | .148 | 1.846 | .067 | |
| Race | −.131 | −1.605 | .111 | ||
| Alcohol Lifetime | .135 | 1.523 | .130 | ||
| Tobacco Lifetime | −.051 | −.567 | .572 | ||
| Total Adult Trauma Exposure (TEI) | −.106 | −1.175 | .242 | ||
| Childhood Trauma (CTQ Total Score) | −.179 | −2.089 | .038 | ||
|
| |||||
| Female | 1 | Age | .010 | .146 | .884 |
| Race | .021 | .314 | .754 | ||
|
| |||||
| 2 | Age | −.001 | −.013 | .990 | |
| Race | .001 | .019 | .985 | ||
| Alcohol Lifetime | .193 | 2.496 | .013 | ||
| Tobacco Lifetime | −.142 | −1.829 | .069 | ||
|
| |||||
| 3 | Age | −.002 | −.030 | .976 | |
| Race | .006 | .084 | .933 | ||
| Alcohol Lifetime | .195 | 2.505 | .013 | ||
| Tobacco Lifetime | −.138 | −1.730 | .085 | ||
| Total Adult Trauma Exposure (TEI) | −.019 | −.268 | .789 | ||
|
| |||||
| 4 | Age | .000 | −.007 | .994 | |
| Race | .007 | .095 | .924 | ||
| Alcohol Lifetime | .196 | 2.508 | .013 | ||
| Tobacco Lifetime | −.146 | −1.794 | .074 | ||
| Total Adult Trauma Exposure (TEI) | −.029 | −.391 | .696 | ||
| Childhood Trauma (CTQ Total Score) | .037 | .510 | .611 | ||
Results
3.1. Demographic Characteristics, Past Substance Abuse, and Psychiatric Symptom Scores of Subjects with Low and High Levels of Childhood Abuse
In our initial analysis, we used data from the CTQ to dichotomize our sample of (N=452) male and female subjects into two groups composed of subjects with either low (N=238) or high (N=214) levels of childhood abuse. Table 1 outlines the demographic characteristics and levels of depressive and post-traumatic stress symptoms in these subjects. There was a significantly (p<0.001) greater percentage of female subjects in the high childhood abuse category (68.7%) than in the low abuse category (52.5%). We did not observe significant between-group differences with respect to subject age, race, level of education, and monthly household income. In addition, a significantly (p<0.026) greater percentage of subjects with a history of high child abuse exposure (41.6%) self-reported a past history of drug abuse than subjects with a history of low child abuse exposure (31.5%). Finally, subjects with high levels of child abuse exposure had significantly higher current depressive (F(1,452)=37.53, p<0.001) and post-traumatic stress (F(1,452)=59.96, p<0.001) symptom scores compared to subjects with low levels of child abuse exposure replicating (34, 51) and others (7, 18–19) previous findings.
Table 1.
Demographic Characteristics, Past Substance Abuse, Psychiatric Symptoms, and Cardiovascular Risk in all Subjects, divided by Low vs High Levels of Child Abuse
| Demographic Variable (units) | Low Child Abuse Mean ± sem, N |
High Child Abuse Mean ± sem, N |
F value | P value |
|---|---|---|---|---|
| Sex (% Female) | 52.5 ± 3.2, 238 | 68.7 ± 3.2, 214 | 12.58 | <0.001 |
| Age (years) | 43.3 ± .85, 238 | 41.2 ± .82, 214 | 3.06 | 0.081 |
| Race (% African Am.) | 92%, 238 | 90.2%, 214 | 0.87 | 0.35 |
| Education (Highest Grade) (0–6 scale)* | 1.57 ± .09, 238 | 1.66 ± .10, 214 | 0.46 | 0.50 |
| Income (monthly) (0–4 scale)** | 1.50 ± .08, 232 | 1.60 ± .09, 209 | 0.64 | 0.43 |
| Substance Abuse in past (%) | 31.5 ± 3.0, 238 | 41.6 ± 3.4, 214 | 4.98 | 0.026 |
| Depression symptoms (BDI score) | 12.5 ± .65, 238 | 19.4 ± .93, 214 | 37.5 | <0.001 |
| PTSD symptoms (mPSS score) | 10.8 ± .70, 238 | 19.2 ± .85, 214 | 60.0 | <0.001 |
| Cigarette Smoker (% lifetime) | 0.49 ± .05, 87 | 0.52 ± .06, 85 | 0.09 | 0.76 |
| Weight (kg) | 91.2 ± 1.7, 226 | 94.2 ± 2.0, 204 | 1.38 | 0.24 |
| Body Mass Index (BMI) | 31.9 ± .58, 220 | 33.8 ± .69, 199 | 4.71 | 0.03 |
| Statins (% prescribed) | 21.0 ± 3.0, 146 | 16.0 ± 3.0, 137 | 1.24 | 0.27 |
| Nonstatin Lipid Medication (% prescribed) | 3.0 ± 1.0, 146 | 1.0 ± 1.0, 136 | 1.10 | 0.29 |
| Blood Pressure (systolic mmHG) | 127.6 ± 1.4, 228 | 127.5 ± 1.4, 204 | .003 | 0.96 |
| Blood Pressure (diasystolic mmHG) | 75.9 ± .80, 228 | 76.1 ± .86, 204 | .025 | 0.87 |
Highest grade completed variable is coded 0–6 based on completion of some grade school to advanced degrees.
Household monthly income is divided into categories of monthly income of: 0=<$250; 1=<$500; 2=<$1000; 3=<$2000; 4=>$2000.
3.2. Cardiovascular Risk and Prescription Medication Characteristics of Subjects with Low and High Levels of Childhood Abuse
We next examined between-group differences in subjects with low and high levels of childhood abuse exposure with respect to the prevalence and extent of cardiovascular disease risk factors and the prevalence of receipt of a prescription for a medication used to treat dyslipidemia. With the possible exception of BMI (F(1,419)=4.71, p=0.03), we did not identify significant differences (Table 1) between the low and high childhood abuse groups on measures of cardiovascular risk factors (BMI, systolic/diastolic blood pressure, lifetime history of smoking) and receipt of a prescription for medications used to treat dyslipidemia.
3.3. Effects of Sex and Childhood Abuse Severity on Cholesterol Levels
We next wished to examine continuous measures of child abuse, in contrast to the categorical definitions used above. In male, but not female subjects, continuous measures of childhood abuse were negatively correlated with HDL/LDL ratio (N=180, Pearson r= −0.183, p=0.01). This effect was observed in all subtypes of child abuse, but the continuous measure of physical abuse was the most strongly inversely correlated with HDL/LDL ratio (N=180, r=−0.217, p=0.003). There were no significant correlations between child abuse and HDL/LDL ratio in the female cohort.
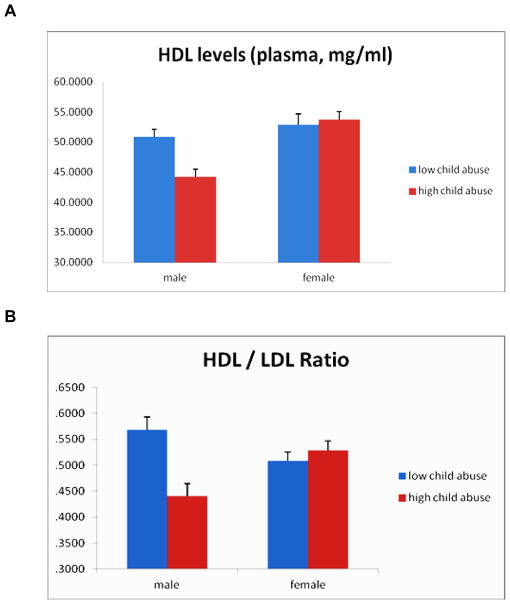
A comparative group analysis was next performed to further explore childhood abuse history and its relationship to circulating levels of HDL and HDL/LDL ratio. Male subjects with a history of high levels of child abuse were found to have significantly lower levels of circulating HDL (mean = 44.5 mg/dL) compared to males with little to no child abuse history (mean= 50.3 mg/dL; F(1, 180)=6.80, p=0.01; Figure 1A). In contrast, female HDL levels were not significantly different based on child abuse history. When HDL/LDL ratios were examined, a similar but more pronounced effect was seen for male HDL/LDL ratio based on abuse history. Males with history of high levels of child abuse have considerably lower HDL/LDL ratios (mean= 0.44) compared to males with little to no child abuse history (mean= 0.56; F(1, 180)=12.0, p=0.001; Figure 1B), resulting in an approximately 22% decrease in lipid ratio as a function of child abuse levels. Female HDL/LDL lipid levels were not significantly different based on child abuse history. Notably, these effects of child abuse history on HDL and HDL/LDL lipid levels were not accounted for by BMI, since when we covary for BMI, these main effects are maintained (Childhood trauma covarying for BMI: HDL, p≤0.05, HDL/LDL ratio, p≤0.01).
Figure 1. HDL and HDL/LDL ratios are lower in men with a history of child abuse.
(A) When looking specifically at circulating HDLs, males with a moderate to severe child abuse history have markedly decreased levels of this protective lipid compared to males with little to no child abuse history. Female HDL lipid levels were not significantly different based on child abuse history. (B) Males with moderate to severe child abuse history have considerably lower HDL/LDL ratios compared to males with little to no child abuse history. Female HDL/LDL lipid levels were not significantly different based on child abuse history. Bar graphs show mean + s.e.m.
3.4. Childhood Abuse Severity as a Predictor of Lipid Levels
Using a univariate ANOVA model, the ability of child abuse history to predict lipid levels was explored when also accounting for the other potential trauma-related variables outlined in Table 1. We found that despite controlling for age, race, levels of adult trauma exposure (TEI), PTSD symptoms (PSS) and depression symptoms (BDI), the effect of childhood abuse (low vs. high as outlined above) was associated with HDL/LDL ratio in men but not women (Men: F(1, 180)=6.0, p=0.015; Women: F(1, 272)=2.0, p=0.160).
To further examine the effect of child abuse history on the HDL/LDL ratio as a function of sex, and other potential risk variables, a model was created accounting for lifetime alcohol and tobacco use, depression symptoms, and PTSD symptoms. Childhood abuse level and sex show a significant interaction on HDL/LDL ratios (abuse x sex interaction F(1, 369)=13.0, p=.0004) with males that have a moderate to severe abuse history displaying a low ratio of HDL/LDL (mean = 0.44) and males with little to no abuse history displaying a ratio of (mean =0.57). Females with little to no abuse history trended towards an opposite effect, but without significance effect as a function of abuse.
3.5. Childhood Abuse Predicts Lipid Levels when Controlling for Cardiometabolic Confounders
A possible reason for these findings is that subjects with a history of childhood trauma may be more likely to have dyslipidemia due to decreased self-care, and thus decreased likelihood of preventative cardiovascular healthcare, as well as increased risk of other factors associated with disease such as smoking, alcohol, and trauma exposure.
Thus, we next examined whether level of childhood abuse would predict HDL/LDL ratios when examined controlling for other cardiovascular risk factors and examining continuously instead of categorically. To control for the potential effects of lipid lowering drugs, we did this by performing a linear regression to predict HDL/LDL ratios, with prescription of statin and non-statin lipid-lowering drugs in the first model, followed by adding total CTQ score as a continuous predictor in the second model (Table 2). We found that in men, but not women, continuous CTQ total score inversely predicted HDL/LDL ratio, even after controlling for medications which target dyslipidemia (standardized β=−0.234, p<0.05).
Finally, we examined whether the continuous childhood trauma measure was associated with continuous HDL/LDL ratios when controlling for other potential demographic and trauma variables frequently associated with dyslipidemia. In this linear regression predicting HDL/LDL ratio, step 1 included age and race; step 2 added lifetime alcohol and tobacco; step 3 added total adult trauma exposure; and step 4 added the childhood trauma level (CTQ) as above. We found that in men, but not women, continuous childhood trauma levels inversely predicted HDL/LDL ratio despite all other variables (standardized β=−0.179, p<0.05, Table 3).
Discussion
Using data obtained from (N=452)male and female subjects from an at-risk low SES population recruited from the general medical clinics of a large urban hospital, we found that exposure to child abuse was associated with low HDL/LDL ratios and low HDL levels in male but not female subjects. Even after evaluating multiple potentially confounding variables that were differentially associated with childhood trauma in our cohort (substance abuse, depression symptoms, PTSD symptoms, and BMI) as well as separately controlling for other a priori variables that have previously been associated with cardiometabolic risk(age, race, level of adult trauma exposure, alcohol use, tobacco use, and prescribed lipid-lowering medications), this association remained significant.
These data suggest that the previously separately observed increased rates of childhood trauma exposure and cardiovascular risk factors found in low-income African Americans, may be related through heightened rates of cardiometabolic risk, in particular dyslipidemia. The results are similar to those found in other studies exploring the association between trauma and elevated lipids and increased rates of cardiovascular disease in very different populations. For example, studies of civilian police officers with significant trauma exposure have also identified associations between a diagnosis of PTSD and the presence of lipid abnormalities (29).
However, this is the first study that we are aware of in which the role of childhood trauma exposure has been examined in a low SES population with known health disparities in cardiovascular disease. It is possible that this association may underlie some of the known correlations between stress, trauma and adverse cardiovascular events. Although it is also possible that low childhood maltreatment may serve as a protective factor against other SES-associated risk factors. This cohort also experiences high rates of childhood maltreatment, abuse and neglect. Long-term impacts of childhood maltreatment include higher rates of unemployment and poverty. Additionally, adults who are physically abused, sexually abused, or severely neglected as children are significantly more likely to be unemployed and to live below the poverty line than people without a history of childhood maltreatment. Notably, having experienced more than one type of maltreatment increases these risks even further (46).
Several limitations are also important to note from this work. The childhood trauma exposure data were obtained with an entirely retrospective, self-report measure from an adult population, which carries the risk of potential biases. However, many prior studies have addressed this potential concern within this and other cohorts, without evidence for it as an important confounding variable, and in fact, for some of the specific child maltreatment variables our data may still provide an underestimate. Additionally, many well-known risk factors for dyslipidemia include lifestyle variables such as diet and exercise, which we did not collect in our sample. Future studies should examine the associations between trauma history and dyslipidemia while controlling for the important potential effects of diet and exercise.
Interestingly, in our study, we found the association between childhood maltreatment and lipid-related cardiovascular risks to be only significant for males. While at this point we do not have data to suggest why we do not see this effect in women, we can hypothesize that females may be partially protected from the dyslipidemia effects of childhood trauma due to estrogen, progesterone, or other sex-specific protective hormones.
African-American men are the highest risk population within the United States for myocardial infarction, stroke, and other severe medical comorbidities related to dyslipidemia and cardiovascular disease. An often overlooked risk factor for these processes is the increased rate of chronic, lifetime, and childhood stress and trauma that this cohort is also exposed to, which is in large part associated with poverty. Although replications and extensions are needed, such as exploring the influence of diet and exercise on this effect, our data suggest a link between childhood trauma exposure and dyslipidemia in this at-risk urban male population which may contribute to their increased lifetime risk for cardiovascular disease.
Acknowledgments
This work was primarily supported by National Institutes of Mental Health (MH071537). Support was also received from National Institute of Mental Health (MH082256 to CFG), Emory and Grady Memorial Hospital General Clinical Research Center, NIH National Centers for Research Resources (M01RR00039 and P20RR16435), NARSAD (CFG), the American Foundation for Suicide Prevention (BB) and the Burroughs Welcome Fund (KJR). We thank staff of the Grady Trauma Project, especially Allen Graham, Angelo Brown, and Maria Nylocks, as well as Marucs J. (Bo) Houston, Carla Moore, and Shelawa Kadree for excellent support.
Abbreviations
- HDL
high density lipoproteins
- LDL
low density lipoproteins
- BMI
body mass index
- CAPS
Clinician-Administered PTSD Scale for DSM-IV
- TG
triglyceride
- SES
socioeconomic status
- PTSD
posttraumatic stress disorder
- TEI
Traumatic Events Inventory
- CTQ
Childhood Trauma Questionnaire
- KMSK
Kreek-McHugh-Schluger-Kellogg Scale
- MPSS
Modified PTSD Symptom Scale
- BDI
Beck Depression Inventory
- SD
standard deviation
Footnotes
Financial Disclosure Statement: There were no commercial sponsors or commercial relationships related to the current work. All additional financial ties of the investigators within the last 3 years are disclosed herein: Dr. Gillespie has received funding from APIRE/Wyeth, NARSAD, NIDA, and NIMH. Dr. Ressler has received awards and/or funding support related to other studies from Burroughs Wellcome Foundation, NARSAD, NIMH, NIDA, and is a cofounder of Extinction Pharmaceuticals for use of NMDA-based therapeutics with Psychotherapy.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sanchez MM. The impact of early adverse care on HPA axis development: non human primate models. Horm Behav. 2006;50(4):623–631. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27(1–2):199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- 3.Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Horm Behav. 2006;50(4):632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Carrion VG, Weems CF, et al. Diurnal salivary cortisol in pediatric posttraumatic stress disorder. Biol Psychiatry. 2002;51(7):575–582. doi: 10.1016/s0006-3223(01)01310-5. [DOI] [PubMed] [Google Scholar]
- 5.Williamson DF, Thompson TJ, Anda RF, Dietz WH, Felitti V. Body weight and obesity in adults and self-reported abuse in childhood. Int J Obes Relat Metab Disord. 2002;26(8):1075–1082. doi: 10.1038/sj.ijo.0802038. [DOI] [PubMed] [Google Scholar]
- 6.Gustafson TB, Sarwer DB. Childhood sexual abuse and obesity. Obes Rev. 2004;5(3):129–135. doi: 10.1111/j.1467-789X.2004.00145.x. [DOI] [PubMed] [Google Scholar]
- 7.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the adverse childhood experiences (ACE) study. American Journal of Preventative Medicine. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 8.Gunstad J, Paul RH, Spitznagel MB, Cohen RA, Williams LM, Kohn M, Gordon E. Exposure to early life trauma is associated with adult obsetity. Psychiatry Research. 2006;142(1):31–37. doi: 10.1016/j.psychres.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Hemmingsson T, Lundberg I. How far are socioeconomic differences in coronary heart disease hospitalization, all-cause mortality and cardiovascular mortality among adult Swedish males attributable to negative childhood circumstances and behaviour in adolescence? Int. J Epidemiol. 2005;34(2):260–267. doi: 10.1093/ije/dyh314. [DOI] [PubMed] [Google Scholar]
- 10.Thomas C, Hyppönen E, Power C. Obesity and type 2 diabetes risk in midadult life: the role of childhood adversity. PEDIATRICS. 2008;121(5):1240–1249. doi: 10.1542/peds.2007-2403. [DOI] [PubMed] [Google Scholar]
- 11.Chan PS, Nichol G, Krumholz HM, Spertus JA, Jones PG, Peterson ED, Rathore SS, Nallamothu BK. Racial differences in survival after in-hospital cardiac arrest. JAMA. 2009;302(11):1195–1201. doi: 10.1001/jama.2009.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark LT, Ferdinand KC, Flack JM, Gavin JR, 3rd, Hall WD, Kumanyika SK, Reed JW, Saunders E, Valantine HA, Watson K, Wenger NK, Wright JT. Coronary heart disease in African Americans. Heart Dis. 2001;3(2):97–108. doi: 10.1097/00132580-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Cook N, Albert M. Regarding REGARDS: does inflammation explain racial and regional differences in cardiovascular disease risk? Clin Chem. 2009;55(9):1603–1605. doi: 10.1373/clinchem.2009.131185. [DOI] [PubMed] [Google Scholar]
- 14.Duprez DA, Jacobs DR, Jr, Lutsey PL, Herrington D, Prime D, Ouyang P, Barr RG, Bluemke DA. Race/ethnic and sex differences in large and small artery elasticity—results of the multi-ethnic study of atherosclerosis (MESA) Ethn Dis. 2009;19(3):243–250. [PMC free article] [PubMed] [Google Scholar]
- 15.Mensah G, Mokdad A, Ford E, Greenlund K, Croft J. State of disparities in cardiovascular health in the United States. Circulation. 2005;111(10):1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 16.Link CL, McKinlay JB. Disparities in the prevalence of diabetes: is it race/ethnicity or socioeconomic status? Results from the Boston Area Community Health (BACH) survey. Ethn Dis. 2009;19(3):288–292. [PMC free article] [PubMed] [Google Scholar]
- 17.Shonkoff J, Boyce W, McEwen B. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301 (21):2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 18.Alim TN, Graves E, Meilman TA, Aigbogun N, Gray E, Lawson W, Charney DS. Trauma exposure, posttraumatic stress disorder and depression in an African-American primary care population. J Natl Med Assoc. 2006;98(10):1630–1636. [PMC free article] [PubMed] [Google Scholar]
- 19.Breslau N, Wilcox H, Storr C, Lucia V, Anthony J. Trauma exposure and posttraumatic stress disorder: a study of youths in urban America. J Urban Health. 2004;81(4):530–544. doi: 10.1093/jurban/jth138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, Weiss T, Schwartz AC, Cubells JF, Ressler KJ. Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry. 2009;31(6):505–514. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz A, Bradley R, Sexton M, Sherry A, Ressler K. Posttraumatic stress disorder among African Americans in an inner city mental health clinic. Psychiatr Serv. 2005;56(2):212. doi: 10.1176/appi.ps.56.2.212. [DOI] [PubMed] [Google Scholar]
- 22.Boscarino J. Posttraumatic stress disorder and physical illness. Ann NY Acad Sci. 2004;1032:141–153. doi: 10.1196/annals.1314.011. [DOI] [PubMed] [Google Scholar]
- 23.Boscarino J. Posttraumatic stress disorder and mortality among US Army veterans 30 years after military service. Ann Epidemiol. 2006;16(4):248–256. doi: 10.1016/j.annepidem.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Krause N, Shaw BA, Cairney J. A descriptive epidemiology of lifetime trauma and the physical health status of older adults. Psychol Aging. 2004;19(4):637–648. doi: 10.1037/0882-7974.19.4.637. [DOI] [PubMed] [Google Scholar]
- 25.Vieweg WV, Julius DA, Fernandez A, Tassone DM, Narla SN, Pandurangi AK. Posttraumatic stress disorder in male military veterans with comorbid overweight and obesity: psychotropic, antihypertensive, and metabolic medications. Prim Care Companion. J Clin Psychiatry. 2006;8(1):25–31. doi: 10.4088/pcc.v08n0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jakovljevic M, Babic D, Crncevic Z, Martinac M, Maslov B, Topic R. Metabolic syndrome and depression in war veterans with posttraumatic stress disorder. Psychiatr Danub. 2008;20(3):406–410. [PubMed] [Google Scholar]
- 27.Jakovljevic M, Crncevic Z, Ljubicic D, Babic D, Topic R, Saric M. Mental disorders and metabolic syndrome: a fatamorgana or warning reality? Psychiatr Danub. 2007;19(1–2):76–86. [PubMed] [Google Scholar]
- 28.Jakovljevic M, Šaric M, Nadj S, Topic R, Vuksan-Cusa B. Metabolic syndrome, somatic and psychiatric comorbidity in war veterans with post-traumatic stress disorder: preliminary findings. Psychiatr Danub. 2006;18(3–4):169–176. [PubMed] [Google Scholar]
- 29.Jin H, Lanouette NM, Mudaliar S, Henry R, Folsom DP, Khandrika S, Glorioso DK, Jeste D. Association of posttraumatic stress disorder with increased prevalence of metabolic syndrome. J Clin Psychopharmacol. 2009;29(3):210–215. doi: 10.1097/JCP.0b013e3181a45ed0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maia DB, Marmar CR, Mendlowicz MV, Metzler T, Nóbrega A, Peres MC, Coutinho ES, Volchan E, Figueira I. Abnormal serum lipid profile in Brazilian police officers with post-traumatic stress disorder. J Affect Disord. 2008;107(1–3):259–63. doi: 10.1016/j.jad.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solter V, Thaller V, Karlovic D, Crnkovic D. Elevated serum lipids in veterans with combat-related chronic posttraumatic stress disorder. Croat Med J. 2002;43(6):685–689. [PubMed] [Google Scholar]
- 32.Schnurr PP, Jankowski MK. Physical health and post-traumatic stress disorder: review and synthesis. Semin Clin Neuropsychiatry. 1999;4 (4):295–304. doi: 10.153/SCNP00400295. [DOI] [PubMed] [Google Scholar]
- 33.Trief PM, Ouimette P, Wade M, Shanahan P, Weinstock RS. Posttraumatic stress disorder and diabetes: co-morbidity and outcomes in a male veterans sample. J Behav Med. 2006;29(5):411–418. doi: 10.1007/s10865-006-9067-2. [DOI] [PubMed] [Google Scholar]
- 34.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299(11):1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cusack K, Frueh B, Brady K. Trauma history screening in a community mental health center. Psychiatr Serv. 2004;55(2):157–162. doi: 10.1176/appi.ps.55.2.157. [DOI] [PubMed] [Google Scholar]
- 36.Norris F, Hamblen J. Standardized self-report measures of civilian trauma and PTSD. In: Wilson John P, Keane Terence M, editors. Assessing psychological trauma and PTSD. New York: The Guilford Press; 2004. p. 63. [Google Scholar]
- 37.Blake DD, Weathers FW, Nagy LM, et al. The development of a clinician administered PTSD scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 38.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. A clinician rating scale for assessing current lifetime PTSD: the CAPS-1. Behav Ther. 1990;13:187–188. [Google Scholar]
- 39.Weathers FW, Keane TM, Davidson JRT. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety. 2001;13:132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- 40.Kellogg SH, McHugh PF, Bell K, Schluger JH, Schluger RP, LaForge KS, Ho A, Kreek MJ. The Kreek–McHugh–Schluger–Kellogg scale: a new, rapid method for quantifying substance abuse and its possible applications. Drug and Alcohol Dependence. 2003;69(2):137–150. doi: 10.1016/s0376-8716(02)00308-3. [DOI] [PubMed] [Google Scholar]
- 41.Coffey SF, Dansky BS, Falsetti SA, Saladin ME, Brady KT. Screening for PTSD in a substance abuse sample: psychometric properties of a modified version of the PTSD Symptom Scale Self-Report. Posttraumatic stress disorder. J Trauma Stress. 1998 Apr;11(2):393–9. doi: 10.1023/A:1024467507565. [DOI] [PubMed] [Google Scholar]
- 42.Foa EB, Tolin DF. Comparison of the PTSD Symptom Scale-Interview Version and the Clinician-Administered PTSD scale. J Trauma Stress. 2000 Apr;13(2):181–91. doi: 10.1023/A:1007781909213. [DOI] [PubMed] [Google Scholar]
- 43.Storch EA, Roberti JW, Roth DA. Factor structure, concurrent validity, and internal consistency of the beck depression inventory—second edition in a sample of college students. Depress Anxiety. 2004;19:187–189. doi: 10.1002/da.20002. [DOI] [PubMed] [Google Scholar]
- 44.Lasa L, Ayuso-Mateos JL, Vázquez-Barquero JL, Díez-Manrique FJ, Dowrick CF. The use of the Beck Depression Inventory to screen for depression in the general population: a preliminary analysis. Journal of Affective Disorders. 2000;57(1–3):261–265. doi: 10.1016/s0165-0327(99)00088-9. [DOI] [PubMed] [Google Scholar]
- 45.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders, (Research Version, 2/96 Final) New York: NY Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 46.Zielinski DS. Child maltreatment and adult socioeconomic well-being. Child Abuse Negl. 2009;33(10):666–678. doi: 10.1016/j.chiabu.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J Am Acad Child Adolesc Psychiatry. 1997;36(3):340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 48.Bernstein DP, Fink L. Childhood trauma questionnaire manual. San Antonio, TX: Psychological Corporation; 1998. [Google Scholar]
- 49.Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151(8):1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 50.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27(2):169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 51.Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Ressler KJ. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65(2):190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]