Abstract
Aneuploidy, the state of having a chromosome number different from a multiple of the haploid number, has been associated with diseases and developmental disorders. The role of aneuploidy in human disease pathology, especially in cancer, has been a subject of much attention and debate over the last century due to the intrinsic complexity of the phenomena and experimental challenges. Over the last decade, yeast has been an invaluable model for driving discoveries about the genetic and molecular aspects of aneuploidy. The understanding of aneuploidy has been significantly improved owing to the methods for selectively generating aneuploid yeast strains without causing other genetic changes, techniques for detecting aneuploidy, and cutting-edge genetics and ‘omics’ approaches. In this review, we discuss the contribution of studies in yeast to current knowledge about aneuploidy. Special emphasis is placed on experimental features which make yeast a simpler and efficient model to investigate the complex questions in the field of aneuploidy.
Introduction
Eukaryotic cell division is a highly complex and regulated process involving a robust surveillance mechanism, called mitotic checkpoint (or spindle assembly checkpoint), to ensure the fidelity of chromosome segregation. Alterations in mitotic checkpoint and components of the chromosome segregation machinery often result in an unbalanced genomic state called aneuploidy (Aguilera & Gomez-Gonzalez, 2008). Aneuploidy exists in somatic cells such as normal human brain (Rehen, et al., 2005) and liver (Duncan, 2013). Aneuploidy at the organismal level in humans causes embryonic lethality; few viable aneuploidies cause genetic disorders such as Down's syndrome (trisomy 21) and Edwards syndrome (trisomy 18) (Nagaoka, et al., 2012). Aneuploidy has also been recognized as a common characteristic of cancer cells for more than 100 years (Boveri, 1914, Holland & Cleveland, 2009). Cancer is a dynamic evolutionary system where cells are continuously selected against restrictive conditions ranging from tissue-specific growth regulators to various drug treatments. The adaptability and evolvability of cancer cells could be attributed to their genomic diversity conferred by aneuploidy and other forms of mutations (Nowell, 1976, Pavelka, et al., 2010, Thomas, et al., 2013).
The implications of aneuploidy in cancer, drug resistance, pathogenicity, as well as the existence of aneuploidy in normal human brain and liver, are the subjects of recent attention. Considering the continual karyotypic changes and heterogeneity in aneuploid cell populations and the difficulty of separating the effect of aneuploidy from other types of genetic aberrations, the molecular mechanisms underlying the diverse phenotypic effects of aneuploidy remain poorly understood. Despite the large effort devoted to the elucidation of the causes and consequences of aneuploidy, it is hindered by inherent scientific and technical difficulties (McGranahan, et al., 2012). The study of aneuploidy in cancer cells is complicated by the presence of numerous point mutations and a variety of other chromosomal abnormalities such as inversions and translocations. In addition, investigation of the physiological and pathological effects of aneuploidy in multi-cellular organisms is limited by difficulties in generating isogenic and stable aneuploid cell populations. Adding to these difficulties are the low-throughput, labor intensive and expensive methods currently available for accurate karyotyping. These experimental challenges underscore the need for model organisms and more efficient experimental techniques to facilitate the study of the diverse aspects of aneuploidy at the cellular and molecular levels.
A simple model organism, such as the budding yeast S. cerevisiae, has emerged as a robust and versatile model system for studying the effects of genetic alterations (Botstein & Fink, 2011). A unicellular eukaryote, the budding yeast is particularly suitable for studying the effects of aneuploidy on cellular physiology because of its small genome, divided into sixteen chromosomes and is well tolerant of aneuploidy (Parry & Cox, 1970). The budding yeast is naturally a diploid, and on a diploid background aneuploidies can be more easily tolerated; loss of chromosome in a haploid background causes lethality. In addition, it is possible to generate isogenic aneuploid yeast strains without causing other genomic changes. Yeast studies of aneuploidy using genomics and proteomics approaches have significantly improved the understanding of the causes and consequences of aneuploidy. Many yeast biochemical pathways are conserved across the higher eukaryotic species justifying the use of yeast as a simple model system for studying complex biological processes. In this review, we summarize the contribution of studies in yeast to the current understanding of the causes and consequences of aneuploidy. Special emphasis is given to the methods for the generation of a variety of aneuploid yeast strains and karyotyping.
Causes of aneuploidy in yeast
Chromosome segregation and replication errors are the causes of aneuploidy. The frequency of spontaneous chromosome gain or loss in laboratory strains of budding yeast can be considered quite high by the fact that in S. cerevisiae the mutation rate is ~0.33*10^-9 per cell division (Lynch, 2010). In standard laboratory conditions, the spontaneous loss rate of chromosome V in diploid budding yeast S. cerevisiae is around 2–8 per 106 cell divisions (Hartwell & Smith, 1985, Klein, 2001). Also, aneuploidy acts upon a very narrow genomic space of 16 chromosomes compared to the length of the genome for point mutations. Accordingly, the chance of specific aneuploidy to occur is much higher compared to other types of mutations. Proper functioning of the mitotic spindle apparatus and the correct structural organization of the duplicated chromosomes are essential for the fidelity of chromosome segregation in mitosis (Page & Snyder, 1993). Furthermore, the spindle assembly checkpoint serves as a surveillance mechanism to prevent chromosome missegregation in mitosis (Musacchio & Salmon, 2007). Defects in any of these biological processes could compromise the accuracy of chromosome segregation producing an aneuploid progeny. Genetic screens in budding yeast have shown at least 10% of its genome is involved in the maintenance of chromosome stability (Ouspenski, et al., 1999, Smith, et al., 2004, Kanellis, et al., 2007, Yuen, et al., 2007, Stirling, et al., 2011). These genes are referred as CIN genes since their mutations cause chromosome instability (CIN). Many of these CIN genes are known to function in processes such as kinetochore and spindle microtubule interaction, DNA replication, repair, condensation, and the spindle assembly checkpoint (Stirling, et al., 2011). It is essential to distinguish true CIN genes from genes whose mutations may not necessarily cause CIN, but pose a strong selection for certain aneuploid karyotypes that help alleviate to growth deficiencies caused by gene mutations (Hughes, et al., 2000, Rancati, et al., 2008). For example, big colonies isolated from rnr1Δ strain were found to have an extra copy of chromosome IX. This could be the result of a selection for the improved growth by increasing dosage of a paralog gene RNR3 on chromosome IX, but, the deletion of RNR1 may not necessarily cause CIN.
Environmental stress has also been shown to induce chromosome missegregation in yeast. Exposure of the pathogenic yeast, C. albicans, to heat stress and antifungal drugs elevates the frequency of chromosome loss (Forche, et al., 2011). Also in C. neoformans, high-dose of fluconazole treatment has been suggested to cause chromosomal instability (Sionov, et al., 2010). It was noticed that the high frequency (0.3 to 0.6%) at which aneuploidy occurs in C. neoformans under fluconazole stress could not be the result of spontaneous aneuploidy formation. A recent study investigating how diverse stress conditions affect CIN in budding yeast through monitoring the loss of a minichromosome found that many stress conditions were shown to promote CIN (Chen, et al., 2012). In particular, inhibition of the Hsp90 chaperone by various means markedly increased the loss rate of artificial chromosome compared to stress-free culture condition. This effect was linked to the crucial role of Hsp90 in kinetochore assembly.
Another route to aneuploidy in yeast is through polyploidization. Meiosis of triploid or pentaploid cells gives rise to an almost exclusively aneuploid progeny. Mitosis of polyploid cells is also known to be error-prone. Tetraploid S. cerevisiae loses chromosomes at a higher rate than diploid (Mayer & Aguilera, 1990). This is thought to be caused by an increased incidence of syntelic (mono-polar) kinetochore attachments, which arise due to an altered spindle geometry in tetraploids (Storchova, et al., 2006). The tetraploid C. albicans can undergo dramatic chromosome loss when growing on S. cerevisiae 'pre-sporulation' media and sorbose media. This often results in a diploid or near-diploid aneuploid progeny (Bennett & Johnson, 2003).
The effect of aneuploidy on gene expression
Recent studies in yeast suggest that phenotypic effects of aneuploidy are directly linked to changes in the expression of many genes (Torres, et al., 2007, Rancati, et al., 2008, Pavelka, et al., 2010, Chen, et al., 2012). There are several possible mechanisms by which aneuploidy could affect gene expression and phenotype (Birchler, 2010, Pavelka, et al., 2010).
The effects of aneuploidy on gene expression can be divided as those proportional to DNA dosage, known as “inlier” changes; or as those far beyond the DNA dosage, known as “outlier” changes. The “inlier” gene expression change is often moderate as it is chromosome copy-number driven and occurs to most genes encoded on aneuploid chromosomes. For example, in haploid aneuploid strains carrying an extra copy of one of the sixteen S. cerevisiae chromosomes (disomies), expression of most of the genes on an aneuploid chromosome was found to be increased two-fold as compared to the haploid control (Torres, et al., 2007). A similar observation came from analysis of a set of relatively stable aneuploid S. cerevisiae strains generated by triploid meiosis, where the relative level of mRNA (compared to euploid) for most of the genes encoded on aneuploid chromosomes directly correlates to the relative gene copy-number, but a small number genes are found to be robust to their copy-number and do not change their expression although the DNA copy-number has changed (Pavelka, et al., 2010). A direct DNA dosage effect on gene expression was observed in 23 aneuploid strains from a yeast deletion collection (Sheltzer, et al., 2012). In other studies, such as in the case of fluconazole-resistant C. albicans isolates, the RNA expression change (compared to euploid) was found to correlate with the DNA copy-number change (Bouchonville, et al., 2009). From the observation that more than 90% of genes react quite linearly to changes in their DNA copy-number, whole chromosome and large segmental aneuploidy can be detected easily and reliably by plotting gene expression data according to their chromosomal location (Hughes, et al., 2000, Bouchonville, et al., 2009).
However, the impact of aneuploidy on gene expression is not limited to a simple dosage effect. Observed in every aneuploid yeast strain analyzed in our studies, there were a small number of genes with expression changes greater than the several standard deviations from the average chromosome expression change. These genes with “outlier” expression changes are distributed throughout the genome (Rancati, et al., 2008, Pavelka, et al., 2010). Yeast transcriptional network analysis found that “outlier” genes are enriched as functional targets of the regulatory factors encoded on aneuploid chromosomes. This implies that a substantial subset of outlier gene expression changes can be viewed as a downstream consequence of the inlier gene expression changes due to karyotypic changes (Rancati, et al., 2008).
Do the apparent effects of aneuploidy on transcriptome translate to similar effects on the proteome? In S. cerevisiae aneuploid strains generated from triploid meiosis, quantitative proteomic analysis using multidimensional protein identification technology (MudPIT) revealed that the relative expression of a majority of proteins encoded on aneuploid chromosomes scaled proportionally to DNA and mRNA dosage. Using the method of stable isotope labeling by amino acids in cell culture (SILAC), proteomic analysis performed in disomic budding yeast aneuploid strains arrived at a similar conclusion. However, in the latter study, approximately 20% of the proteins analyzed did not show a proportional increase in accordance with the chromosome number and the gene expression change, leading the authors to conclude that dosage compensation occurs in aneuploid yeast only for some genes. Further analysis of proteins that did not follow the trend of copy-number change showed that a majority of these proteins were components of various protein complexes (Torres, et al., 2010). Although the mechanism underlying the observed dosage compensation remains to be elucidated, it has been suggested that the reduced stability of proteins encoded on gained chromosomes may be due to insufficient incorporation of these proteins into their native complexes (Torres, et al., 2007, Torres, et al., 2010). The study characterizing the effects of heterozygous deletions on protein expression in budding yeast showed a general lack of compensation mechanism at the protein level (Springer, et al., 2010).
Phenotypic effects of aneuploidy
The euploid chromosome number in a given species is an optimum acquired during evolution of that species. Generally aneuploidy is not well tolerated in nature, manifested by impaired fitness at the cellular and organismal level. Systematic analysis of budding yeast disomic strains showed slower cell proliferation under normal conditions compared to euploid strains (Torres, et al., 2007). Similar poor proliferative capacity under standard growth parameters is exhibited by aneuploid strains generated from triploid meiosis in S. cerevisiae and S. pombe (Niwa, et al., 2006, Pavelka, et al., 2010). Studies in S. cerevisiae and S. pombe aneuploid strains have shown that aneuploidy causes delay in the G1 phase of their cell cycle. (Niwa, et al., 2006, Torres, et al., 2007, Pavelka, et al., 2010). Recent study has characterized the G1 delay in aneuploid cells as a consequence of a slower accumulation of G1 cyclins (Thorburn, et al., 2013).
Even though aneuploidy impairs growth and fitness under stress-free conditions, its adaptive value becomes apparent under conditions detrimental to euploid yeast strains. Emerging evidence suggests that aneuploidy is a form of genome alteration that promotes adaptive evolution of cells in response to harsh environments or genetic perturbations (Table 1). For example, certain aneuploid karyotypes in budding yeast enable them to overcome nutrient limitations such as low glucose, high-phosphate, or sulphate media (Gresham, et al., 2008). In pathogenic fungi, aneuploidy is widely known to be associated with drug resistance and increased pathogenicity (Polakova, et al., 2009, Sionov, et al., 2009, Hu, et al., 2011, Silva, et al., 2012) (Morrow & Fraser, 2013).
Table 1.
Aneuploidy as an adaptive mechanism under different types of stress in yeast.
| Type of stress | Species | Aneuploidy and implicated genes |
Adaptive strategy | Reference |
|---|---|---|---|---|
|
Therapeutic drug: |
||||
| Fluconazole (FLC) |
C. albicans | i5: ERG11, TAC1 |
Resistance is acquired by up- regulation of ERG11, encodes FLC target and TAC1, encodes for a regulator of the drug efflux system. |
(Selmecki, et al., 2006, Coste, et al., 2007, Selmecki, et al., 2008) |
|
C neoformins |
Disomy I: ERG11, AFR1 |
Acquired resistance is attributed to up-regulation of ERG11, encodes drug target and AFR1, encodes major transporter of azoles. |
(Sionov, et al., 2010) | |
| Disomy IV: SEY1, GLO3, GCS3 |
Up-regulation of genes SEY1, GLO3, GCS3 encoding a GTPase, linked with morphology and integrity of endoplasmic reticulum, a site of sterol synthesis. |
(Ngamskulrungroj, et al., 2012) | ||
| C. glabrata | Chromosome M: CDR1 |
Elevated drug efflux by up- regulation of CDR1. |
(Polakova, et al., 2009) | |
|
Proteotoxic stress: |
||||
| Radicicol | S. cerevisiae | Disomy XV: STI1, PDR5 |
Resistance to radicicol is acquired by improved protein folding by up-regulation of the Hsp90 co-chaperone through increased expression of STI1. Over-expression of PDR5 improves drug efflux system. |
(Chen, et al., 2012) |
| DNA damage: | ||||
| 4-NQO | S. cerevisiae | Disomy XIII: ATR1 |
Improved drug efflux by up-regulation of ATR1 conferred resistance to 4-NQO. |
(Pavelka, et al., 2010) |
|
Genetic perturbations: |
||||
| MYO1 deletion | S. cerevisiae | Trisomy and tetrasomy XVI: RLM1, MKK2 |
RLM1 MKK2 mediated up- regulation of genes involved in cell wall biogenesis and bud neck constriction restoring cytokinesis. |
(Rancati, et al., 2008) |
| Deletion of RPS 24A and RNR1 on Chr. V |
S. cerevisiae | Disomy IX: RPS24B and RNR3 |
It is suggested that gain of chromosome IX might have been a result of a selection for growth advantage by increasing gene dosage of the paralog of the deleted gene. |
(Hughes, et al., 2000) |
|
Nutrient Limitations: |
||||
| Sulphate limitation | S. cerevisiae | Segmental gain of chromosome II: SUL1 |
Improved drug efflux by up- regulation of SulP anion transporter through elevated SUL1 level. |
(Gresham, et al., 2008) |
| Carbon source manipulation, l-sorbose/ d-arabinose |
C. albicans | Monosomy V: SOU1 |
Monosomy of chromosome 5 activates SOU1 expression enabling l-sorbose. |
(Rustchenko, et al., 1994, Janbon, et al., 1998) |
|
High temperature |
S. cerevisiae | Gain of chromosome III |
--- | (Yona, et al., 2012) |
| High pH | S. cerevisiae | Gain of chromosome V |
--- | (Yona, et al., 2012) |
The selection for aneuploidy under stress is affected by the cost of aneuploidy. Given that the cost of aneuploidy is proportional to the chromosomal length (Tang & Amon, 2013) it is conceivable that under some stresses there are many genes whose altered expression can be beneficial. Yet, a particular chromosome that harbors some of these genes and is associated with less cost would have a higher probability to be selected. The adaptive benefits of aneuploidy can be attributed to the altered dosage of single or multiple genes on an aneuploid chromosome. For example, in the systematic analysis of 38 aneuploid S. cerevisiae strains, some aneuploid variants showed improved fitness compared to parental euploid strains under adverse conditions such as treatment with the tumorigenic compound 4-nitroquinoline-oxide (4-NQO). The improved fitness in the presence of 4-NQO was attributed to the increased expression of the gene ATR1, whose copy-number was increased through the gain of chromosome XIII (Pavelka, et al., 2010). ATR1 encodes a transporter protein known to confer 4-NQO-resistance when overexpressed (Mack, et al., 1988). Another example is radicicol resistance associated with chromosome XV gain in S. cerevisiae. This was attributed to the synergistic effect of the increased dosage of two genes, STI1 and PDR5 (encoding an Hsp90 co-chaperone and a drug pump, respectively), encoded on chromosome XV (Chen, et al., 2012).
A remarkable adaptive effect of aneuploidy in budding yeast was observed in the case of myo1Δ. MYO1 encodes the myosin-II motor protein that normally drives bud neck constriction during cytokinesis. Whereas deletion of MYO1 results in massive cytokinesis failure and lethality in most cells (Tolliday, et al., 2003), a few myo1Δ survivors were able to evolve alternative cytokinesis mechanisms through changes in chromosome stoichiometry. One class of the myo1Δ “evolvants” restores cytokinesis by cell wall thickening in the bud neck region by upregulating the set of genes involved in the cell wall biogenesis. Located on both euploid and aneuploid chromosomes, many of these showed outlier gene expression (Rancati, et al., 2008). Further analysis demonstrated that these gene expression changes were due to extra copies of two genes - RLM1 (a transcription factor) and MKK2 (activator of RLM1) located on chromosome XVI. By simply introducing extra copies of RLM1 and MKK2 into myo1Δ, the evolved mechanism of cytokinesis was recapitulated. Well-annotated functional genomics data in yeast have been particularly helpful in unraveling the molecular mechanisms by which an aneuploid karyotype confers a particular phenotype. In aneuploid cancer cells, identifying specific genes on an aneuploid chromosome that could account for the growth advantage is more challenging.
Aneuploidy and chromosomal instability in yeast
While aneuploidy is a product of chromosome instability, it also correlates with elevated chromosomal instability observed as gaining or losing chromosomes at a high frequency. This vicious cycle is thought to underlie cancer “genome chaos” (Potapova, et al., 2013). Studying the relationship between aneuploidy and chromosomal instability in cancer cells is complicated by the presence of a variety of other genetic changes. Therefore, a simple and genetically tractable model system, such as yeast, holds many advantages for studying this phenomenon. The observation of chromosomal instability in aneuploid yeast cells was made more than four decades ago (Parry & Cox, 1970). Extra chromosomes in aneuploid strains generated from triploid meiosis were found to have a higher loss rate, generating karyotypically diverse cell populations (St Charles, et al., 2010). High rates of chromosomal instability were also seen in aneuploid C. albicans strains exposed to the stresses of routine experimental techniques such as transformation (Bouchonville, et al., 2009). Recently, the link between chromosomal instability and aneuploidy was addressed systematically using several approaches. The rate of chromosome missegregation inferred from the loss of artificial chromosome was found to increase in 9 out of 13 yeast disomic strains (Sheltzer, et al., 2011). Similarly, monosomic budding yeast cells showed an increased chromosomal instability and were predisposed to return to a diploid karyotype when cultured under standard conditions (Waghmare & Bruschi, 2005, Chen, et al., 2012). For example, chromosome XVI monosomic strain was found to be karyotypically unstable under normal conditions, but when maintained under tunicamycin selection, appeared stable in terms of population homogeneity. This underscores the challenge of finding the right conditions to separate selection vs. intrinsic genomic stability (Chen, et al., 2012). A recent study demonstrated a positive correlation of chromosome instability with the degree of aneuploidy as well as with the presence of specific aneuploid chromosomes and dosage imbalance between specific chromosome pairs (Zhu, et al., 2012). The “genome chaos” created by aneuploidy-associated chromosome instability may be a powerful mechanism for the rapid generation of karyotypic diversity in the population, providing the substrate for evolutionary selection of adaptive genomes (Nowell, 1976, Merlo, et al., 2006, Selmecki, et al., 2006, Rancati, et al., 2008, Selmecki, et al., 2009, Chen, et al., 2012).
Experimental approaches in the study of aneuploidy using yeasts as a model organism
Methods for generating aneuploidy in yeast
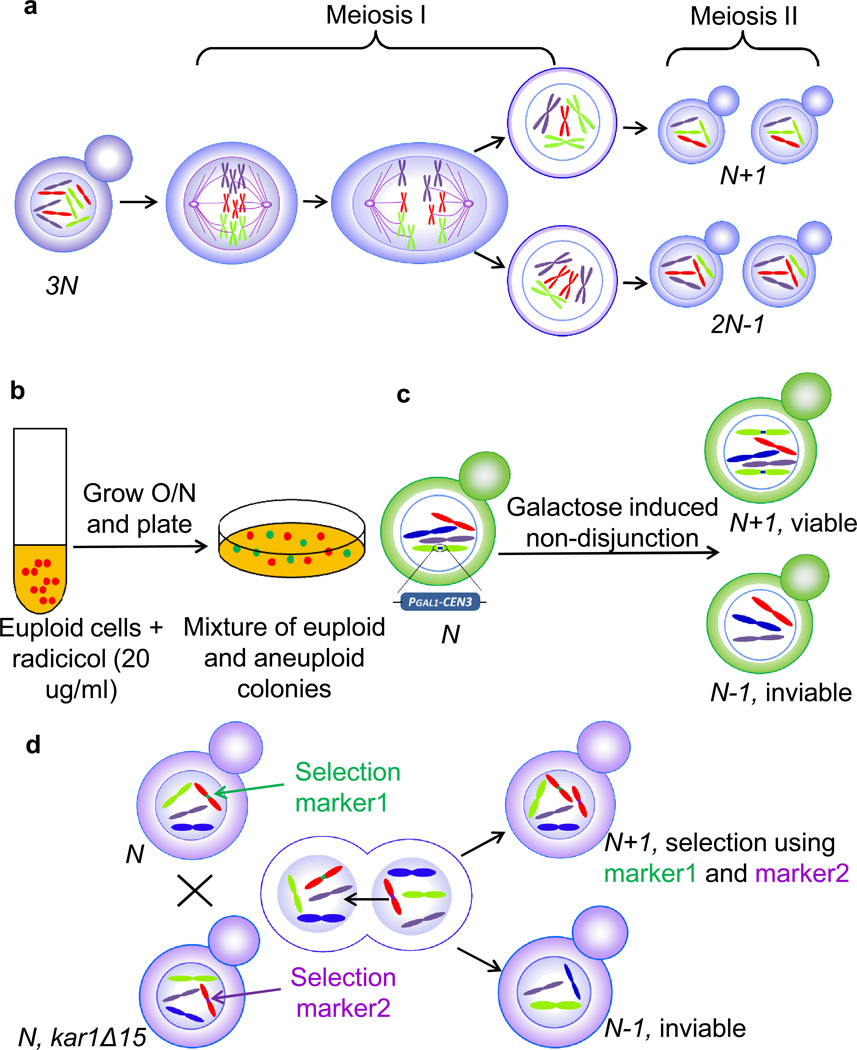
A number of different methods has been developed to generate aneuploidy in yeast. An efficient way for obtaining random aneuploid strains is by sporulation of polyploid strains with an odd ploidy. This method takes an advantage of the fact that, in triploid and pentaploid strains, homologous chromosomes segregate randomly in the first meiotic division thereby giving rise to mostly aneuploid spores (Fig. 1a). It has been used successfully in generating budding yeast strains with diverse karyotypes and for generating fission yeast with disomy III (Niwa & Yanagida, 1985, Niwa, et al., 2006, Rancati, et al., 2008, Charles, et al., 2010, Pavelka, et al., 2010). As discussed above, aneuploid strains derived from this method have various levels of karyotypic instability; relatively stable karyotypes could be obtained by screening for strains with low level of intra-population karyotype heterogeneity (Pavelka, et al., 2010, Zhu, et al., 2012). Another method for obtaining aneuploid budding yeast strains with random chromosome stoichiometry is treatment with a low concentration of radicicol thereby disrupting normal kinetochore function (Chen, et al., 2012). When a diploid strain was treated with 20 µg/ml radicicol for two days, about one-third of the population was found to be an aneuploid with diverse karyotypes (Fig. 1b) (Chen, et al., 2012). This is one simple way to generate yeast aneuploid strains as it does not require a complicated genetic manipulation.
Figure 1.
Experimental methods for the generation of aneuploidy in yeast.
Defined aneuploid strains with simple karyotypes could be obtained by two other means. One is through the use of a conditional centromere where a GAL1 promoter is inserted adjacent to centromere sequences (Hill & Bloom, 1987, Reid, et al., 2008, Anders, et al., 2009). Transcriptional induction of the promoter abrogates centromere function and causes chromosome nondisjunction (Fig. 1c). Both disomies and monosomies have been isolated using this approach. Another method for isolating disomies is through chromoduction (Conde & Fink, 1976, Nilsson-Tillgren, et al., 1980, Torres, et al., 2007). This method utilizes the rare chromosome transfer between two haploid strains during abortive mating (Fig. 1d). The disomic chromosome can be selected by introducing selectable markers to both homologs. This selection prevents only the loss but not the gain of disomic or any other yeast chromosomes in the strain. The manipulation of the carbon source can also be used to generate aneuploidy in Candida species. For example, sorbose is used as the sole carbon source to generate monosomy and subsequently homozygosity of chromosome V and mating type in C. albicans (Magee & Magee, 2000) and C. tropicalis (Porman, et al., 2011). Strains with complex and simple aneuploid karyotypes have proven to be complementary in the study of the aneuploidy. Of practical note, because of the intrinsic instability of aneuploid strains, it is essential to frequently check the karyotype (see methods below) of the strains during passages and especially after revival from frozen stocks.
Methods for detection of aneuploidy in yeast
Recently developed genomic analysis techniques have advanced our ability to monitor karyotype changes and determine chromosome copy-numbers in yeast. Here, we discuss various approaches used to detect whole chromosome aneuploidy. These approaches include electrophoresis-based methods, flow cytometry, array-comparative genomic hybridization (a-CGH), qPCR karyotyping and Next-Generation sequencing. Besides the determination of chromosome copy-number variations, these karyotyping methods allow assessment of the size of chromosomes, genome size and ploidy level, study of genome dynamics, identification of gross chromosomal rearrangements and associated chromosomal polymorphism. Considering the scope of this article, these methods are focused for karyotyping yeast cells but are generally applicable to other eukaryotic organisms.
Electrophoresis based karyotyping
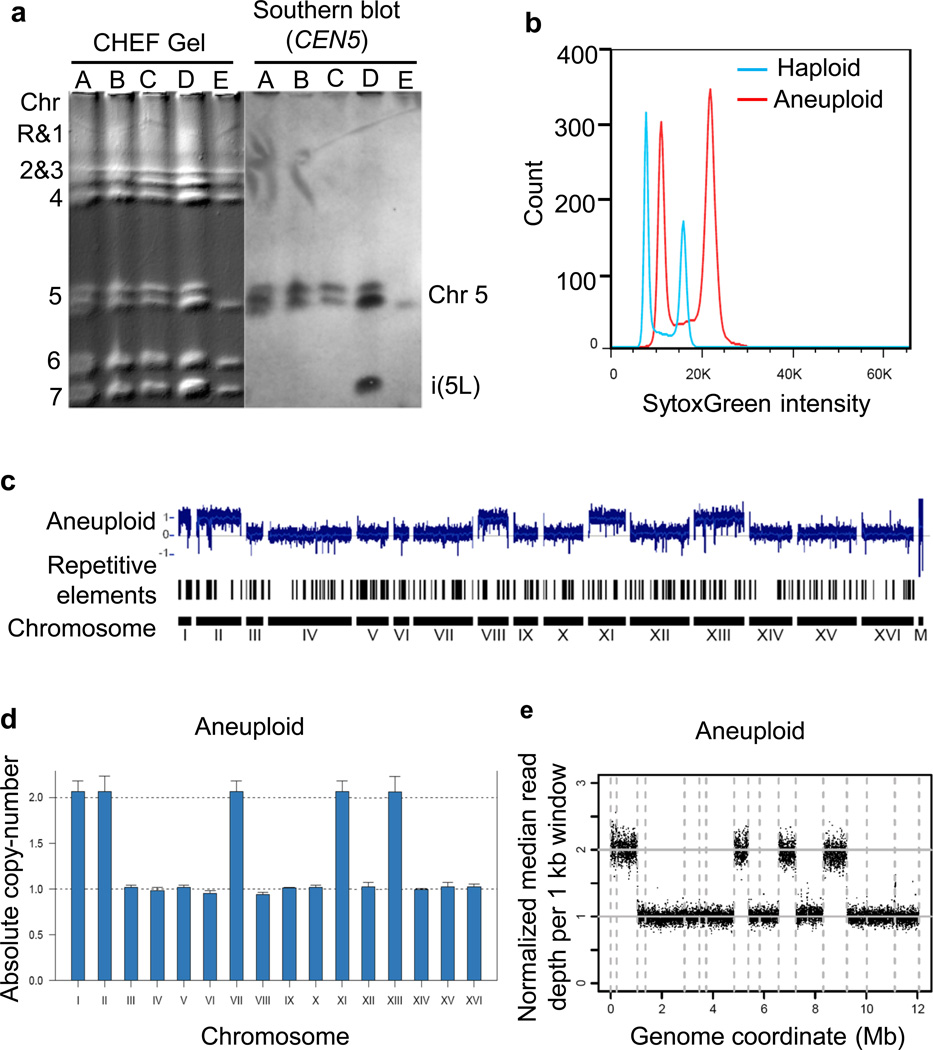
Pulsed-field gel electrophoresis (PFGE) has been widely used in epidemiological studies of pathogenic yeasts and optimized for the separation of C. albicans and S. cerevisiae chromosomes (Doi, et al., 1992, Maringele & Lydall, 2006). Although PFGE has been mainly used to detect gross chromosomal rearrangements (Pardo & Aguilera, 2012, Reis, et al., 2012), quantitative southern blotting after the separation of chromosomes by PFGE can be used to detect alterations in the relative copy-number of different chromosomes (Fig 2a) (Chen, et al., 2004, Bouchonville, et al., 2009). A detailed protocol can be obtained from the cited reference (O'Brien, et al., 2006). Another method for chromosome copy-number determination is the multiplex PCR method with micro-capillary electrophoresis where chromosomal DNA is separated based upon the size-to-charge ratio in the interior of a small electrolyte filled capillary. Further, using a bioanalyzer, aneuploidy in the test sample can be determined by the relative ratio of the peak height of the test and euploid control DNA fragments in the chromatogram (Arbour, et al., 2009, Mitchell, et al., 2013).
Figure 2.
Characteristic examples of karyotyping methods.
Flow cytometry
Flow cytometry is a convenient and least expensive method for assessment of ploidy variation in the population. This technique provides an overview of cellular DNA content rather than information on the gain or loss of specific chromosomes. Estimation of DNA content is based on the use of an intercalating fluorescent dye such as SYTOX® Green dye, that binds proportionally to DNA, allowing estimation of DNA cell cycle distribution and ploidy for thousands of cells per second (Fig 2b). This method has been optimized and routinely used for both Candida and Saccharomyces species. A detailed protocol can be obtained from cited references (Haase & Lew, 1997, Ibrahim, et al., 2005, Darzynkiewicz, et al., 2010, Zhu, et al., 2012). This technique is also highly adaptable to a high-throughput format.
Array-comparative genomic hybridization (a-CGH)
Whereas, electrophoresis and flow cytometry-based methods allow rapid detection of aneuploid genome, a-CGH provides a high-resolution map of both DNA copy-number changes and possible structural chromosomal aberrations (Pinkel & Albertson, 2005). In typical a-CGH measurements, total genomic DNA from a test sample and a normal reference sample are hybridized to an array of fluorescent probes spanning the entire genome. The fluorescence intensity is then measured and compared to reference samples, indicating chromosome copy-number changes as well as the gain or loss of specific loci. A variety of studies has used this method to monitor whole and segmental chromosome aneuploidy (Fig 2c) (Pinkel & Albertson, 2005, Torres, et al., 2007, Rancati, et al., 2008, Arbour, et al., 2009, Selmecki, et al., 2009, Pavelka, et al., 2010, Chen, et al., 2012). A detailed protocol can be referred from cited reference (Dion & Brown, 2009). An advanced form a-CGH based method involves the use of SNP-array. It is a rapid, high-resolution, and cost-effective tool used for the characterization of changes in allele diversity as well as chromosome copy-number in C. albicans (Abbey, et al., 2011) and also in S. cerevisiae (Song & Petes, 2012).
qPCR-karyotyping
Although a-CGH offers a high-resolution map of the genome; its limitations include low-throughput, high costs and cumbersome sample preparation. qPCR-based karyotyping is a more convenient and high-throughput, although less accurate method, for monitoring whole chromosome aneuploidy. The existing protocol employs primers recognizing a non-coding region of each arm of a chromosome and the amplified PCR product can be detected using several fluorescent intercalating dyes, such as SYBR® Green in a real-time manner. With the use of liquid handling robotics, the entire procedure, from genomic DNA extraction to the q-PCR, can be easily performed in a high-throughput format. This method has been used successfully in several yeast aneuploidy studies (Fig 2d) (Pavelka, et al., 2010, Chen, et al., 2012, Zhu, et al., 2012).
Next Generation sequencing (NGS)-karyotyping
NGS-karyotyping is based upon massive parallel sequencing of the whole yeast genome. In brief, genomic DNA is used to create a library of smaller fragments, and then sequenced by millions of parallel reactions generating nucleotide reads. The differential coverage abundance across segments of the reference genome is used to detect chromosome copy-number variation (Dudarewicz, et al., 2005, Didelot, et al., 2012, Nekrutenko & Taylor, 2012). Beside copy-number information, determination of single-nucleotide polymorphisms (SNP) by sequencing also allows consideration of whether the presence of SNP contributes to phenotypic changes associated with an aneuploid strain (Fig 2e) (Rancati, et al., 2008, Pavelka, et al., 2010, Torres, et al., 2010). A recent study has used RAD-Seq as a cost effective form of NGS for karyotyping. In this method, whole genome is cut using at least one restriction enzyme and sequenced using Illumina high-throughput sequencing technology. An alteration in chromosome copy-number can be detected from the alignment of resultant reads of the test sample to a reference genome (Baird, et al., 2008, Hohenlohe, et al., 2010, Tan, et al., 2013). The throughput of Next Generation sequencing machines has improved dramatically over the past few years. A recent study revealed the genotyping of 1000 yeast strains, at the cost of less than 15 euros per sample, in a few weeks (Wilkening, et al., 2013). Also, due to the recent development of single-cell whole genome amplification of an individual human cell, high coverage single-cell sequencing is now possible (Zong, et al., 2012).
Future Perspective
In this review, we discussed recent progress in understanding of the causes and consequences of aneuploidy through studies in yeast. We also highlighted the methods to generate and detect aneuploidy in yeast. Yeast has proven to be a useful model in studying aneuploidy on account of its powerful genetics. For instance, the gain of chromosome XVI converts a budding yeast colony from a ‘fluffy’ to a ‘smooth’ morphology. Further, the gene responsible for the phenotypic switch was easily identified (Tan, et al., 2013). This exemplifies the benefit of well-annotated functional genomics data and a plasmid library of the yeast genome (Hvorecny & Prelich, 2010) in unraveling the molecular mechanism by which an aneuploid karyotype confers a particular phenotype.
However, many questions remain unanswered. For example, as a large portion of yeast CIN genes involved in cellular pathways is not connected to chromosome stability, additional mechanisms have yet to be identified that would lead to aneuploidy (Stirling, et al., 2011). Existing data has clearly demonstrated the adaptive potential of aneuploidy under acute stress, but whether aneuploidy could contribute to long term adaptive evolution is less clear. A recent experimental evolution study suggested that aneuploidy per se might not serve as a stable and sustainable evolutionary solution (Yona, et al., 2012). Yona et al. observed that when diploid budding yeast grew under heat and high pH conditions, aneuploids were observed first but were eventually replaced by gene mutations. Thus, aneuploidy may be a quick and rough fix under strong selective pressure, allowing sufficient propagation of the population for the emergence of adaptive mutations with less fitness cost than aneuploidy. Giving the constantly changing environment where unicellular organisms live, it is tempting to speculate that natural selection might favor the evolution of mechanisms that would modulate chromosome segregation fidelity based on the presence of environmental stress, such as critically involving a stress-handling chaperone (Hsp90) in the kinetochore function.
Another key question is, to what extent can the knowledge we have gained about aneuploidy in yeast be applied to multicellular organisms? As chromosome segregation is a highly conserved cellular process in eukaryotes, it is safe to say that the mechanisms of CIN in yeast could also be applied to human. Multicellular organisms may respond differently to aneuploidy as compared to yeast. For instance, whole chromosome aneuploidy is largely detrimental in multicellular species (Williams, et al., 2008). At the cellular level, it has been suggested that the proliferation of aneuploid mouse and human cells could be limited through the p53 pathway, which do not exist in yeast (Belyi, et al., 2010, Thompson & Compton, 2010). Nonetheless, most cancer cells inactivate the p53 pathway and could evolve through the production of aneuploidy (Belyi, et al., 2010, Navin, et al., 2011). Besides aneuploidy, cancer cells also hold mutations and epigenetic de-regulation. Nevertheless, yeast will remain a useful experimental model for future elucidation of the interplay between small and large genetic changes and their influence on the epigenome and vice versa.
Acknowledgments
We would like to thank Tamara Potapova, Guangbo Chen, Hung-Ji Tsai and Sarah Smith for comments on the manuscript, and Rosella Partida-Prins for editorial assistance. We are grateful to the other Rong Li lab members for insightful discussions. This work was supported by the NIH grant RO1-GM059964 to Rong Li. Jin Zhu is a graduate student registered with the Open University.
References
- Abbey D, Hickman M, Gresham D, Berman J. High-Resolution SNP/CGH Microarrays Reveal the Accumulation of Loss of Heterozygosity in Commonly Used Candida albicans Strains. G3 (Bethesda) 2011;1:523–530. doi: 10.1534/g3.111.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera A, Gomez-Gonzalez B. Genome instability: a mechanistic view of its causes and consequences. Nat Rev Genet. 2008;9:204–217. doi: 10.1038/nrg2268. [DOI] [PubMed] [Google Scholar]
- Anders K, Kudrna J, Keller K, et al. A strategy for constructing aneuploid yeast strains by transient nondisjunction of a target chromosome. BMC Genetics. 2009;10:36. doi: 10.1186/1471-2156-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbour M, Epp E, Hogues H, et al. Widespread occurrence of chromosomal aneuploidy following the routine production of Candida albicans mutants. FEMS Yeast Res. 2009;9:1070–1077. doi: 10.1111/j.1567-1364.2009.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird NA, Etter PD, Atwood TS, et al. Rapid SNP discovery and genetic mapping using sequenced RAD markers. PLoS One. 2008;3:e3376. doi: 10.1371/journal.pone.0003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyi VA, Ak P, Markert E, Wang H, Hu W, Puzio-Kuter A, Levine AJ. The origins and evolution of the p53 family of genes. Cold Spring Harb Perspect Biol. 2010;2:a001198. doi: 10.1101/cshperspect.a001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett RJ, Johnson AD. Completion of a parasexual cycle in Candida albicans by induced chromosome loss in tetraploid strains. EMBO J. 2003;22:2505–2515. doi: 10.1093/emboj/cdg235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchler JA. Reflections on studies of gene expression in aneuploids. Biochem J. 2010;426:119–123. doi: 10.1042/BJ20091617. [DOI] [PubMed] [Google Scholar]
- Botstein D, Fink GR. Yeast: an experimental organism for 21st Century biology. Genetics. 2011;189:695–704. doi: 10.1534/genetics.111.130765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchonville K, Forche A, Tang KE, Selmecki A, Berman J. Aneuploid chromosomes are highly unstable during DNA transformation of Candida albicans. Eukaryot Cell. 2009;8:1554–1566. doi: 10.1128/EC.00209-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveri T. Zur Frage der Entstehung maligner Tumoren. Jena, Gustav Fisher Verlag, Germany. 1914 [Google Scholar]
- Charles JS, Hamilton ML, Petes TD. Meiotic Chromosome Segregation in Triploid Strains of Saccharomyces cerevisiae. Genetics. 2010;186:537–550. doi: 10.1534/genetics.110.121533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Bradford WD, Seidel CW, Li R. Hsp90 stress potentiates rapid cellular adaptation through induction of aneuploidy. Nature. 2012;482:246–250. doi: 10.1038/nature10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Magee BB, Dawson D, Magee PT, Kumamoto CA. Chromosome 1 trisomy compromises the virulence of Candida albicans. Mol Microbiol. 2004;51:551–565. doi: 10.1046/j.1365-2958.2003.03852.x. [DOI] [PubMed] [Google Scholar]
- Conde J, Fink GR. A mutant of Saccharomyces cerevisiae defective for nuclear fusion. Proceedings of the National Academy of Sciences. 1976;73:3651–3655. doi: 10.1073/pnas.73.10.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste A, Selmecki A, Forche A, et al. Genotypic evolution of azole resistance mechanisms in sequential Candida albicans isolates. Eukaryot Cell. 2007;6:1889–1904. doi: 10.1128/EC.00151-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z, Halicka HD, Zhao H. Analysis of cellular DNA content by flow and laser scanning cytometry. Adv Exp Med Biol. 2010;676:137–147. doi: 10.1007/978-1-4419-6199-0_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didelot X, Bowden R, Wilson DJ, Peto TE, Crook DW. Transforming clinical microbiology with bacterial genome sequencing. Nat Rev Genet. 2012;13:601–612. doi: 10.1038/nrg3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion B, Brown GW. Comparative genome hybridization on tiling microarrays to detect aneuploidies in yeast. Methods Mol Biol. 2009;548:1–18. doi: 10.1007/978-1-59745-540-4_1. [DOI] [PubMed] [Google Scholar]
- Doi M, Homma M, Chindamporn A, Tanaka K. Estimation of chromosome number and size by pulsed-field gel electrophoresis (PFGE) in medically important Candida species. J Gen Microbiol. 1992;138:2243–2251. doi: 10.1099/00221287-138-10-2243. [DOI] [PubMed] [Google Scholar]
- Dudarewicz L, Holzgreve W, Jeziorowska A, Jakubowski L, Zimmermann B. Molecular methods for rapid detection of aneuploidy. J Appl Genet. 2005;46:207–215. [PubMed] [Google Scholar]
- Duncan AW. Aneuploidy, polyploidy and ploidy reversal in the liver. Semin Cell Dev Biol. 2013;24:347–356. doi: 10.1016/j.semcdb.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Forche A, Abbey D, Pisithkul T, et al. Stress Alters Rates and Types of Loss of Heterozygosity in Candida albicans. mBio. 2011;2 doi: 10.1128/mBio.00129-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresham D, Desai MM, Tucker CM, et al. The repertoire and dynamics of evolutionary adaptations to controlled nutrient-limited environments in yeast. PLoS Genet. 2008;4:e1000303. doi: 10.1371/journal.pgen.1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase SB, Lew DJ. Flow cytometric analysis of DNA content in budding yeast. Methods Enzymol. 1997;283:322–332. doi: 10.1016/s0076-6879(97)83026-1. [DOI] [PubMed] [Google Scholar]
- Hartwell LH, Smith D. Altered fidelity of mitotic chromosome transmission in cell cycle mutants of S. cerevisiae. Genetics. 1985;110:381–395. doi: 10.1093/genetics/110.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A, Bloom K. Genetic manipulation of centromere function. Molecular and Cellular Biology. 1987;7:2397–2405. doi: 10.1128/mcb.7.7.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenlohe PA, Bassham S, Etter PD, Stiffler N, Johnson EA, Cresko WA. Population genomics of parallel adaptation in threespine stickleback using sequenced RAD tags. PLoS Genet. 2010;6:e1000862. doi: 10.1371/journal.pgen.1000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland AJ, Cleveland DW. Boveri revisited: chromosomal instability, aneuploidy and tumorigenesis. Nat Rev Mol Cell Biol. 2009;10:478–487. doi: 10.1038/nrm2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Wang J, Choi J, et al. Variation in chromosome copy number influences the virulence of Cryptococcus neoformans and occurs in isolates from AIDS patients. BMC Genomics. 2011;12:526. doi: 10.1186/1471-2164-12-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TR, Roberts CJ, Dai H, et al. Widespread aneuploidy revealed by DNA microarray expression profiling. Nat Genet. 2000;25:333–337. doi: 10.1038/77116. [DOI] [PubMed] [Google Scholar]
- Hvorecny KL, Prelich G. A systematic CEN library of the Saccharomyces cerevisiae genome. Yeast. 2010;27:861–865. doi: 10.1002/yea.1783. [DOI] [PubMed] [Google Scholar]
- Ibrahim AS, Magee BB, Sheppard DC, et al. Effects of ploidy and mating type on virulence of Candida albicans. Infect Immun. 2005;73:7366–7374. doi: 10.1128/IAI.73.11.7366-7374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janbon G, Sherman F, Rustchenko E. Monosomy of a specific chromosome determines L-sorbose utilization: a novel regulatory mechanism in Candida albicans. Proc Natl Acad Sci U S A. 1998;95:5150–5155. doi: 10.1073/pnas.95.9.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanellis P, Gagliardi M, Banath JP, et al. A Screen for Suppressors of Gross Chromosomal Rearrangements Identifies a Conserved Role for PLP in Preventing DNA Lesions. PLoS Genet. 2007;3:e134. doi: 10.1371/journal.pgen.0030134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein HL. Spontaneous Chromosome Loss in Saccharomyces cerevisiae Is Suppressed by DNA Damage Checkpoint Functions. Genetics. 2001;159:1501–1509. doi: 10.1093/genetics/159.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M. Evolution of the mutation rate. Trends Genet. 2010;26:345–352. doi: 10.1016/j.tig.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack M, Gompel-Klein P, Haase E, Hietkamp J, Ruhland A, Brendel M. Genetic characterization of hyperresistance to formaldehyde and 4-nitroquinoline-N-oxide in the yeast Saccharomyces cerevisiae. Mol Gen Genet. 1988;211:260–265. doi: 10.1007/BF00330602. [DOI] [PubMed] [Google Scholar]
- Magee BB, Magee PT. Induction of mating in Candida albicans by construction of MTLa and MTLalpha strains. Science. 2000;289:310–313. doi: 10.1126/science.289.5477.310. [DOI] [PubMed] [Google Scholar]
- Maringele L, Lydall D. Pulsed-field gel electrophoresis of budding yeast chromosomes. Methods Mol Biol. 2006;313:65–73. doi: 10.1385/1-59259-958-3:065. [DOI] [PubMed] [Google Scholar]
- Mayer VW, Aguilera A. High levels of chromosome instability in polyploids of Saccharomyces cerevisiae. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 1990;231:177–186. doi: 10.1016/0027-5107(90)90024-x. [DOI] [PubMed] [Google Scholar]
- McGranahan N, Burrell RA, Endesfelder D, Novelli MR, Swanton C. Cancer chromosomal instability: therapeutic and diagnostic challenges. EMBO Rep. 2012;13:528–538. doi: 10.1038/embor.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo LM, Pepper JW, Reid BJ, Maley CC. Cancer as an evolutionary and ecological process. Nat Rev Cancer. 2006;6:924–935. doi: 10.1038/nrc2013. [DOI] [PubMed] [Google Scholar]
- Mitchell AP, Ni M, Feretzaki M, et al. Unisexual and Heterosexual Meiotic Reproduction Generate Aneuploidy and Phenotypic Diversity De Novo in the Yeast Cryptococcus neoformans. PLoS Biology. 2013;11:e1001653. doi: 10.1371/journal.pbio.1001653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow CA, Fraser JA. Ploidy variation as an adaptive mechanism in human pathogenic fungi. Semin Cell Dev Biol. 2013 doi: 10.1016/j.semcdb.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- Nagaoka SI, Hassold TJ, Hunt PA. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet. 2012;13:493–504. doi: 10.1038/nrg3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navin N, Kendall J, Troge J, et al. Tumour evolution inferred by single-cell sequencing. Nature. 2011;472:90–94. doi: 10.1038/nature09807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekrutenko A, Taylor J. Next-generation sequencing data interpretation: enhancing reproducibility and accessibility. Nat Rev Genet. 2012;13:667–672. doi: 10.1038/nrg3305. [DOI] [PubMed] [Google Scholar]
- Ngamskulrungroj P, Chang Y, Hansen B, Bugge C, Fischer E, Kwon-Chung KJ. Characterization of the chromosome 4 genes that affect fluconazole-induced disomy formation in Cryptococcus neoformans. PLoS One. 2012;7:e33022. doi: 10.1371/journal.pone.0033022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson-Tillgren T, Petersen JL, Holmberg S, Kielland-Brandt M. Transfer of chromosome III during kar mediated cytoduction in yeast. Carlsberg Research Communications. 1980;45:113–117. [Google Scholar]
- Niwa O, Yanagida M. Triploid meiosis and aneuploidy in Schizosaccharomyces pombe: an unstable aneuploid disomic for chromosome III. Current Genetics. 1985;9:463–470. [Google Scholar]
- Niwa O, Tange Y, Kurabayashi A. Growth arrest and chromosome instability in aneuploid yeast. Yeast. 2006;23:937–950. doi: 10.1002/yea.1411. [DOI] [PubMed] [Google Scholar]
- Niwa O, Tange Y, Kurabayashi A. Growth arrest and chromosome instability in aneuploid yeast. Yeast. 2006;23:937–950. doi: 10.1002/yea.1411. [DOI] [PubMed] [Google Scholar]
- Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- O'Brien FG, Udo EE, Grubb WB. Contour-clamped homogeneous electric field electrophoresis of Staphylococcus aureus. Nat Protoc. 2006;1:3028–3033. doi: 10.1038/nprot.2006.382. [DOI] [PubMed] [Google Scholar]
- Ouspenski II, Elledge SJ, Brinkley BR. New yeast genes important for chromosome integrity and segregation identified by dosage effects on genome stability. Nucleic Acids Research. 1999;27:3001–3008. doi: 10.1093/nar/27.15.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page BD, Snyder M. Chromosome Segregation in Yeast. Annual Review of Microbiology. 1993;47:231–261. doi: 10.1146/annurev.mi.47.100193.001311. [DOI] [PubMed] [Google Scholar]
- Pardo B, Aguilera A. Complex chromosomal rearrangements mediated by break-induced replication involve structure-selective endonucleases. PLoS Genet. 2012;8:e1002979. doi: 10.1371/journal.pgen.1002979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry EM, Cox BS. The tolerance of aneuploidy in yeast. Genetics Research. 1970;16:333–340. doi: 10.1017/s0016672300002597. [DOI] [PubMed] [Google Scholar]
- Pavelka N, Rancati G, Li R. Dr Jekyll and Mr Hyde: role of aneuploidy in cellular adaptation and cancer. Curr Opin Cell Biol. 2010;22:809–815. doi: 10.1016/j.ceb.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavelka N, Rancati G, Zhu J, et al. Aneuploidy confers quantitative proteome changes and phenotypic variation in budding yeast. Nature. 2010;468:321–325. doi: 10.1038/nature09529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkel D, Albertson DG. Array comparative genomic hybridization and its applications in cancer. Nat Genet. 2005;37(Suppl):S11–S17. doi: 10.1038/ng1569. [DOI] [PubMed] [Google Scholar]
- Polakova S, Blume C, Zarate JA, Mentel M, Jorck-Ramberg D, Stenderup J, Piskur J. Formation of new chromosomes as a virulence mechanism in yeast Candida glabrata. Proc Natl Acad Sci U S A. 2009;106:2688–2693. doi: 10.1073/pnas.0809793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porman AM, Alby K, Hirakawa MP, Bennett RJ. Discovery of a phenotypic switch regulating sexual mating in the opportunistic fungal pathogen Candida tropicalis. Proc Natl Acad Sci U S A. 2011;108:21158–21163. doi: 10.1073/pnas.1112076109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potapova T, Zhu J, Li R. Aneuploidy and chromosomal instability: a vicious cycle driving cellular evolution and cancer genome chaos. Cancer and Metastasis Reviews. 2013:1–13. doi: 10.1007/s10555-013-9436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancati G, Pavelka N, Fleharty B, et al. Aneuploidy underlies rapid adaptive evolution of yeast cells deprived of a conserved cytokinesis motor. Cell. 2008;135:879–893. doi: 10.1016/j.cell.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehen SK, Yung YC, McCreight MP, et al. Constitutional aneuploidy in the normal human brain. J Neurosci. 2005;25:2176–2180. doi: 10.1523/JNEUROSCI.4560-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RJD, Sunjevaric I, Voth WP, et al. Chromosome-Scale Genetic Mapping Using a Set of 16 Conditionally Stable Saccharomyces cerevisiae Chromosomes. Genetics. 2008;180:1799–1808. doi: 10.1534/genetics.108.087999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis CC, Batista S, Ferreira MG. The fission yeast MRN complex tethers dysfunctional telomeres for NHEJ repair. EMBO J. 2012;31:4576–4586. doi: 10.1038/emboj.2012.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustchenko EP, Howard DH, Sherman F. Chromosomal alterations of Candida albicans are associated with the gain and loss of assimilating functions. J Bacteriol. 1994;176:3231–3241. doi: 10.1128/jb.176.11.3231-3241.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmecki A, Forche A, Berman J. Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Science. 2006;313:367–370. doi: 10.1126/science.1128242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmecki A, Gerami-Nejad M, Paulson C, Forche A, Berman J. An isochromosome confers drug resistance in vivo by amplification of two genes, ERG11 and TAC1. Mol Microbiol. 2008;68:624–641. doi: 10.1111/j.1365-2958.2008.06176.x. [DOI] [PubMed] [Google Scholar]
- Selmecki AM, Dulmage K, Cowen LE, Anderson JB, Berman J. Acquisition of aneuploidy provides increased fitness during the evolution of antifungal drug resistance. PLoS Genet. 2009;5:e1000705. doi: 10.1371/journal.pgen.1000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheltzer JM, Torres EM, Dunham MJ, Amon A. Transcriptional consequences of aneuploidy. Proc Natl Acad Sci U S A. 2012;109:12644–12649. doi: 10.1073/pnas.1209227109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheltzer JM, Blank HM, Pfau SJ, et al. Aneuploidy drives genomic instability in yeast. Science. 2011;333:1026–1030. doi: 10.1126/science.1206412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol Rev. 2012;36:288–305. doi: 10.1111/j.1574-6976.2011.00278.x. [DOI] [PubMed] [Google Scholar]
- Sionov E, Chang YC, Garraffo HM, Kwon-Chung KJ. Heteroresistance to fluconazole in Cryptococcus neoformans is intrinsic and associated with virulence. Antimicrob Agents Chemother. 2009;53:2804–2815. doi: 10.1128/AAC.00295-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sionov E, Lee H, Chang YC, Kwon-Chung KJ. Cryptococcus neoformans overcomes stress of azole drugs by formation of disomy in specific multiple chromosomes. PLoS Pathog. 2010;6:e1000848. doi: 10.1371/journal.ppat.1000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Hwang J-Y, Banerjee S, Majeed A, Gupta A, Myung K. Mutator genes for suppression of gross chromosomal rearrangements identified by a genome-wide screening in Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:9039–9044. doi: 10.1073/pnas.0403093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Petes TD. Haploidization in Saccharomyces cerevisiae induced by a deficiency in homologous recombination. Genetics. 2012;191:279–284. doi: 10.1534/genetics.111.138180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M, Weissman JS, Kirschner MW. A general lack of compensation for gene dosage in yeast. Mol Syst Biol. 2010;6:368. doi: 10.1038/msb.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Charles J, Hamilton ML, Petes TD. Meiotic chromosome segregation in triploid strains of Saccharomyces cerevisiae. Genetics. 2010;186:537–550. doi: 10.1534/genetics.110.121533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling PC, Bloom MS, Solanki-Patil T, et al. The Complete Spectrum of Yeast Chromosome Instability Genes Identifies Candidate CIN Cancer Genes and Functional Roles for ASTRA Complex Components. PLoS Genet. 2011;7:e1002057. doi: 10.1371/journal.pgen.1002057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storchova Z, Breneman A, Cande J, Dunn J, Burbank K, O'Toole E, Pellman D. Genome-wide genetic analysis of polyploidy in yeast. Nature. 2006;443:541–547. doi: 10.1038/nature05178. [DOI] [PubMed] [Google Scholar]
- Tan Z, Hays M, Cromie GA, et al. Aneuploidy underlies a multicellular phenotypic switch. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1301047110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YC, Amon A. Gene copy-number alterations: a cost-benefit analysis. Cell. 2013;152:394–405. doi: 10.1016/j.cell.2012.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas F, Fisher D, Fort P, et al. Applying ecological and evolutionary theory to cancer: a long and winding road. Evol Appl. 2013;6:1–10. doi: 10.1111/eva.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SL, Compton DA. Proliferation of aneuploid human cells is limited by a p53-dependent mechanism. J Cell Biol. 2010;188:369–381. doi: 10.1083/jcb.200905057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn RR, Gonzalez C, Brar GA, et al. Aneuploid yeast strains exhibit defects in cell growth and passage through START. Mol Biol Cell. 2013;24:1274–1289. doi: 10.1091/mbc.E12-07-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolliday N, Pitcher M, Li R. Direct evidence for a critical role of myosin II in budding yeast cytokinesis and the evolvability of new cytokinetic mechanisms in the absence of myosin II. Mol Biol Cell. 2003;14:798–809. doi: 10.1091/mbc.E02-09-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres EM, Sokolsky T, Tucker CM, Chan LY, Boselli M, Dunham MJ, Amon A. Effects of aneuploidy on cellular physiology and cell division in haploid yeast. Science. 2007;317:916–924. doi: 10.1126/science.1142210. [DOI] [PubMed] [Google Scholar]
- Torres EM, Dephoure N, Panneerselvam A, et al. Identification of aneuploidy-tolerating mutations. Cell. 2010;143:71–83. doi: 10.1016/j.cell.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghmare SK, Bruschi CV. Differential chromosome control of ploidy in the yeast Saccharomyces cerevisiae. Yeast. 2005;22:625–639. doi: 10.1002/yea.1226. [DOI] [PubMed] [Google Scholar]
- Wilkening S, Tekkedil MM, Lin G, et al. Genotyping 1000 yeast strains by next-generation sequencing. BMC Genomics. 2013;14:90. doi: 10.1186/1471-2164-14-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BR, Prabhu VR, Hunter KE, Glazier CM, Whittaker CA, Housman DE, Amon A. Aneuploidy affects proliferation and spontaneous immortalization in mammalian cells. Science. 2008;322:703–709. doi: 10.1126/science.1160058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yona AH, Manor YS, Herbst RH, et al. Chromosomal duplication is a transient evolutionary solution to stress. Proc Natl Acad Sci U S A. 2012;109:21010–21015. doi: 10.1073/pnas.1211150109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen KWY, Warren CD, Chen O, Kwok T, Hieter P, Spencer FA. Systematic genome instability screens in yeast and their potential relevance to cancer. Proceedings of the National Academy of Sciences. 2007;104:3925–3930. doi: 10.1073/pnas.0610642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Pavelka N, Bradford WD, Rancati G, Li R. Karyotypic determinants of chromosome instability in aneuploid budding yeast. PLoS Genet. 2012;8:e1002719. doi: 10.1371/journal.pgen.1002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong C, Lu S, Chapman AR, Xie XS. Genome-wide detection of single-nucleotide and copy-number variations of a single human cell. Science. 2012;338:1622–1626. doi: 10.1126/science.1229164. [DOI] [PMC free article] [PubMed] [Google Scholar]