Abstract
Papillomaviruses enter basal cells of stratified epithelia. Assembly of new virions occurs in infected cells during terminal differentiation. This unique biology is reflected in the mechanism of entry. Extracellularly, the interaction of nonenveloped capsids with several host cell proteins, after binding, results in discrete conformational changes. Asynchronous internalization occurs over several hours by an endocytic mechanism related to, but distinct from macropinocytosis. Intracellular trafficking leads virions through the endosomal system, and from late endosomes to the trans-Golgi-network, prior to nuclear delivery. Here, we discuss the existing data with the aim to synthesize an integrated model of the stepwise process of entry, thereby highlighting key open questions. Additionally, we relate data from experiments with cultured cells to in vivo results.
INTRODUCTION
Papillomaviruses (PV) are a large family of viruses with transforming potential, several of which are implicated in anogenital cancers and tumors of the head and neck [1]. PV particles are nonenveloped icosahedrons (T=7) with a diameter of 50-55 nm. This capsid is formed by 72 pentamers of the major structural protein, L1, and variable amounts (up to 72 copies) of the minor structural protein, L2 [2,3]. The encapsidated genome is a circular, double-stranded DNA. These particles mediate transmission and entry through mechanisms that are currently unique amongst viruses. A particularly interesting feature is the protracted residence of viral particles on the cell surface prior to endocytic uptake and the extended time until infection is established [4-8]. Many of the atypical aspects of PV infections are likely adaptations due to the restriction of the productive life cycle to the terminally differentiating stratified squamous epithelium, and the ability to avoid induction of a host immune response [1]. The former issue has presented an experimental challenge as authentic viruses are not readily available for entry studies (see below).
A variety of in vitro systems have been used to produce surrogate viral particles. Non-infectious virus-like particles (VLPs) formed by L1 or L1 and L2, mimic the conformation of authentic virus [9]. They are the basis for the current vaccines, which attests to their authenticity at an immunologic level. Pseudovirions (PsV), harbor a plasmid, which encodes a reporter protein and serves as a viral pseudogenome [10]. Entry of VLPs can be followed by biochemical methods and microscopy, whereas expression of the PsV reporter indicates a successful “pseudoinfection”. Therefore PsV are used for the majority of current PV research that is focused on entry, both in vitro and in vivo. In another in vitro PV production system the viral genome is transfected into primary keratinocytes, which are subsequently grown to differentiate into three-dimensional epithelium termed an organotypic raft. Virions are produced in the upper layers of the raft culture which mimics the natural situation [11]. However, with this method it is difficult to obtain particles of sufficient purity to adequately perform microscopic analyses of virus entry.
The range of methods to produce proxy PV virions poses a caveat to the comparison of different studies, as the purity and quality of the particles varies. If many defective or empty particles are added alongside legitimate particles, the high total dose may affect the outcome of infection. Many studies within the existing literature lack information on particle quality and quantity. We propose that subsequent work should document measures such as viral genome equivalents and viral protein amounts (or particle number) per cell.
In this review, we outline the emerging concepts of how incoming PV engage receptors, induce endocytosis, traffic intracellularly to the nuclear site of replication, and how structural alterations of the capsid may facilitate these processes and the release of the viral genome for eventual replication. Moreover, the data from in vivo studies is highlighted. Please refer to several recent reviews for a more detailed discussion on particular aspects of PV entry [1,12-20].
BINDING
Several lines of evidence have established that PV initially bind to the glycosaminoglycan (GAG) chains of heparan sulfate proteoglycans (HSPG). Early work showed that L1 VLPs interact with immobilized heparin, and that soluble heparin inhibits VLP binding to cells [21]. Later work demonstrated the importance of this interaction for PsV infection of cultured cells [6]. PV can also bind to the extracellular matrix (ECM) of cultured cells through interactions with HSPG and laminin-332 [22-25]. Laminin-332 can serve as a transient binding receptor, but appears to be dispensable for infection of cultured cells.
Mutational analysis and x-ray crystallography of L1 capsomers indicated charge-based interactions of PV with heparin at minimally four different sites [30,31]. It appears that PV do not require a specific HSPG protein core for binding and infection [32,33]. Since O-sulfation but not N-sulfation of HS moieties is required for infection [34,35], specificity for binding and entry is instead conferred by the sequence of GAGs and their sulfation pattern [22].
Although the majority of the PV literature stresses the importance of PV interaction with HS moieties for binding and infection, a few studies suggest that certain PV types or virion preparations may infect cells independent of HSPGs [26-29].
EXTRACELLULAR STRUCTURAL CHANGES
After initial binding, several discrete structural changes in human PV (HPV virions) have been described. Post-binding conformational changes were first suggested by a shift from a heparin-sensitive to a heparin-insensitive form of the virus [6]. PV interaction with HS-GAGs induces the exposure of a linear epitope located in the cleft between capsomers [22]. Additionally, a critical conformational change exposes the amino terminus of L2, which is originally buried within the capsid. This exposure appears to require extracellular cyclophilin B (CyPB), a cellular petidyl-prolyl cis/trans isomerase [36]. The exposed L2 N-terminus contains a furin/proprotein convertase cleavage site, which is conserved amongst most PV [37]. In vitro, this site is proteolytically cleaved on the cell surface and cleavage is essential for successful infection. Importantly, these early, HSPG-dependent events have been shown to occur on the extracellular basement membrane (BM) in the murine cervico-vaginal model of HPV16 infection [38].
Current data indicate that these structural alterations cause a reduced affinity of virus to HSPG that is required for subsequent engagement of a secondary receptor and primes the virus for later steps in entry, including uncoating and membrane penetration [35]. If HSPG release is blocked, the virus is channeled into a non-infectious uptake pathway [25]. This suggests that, following HSPG interaction, the virus engages a second, HSPG-independent cellular receptor. Further support for a HSPG-independent receptor stems from the finding that virions in which L2 has been precleaved by furin can bind to and infect HSPG-deficient cells [39]. The interaction with the putative secondary receptor may trigger infectious uptake. The cleavage of L2 appears to further facilitate membrane penetration, a later step during entry [37].
INTERNALIZATION RECEPTOR
The identity of the putative secondary receptor has remained elusive despite extensive efforts to identify it. This could indicate that it is not a single molecule. The candidate receptors thus far include alpha 6 integrin, tetraspanin CD151, and annexin A2 heterotetramer [24,40-46]. All of these have the attractive feature of high expression within the basal epithelium, the in vivo target cells for PV infection. All are also known to be associated with HSPG complexes. However, alpha 6 integrin and annexin A2 cannot be considered obligate receptors as deficient cell lines can be infected.
A rather unconventional mode of receptor engagement has been suggested, in which PVs and growth factors attached to HSPG mediate PV entry [47]. This idea entails attachment of particles to HSPG on cells, removal of the HSPG-virus complex by host metalloproteinases, and reattachment of these complexes to growth factor receptors via growth factors attached to the shed virus-HSPG complex. Although experimental evidence exists that cells can be infected in vitro by this mechanism, it is unclear to what extent this mechanism contributes to infection versus direct cell surface interaction and entry. It is not an obligate entry step as ECM-bound virus that is cleaved by furin can efficiently infect HSPG-deficient cells [39]. There is currently no evidence that this mechanism contributes to infection in vivo.
DYNAMICS OF CELL SURFACE INTERACTIONS
There is limited information on the dynamics of PV interactions prior to internalization. Data for virus ensembles suggests that binding is relatively quick but PV endocytose rather slowly [4-8]. The reason for this protracted cell surface residence is undetermined. Single virus tracking revealed different types of lateral movement on the plasma membrane including diffusive and directed motions prior to confinement [48]. The directed motion (surfing) is most prominent on filopodia [48,49]. Powered by actin retrograde flow, this motion propels bound virions towards the cell body [48]. It is unclear how important this motion is for infection in vivo, but it is well established that filopodia play a major role in epithelial wound healing which appears to be critical for in vivo infection [50] A second study showed that single endocytic events can occur within two minutes of confinement in live cells [7]. This suggested that the formation of an endocytic vesicle is quick, and that the slow uptake reflects an asynchronous mode of internalization is likely to be due to a stochastic and rate-limiting nature of conformational changes and/or of secondary receptor engagement.
ENDOCYTOSIS
The early literature on PV endocytosis has little concurrence and indicated uptake by both clathrin-mediated and clathrin-independent mechanisms [8,51-55], likely due to a combination of factors.These include the variety of proxy particles used, pleiotropic inhibitor effects, the potential cell type-dependent use of different mechanisms, and the complexity of endocytic pathways [56]. A recent comprehensive analysis focused on HPV16 endocytosis into keratinocyte-derived cells, using PsV-based assays, indicates vesicle formation during PV endocytosis is clathrin-, caveolin-, and lipid raft-independent [7]. The cellular machinery facilitating HPV16 endocytosis is most congruent with macropinocytosis, as it involves actin dynamics, receptor tyrosine kinase and p21-activated kinase signaling, and as it is sensitive to amiloride. However, in contrast to macropinocytosis, HPV16 endocytosis forms small, uncoated vesicles rather than large membrane protrusions. Thus, PV particles likely are internalized by a novel, ligand-activated endocytic pathway.
This is supported by studies that report an actin-dependent, clathrin-, caveolin-, and lipid raft-independent uptake of HPV16, and that implicate tetraspanin-enriched microdomains (TEM) as entry mediators [44,57]. Live cell microscopy revealed that HPV16 co-migrates with the tetraspanin CD151 and, moreover, co-internalizes with HPV16 into cells [43]. Interestingly, uptake occurs independently of the tyrosine-based sorting motif of CD151 necessary for sorting into clathrin-coated pits, but requires motifs related to integrin-associated functions. This uptake mechanism is common for several PV types [45].
SIGNALING
Virus binding to receptors often triggers signaling events that facilitate infection. Consistent with a macropinocytosis-like pathway, inhibition of receptor tyrosine kinases, protein kinase C (PKC), PI3 kinase (PI3K), and p21-activated kinase (PAK-1) prevents uptake into cells and infection [7,47]. Other work has implicated signaling by the focal adhesion kinase (FAK), but not Src family kinases, in HPV uptake and infection [58].
Several studies describe the rapid and transient activation of signaling cascades after cell exposure to VLPs and PsV. The activation of the Ras/MAPK pathway, and their downstream targets, by VLPs has been suggested to induce cell proliferation early after binding [59]. A recent study reports the activation of growth factor receptor kinases as early as 5 min post virus-host cell interaction, which appears to be responsible for the induction of the Ras/MAPK pathway [47]. Further receptor kinase targets are PI3K and its effector Akt, which are activated 10 min post VLP binding [60]. A recent study suggests that activation of the PI3K downstream effectors PTEN and mTOR leads to inhibition of autophagy [61]. The observation that inhibition of autophagy facilitates infection suggests that autophagy is a cellular mechanism to restrict infection [61,62]. In addition, a rapid, transient activation of FAK, involving integrins, has been seen to occur as early as 10 min after virus addition [58].
Despite the finding that similar signaling molecules are activated after PV binding and are required for PV endocytosis, it is troubling that the kinetics of activation and uptake are inherently different. The underlying cause for this discrepancy is not clear. One reason may be the nonphysiologically high viral dose used in activation studies. Alternatively, it may indicate a biphasic activation of cellular signaling cascades during HPV infection: a fast activation caused by binding of PV to cells that remains in search of a function, and an asynchronous activation caused by secondary receptor engagement required for internalization.
VESICULAR TRAFFICKING AND UNCOATING
Following endocytosis, PV are trafficked through the endosomal system. This was initially suggested by the requirement for a low pH-dependent step in infection [7,44,52,55,57]. This step occurs on average 2-3h post internalization indicating prolonged residence in the endosomal system [7]. PV require delivery to early compartments such as early endosomes or the macropinosomes, as infectivity depends on Rab5 [7,55], a member of the Rab GTPase family that mark distinct endosomes, and that facilitate sorting between endosomes and maturation of the endosomal system [63]. In live cells, PV associate only briefly with Rab5-positive structures [7]. Then, PV particles are detected in multivesicular endosomes and late endosomes (LE)/lysosomes, supporting the idea that they follow the degradative arm of the endosomal system [7,44]. In the presence of lysosomotropic agents, PV accumulate in LE. Interestingly, if the inhibitor is removed after PV accumulation, infection is unperturbed, suggesting that LE are viable compartments for infection [7]. As several studies report that Rab7a, responsible for endosome maturation and vesicular transport to late compartments, is not required for infection [7,55,64], it appears that PV uses an unconventional mode of delivery to LE like e.g. lymphocytic choriomeningitis virus [65].
Within endosomes further structural changes occur that result in at least partial uncoating of the virus. Two assays have been developed to examine PV uncoating. They detect exposure of either an interior region of L2 or the viral pseudogenome labeled with BrdU during production [66]. Although it is not entirely clear what triggers uncoating, the need for a low pH-dependent step has been suggested. Additionally, co-internalized CyBP facilitates the segregation of L1 and the pseudogenome within endosomes [67]. A later study showed that L1 is retained within LE, and that a subviral structure composed of L2 and the pseudogenome is transferred to the trans-Golgi network (TGN) [64]. This study also showed that the transit of L2 and the genome from LE to the TGN is dependent on both Rab7b and Rab9a. Consistent with previous reports, no role for Rab7a was found. A second study on Golgi involvement used a genome-wide RNA interference screen to identify novel factors in HPV entry [68]. These results indicated the requirement of retromer-mediated processes, in addition to Rab9a, Rab7b and Rab7a, for transit to the TGN. The retromer complex mediates cargo delivery from the early endosome to the TGN [69]. It is unclear whether PV uses both mechanisms independently, or whether perturbation of one of the pathways interferes with the function of the other. Based on results from a very sensitive proximity ligation assay, this study also suggests that despite L1 and L2 segregation within the endosomal system a portion of L1 may reach the TGN [68].
Besides the dependency of Rab-mediated sorting events, sorting nexin 17 (SNX17) has been reported to interact with L2 of several HPV [70,71]. SNX are involved in several endosomal sorting events. Specifically, SNX17 is involved in preventing degradation of membrane receptors by facilitating their recycling to the plasmamembrane [72-74]. Depletion of SNX17 induces increased lysosomal degradation of L2 and interferes with infection [71]. The potential of L2 to biochemically interact with SNX17 has led to the hypothesis that this physical interaction prevents routing of virions to the lysosomes. It is therefore interesting that the mapped interaction site on L2 would require the translocation of a substantial part of L2 to the cytosolic leaflet of endosomal membranes in order to physically interact with the cytosolic SNX17.
The carboxyl terminus of L2 has been described to mediate membrane penetration of both L2 and the genome from the endosomal system [75]. How this observation meshes with the hypothesized interaction with SNX17 remains to be determined as, in this scenario, only a small portion of L2 would function as a transmembrane domain. It has been suggested that insertion of this L2 C-terminal domain into membranes would allow for membrane penetration [76]. However, to date, there is no evidence for a membrane insertion of L2 during entry.
The involvement of microtubules in PV intracellular trafficking has been established by multiple studies. The microtubule disrupting drug nocodazole inhibits PV infection until a late step in infection [7,52,57]. This is unsurprising as endosomal trafficking occurs along microtubules. However, since L2 of HPV16 and HPV33 can interact with the minus-end directed, microtubular motor protein dynein, PV subviral structures are likely transported along microtubules within the cytosol [77]. More specifically, it was found that L2 interacts with the dynein light chains DYNLT1 and DYNLT3 that mediate specific cargo binding in the motor complex [78].
NUCLEAR IMPORT AND NUCLEAR EVENTS
How nuclear import of PV genomes occurs is presently undetermined. L2 harbors nuclear localisation signals for karyopherin-mediated import, which may allow import via nuclear pore complexes [79,80]. An alternative scenario involves progression of the host cell through early mitosis, which is required for infection [81]. Many cellular events are linked to early mitosis including changes in cytoskeletal rearrangement, membrane trafficking, gene expression profiles, nuclear envelope breakdown, and subnuclear restructuring. It is presently unclear, which mechanism(s) facilitate(s) PV entry, but it is conceivable that the breakdown of the nuclear envelope aids PV genomes in accessing the nuclear lumen.
After entry into the nucleoplasm, L2 and the viral genome colocalise at ND10 domains. This localisation is critical for the establishment of infection, as early PV transcription requires either intact ND10 or expression of the PML protein [66]. Since the ND10 nuclear subdomains are dramatically restructured during mitosis [82], early mitotic events may be required for correct localisation to these domains.
SUMMARY AND OUTLOOK
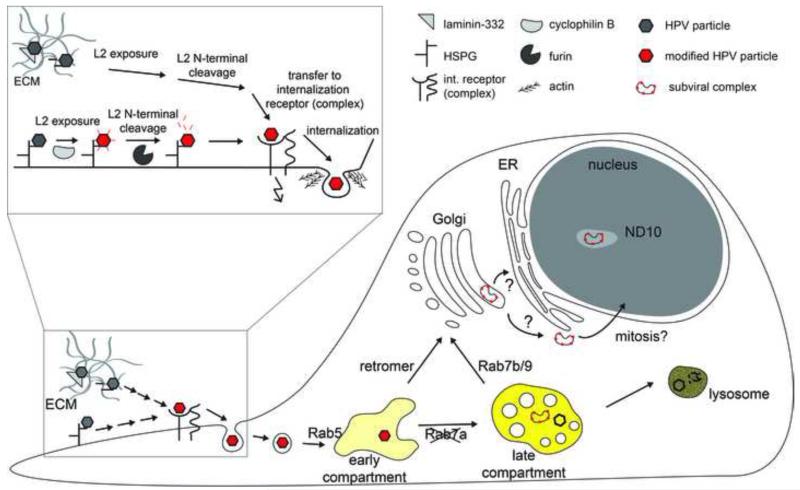
Recent years have seen a tremendous advance in PV entry research, which can be synthesized in an integrated model of entry (depicted in Figure 1). The model probably most consistent with the literature involves initial binding to HS-GAG on the cell surface or ECM, after which a series of structural changes primes the viral particle for uncoating and membrane penetration. Interaction with an elusive internalization receptor triggers uptake by a potentially novel endocytic mechanism. Delivery to an endosomal compartment allows shedding of the L1 capsomers from a subviral particle, which is then transported to the TGN where membrane penetration may occur. After nuclear import, the virus locates to ND10 structures to initiate transcription, thus beginning the post entry program.
Figure 1. Schematic depiction of PV entry.
Depicted are certain steps of PV entry into host cells. The initial interaction with cellular receptors and structural modifications on the cell surface or ECM/basement membrane are enlarged for a better view. Inidcated is also the uptake by a novel, ligand-activated endocytic mechanism where actin polymerization assists in vesicle scission from the plasma membrane. After internalization, the structurally modified PV particles are delivered to an early endosomal compartment, i.e. early endosome or macropinosome-like endosome. Delivery of a subviral complex consisting of L2 and the vDNA to the TGN occurs after separation of most of the L1 capsomers through Rab7b/Rab9- and/or retromer-mediated vesicular trafficking. After membrane penetration and nuclear import the subviral complex locates to ND10 in the nucleus. Indicated is the need for acidification of endosomal organelles by the yellow shading of organelles.
We are just starting to understand how certain steps in the PV entry program are triggered and how they are linked. The use of an atypical endocytic mechanism prompts us to investigate how uptake is mediated mechanistically. Additionally, we need to understand how sorting decisions from early endosomal compartments lead to TGN delivery and how membrane penetration occurs.
Another critical issue that is still in its infancy is how in vitro results correspond to the in vivo situation. This has implications as to what host cells, virus types, methods of production and harvest, most closely follow the natural situation. In vivo work has largely been performed with HPV16 PsV in the murine cervicovaginal model. These studies have highlighted differences between in vitro and in vivo systems [28,38,83]. Importantly, in vitro the ECM and cell surface provide the initial attachment sites, whereas in vivo this occurs on the extracellular BM. Unfortunately, it is nearly impossible to know if HPV16 interacts with human tissues in the same manner. It has recently become possible to look at species-matched infection events. The newly described MusPV1 provides an opportunity to use authentic virions for both in vitro and in vivo systems [84,85]. However, MusPV1 is a PV of the pi types and may not be a useful model to study the more medically important alpha types [86,87].
Despite the in vivo advances, it remains clear that for the time being detailed study of PV entry will continue to use in vitro systems, especially as the manipulation of in vivo infection is not possible to the same extent as in vitro. However it is critical to apply the in vitro discoveries to the available in vivo models to the extent possible.
-
-
Papillomaviruses show a protracted time course of entry
-
-
Several extracellular interactions trigger conformational changes in virions
-
-
Internalization occurs by a potentially novel, ligand-activated, endocytic pathway
-
-
Separation of L1 capsomers and a subviral complex of L2/genome occurs in endosomes
-
-
The subviral complex is trafficked to the trans-Golgi-network
ACKNOWLEDGEMENTS
We would like to thank Lena Kühling for assistance with generating a schematic depiction of PV entry and members of the Schelhaas lab for critical comments on the manuscript. We apologize to all individuals who have advanced the field of PV entry and who were not mentioned in the manuscript due to space limitations. Research in the laboratory of M. S. is supported by the German Research Foundation (DFG, grants SCHE 1552/2-1, SFB629/A16, and partly by EXC 1003).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR, Stanley MA. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30(Suppl 5):F55–70. doi: 10.1016/j.vaccine.2012.06.083. [DOI] [PubMed] [Google Scholar]
- 2.Baker TS, Newcomb WW, Olson NH, Cowsert LM, Olson C, Brown JC. Structures of bovine and human papillomaviruses. Analysis by cryoelectron microscopy and three-dimensional image reconstruction. Biophys J. 1991;60:1445–1456. doi: 10.1016/S0006-3495(91)82181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buck CB, Cheng N, Thompson CD, Lowy DR, Steven AC, Schiller JT, Trus BL. Arrangement of L2 within the Papillomavirus capsid. J Virol. 2008;82:5190–5197. doi: 10.1128/JVI.02726-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christensen ND, Cladel NM, Reed CA. Postattachment neutralization of papillomaviruses by monoclonal and polyclonal antibodies. Virology. 1995;207:136–142. doi: 10.1006/viro.1995.1059. Describes the ability to prevent PV infection with the addition of neutralizing antibodies at late times following the addition of infectious PV to host cells. These were the first results to imply that papillomaviruses attach to but do not internalize into host cells for an unusually extended time.
- 5.Culp TD, Christensen ND. Kinetics of in vitro adsorption and entry of papillomavirus virions. Virology. 2004;319:152–161. doi: 10.1016/j.virol.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Giroglou T, Florin L, Schafer F, Streeck RE, Sapp M. Human papillomavirus infection requires cell surface heparan sulfate. J Virol. 2001;75:1565–1570. doi: 10.1128/JVI.75.3.1565-1570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schelhaas M, Shah B, Holzer M, Blattmann P, Kuhling L, Day PM, Schiller JT, Helenius A. Entry of human papillomavirus type 16 by actin-dependent, clathrin- and lipid raft-independent endocytosis. PLoS Pathog. 2012;8:e1002657. doi: 10.1371/journal.ppat.1002657. Represents a comprehensive analysis of HPV16 endocytosis combining infection and endocytosis studies with electron, light, and video microscopy. Concludes that HPV16 is endocytosed by a potentially novel clathrin-, caveolin- and lipid raft-independent mechanism that most closely resembles macropinocytosis yet exhibits distinctive differences.
- 8.Smith JL, Campos SK, Ozbun MA. Human papillomavirus type 31 uses a caveolin 1- and dynamin 2-mediated entry pathway for infection of human keratinocytes. J Virol. 2007;81:9922–9931. doi: 10.1128/JVI.00988-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirnbauer R, Taub J, Greenstone H, Roden R, Durst M, Gissmann L, Lowy DR, Schiller JT. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J Virol. 1993;67:6929–6936. doi: 10.1128/jvi.67.12.6929-6936.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buck CB, Thompson CD. Production of papillomavirus-based gene transfer vectors. Curr Protoc Cell Biol. 2007 doi: 10.1002/0471143030.cb2601s37. Chapter 26:Unit 26 21. [DOI] [PubMed] [Google Scholar]
- 11.Meyers C, Frattini MG, Hudson JB, Laimins LA. Biosynthesis of human papillomavirus from a continuous cell line upon epithelial differentiation. 1992;257:971–973. doi: 10.1126/science.1323879. [DOI] [PubMed] [Google Scholar]
- 12.Buck CB, Day PM, Trus BL. The papillomavirus major capsid protein L1. Virology. 2013 doi: 10.1016/j.virol.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buck CB, Trus BL. The papillomavirus virion: a machine built to hide molecular Achilles’ heels. Adv Exp Med Biol. 2012;726:403–422. doi: 10.1007/978-1-4614-0980-9_18. [DOI] [PubMed] [Google Scholar]
- 14.Cerqueira C, Schelhaas M. Principles of polyoma- and papillomavirus uncoating. Med Microbiol Immunol. 2012;201:427–436. doi: 10.1007/s00430-012-0262-1. [DOI] [PubMed] [Google Scholar]
- 15.Florin L, Sapp M, Spoden GA. Host-cell factors involved in papillomavirus entry. Med Microbiol Immunol. 2012;201:437–448. doi: 10.1007/s00430-012-0270-1. [DOI] [PubMed] [Google Scholar]
- 16.Raff AB, Woodham AW, Raff LM, Skeate JG, Yan L, Da Silva DM, Schelhaas M, Kast WM. The evolving field of human papillomavirus receptor research: a review of binding and entry. J Virol. 2013;87:6062–6072. doi: 10.1128/JVI.00330-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sapp M, Bienkowska-Haba M. Viral entry mechanisms: human papillomavirus and a long journey from extracellular matrix to the nucleus. FEBS J. 2009;276:7206–7216. doi: 10.1111/j.1742-4658.2009.07400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sapp M, Day PM. Structure, attachment and entry of polyoma- and papillomaviruses. Virology. 2009;384:400–409. doi: 10.1016/j.virol.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 19.Schiller JT, Day PM, Kines RC. Current understanding of the mechanism of HPV infection. Gynecol Oncol. 2010;118:S12–17. doi: 10.1016/j.ygyno.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang JW, Roden RB. L2, the minor capsid protein of papillomavirus. Virology. 2013 doi: 10.1016/j.virol.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joyce JG, Tung JS, Przysiecki CT, Cook JC, Lehman ED, Sands JA, Jansen KU, Keller PM. The L1 major capsid protein of human papillomavirus type 11 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. J Biol Chem. 1999;274:5810–5822. doi: 10.1074/jbc.274.9.5810. [DOI] [PubMed] [Google Scholar]
- 22.Cerqueira C, Liu Y, Kuhling L, Chai W, Hafezi W, van Kuppevelt TH, Kuhn JE, Feizi T, Schelhaas M. Heparin increases the infectivity of Human Papillomavirus Type 16 independent of cell surface proteoglycans and induces L1 epitope exposure. Cell Microbiol. 2013 doi: 10.1111/cmi.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Culp TD, Budgeon LR, Christensen ND. Human papillomaviruses bind a basal extracellular matrix component secreted by keratinocytes which is distinct from a membrane-associated receptor. Virology. 2006;347:147–159. doi: 10.1016/j.virol.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 24.Culp TD, Budgeon LR, Marinkovich MP, Meneguzzi G, Christensen ND. Keratinocyte-secreted laminin 5 can function as a transient receptor for human papillomaviruses by binding virions and transferring them to adjacent cells. J Virol. 2006;80:8940–8950. doi: 10.1128/JVI.00724-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selinka HC, Florin L, Patel HD, Freitag K, Schmidtke M, Makarov VA, Sapp M. Inhibition of transfer to secondary receptors by heparan sulfate-binding drug or antibody induces noninfectious uptake of human papillomavirus. J Virol. 2007;81:10970–10980. doi: 10.1128/JVI.00998-07. Demonstrates that the HS-binding drug leads to non-infectious uptake of PV together with HSPGs suggesting that PV interact with a secondary receptor molecule.
- 26.Buck CB, Thompson CD, Roberts JN, Muller M, Lowy DR, Schiller JT. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006;2:e69. doi: 10.1371/journal.ppat.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cruz L, Meyers C. Differential Dependence on Host Cell Glycosaminoglycans for Infection of Epithelial Cells by High-Risk HPV Types. PLoS One. 2013;8:e68379. doi: 10.1371/journal.pone.0068379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson KM, Kines RC, Roberts JN, Lowy DR, Schiller JT, Day PM. Role of heparan sulfate in attachment to and infection of the murine female genital tract by human papillomavirus. J Virol. 2009;83:2067–2074. doi: 10.1128/JVI.02190-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patterson NA, Smith JL, Ozbun MA. Human papillomavirus type 31b infection of human keratinocytes does not require heparan sulfate. 2005;79:6838–6847. doi: 10.1128/JVI.79.11.6838-6847.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dasgupta J, Bienkowska-Haba M, Ortega ME, Patel HD, Bodevin S, Spillmann D, Bishop B, Sapp M, Chen XS. Structural basis of oligosaccharide receptor recognition by human papillomavirus. J Biol Chem. 2011;286:2617–2624. doi: 10.1074/jbc.M110.160184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knappe M, Bodevin S, Selinka HC, Spillmann D, Streeck RE, Chen XS, Lindahl U, Sapp M. Surface-exposed amino acid residues of HPV16 L1 protein mediating interaction with cell surface heparan sulfate. J Biol Chem. 2007;282:27913–27922. doi: 10.1074/jbc.M705127200. [DOI] [PubMed] [Google Scholar]
- 32.Shafti-Keramat S, Handisurya A, Kriehuber E, Meneguzzi G, Slupetzky K, Kirnbauer R. Different heparan sulfate proteoglycans serve as cellular receptors for human papillomaviruses. J Virol. 2003;77:13125–13135. doi: 10.1128/JVI.77.24.13125-13135.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang HS, Lambert PF. Use of an in vivo animal model for assessing the role of integrin alpha(6)beta(4) and syndecan-1 in early steps in papillomavirus infection. Virology. 2012;433:395–400. doi: 10.1016/j.virol.2012.08.032. The in vivo analyses in this study show that neither alpha6 beta4 integrin nor syndecan 1 are required for pseudovirus infection in the murine cervicovaginal model. It is unlikely that HSPG core proteins contribute to PV binding.
- 34.Schowalter RM, Pastrana DV, Buck CB. Glycosaminoglycans and sialylated glycans sequentially facilitate Merkel cell polyomavirus infectious entry. PLoS Pathog. 2011;7:e1002161. doi: 10.1371/journal.ppat.1002161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selinka HC, Giroglou T, Nowak T, Christensen ND, Sapp M. Further evidence that papillomavirus capsids exist in two distinct conformations. J Virol. 2003;77:12961–12967. doi: 10.1128/JVI.77.24.12961-12967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bienkowska-Haba M, Patel HD, Sapp M. Target cell cyclophilins facilitate human papillomavirus type 16 infection. PLoS Pathog. 2009;5:e1000524. doi: 10.1371/journal.ppat.1000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richards RM, Lowy DR, Schiller JT, Day PM. Cleavage of the papillomavirus minor capsid protein, L2, at a furin consensus site is necessary for infection. Proc Natl Acad Sci U S A. 2006;103:1522–1527. doi: 10.1073/pnas.0508815103. Secreted, extracellular furin cleaves a conserved consensus site in the L2 protein of PV. Cleavage is essential for infection, probably for membrane penetration. First report implicating furin in entry of a virus.
- 38.Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc Natl Acad Sci U S A. 2009;106:20458–20463. doi: 10.1073/pnas.0908502106. This manuscript shows how the early events described to occur on the cell surface in cultured cells are transposed to the acellular basement membrane in the murine cervicovaginal PV model. These include HSPG interaction and the subsequent conformational changes and furin cleavage of L2.
- 39.Day PM, Lowy DR, Schiller JT. Heparan sulfate-independent cell binding and infection with furin-precleaved papillomavirus capsids. J Virol. 2008;82:12565–12568. doi: 10.1128/JVI.01631-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dziduszko A, Ozbun MA. Annexin A2 and S100A10 regulate human papillomavirus type 16 entry and intracellular trafficking in human keratinocytes. J Virol. 2013;87:7502–7515. doi: 10.1128/JVI.00519-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evander M, Frazer IH, Payne E, Qi YM, Hengst K, McMillan NA. Identification of the alpha6 integrin as a candidate receptor for papillomaviruses. J Virol. 1997;71:2449–2456. doi: 10.1128/jvi.71.3.2449-2456.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McMillan NA, Payne E, Frazer IH, Evander M. Expression of the alpha6 integrin confers papillomavirus binding upon receptor-negative B-cells. Virology. 1999;261:271–279. doi: 10.1006/viro.1999.9825. [DOI] [PubMed] [Google Scholar]
- 43.Scheffer KD, Gawlitza A, Spoden GA, Zhang XA, Lambert C, Berditchevski F, Florin L. Tetraspanin CD151 mediates papillomavirus type 16 endocytosis. J Virol. 2013;87:3435–3446. doi: 10.1128/JVI.02906-12. Demonstrates that the tetraspanin CD151 co-migrates and co-internalizes with HPV16 and that papillomaviruses may use tetraspanin-enriched microdomains and associated integrins as a platform for viral entry.
- 44.Spoden G, Freitag K, Husmann M, Boller K, Sapp M, Lambert C, Florin L. Clathrin- and caveolin-independent entry of human papillomavirus type 16--involvement of tetraspanin-enriched microdomains (TEMs) PLoS One. 2008;3:e3313. doi: 10.1371/journal.pone.0003313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spoden G, Kuhling L, Cordes N, Frenzel B, Sapp M, Boller K, Florin L, Schelhaas M. Human papillomavirus types 16, 18, and 31 share similar endocytic requirements for entry. J Virol. 2013;87:7765–7773. doi: 10.1128/JVI.00370-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woodham AW, Da Silva DM, Skeate JG, Raff AB, Ambroso MR, Brand HE, Isas JM, Langen R, Kast WM. The S100A10 subunit of the annexin A2 heterotetramer facilitates L2-mediated human papillomavirus infection. PLoS One. 2012;7:e43519. doi: 10.1371/journal.pone.0043519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Surviladze Z, Dziduszko A, Ozbun MA. Essential roles for soluble virion-associated heparan sulfonated proteoglycans and growth factors in human papillomavirus infections. PLoS Pathog. 2012;8:e1002519. doi: 10.1371/journal.ppat.1002519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schelhaas M, Ewers H, Rajamaki ML, Day PM, Schiller JT, Helenius A. Human papillomavirus type 16 entry: retrograde cell surface transport along actin-rich protrusions. PLoS Pathog. 2008;4:e1000148. doi: 10.1371/journal.ppat.1000148. Describes the dynamics of HPV16-receptor complexes within the plasmamembrane, particularly the directed motion along filopodia (surfing).
- 49.Smith JL, Lidke DS, Ozbun MA. Virus activated filopodia promote human papillomavirus type 31 uptake from the extracellular matrix. Virology. 2008;381:16–21. doi: 10.1016/j.virol.2008.08.040. Adds to [48] that filopodia may assist in transfer of virions from the ECM to cells possibly during wound healing.
- 50.Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 51.Bousarghin L, Touze A, Sizaret PY, Coursaget P. Human papillomavirus types 16, 31, and 58 use different endocytosis pathways to enter cells. J Virol. 2003;77:3846–3850. doi: 10.1128/JVI.77.6.3846-3850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Day PM, Lowy DR, Schiller JT. Papillomaviruses infect cells via a clathrin-dependent pathway. Virology. 2003;307:1–11. doi: 10.1016/s0042-6822(02)00143-5. [DOI] [PubMed] [Google Scholar]
- 53.Laniosz V, Dabydeen SA, Havens MA, Meneses PI. Human papillomavirus type 16 infection of human keratinocytes requires clathrin and caveolin-1 and is brefeldin a sensitive. J Virol. 2009;83:8221–8232. doi: 10.1128/JVI.00576-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laniosz V, Holthusen KA, Meneses PI. Bovine papillomavirus type 1: from clathrin to caveolin. J Virol. 2008;82:6288–6298. doi: 10.1128/JVI.00569-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith JL, Campos SK, Wandinger-Ness A, Ozbun MA. Caveolin-1-dependent infectious entry of human papillomavirus type 31 in human keratinocytes proceeds to the endosomal pathway for pH-dependent uncoating. J Virol. 2008;82:9505–9512. doi: 10.1128/JVI.01014-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mercer J, Schelhaas M, Helenius A. Virus Entry by Endocytosis. Annu Rev Biochem. 2010 doi: 10.1146/annurev-biochem-060208-104626. [DOI] [PubMed] [Google Scholar]
- 57.Selinka HC, Giroglou T, Sapp M. Analysis of the infectious entry pathway of human papillomavirus type 33 pseudovirions. Virology. 2002;299:279–287. doi: 10.1006/viro.2001.1493. [DOI] [PubMed] [Google Scholar]
- 58.Abban CY, Meneses PI. Usage of heparan sulfate, integrins, and FAK in HPV16 infection. Virology. 2010;403:1–16. doi: 10.1016/j.virol.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Payne E, Bowles MR, Don A, Hancock JF, McMillan NA. Human papillomavirus type 6b virus-like particles are able to activate the Ras-MAP kinase pathway and induce cell proliferation. J Virol. 2001;75:4150–4157. doi: 10.1128/JVI.75.9.4150-4157.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fothergill T, McMillan NA. Papillomavirus virus-like particles activate the PI3-kinase pathway via alpha-6 beta-4 integrin upon binding. Virology. 2006;352:319–328. doi: 10.1016/j.virol.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 61.Surviladze Z, Sterk RT, DeHaro SA, Ozbun MA. Cellular entry of human papillomavirus type 16 involves activation of the phosphatidylinositol 3-kinase/Akt/mTOR pathway and inhibition of autophagy. J Virol. 2013;87:2508–2517. doi: 10.1128/JVI.02319-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Griffin LM, Cicchini L, Pyeon D. Human papillomavirus infection is inhibited by host autophagy in primary human keratinocytes. Virology. 2013;437:12–19. doi: 10.1016/j.virol.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 64.Day PM, Thompson CD, Schowalter RM, Lowy DR, Schiller JT. Identification of a role for the trans-Golgi network in human papillomavirus 16 pseudovirus infection. J Virol. 2013;87:3862–3870. doi: 10.1128/JVI.03222-12. Initial study implicating a role of Rab9a- and Rab7b-mediated transfer to the TGN in PV entry.
- 65.Quirin K, Eschli B, Scheu I, Poort L, Kartenbeck J, Helenius A. Lymphocytic choriomeningitis virus uses a novel endocytic pathway for infectious entry via late endosomes. Virology. 2008;378:21–33. doi: 10.1016/j.virol.2008.04.046. [DOI] [PubMed] [Google Scholar]
- 66.Day PM, Baker CC, Lowy DR, Schiller JT. Establishment of papillomavirus infection is enhanced by promyelocytic leukemia protein (PML) expression. Proc Natl Acad Sci U S A. 2004;101:14252–14257. doi: 10.1073/pnas.0404229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bienkowska-Haba M, Williams C, Kim SM, Garcea RL, Sapp M. Cyclophilins Facilitate Dissociation of the HPV16 Capsid Protein L1 from the L2/DNA Complex Following Virus Entry. J Virol. 2012 doi: 10.1128/JVI.00980-12. Demonstrates in a cell free assay and in cells that the host cell chaperone cyclophilin B mediates the dissociation of L1 capsomers from the L2/viral genome complex during virus uncoating.
- 68.Lipovsky A, Popa A, Pimienta G, Wyler M, Bhan A, Kuruvilla L, Guie MA, Poffenberger AC, Nelson CD, Atwood WJ, et al. Genome-wide siRNA screen identifies the retromer as a cellular entry factor for human papillomavirus. Proc Natl Acad Sci U S A. 2013;110:7452–7457. doi: 10.1073/pnas.1302164110. Identifies in addition to the findings from [64] the retromer complex as a determinant for PV entry into host cells. Applies genome-wide RNAi screening and discloses numerous potential cellular determinants for HPV16 infection.
- 69.Bonifacino JS, Hurley JH. Retromer. Curr Opin Cell Biol. 2008;20:427–436. doi: 10.1016/j.ceb.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bergant M, Banks L. SNX17 facilitates infection with diverse papillomavirus types. J Virol. 2013;87:1270–1273. doi: 10.1128/JVI.01991-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bergant Marusic M, Ozbun MA, Campos SK, Myers MP, Banks L. Human papillomavirus L2 facilitates viral escape from late endosomes via sorting nexin 17. Traffic. 2012;13:455–467. doi: 10.1111/j.1600-0854.2011.01320.x. Identifies the biochemical interaction of SNX17 with L2 and establishes the importance of SNX17 for intracellular trafficking.
- 72.Bottcher RT, Stremmel C, Meves A, Meyer H, Widmaier M, Tseng HY, Fassler R. Sorting nexin 17 prevents lysosomal degradation of beta1 integrins by binding to the beta1-integrin tail. Nat Cell Biol. 2012;14:584–592. doi: 10.1038/ncb2501. [DOI] [PubMed] [Google Scholar]
- 73.Lee J, Retamal C, Cuitino L, Caruano-Yzermans A, Shin JE, van Kerkhof P, Marzolo MP, Bu G. Adaptor protein sorting nexin 17 regulates amyloid precursor protein trafficking and processing in the early endosomes. J Biol Chem. 2008;283:11501–11508. doi: 10.1074/jbc.M800642200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Farfan P, Lee J, Larios J, Sotelo P, Bu G, Marzolo MP. A sorting nexin 17-binding domain within the LRP1 cytoplasmic tail mediates receptor recycling through the basolateral sorting endosome. Traffic. 2013;14:823–838. doi: 10.1111/tra.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kamper N, Day PM, Nowak T, Selinka HC, Florin L, Bolscher J, Hilbig L, Schiller JT, Sapp M. A membrane-destabilizing peptide in capsid protein L2 is required for egress of papillomavirus genomes from endosomes. J Virol. 2006;80:759–768. doi: 10.1128/JVI.80.2.759-768.2006. Describes a short C-terminal region of L2 that displays membrane-disrupting activity. Mutations within this region prevented endosome escape of L2 and viral genome.
- 76.Bronnimann MP, Chapman JA, Park CK, Campos SK. A transmembrane domain and GxxxG motifs within L2 are essential for papillomavirus infection. J Virol. 2013;87:464–473. doi: 10.1128/JVI.01539-12. Describes a potential transmembrane domain within L2 and suggests that multiple L2 molecules may participate via this domain to mediate membrane translocation of the viral genome.
- 77.Florin L, Becker KA, Lambert C, Nowak T, Sapp C, Strand D, Streeck RE, Sapp M. Identification of a dynein interacting domain in the papillomavirus minor capsid protein l2. J Virol. 2006;80:6691–6696. doi: 10.1128/JVI.00057-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schneider MA, Spoden GA, Florin L, Lambert C. Identification of the dynein light chains required for human papillomavirus infection. Cell Microbiol. 2011;13:32–46. doi: 10.1111/j.1462-5822.2010.01515.x. [DOI] [PubMed] [Google Scholar]
- 79.Darshan MS, Lucchi J, Harding E, Moroianu J. The l2 minor capsid protein of human papillomavirus type 16 interacts with a network of nuclear import receptors. J Virol. 2004;78:12179–12188. doi: 10.1128/JVI.78.22.12179-12188.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mamoor S, Onder Z, Karanam B, Kwak K, Bordeaux J, Crosby L, Roden RB, Moroianu J. The high risk HPV16 L2 minor capsid protein has multiple transport signals that mediate its nucleocytoplasmic traffic. Virology. 2012;422:413–424. doi: 10.1016/j.virol.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pyeon D, Pearce SM, Lank SM, Ahlquist P, Lambert PF. Establishment of human papillomavirus infection requires cell cycle progression. PLoS Pathog. 2009;5:e1000318. doi: 10.1371/journal.ppat.1000318. Demonstrates the need for cell cycle progression to establish HPV infection.
- 82.Everett RD, Lomonte P, Sternsdorf T, van Driel R, Orr A. Cell cycle regulation of PML modification and ND10 composition. J Cell Sci. 1999;112(Pt 24):4581–4588. doi: 10.1242/jcs.112.24.4581. [DOI] [PubMed] [Google Scholar]
- 83.Day PM, Kines RC, Thompson CD, Jagu S, Roden RB, Lowy DR, Schiller JT. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe. 2010;8:260–270. doi: 10.1016/j.chom.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cladel NM, Budgeon LR, Cooper TK, Balogh KK, Hu J, Christensen ND. Secondary Infections, Expanded Tissue Tropism, and Evidence for Malignant Potential in Immunocompromised Mice Infected with Mus musculus Papillomavirus 1 DNA and Virus. J Virol. 2013;87:9391–9395. doi: 10.1128/JVI.00777-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ingle A, Ghim S, Joh J, Chepkoech I, Bennett Jenson A, Sundberg JP. Novel laboratory mouse papillomavirus (MusPV) infection. Vet Pathol. 2011;48:500–505. doi: 10.1177/0300985810377186. [DOI] [PubMed] [Google Scholar]
- 86.Joh J, Jenson AB, King W, Proctor M, Ingle A, Sundberg JP, Ghim SJ. Genomic analysis of the first laboratory-mouse papillomavirus. J Gen Virol. 2011;92:692–698. doi: 10.1099/vir.0.026138-0. [DOI] [PubMed] [Google Scholar]
- 87.Schulz E, Gottschling M, Ulrich RG, Richter D, Stockfleth E, Nindl I. Isolation of three novel rat and mouse papillomaviruses and their genomic characterization. PLoS One. 2012;7:e47164. doi: 10.1371/journal.pone.0047164. [DOI] [PMC free article] [PubMed] [Google Scholar]