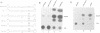Abstract
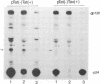
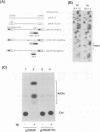
Thirteen genetically altered HIV-1 proviruses were created. These various genomes can be segregated into three groups: (i) a set of tat(-) viruses that have a functional HTLV-I Tax inserted in nef; (ii) a set of tat(-) viruses with Gal4 binding sites inserted in U3 and a Gal4-VP16 cDNA inserted in nef; and (iii) a set of tat(+) HIV genomes that are 5' and 3' TAR(-) and are Gal4-binding-site(+) in U3 and Gal4-VP16(+) in nef. We found that viruses in groups (i) and (ii), although tat(-), were fully complemented for viral gene expression based on quantitative measurements of viral protein synthesis and on the visualization by electron microscopy of the proper assembly of morphologically correct virions. Interestingly, group (i) and (ii) virions were defective in a spreading cytopathic infection when assayed in T-lymphocytes. Group (iii) viruses, although capable of producing intact Tat protein, also could not use Tat for transcription/gene expression because of the TAR(-) genotype. However, this class of viral genomes produced viruses that were highly infectious and cytopathic in primary and in continuously propagated T-lymphocytes. These three groups of viruses are all transcriptionally Tat-TAR independent. Their distinct differences in infectivity/cytopathicity provide genetic evidence that Tat provides a transcriptionally independent function in determining infectivity and cytopathicity in the setting of a spreading viral infection. Given that all HIV virions normally contain four intact copies of TAR RNA, our findings suggest a re-examination of whether Tat could be a virion-TAR-associated protein and the possible implications of this for virus infectivity/cytopathicity.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Gendelman H. E., Koenig S., Folks T., Willey R., Rabson A., Martin M. A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986 Aug;59(2):284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asjö B., Morfeldt-Månson L., Albert J., Biberfeld G., Karlsson A., Lidman K., Fenyö E. M. Replicative capacity of human immunodeficiency virus from patients with varying severity of HIV infection. Lancet. 1986 Sep 20;2(8508):660–662. [PubMed] [Google Scholar]
- Berkhout B., Gatignol A., Silver J., Jeang K. T. Efficient trans-activation by the HIV-2 Tat protein requires a duplicated TAR RNA structure. Nucleic Acids Res. 1990 Apr 11;18(7):1839–1846. doi: 10.1093/nar/18.7.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkhout B., Jeang K. T. Functional roles for the TATA promoter and enhancers in basal and Tat-induced expression of the human immunodeficiency virus type 1 long terminal repeat. J Virol. 1992 Jan;66(1):139–149. doi: 10.1128/jvi.66.1.139-149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkhout B., Jeang K. T. trans activation of human immunodeficiency virus type 1 is sequence specific for both the single-stranded bulge and loop of the trans-acting-responsive hairpin: a quantitative analysis. J Virol. 1989 Dec;63(12):5501–5504. doi: 10.1128/jvi.63.12.5501-5504.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkhout B., Silverman R. H., Jeang K. T. Tat trans-activates the human immunodeficiency virus through a nascent RNA target. Cell. 1989 Oct 20;59(2):273–282. doi: 10.1016/0092-8674(89)90289-4. [DOI] [PubMed] [Google Scholar]
- Biggar R. J. AIDS incubation in 1891 HIV seroconverters from different exposure groups. International Registry of Seroconverters. AIDS. 1990 Nov;4(11):1059–1066. doi: 10.1097/00002030-199011000-00002. [DOI] [PubMed] [Google Scholar]
- Braddock M., Chambers A., Wilson W., Esnouf M. P., Adams S. E., Kingsman A. J., Kingsman S. M. HIV-1 TAT "activates" presynthesized RNA in the nucleus. Cell. 1989 Jul 28;58(2):269–279. doi: 10.1016/0092-8674(89)90841-6. [DOI] [PubMed] [Google Scholar]
- Calnan B. J., Biancalana S., Hudson D., Frankel A. D. Analysis of arginine-rich peptides from the HIV Tat protein reveals unusual features of RNA-protein recognition. Genes Dev. 1991 Feb;5(2):201–210. doi: 10.1101/gad.5.2.201. [DOI] [PubMed] [Google Scholar]
- Caputo A., Sodroski J. G., Haseltine W. A. Constitutive expression of HIV-1 tat protein in human Jurkat T cells using a BK virus vector. J Acquir Immune Defic Syndr. 1990;3(4):372–379. [PubMed] [Google Scholar]
- Chang Y. N., Jeang K. T. The basic RNA-binding domain of HIV-2 Tat contributes to preferential trans-activation of a TAR2-containing LTR. Nucleic Acids Res. 1992 Oct 25;20(20):5465–5472. doi: 10.1093/nar/20.20.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C., Seto D., Tateno M., Levy J. A. Biologic features of HIV-1 that correlate with virulence in the host. Science. 1988 Apr 1;240(4848):80–82. doi: 10.1126/science.2832945. [DOI] [PubMed] [Google Scholar]
- Cheng-Mayer C., Shioda T., Levy J. A. Host range, replicative, and cytopathic properties of human immunodeficiency virus type 1 are determined by very few amino acid changes in tat and gp120. J Virol. 1991 Dec;65(12):6931–6941. doi: 10.1128/jvi.65.12.6931-6941.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow Y. K., Hirsch M. S., Merrill D. P., Bechtel L. J., Eron J. J., Kaplan J. C., D'Aquila R. T. Use of evolutionary limitations of HIV-1 multidrug resistance to optimize therapy. Nature. 1993 Feb 18;361(6413):650–654. doi: 10.1038/361650a0. [DOI] [PubMed] [Google Scholar]
- Churcher M. J., Lamont C., Hamy F., Dingwall C., Green S. M., Lowe A. D., Butler J. G., Gait M. J., Karn J. High affinity binding of TAR RNA by the human immunodeficiency virus type-1 tat protein requires base-pairs in the RNA stem and amino acid residues flanking the basic region. J Mol Biol. 1993 Mar 5;230(1):90–110. doi: 10.1006/jmbi.1993.1128. [DOI] [PubMed] [Google Scholar]
- Cloyd M. W., Moore B. E. Spectrum of biological properties of human immunodeficiency virus (HIV-1) isolates. Virology. 1990 Jan;174(1):103–116. doi: 10.1016/0042-6822(90)90059-z. [DOI] [PubMed] [Google Scholar]
- Cohen J. Early AZT takes a pounding in French-British 'Concorde' trial. Science. 1993 Apr 9;260(5105):157–157. doi: 10.1126/science.8469966. [DOI] [PubMed] [Google Scholar]
- Connor R. I., Mohri H., Cao Y., Ho D. D. Increased viral burden and cytopathicity correlate temporally with CD4+ T-lymphocyte decline and clinical progression in human immunodeficiency virus type 1-infected individuals. J Virol. 1993 Apr;67(4):1772–1777. doi: 10.1128/jvi.67.4.1772-1777.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousens D. J., Greaves R., Goding C. R., O'Hare P. The C-terminal 79 amino acids of the herpes simplex virus regulatory protein, Vmw65, efficiently activate transcription in yeast and mammalian cells in chimeric DNA-binding proteins. EMBO J. 1989 Aug;8(8):2337–2342. doi: 10.1002/j.1460-2075.1989.tb08361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R. Does HIV-1 Tat induce a change in viral initiation rights? Cell. 1993 May 7;73(3):417–420. doi: 10.1016/0092-8674(93)90126-b. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Kirchhoff F., Czajak S. C., Sehgal P. K., Desrosiers R. C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992 Dec 18;258(5090):1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- Dayton A. I., Sodroski J. G., Rosen C. A., Goh W. C., Haseltine W. A. The trans-activator gene of the human T cell lymphotropic virus type III is required for replication. Cell. 1986 Mar 28;44(6):941–947. doi: 10.1016/0092-8674(86)90017-6. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C. HIV with multiple gene deletions as a live attenuated vaccine for AIDS. AIDS Res Hum Retroviruses. 1992 Mar;8(3):411–421. doi: 10.1089/aid.1992.8.411. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C. The simian immunodeficiency viruses. Annu Rev Immunol. 1990;8:557–578. doi: 10.1146/annurev.iy.08.040190.003013. [DOI] [PubMed] [Google Scholar]
- Dimitrov D. S., Willey R. L., Sato H., Chang L. J., Blumenthal R., Martin M. A. Quantitation of human immunodeficiency virus type 1 infection kinetics. J Virol. 1993 Apr;67(4):2182–2190. doi: 10.1128/jvi.67.4.2182-2190.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C., Ernberg I., Gait M. J., Green S. M., Heaphy S., Karn J., Lowe A. D., Singh M., Skinner M. A., Valerio R. Human immunodeficiency virus 1 tat protein binds trans-activation-responsive region (TAR) RNA in vitro. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6925–6929. doi: 10.1073/pnas.86.18.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman M., Guyader M., Montagnier L., Baltimore D., Muesing M. A. The specificity of the human immunodeficiency virus type 2 transactivator is different from that of human immunodeficiency virus type 1. EMBO J. 1987 Dec 1;6(12):3755–3760. doi: 10.1002/j.1460-2075.1987.tb02710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S., Holland E. C. HIV-1 tat trans-activation requires the loop sequence within tar. Nature. 1988 Jul 14;334(6178):165–167. doi: 10.1038/334165a0. [DOI] [PubMed] [Google Scholar]
- Fisher A. G., Feinberg M. B., Josephs S. F., Harper M. E., Marselle L. M., Reyes G., Gonda M. A., Aldovini A., Debouk C., Gallo R. C. The trans-activator gene of HTLV-III is essential for virus replication. 1986 Mar 27-Apr 2Nature. 320(6060):367–371. doi: 10.1038/320367a0. [DOI] [PubMed] [Google Scholar]
- Garcia J. A., Harrich D., Soultanakis E., Wu F., Mitsuyasu R., Gaynor R. B. Human immunodeficiency virus type 1 LTR TATA and TAR region sequences required for transcriptional regulation. EMBO J. 1989 Mar;8(3):765–778. doi: 10.1002/j.1460-2075.1989.tb03437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Montagnier L., Peden K. VPX mutants of HIV-2 are infectious in established cell lines but display a severe defect in peripheral blood lymphocytes. EMBO J. 1989 Apr;8(4):1169–1175. doi: 10.1002/j.1460-2075.1989.tb03488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori N., Michaels F., Fargnoli K., Marcon L., Gallo R. C., Franchini G. The human immunodeficiency virus type 2 vpr gene is essential for productive infection of human macrophages. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8080–8084. doi: 10.1073/pnas.87.20.8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber J., Cullen B. R. Mutational analysis of the trans-activation-responsive region of the human immunodeficiency virus type I long terminal repeat. J Virol. 1988 Mar;62(3):673–679. doi: 10.1128/jvi.62.3.673-679.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howcroft T. K., Strebel K., Martin M. A., Singer D. S. Repression of MHC class I gene promoter activity by two-exon Tat of HIV. Science. 1993 May 28;260(5112):1320–1322. doi: 10.1126/science.8493575. [DOI] [PubMed] [Google Scholar]
- Huang L. M., Jeang K. T. Increased spacing between Sp1 and TATAA renders human immunodeficiency virus type 1 replication defective: implication for Tat function. J Virol. 1993 Dec;67(12):6937–6944. doi: 10.1128/jvi.67.12.6937-6944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobovits A., Smith D. H., Jakobovits E. B., Capon D. J. A discrete element 3' of human immunodeficiency virus 1 (HIV-1) and HIV-2 mRNA initiation sites mediates transcriptional activation by an HIV trans activator. Mol Cell Biol. 1988 Jun;8(6):2555–2561. doi: 10.1128/mcb.8.6.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeang K. T., Berkhout B. Kinetics of HIV-1 long terminal repeat trans-activation. Use of intragenic ribozyme to assess rate-limiting steps. J Biol Chem. 1992 Sep 5;267(25):17891–17899. [PubMed] [Google Scholar]
- Jeang K. T., Chun R., Lin N. H., Gatignol A., Glabe C. G., Fan H. In vitro and in vivo binding of human immunodeficiency virus type 1 Tat protein and Sp1 transcription factor. J Virol. 1993 Oct;67(10):6224–6233. doi: 10.1128/jvi.67.10.6224-6233.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi A., Jeang K. T. Reduction in growth temperature minimizes instability of large plasmids containing HIV-1 proviral genomes. Biotechniques. 1993 Jun;14(6):880, 884-6. [PubMed] [Google Scholar]
- Kamine J., Subramanian T., Chinnadurai G. Sp1-dependent activation of a synthetic promoter by human immunodeficiency virus type 1 Tat protein. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8510–8514. doi: 10.1073/pnas.88.19.8510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestler H. W., 3rd, Ringler D. J., Mori K., Panicali D. L., Sehgal P. K., Daniel M. D., Desrosiers R. C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991 May 17;65(4):651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- Lang S. M., Weeger M., Stahl-Hennig C., Coulibaly C., Hunsmann G., Müller J., Müller-Hermelink H., Fuchs D., Wachter H., Daniel M. M. Importance of vpr for infection of rhesus monkeys with simian immunodeficiency virus. J Virol. 1993 Feb;67(2):902–912. doi: 10.1128/jvi.67.2.902-912.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laspia M. F., Rice A. P., Mathews M. B. HIV-1 Tat protein increases transcriptional initiation and stabilizes elongation. Cell. 1989 Oct 20;59(2):283–292. doi: 10.1016/0092-8674(89)90290-0. [DOI] [PubMed] [Google Scholar]
- LeGuern M., Shioda T., Levy J. A., Cheng-Mayer C. Single amino acid change in Tat determines the different rates of replication of two sequential HIV-1 isolates. Virology. 1993 Aug;195(2):441–447. doi: 10.1006/viro.1993.1394. [DOI] [PubMed] [Google Scholar]
- Levy J. A. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993 Mar;57(1):183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Madore S. J., Parslow T. G., Cullen B. R., Peterlin B. M. Functional analysis of interactions between Tat and the trans-activation response element of human immunodeficiency virus type 1 in cells. J Virol. 1993 Sep;67(9):5617–5622. doi: 10.1128/jvi.67.9.5617-5622.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore S. J., Cullen B. R. Genetic analysis of the cofactor requirement for human immunodeficiency virus type 1 Tat function. J Virol. 1993 Jul;67(7):3703–3711. doi: 10.1128/jvi.67.7.3703-3711.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merson M. H. Slowing the spread of HIV: agenda for the 1990s. Science. 1993 May 28;260(5112):1266–1268. doi: 10.1126/science.8493570. [DOI] [PubMed] [Google Scholar]
- Montefiori D. C., Lefkowitz L. B., Jr, Keller R. E., Holmberg V., Sandstrom E., Phair J. P. Absence of a clinical correlation for complement-mediated, infection-enhancing antibodies in plasma or sera from HIV-1-infected individuals. Multicenter AIDS Cohort Study Group. AIDS. 1991 May;5(5):513–517. doi: 10.1097/00002030-199105000-00006. [DOI] [PubMed] [Google Scholar]
- Nowak M. A., Anderson R. M., McLean A. R., Wolfs T. F., Goudsmit J., May R. M. Antigenic diversity thresholds and the development of AIDS. Science. 1991 Nov 15;254(5034):963–969. doi: 10.1126/science.1683006. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Fauci A. S. New concepts in the immunopathogenesis of human immunodeficiency virus infection. N Engl J Med. 1993 Feb 4;328(5):327–335. doi: 10.1056/NEJM199302043280508. [DOI] [PubMed] [Google Scholar]
- Racaniello V. R. Poliovirus neurovirulence. Adv Virus Res. 1988;34:217–246. doi: 10.1016/s0065-3527(08)60519-9. [DOI] [PubMed] [Google Scholar]
- Richman D. D. HIV drug resistance. AIDS Res Hum Retroviruses. 1992 Jun;8(6):1065–1071. doi: 10.1089/aid.1992.8.1065. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Haseltine W. A. The location of cis-acting regulatory sequences in the human T cell lymphotropic virus type III (HTLV-III/LAV) long terminal repeat. Cell. 1985 Jul;41(3):813–823. doi: 10.1016/s0092-8674(85)80062-3. [DOI] [PubMed] [Google Scholar]
- Roy S., Parkin N. T., Rosen C., Itovitch J., Sonenberg N. Structural requirements for trans activation of human immunodeficiency virus type 1 long terminal repeat-directed gene expression by tat: importance of base pairing, loop sequence, and bulges in the tat-responsive sequence. J Virol. 1990 Mar;64(3):1402–1406. doi: 10.1128/jvi.64.3.1402-1406.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H., Sakuragi J., Sakuragi S., Shibata R., Adachi A. Functional analysis of biologically distinct genetic variants of simian immunodeficiency virus isolated from a mandrill. Virology. 1992 Jul;189(1):161–166. doi: 10.1016/0042-6822(92)90691-h. [DOI] [PubMed] [Google Scholar]
- Salk J., Bretscher P. A., Salk P. L., Clerici M., Shearer G. M. A strategy for prophylactic vaccination against HIV. Science. 1993 May 28;260(5112):1270–1272. doi: 10.1126/science.8098553. [DOI] [PubMed] [Google Scholar]
- Selby M. J., Peterlin B. M. Trans-activation by HIV-1 Tat via a heterologous RNA binding protein. Cell. 1990 Aug 24;62(4):769–776. doi: 10.1016/0092-8674(90)90121-t. [DOI] [PubMed] [Google Scholar]
- Semmes O. J., Jeang K. T. Mutational analysis of human T-cell leukemia virus type I Tax: regions necessary for function determined with 47 mutant proteins. J Virol. 1992 Dec;66(12):7183–7192. doi: 10.1128/jvi.66.12.7183-7192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Marciniak R. A. HIV TAR: an RNA enhancer? Cell. 1989 Oct 20;59(2):229–230. doi: 10.1016/0092-8674(89)90279-1. [DOI] [PubMed] [Google Scholar]
- Sheldon M., Ratnasabapathy R., Hernandez N. Characterization of the inducer of short transcripts, a human immunodeficiency virus type 1 transcriptional element that activates the synthesis of short RNAs. Mol Cell Biol. 1993 Feb;13(2):1251–1263. doi: 10.1128/mcb.13.2.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekevitz M., Josephs S. F., Dukovich M., Peffer N., Wong-Staal F., Greene W. C. Activation of the HIV-1 LTR by T cell mitogens and the trans-activator protein of HTLV-I. Science. 1987 Dec 11;238(4833):1575–1578. doi: 10.1126/science.2825351. [DOI] [PubMed] [Google Scholar]
- Smith M. R., Greene W. C. Identification of HTLV-I tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1990 Nov;4(11):1875–1885. doi: 10.1101/gad.4.11.1875. [DOI] [PubMed] [Google Scholar]
- Southgate C. D., Green M. R. The HIV-1 Tat protein activates transcription from an upstream DNA-binding site: implications for Tat function. Genes Dev. 1991 Dec;5(12B):2496–2507. doi: 10.1101/gad.5.12b.2496. [DOI] [PubMed] [Google Scholar]
- Southgate C., Zapp M. L., Green M. R. Activation of transcription by HIV-1 Tat protein tethered to nascent RNA through another protein. Nature. 1990 Jun 14;345(6276):640–642. doi: 10.1038/345640a0. [DOI] [PubMed] [Google Scholar]
- Tardy-Panit M., Blondel B., Martin A., Tekaia F., Horaud F., Delpeyroux F. A mutation in the RNA polymerase of poliovirus type 1 contributes to attenuation in mice. J Virol. 1993 Aug;67(8):4630–4638. doi: 10.1128/jvi.67.8.4630-4638.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M. A proposal for a new approach to a preventive vaccine against human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4419–4420. doi: 10.1073/pnas.90.10.4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tersmette M., Lange J. M., de Goede R. E., de Wolf F., Eeftink-Schattenkerk J. K., Schellekens P. T., Coutinho R. A., Huisman J. G., Goudsmit J., Miedema F. Association between biological properties of human immunodeficiency virus variants and risk for AIDS and AIDS mortality. Lancet. 1989 May 6;1(8645):983–985. doi: 10.1016/s0140-6736(89)92628-7. [DOI] [PubMed] [Google Scholar]
- Tiley L. S., Madore S. J., Malim M. H., Cullen B. R. The VP16 transcription activation domain is functional when targeted to a promoter-proximal RNA sequence. Genes Dev. 1992 Nov;6(11):2077–2087. doi: 10.1101/gad.6.11.2077. [DOI] [PubMed] [Google Scholar]
- Weiss R. A. How does HIV cause AIDS? Science. 1993 May 28;260(5112):1273–1279. doi: 10.1126/science.8493571. [DOI] [PubMed] [Google Scholar]
- Willey R. L., Martin M. A. Association of human immunodeficiency virus type 1 envelope glycoprotein with particles depends on interactions between the third variable and conserved regions of gp120. J Virol. 1993 Jun;67(6):3639–3643. doi: 10.1128/jvi.67.6.3639-3643.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey R. L., Smith D. H., Lasky L. A., Theodore T. S., Earl P. L., Moss B., Capon D. J., Martin M. A. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J Virol. 1988 Jan;62(1):139–147. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]