Abstract
Background/Objectives
Coronary artery bypass grafting (CABG) is among the most commonly performed heart surgical procedures for the treatment of ischemic heart disease. Saphenous vein graft failure due to stenosis impedes the longer-term success of CABG. A key cellular event in the process of vein graft stenosis is smooth muscle cell (SMC) hyperplasia. In this study, we evaluated the effect of a DNAzyme (Dz13) targeting the transcription factor c-Jun in a rabbit model of vein graft stenosis after autologous transplantation in a cationic liposomal formulation containing 1,2-dioleoyl-3-trimethylammonium propane (DOTAP)/1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE). Dz13 in DOTAP/DOPE has undergone preclinical toxicological testing, and a Phase I clinical trial we recently conducted in skin cancer patients demonstrates that it is safe and well tolerated after local administration.
Methods
Effects of Dz13 in a formulation containing DOTAP/DOPE on SMC growth and c-Jun expression were assessed. Dz13 transfection was determined by cellular uptake of carboxyfluorescein-labeled Dz13. Autologous jugular vein to carotid artery transplantation was performed in New Zealand White rabbits to investigate the effect of the Dz13 in DOTAP/DOPE formulation on the extent of intimal hyperplasia.
Results
Dz13/DOTAP/DOPE reduced SMC proliferation and c-Jun protein expression in vitro compared with an impotent form of Dz13 bearing a point mutation in its catalytic domain (Dz13.G>C). The Dz13 (500 µg)/DOTAP/DOPE formed lipoplexes that were colloidally stable for up to 1 hour on ice (0°C) or 30 minutes at 37°C, allowing sufficient uptake by the veins. Dz13 (500µg) inhibited neointima formation 28 days after end-to-side transplantation.
Conclusions
This formulation applied to veins prior to transplantation may potentially be useful in efforts to reduce graft failure.
Keywords: smooth muscle cells, intimal thickening, vein bypass graft, autologous end-to-side transplantation, gene silencing, bypass grafts
Introduction
Coronary artery bypass grafting (CABG), which was first introduced in 1960s [1–3] is the most common cardiac surgical procedure with around 1 million CABG procedures performed annually worldwide [4]. It is currently the accepted revascularization strategy to treat appropriate patients with three-vessel or left main coronary artery disease [5, 6]. Saphenous vein grafts remain the most common type of conduits that are used for CABG. Sabik et al reported that of the 10881 coronary artery bypass grafts performed between 1972 and 1999, 8733 used saphenous vein grafts and 2121 used internal mammary arteries [7]. However, vein grafts have limited life expectancy. Sabik et al found at 10 years after CABG, patency of saphenous veins was 57%. Campeau et al published angiographic data demonstrating yearly occlusion rates of saphenous vein grafts of 2.1% per year between years 1 and 5, and 5.2% between years 5 to 7 and 10 to 12 [8, 9]. Fitzgibbon et al showed that, after CABG, the cumulative occlusion rate was 8% at the early stage, 13% at one year, 20% at 5 years, 41% at 7.5 years, 41% at 10 years and 45% at more than 11.5 years [10, 11].
Key to the pathogenesis of vein graft failure after surgery is vascular smooth muscle cell (SMC) proliferation. The resident cells in the grafted vein are exposed to a new microenvironment, with a static-to-shear insult, increased shear stand pulsatile stress, circumferential stress, and radial deformation [12]. The graft has to adapt to higher arterial pressures. SMCs eventually accumulate in the intima [13] and proliferation causes intimal hyperplasia. A potential approach to address this problem is to effectively inhibit SMC proliferation and migration. The statin class of drugs has reduced atherogenesis, however there is no proven effective pharmacologic agent to inhibit smooth muscle cell growth to prevent vein graft failure.
Dz13 is a DNAzyme bearing 9 arms and a 10–23 catalytic domain that binds and cleaves c-jun RNA. Our laboratory has previously demonstrated that c-Jun plays an important role in injury- and shear-inducible transcriptional networks implicated in SMC proliferation and intimal hyperplasia using Dz13 in the proprietary lipid blend FuGENE6 (Roche) [14, 15]. FuGENE6, however, is not currently authorized for clinical use thus preventing the clinical translation of Dz13 in this cardiovascular setting. As an alternative, the transfection agent 1,2-dioleoyl-3-trimethylammonium propane (DOTAP)/1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) has been used in human trials and is safe [16]. We recently used Dz13 in DOTAP/DOPE to cause regression of pre-existing neovascularization in mice [17] and inhibited the growth of squamous cell carcinoma and basal cell carcinoma in murine models [18]. Moreover, recent clinical studies in cancer patients indicate the safety and tolerability of Dz13 in a formulation containing DOTAP/DOPE following local delivery [19]. In this study we determined the effect Dz13/DOTAP/DOPE on SMC growth in vitro and the complex biological process of intimal thickening in a rabbit model of autologous transplantation.
Methods and Materials
SMC culture conditions and proliferation
Rat aortic smooth muscle cells (SMC) (Cell Applications, Inc, San Diego, CA) were cultured in Waymouth’s MB752/1 medium (Invitrogen, NY, USA), containing 10% FBS and antibiotics [20] in a humidified atmosphere of 5% CO2 at 37°C. Cells beyond passage 8 were not used in experiments. In proliferation assays, we used Xcelligence system (ACEA Biosciences, Inc) that quantifies cell growth in real time. SMC were seeded in 96-well plates and arrested in serum-free medium for 24 h, then treated with Dz13/DOTAP/DOPE or Dz13.G>C/DOTAP/DOPE (DNAzyme/carrier ratio: 1.33/1, 0.6µM DNAzyme for 24 h at 37°C then changed to 5% FBS medium).
Rabbits vein autologous end-to-side transplantation bypass graft model
Adult male New Zealand White rabbits (approximately 2.5 kg, on normal diet) were used at 5 per group. The rabbits were anaesthetized with an intramuscular injection of ketamine (25mg/kg) and xylazine (Ilium Xylazil-20, 5 mg/kg) (Parnell Laboratories, Australia) and anaesthesia was maintained throughout the operation via mask with a mixture of 2% isofluorane and 2% O2. After anaesthesia, a cannula (sheathe syringe needle) was inserted into the auricular vein for venous transfusion (5% glucose) during surgery. By midline incision, a 2 cm segment of the right external jugular vein was carefully exposed, flushed and kept moist in heparinised saline. The vein was separated from the surrounding tissues, and the 2 cm vein segment was extracted, flushed with 5 ml heparinised saline and put into a 500 µl tube that contained 300 µl solution comprising 37.5 µl (375 µg) DOTAP/DOPE, 5 µl of 1 mM MgCl2 in serum-free DMEM, pH 7.4 with 500 µg Dz13 or Dz13.G>C, or just the vehicle. The vein segment was incubated in the solution at 37°C for 30 min. During this time, the right common carotid artery was dissected and clamped at the proximal and distal ends. Along the side of the artery an incision was made and flushed with heparinised saline containing 1% lignocaine. A 8/0 Proline uninterrupted suture under 3.5× magnification (Zeiss, Germany) was used to make a reversed vein attachment end-to-side to the artery (proximal end of vein to the distal part of artery and vice versa). Prior to incubation of the veins with Dz13(500µg)/DOTAP/DOPE mixture at 37°C, the mixture was prepared at 22°C and placed on ice (0°C) for 1h that allowed material transport and surgical preparation. The artery segment bypassed by the vein graft was ligated and cut so that unidirectional blood flow was ensured. After graft anastomosis, the incision was closed with 4/0 silk suture (Silkam, Johnson & Johnson). When completing the operation, the patency of anastomosis was confirmed by palpable pulsatile blood flow through the vein. Before removing the cannula, 50 ml of 5% glucose was given intravenously. The rabbits were placed on their sides under warm light and given oxygen until they recovered completely from anaesthesia. Animal experiments were approved by the Animal Care and Ethics Committee of the University of New South Wales. All care was taken to minimize any animal discomfort.
After 28 days, the rabbits were anaesthetized and a midline incision was conducted to expose the vein graft, which was dissected from surrounding tissues. Cannulas were put through the proximal and distal carotid segments and the vein was perfusion-fixed under distending pressure with 10% formalin. The grafts were removed and placed in 10% formalin (Ambion, USA) overnight and stored in 70% ethanol. Rabbits were sacrificed with pentobarbitone.
Vein graft cross sections were stained (VerHoeff elastin stain) and examined for intimal and total vessel areas. The ratio of intimal to total vessel area was obtained for each sample. A mean intimal/total vessel area plus/minus SD was determined for each experimental group. Differences between groups were tested for statistical significance using Mann Whitney test and considered significant at p<0.05.
Stablity of Dz13/DOTAP/DOPE lipoplex
A flow cytometer was used to investigate the the Dz13/DOTAP/DOPE lipoplex stablity over various conditions. Briefly, 500 µg of Dz13 was incubated with 37.5 µl (375 µg) DOTAP/DOPE in a 300 µl solution containing 16.7 µM MgCl2 in serum-free DMEM, pH 7.4, at 22°C for 10 min. To measure the lipoplex stability during material transport and surgical preparation, aliquots of the lipoplexes were transferred onto ice (0°C) and incubated for various times. The lipoplexes were then analyzed by a flow cytometer (LSRII flow cytometer, BD biosciences, San Jose, CA) and were recorded on a forward scatter/side scatter (FSC/SSC) plot, where generally, FSC correlates with the particle size and SSC depends on the inner complexity. To measure the stability of the lipoplexes during incubation with the veins, aliquots of the lipoplexes were incubated at 37°C for various times, and analyzed by flow cytometry.
DNAzyme transfection of veins
Veins were incubated in 300µl transfection solution that contained 500µg FAM-Dz13 (TriLink BioTechnologies, Inc) and 37.5µl DOTAP/DOPE (Avanti Polar Lipids) at 37°C for 30min, then rinsed in PBS pH 7.4 and snap frozen with OCT (Sakura Tissue-Tek® 4583) on dry ice. The blocks were sectioned and placed onto slides and fixed in 95% ethanol. The slides were stained with DAPI to identify nuclei under fluorescence microscopy.
Western blot analysis
Protein samples (in 1xRIPA buffer) were combined with 6µl 4× sodium dodecyl sulfate (SDS) loading buffer and 2µl of 0.5M DTT and boiled for 5min. Samples were snap-cooled on ice and 2µl of 0.5M iodoacetamide (IAM) (Sigma) was added. Proteins were loaded on 4–20% SDS-polyacrylamide gradient gels (BIO-RAD Mini-PROTEAN TGX) and run in a Bio-Rad Trans-Blot® Mini cell system with 1xSDS running buffer at 100V. Proteins were electrotransferred to Immobilon-P polyvinylidene fluoride (PVDF) membranes (Millipore) pre-soaked in ethanol (Merck). Transfer was conducted in pre-cooled transfer buffer at 90 V for 2h and confirmed by staining the gel with Gel Code Blue stain reagent (Pierce). Membranes were blocked for 1 h with 5% skim milk in 0.05% Tween 20 (Sigma) in PBS, pH 7.4 with gentle shaking on a platform shaker at room temperature. Membranes were incubated in the primary antibody (Anti-c-Jun, Santa Cruz H-79, km-1) diluted in 5% skim milk in Tween 20 PBS (1:3000) at 4°C overnight. After repeated washing membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (DAKOCytomation, Denmark) [21] diluted in 5% skim milk-0.05% Tween 20 PBS (1:1000) for 1 h. Membranes were washed 3 times, and chemiluminescence was detected using the Western Lightning Chemiluminescence system (PerkinElmer, MA, USA) and exposed using Hyperfilm-MP (Amersham Pharmacia Biotech, UK).
Immunohistochemistry
Immunohistochemical analysis was performed with c-Jun antibodies (Santa Cruz H-79, km-1, 1:250) and PCNA antibodies (Santa Cruz PC10, 1:200) on consecutive paraffin-embedded sections of formalin-fixed rabbit vein bypass graft. Primary antibodies were omitted as negative controls. The sections were first deparaffinized in xylene for 3 × 5 min, 100% ethanol for 2 × 3 min, and 70% ethanol for 1 × 3 min, and then rehydrated in distilled water for 2 × 5 min. For PCNA staining, antigen retrieval was performed by placing the slides in a glass jar and adding sufficient citrate buffer and heating to 95°C for 30 min in a microwave stove. For c-Jun staining, EDTA, pH 8.0 buffer containing Tween 20 was used. After heating, the slides were removed from the microwave to cool down for 20 min at room temperature and then rinsed in TBS for 5 min. Endogenous peroxidase was blocked with 3% hydrogen peroxide for 30 min, and then rinsed in TBS for 2 × 5 min. Non-specific staining was inhibited with KPL normal goat serum (KPL 71-00-27) for 30 min. For primary antibodies, the c-Jun antibody was diluted in 0.05% saponin, 1% BSA/PBS at 1:250 and incubated at 4°C overnight, PCNA antibody was diluted in 0.05% saponin, 1% BSA/PBS at 1:200 and incubated at 4°C overnight. After rinsing in TBS for 2 × 5 min, the c-Jun and PCNA staining slides were incubated with Dako goat anti-mouse secondary antibodies (Dako E0433) at 1:250 dilution at room temperature for 1 h, and then rinsed again in TBS for 2 × 5 min. KPL streptavidin peroxidase reagent (KPL 71-00-38) was incubated with the slides for 30 min and then rinsed in TBS for 2 × 5 min. Dako liquid diaminobenzidine (DAB) chromagen (Dako k3468) (1 drop DAB/ml of buffer substrate) was applied for 5 min, and the slides were rinsed in water for 5 min. Counter staining was performed in Modified Harris Hematoxylin for 30 s, and the slides were rinsed in water, followed by dipping 5 times in Scotts Blue and being rinsed and dehydrated in 70% ethanol for 1 × 3 min, in 100% ethanol for 2 × 3 min, and in xylene for 3 × 5 min to clear then coverslipped.
Ethics statement
The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology.
Results
Effect of Dz13 and Dz13.G>C in DOTAP/DOPE on serum-inducible vascular SMC proliferation
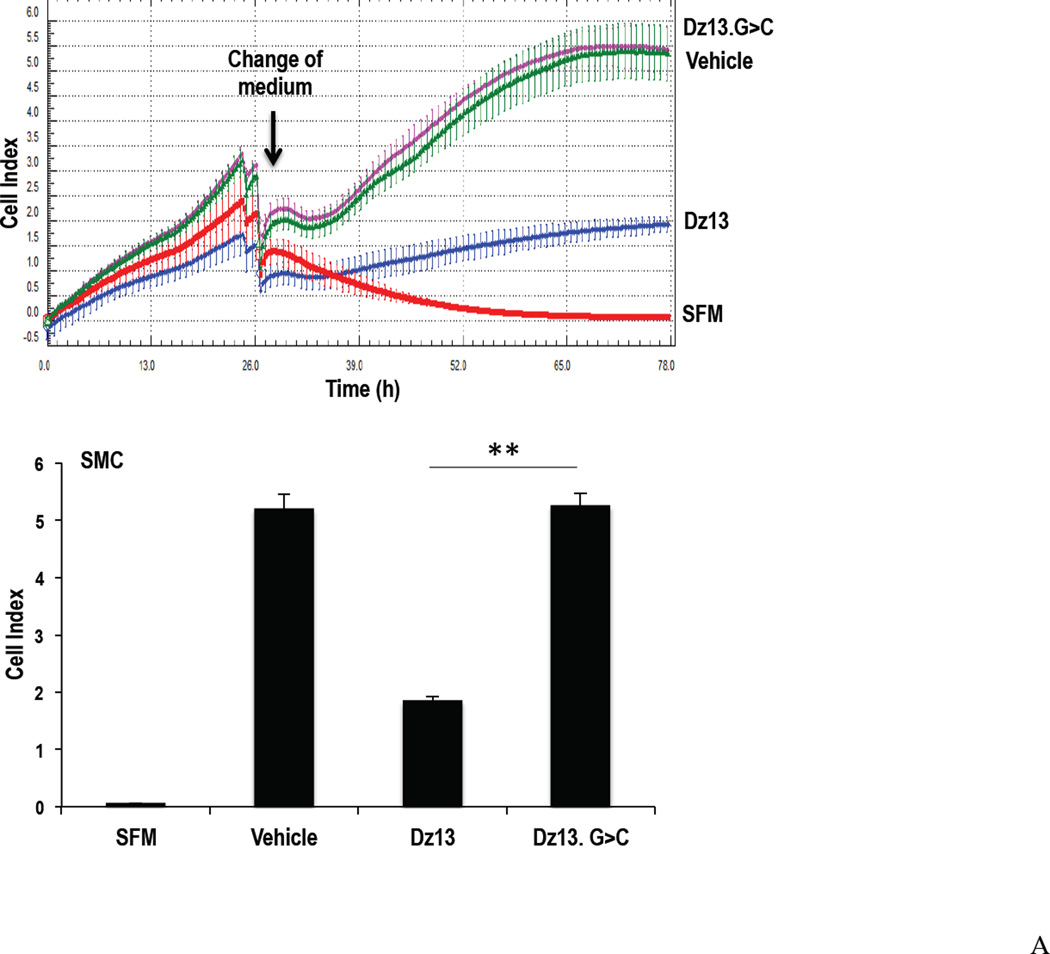
Growth-quiescent cultured SMC were treated with Dz13 or Dz13.G>C (0.6µM) in DOTAP/DOPE and cell numbers in medium-containing serum were determined using the Xcelligence system, which quantifies cell growth in real time by changes in electric impedence at the bottom surface of the microtiter plate. Dz13 in a formulation containing DOTAP/DOPE has recently undergone preclinical toxicological testing [18] and a Phase I clinical trial in skin cancer patients was successfully completed using this combination [19]. Dz13 in DOTAP/DOPE reduced SMC proliferation compared with Dz13.G>C over the course of 48 h (Figure 1A).
Figure 1. Effect of Dz13 in DOTAP/DOPE on SMC proliferation and c-Jun expression in vitro.
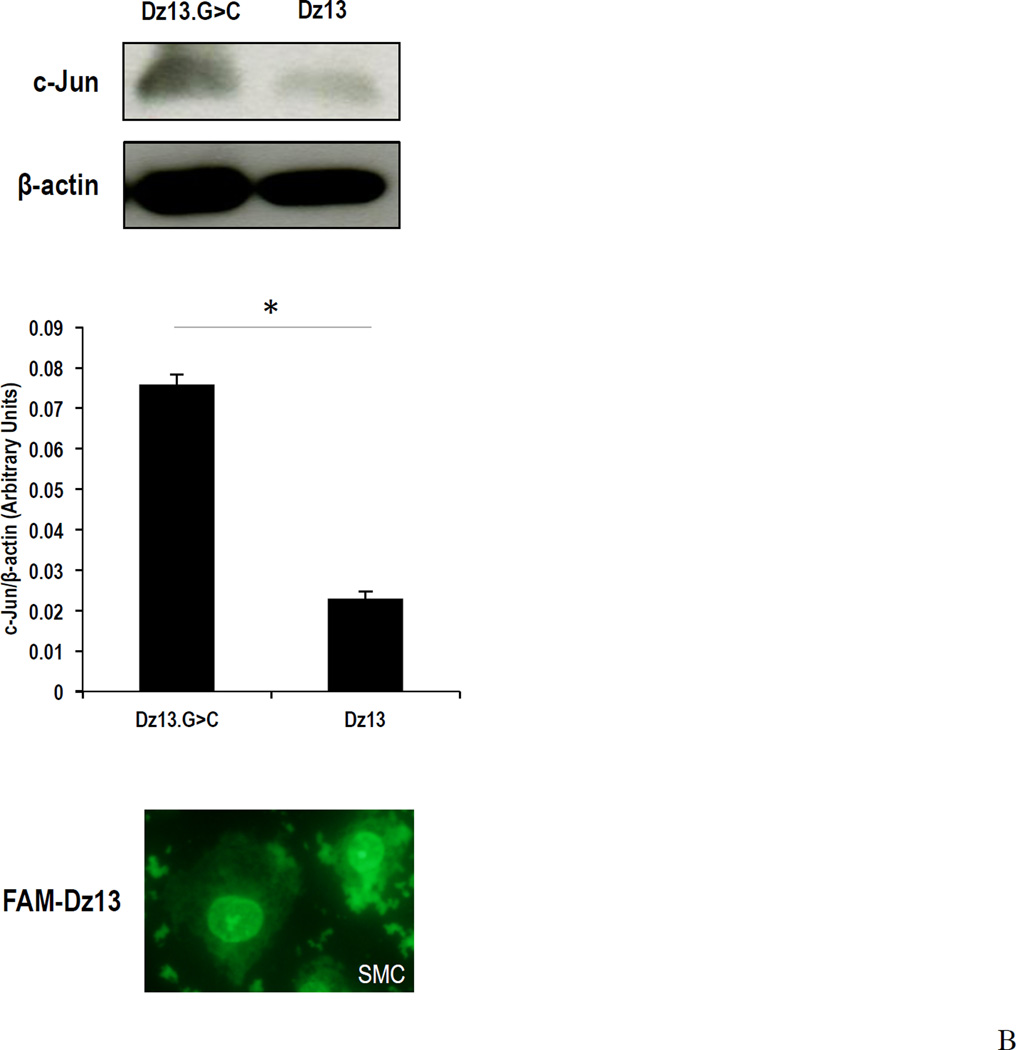
(A) SMCs were grown in 96-well plates, and rendered growth quiescent in serum-free medium for 24h, then treated with Dz13 or Dz13.G>C in DOTAP/DOPE. The medium was changed to 5% FBS medium or serum-free medium. Dz13 in DOTAP/DOPE (0.6µM) reduced SMC proliferation using the Xcelligence system compared with Dz13.G>C. SFM denotes serum-free medium. (B) SMCs were grown in 100 mm petri-dishes, and rendered growth quiescent in serum-free medium for 24h, then treated with Dz13 or Dz13.G>C in DOTAP/DOPE. The medium was then changed to 5% FBS medium for 2 h. Dz13 in DOTAP/DOPE reduced c-Jun protein levels compared with Dz13.G>C. Figure shows typical SMC uptake of FAM-labeled Dz13 under 400× magnification 24 h after transfection with DOTAP/DOPE. * indicates P<0.05, ** indicates P<0.01.
Dz13 in DOTAP/DOPE reduces c-Jun expression in vitro
Growth-quiescent SMCs were treated with Dz13 or Dz13.G>C in DOTAP/DOPE for 24 h, and incubated in medium-containing serum for another 2 h. Western blotting demonstrated that Dz13 in DOTAP/DOPE reduced levels of c-Jun protein compared with Dz13.G>C treatment. Imaging under fluorescence microscopy confirmed cellular uptake of carboxyfluorescein (FAM)-labeled Dz13 (Figure 1B).
Effect of Dz13 in DOTAP/DOPE on intimal hyperplasia 28d after autologous vein bypass grafting
To investigate the effect of the Dz13 in DOTAP/DOPE on intimal hyperplasia in a rabbit model of bypass grafting, we performed autologous end-to-side transplantation of the jugular vein to the carotid artery. The rabbit autologous vein bypass graft model has been widely studied in the process of intimal hyperplasia. Compared with large animal models (ie. baboon, pig, sheep, etc), the rabbit model is considerably cheaper, with low mortality and high graft patency post surgery. Moreover, the entire procedure, including anaesthesia, can be carried out by a single operator. In addition, histological changes in the rabbit autologous vein grafts are similar to those in human vessels after CABG [22]. Indeed, the rabbit model has been used to study risk factors for vein graft failure, including shear stress, dyslipidemia, thrombosis and diabetes [23–27].
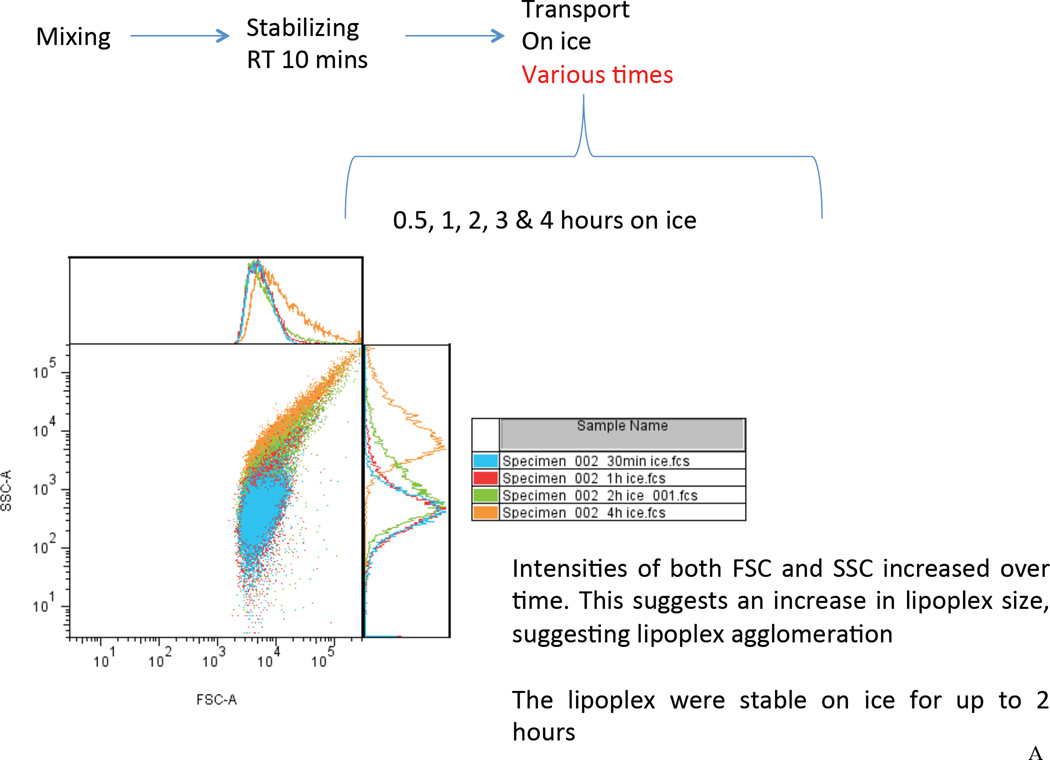
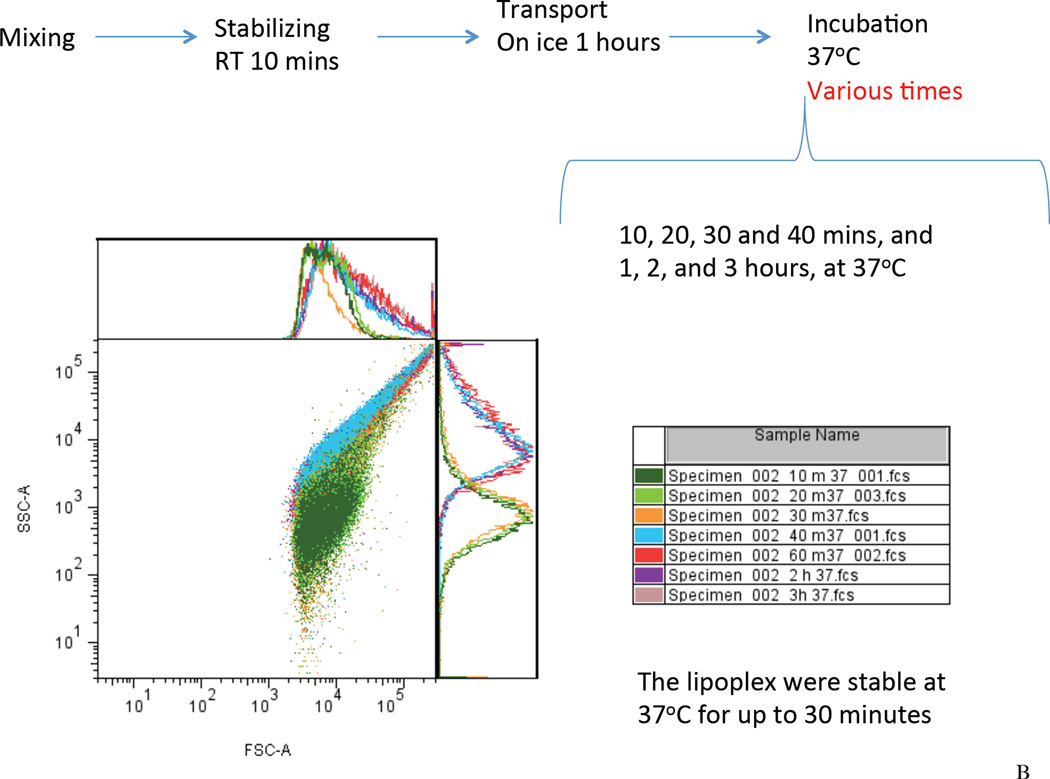
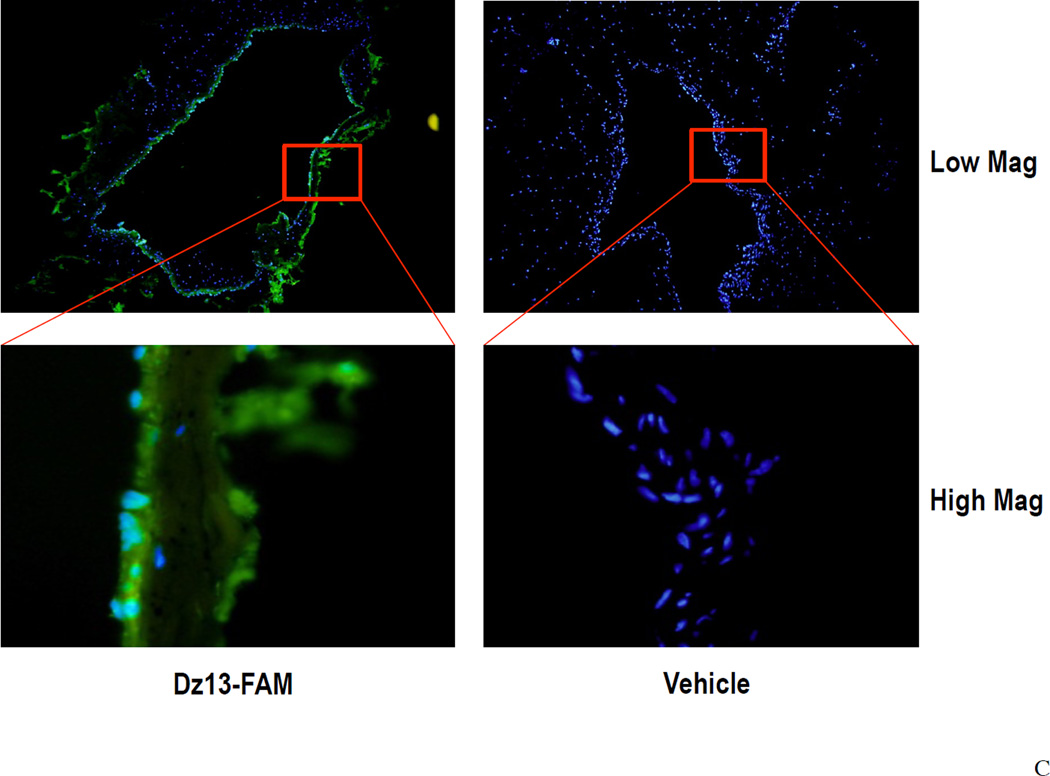
In this study, the Dz13 (500 µg) /DOTAP/DOPE lipoplexes were prepared at 22°C for 10 min. The lipoplexes were then placed on ice (0°C) during material transport and surgical preparation. To assess the lipoplex stability during this procedure, we used flow cytometry to investigate the samples placed on ice for 0.5 hour up to 3 hours. It was found that the scatter plot of the Dz13 lipoplexes remained a single population on the forward scatter/side scatter (FSC/SSC) plot for up to 1 hour, suggesting formation of lipoplexes (Figure 2A). After 2 hours, increasing intensities of the lipoplexes on the FSC and the SSC were seen, indicative of a potential colloidal agglomeration. The result suggested that the lipoplexes were stable for up to 1 hour on ice (0°C). Next, the lipoplexes were incubated with the veins at 37°C allowing cellular uptake. Using flow cytometry, the lipoplex was found to be colloidally stable for up to 30 minutes at 37°C (Figure 2B). To demonstrate the in vivo delivery of Dz13, we incubated the veins with FAM-Dz13 in the DOTAP/DOPE mixture for 30 min at 37°C, following the 1h incubation on ice. Visualization of green fluorescence image against the blue DAPI-stained nuclei revealed that the DNAzyme was delivered to the tissue to be grafted within the 30 min at 37°C (Figure 2C).
Figure 2. Assessment of lipoplex formation and stability by flow cytometry and uptake of Dz13 in DOTAP/DOPE in rabbit veins ex vivo.
(A) To assess the lipoplex stability during material transport and surgical preparation, the Dz13 (500 µg) /DOTAP/DOPE mixture were incubated at 22°C for 10 minutes, followed by incubation on ice (0°C) for 0.5, 1, 2 and 3 hours. The FSC/SSC scatter plot of the lipoplexes at different time points were recorded by flow cytometry. (B) Using flow cytometry, similarly, to assess the lipoplex stability during incubation with the veins, the Dz13 (500 µg) /DOTAP/DOPE mixture were incubated at 22°C for 10 minutes and on ice (0°C) for 1 hour, followed by incubation at 37°C for up to 3 hours. (C) Rabbit jugular veins were incubated in 300µl transfection solution containing 500µg FAM-Dz13 and 37.5µl DOTAP/DOPE at 37°C for 30 min (after incubation of the Dz13/DOTAP/DOPE mixture for 10 min at 22°C, and 1h at 0°C) then rinsed in PBS and snap frozen. Blocks were sectioned, placed onto slides and fixed in 95% ethanol. DAPI nuclear staining (blue) was imaged under fluorescence microscopy. Green fluorescence demonstrates that Dz13 was delivered into rabbit veins.
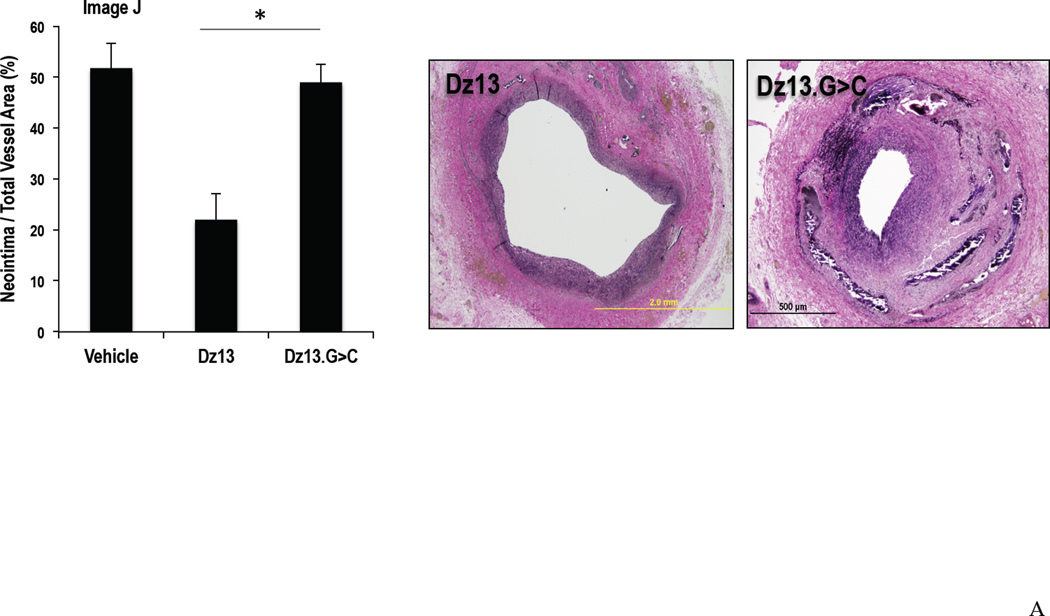
Vein grafts were collected 28 d after treated with DNAzyme and transplantation. Typically these grafts undergo intimal thickening as would occur in human bypass failure. Graft cross sections were stained with VerHoeff elastin stain and examined for intima and total vessel areas, and the ratio of intimal area to total vessel area was obtained for each sample. Intimal thickening in the Dz13 treated group was again around half that of the Dz13.G>C or Vehicle groups (Figure 3A). The inhibitory effect of Dz13 on neointima formation was confirmed by ex vivo optical coherence tomography (data not shown). Moreover, reduced proliferation within the vessel was confirmed by immunostaining for PCNA. Expression of this mitogenesis marker was significantly lower in the Dz13 group than the Dz13.G>C group (data not shown).
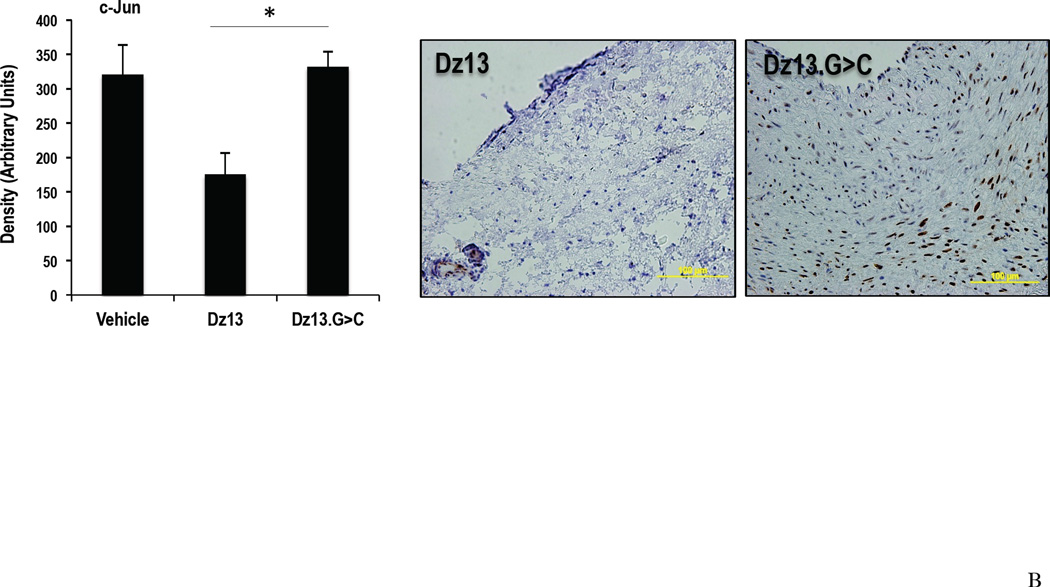
Figure 3. Effect of Dz13 in DOTAP/DOPE on intimal hyperplasia in the rabbit vein bypass grafts by morphometric analysis and c-Jun expression.
(A) Vein grafts 28d after transplantation were collected and cross sections were stained with VerHoeff elastin stain. Intima to total vessel area ratios were determined. (B) c-Jun staining in paraffin-embedded formalin-fixed sections of rabbit vein bypass grafts 28 d after transplantation. * indicates P<0.05.
Decreased c-Jun expression in vein grafts after transplantation
The effect of Dz13 with DOTAP/DOPE on the vessel wall in vivo was further examined through the expression of c-Jun. At 28 d, c-Jun staining in the Dz13 group was significantly lower than that in the Dz13.G>C and Vehicle groups, with the magnitude of the reduction consistent with the extent of Dz13 inhibition of intimal thickening (Figure 3B).
Discussion
DNAzymes have demonstrated potential for use as inhibitory agents in the therapy of various experimental diseases. These synthetic agents are composed entirely of phosphodiester-linked DNA that bind and cleave their target mRNA, destabilizing the mRNA and preventing c-Jun synthesis. Using this gene-silencing approach we, and others, have targeted the basic leucine zipper protein c-Jun in a variety of disease settings such as cancer [18, 28], acute inflammation [29], rheumatoid arthritis [29], arterial thickening [15, 30] and ocular neovascularization [17, 29]. Dz13 also inhibits intimal hyperplasia in human saphenous veins and vein bypass graft stenosis in rabbits using FuGENE6, a transfection agent that cannot be used in humans [14].
In this study, we investigated the biological activity of Dz13 in the context of SMC hyperplasia using a liposomal formulation comprising DOTAP/DOPE. Dz13 in DOTAP/DOPE inhibited SMC proliferation and c-Jun protein expression in vitro. In the rabbit bypass graft model, the jugular vein was transplanted end-to-side to the carotid artery. This triggered neointima formation within 28 d. The vein graft was exposed to Dz13 in DOTAP/DOPE prior to autologous transplantation, analogous to local delivery approach of the E2F decoy oligodeoxynucleotide Edifoligide. That Dz13 was delivered into the vein conduit before the anastomosis was confirmed by visualizing FAM-labeled Dz13 under fluorescence. Morphometric analysis demonstrated that Dz13 in DOTAP/DOPE inhibited intimal thickening. Immunohistochemical analysis further demonstrated that Dz13 inhibited the expression of its target c-Jun in the vessel wall. In all experiments, Dz13 was compared with its size-matched counterpart, Dz13.G>C, which carries the same 9 hybridizing arms and 15nt “10–23” catalytic domain as Dz13 except for a single point mutation in the catalytic domain.
This work differs from our previous study using a similar model in rabbits in that here we evaluated Dz13 delivery and efficacy in a formulation containing DOTAP/DOPE, rather than FuGENE6, where the former has been tested clinically and is safe and well tolerated in a local delivery setting. The latter is a proprietary multi-component lipid-based transfection reagent with its ingredients and concentrations unspecified, thus presenting potential regulatory challenges in clinical translation. The Dz13(500 ug)/DOTAP/DOPE mixture formed lipoplexes and were colloidally stable over the experimental procedures. Furthermore we used a catalytically-inactive DNAzyme rather than a DNAzyme with scrambled hybridization arms to control for Dz13, and evaluated SMC growth in real time. Our studies demonstrate the versatility of Dz13’s inhibitory effect on intimal thickening in vein conduits in relation to lipid carrier and bring Dz13 closer to clinical application for the prevention of CABG failure.
DOTAP and DOPE have been widely studied. DOPE is a zwitterionic lipid, and DOTAP a cationic lipid. Dz13 in a formulation containing DOTAP/DOPE recently underwent preclinical toxicological testing in a range of species including mice, rats, minipigs and monkeys [18] and a Phase I clinical trial in skin cancer patients indicates that it is safe and well tolerated after local delivery [19]. Recent studies in mice by our group using this formulation also show that Dz13 can reduce retinal microvascular density and improve forepaw reach in mice [17]. The present demonstration that Dz13 in DOTAP/DOPE can inhibit intimal thickening in large blood vessels, suggests that this formulation may provide a safe and effective strategy in CABG and other human settings amenable to local delivery.
Acknowledgements
We thank Harsha Dadlani for reviewing the manuscript, and Annie Au-Yeung for invaluable technical support.
Sources of Funding
This work was supported by the National Health and Medical Research Council of Australia, National Heart Foundation of Australia, and Heart Research Australia.
Footnotes
Author Contributions
YL performed the work, analyzed the data and wrote the manuscript; RB designed part of the research and wrote the manuscript; LMK designed and directed the research and wrote the manuscript. LMK is an Australia Fellow of the NHMRC.
Disclosures
UNSW has IP interests in Dz13.
References
- 1.Goetz RH, Rohman M, Haller JD, Dee R, Rosenak SS. Internal mammary-coronary artery anastomosis. A nonsuture method employing tantalum rings. The Journal of thoracic and cardiovascular surgery. 1961;41:378–386. [PubMed] [Google Scholar]
- 2.Kolessov VI. Mammary artery-coronary artery anastomosis as method of treatment for angina pectoris. The Journal of thoracic and cardiovascular surgery. 1967;54:535–544. [PubMed] [Google Scholar]
- 3.Favaloro RG. Saphenous vein graft in the surgical treatment of coronary artery disease. Operative technique. J Thorac Cardiovasc Surg. 1969;58:178–185. [PubMed] [Google Scholar]
- 4.Keenan TD, Abu-Omar Y, Taggart DP. Bypassing the pump: changing practices in coronary artery surgery. Chest. 2005;128:363–369. doi: 10.1378/chest.128.1.363. [DOI] [PubMed] [Google Scholar]
- 5.Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 6.Hlatky MA, Boothroyd DB, Bravata DM, Boersma E, Booth J, Brooks MM, et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet. 2009;373:1190–1197. doi: 10.1016/S0140-6736(09)60552-3. [DOI] [PubMed] [Google Scholar]
- 7.Sabik JF, 3rd, Lytle BW, Blackstone EH, Houghtaling PL, Cosgrove DM. Comparison of saphenous vein and internal thoracic artery graft patency by coronary system. The Annals of thoracic surgery. 2005;79:544–551. doi: 10.1016/j.athoracsur.2004.07.047. discussion -51. [DOI] [PubMed] [Google Scholar]
- 8.Campeau L, Enjalbert M, Lesperance J, Vaislic C, Grondin CM, Bourassa MG. Atherosclerosis and late closure of aortocoronary saphenous vein grafts: sequential angiographic studies at 2 weeks, 1 year, 5 to 7 years, and 10 to 12 years after surgery. Circulation. 1983;68:II1–II7. [PubMed] [Google Scholar]
- 9.Campeau L, Enjalbert M, Lesperance J, Bourassa MG, Kwiterovich P, Jr, Wacholder S, et al. The relation of risk factors to the development of atherosclerosis in saphenous-vein bypass grafts and the progression of disease in the native circulation. A study 10 years after aortocoronary bypass surgery. N Engl J Med. 1984;311:1329–1332. doi: 10.1056/NEJM198411223112101. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgibbon GM, Kafka HP, Leach AJ, Koen WJ, Hooper GD, Burton JR. Coronary bypass graft fate and patient outcome: angiographic follow up of 5065 grafts related to survival and reoperation in 1388 pateints during 25 years. J Am Coll Cardiol. 1996;28:616–626. doi: 10.1016/0735-1097(96)00206-9. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgibbon GM, Leach AJ, Keon WJ, Burton JR, Kafka HP. Coronary bypass graft fate. Angiographic study of 1,179 vein grafts early, one year, and five years after operation. The Journal of thoracic and cardiovascular surgery. 1986;91:773–778. [PubMed] [Google Scholar]
- 12.Dobrin PB, Littooy FN, Endean ED. Mechanical factors predisposing to intimal hyperplasia and medial thickening in autogenous vein grafts. Surgery. 1989;105:393–400. [PubMed] [Google Scholar]
- 13.Parang P, Arora R. Coronary vein graft disease: pathogenesis and prevention. The Canadian journal of cardiology. 2009;25:e57–e62. doi: 10.1016/s0828-282x(09)70486-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ni J, Waldman A, Khachigian LM. c-Jun regulates shear- and injury-inducible Egr-1 expression, vein graft stenosis after autologous end-to-side transplantation in rabbits and intimal hyperplasia in human saphenous veins. J Biol Chem. 2010;285:4038–4048. doi: 10.1074/jbc.M109.078345. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Khachigian LM, Fahmy RG, Zhang G, Bobryshev YV, Kaniaros A. c-Jun regulates vascular smooth muscle cell growth and neointima formation after arterial injury: inhibition by a novel DNAzyme targeting c-Jun. J Biol Chem. 2002;277:22985–22991. doi: 10.1074/jbc.M200977200. [DOI] [PubMed] [Google Scholar]
- 16.Gao X, Huang L. Cationic liposome-mediated gene transfer. Gene therapy. 1995;2:710–722. [PubMed] [Google Scholar]
- 17.Chan CWS, Kaplan W, Parish CR, Khachigian LM. Regression of retinal neovascularization, improvement in forepaw reach, comparative microarray and gene set enrichment analysis with c-jun targeting DNA enzyme. PLoS ONE. 2012;7:e39160. doi: 10.1371/journal.pone.0039160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai H, Santiago FS, Prado-Lourenco L, Patrikakis M, Wang B, Chong BH, et al. DNAzymes targeting c-jun suppress skin cancer growth. Science Translational Medicine. 2012;4:139ra82. doi: 10.1126/scitranslmed.3003960. [DOI] [PubMed] [Google Scholar]
- 19.Cho EA, Moloney FJ, Cai H, Au-Yeung A, China C, Scolyer RA, et al. Phase I first-in-human clinical trial of Dz13 DNAzyme targeting c-jun in patients with nodular basal cell carcinoma (DISCOVER trial) The Lancet. 2013 doi: 10.1016/S0140-6736(12)62166-7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kavurma MM, Khachigian LM. Sp1 inhibits proliferation and induces apoptosis in vascular smooth muscle cells by repressing p21WAF1/Cip1 transcription and cyclin D1/Cdk4/p21WAF1/Cip1 complex formation. J Biol Chem. 2003;278:32537–32543. doi: 10.1074/jbc.M305650200. [DOI] [PubMed] [Google Scholar]
- 21.de Mestre AM, Khachigian LM, Santiago FS, Staykova MA, Hulett MD. Regulation of inducible heparanase gene transcription in activated T cells by early growth response 1. J Biol Chem. 2003;278:50377–50385. doi: 10.1074/jbc.M310154200. [DOI] [PubMed] [Google Scholar]
- 22.Murday AJ, Gershlick AH, Syndercombe-Court YD, Ledingham SJ, Betts NJ, Lewis CT, et al. Intimal hyperplasia in arterial autogenous vein grafts: a new animal model. Cardiovascular research. 1983;17:446–451. doi: 10.1093/cvr/17.8.446. [DOI] [PubMed] [Google Scholar]
- 23.Wong AP, Nili N, Jackson ZS, Qiang B, Leong-Poi H, Jaffe R, et al. Expansive remodeling in venous bypass grafts: novel implications for vein graft disease. Atherosclerosis. 2008;196:580–589. doi: 10.1016/j.atherosclerosis.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 24.Schachner T, Laufer G, Bonatti J. In vivo (animal) models of vein graft disease. Eur J Cardiothorac Surg. 2006;30:451–463. doi: 10.1016/j.ejcts.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Tran-Son-Tay R, Hwang M, Garbey M, Jiang Z, Ozaki CK, Berceli SA. An experiment-based model of vein graft remodeling induced by shear stress. Ann Biomed Eng. 2008;36:1083–1091. doi: 10.1007/s10439-008-9495-y. [DOI] [PubMed] [Google Scholar]
- 26.Kapur NK, Bian C, Lin E, Deming CB, Sperry JL, Hansen BS, et al. Inhibition of transforming growth factor-beta restores endothelial thromboresistance in vein grafts. J Vasc Surg. 2011;54:1117–1123 e1. doi: 10.1016/j.jvs.2011.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morisaki K, Shibata R, Takahashi N, Ouchi N, Maehara Y, Murohara T, et al. Pioglitazone prevents intimal hyperplasia in experimental rabbit vein grafts. J Vasc Surg. 2011;54:1753–1759. doi: 10.1016/j.jvs.2011.06.081. [DOI] [PubMed] [Google Scholar]
- 28.Tan ML, Choong PF, Dass CR. Direct anti-metastatic efficacy by the DNA enzyme Dz13 and downregulated MMP-2, MMP-9 and MT1-MMP in tumours. Cancer Cell Int. 2010;10:9. doi: 10.1186/1475-2867-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fahmy R, Waldman A, Zhang G, Mitchell A, Tedla N, Cai H, et al. Suppression of vascular permeability and inflammation by targeting of the transcription factor c-Jun. Nature Biotech. 2006;24:856–863. doi: 10.1038/nbt1225. [DOI] [PubMed] [Google Scholar]
- 30.Murrell M, Khachigian L, Ward MR. The role of c-jun in PDTC-sensitive flow-dependent restenosis after angioplasty and stenting. Atherosclerosis. 2007;194:364–371. doi: 10.1016/j.atherosclerosis.2006.11.016. [DOI] [PubMed] [Google Scholar]