Abstract
Synapses represent the main junctures of communication between neurons in the nervous system. In many neurotransmitter systems, a fraction of presynaptic terminals fails to release vesicles in response to action potential stimulation and strong calcium influx. These silent presynaptic terminals exhibit a reversible functional dormancy beyond low vesicle release probability, and dormancy status may have important implications in neural function. Recent advances have implicated presynaptic proteins interacting with vesicles downstream of cAMP and protein kinase A signaling cascades in modulating the number of these mute presynaptic terminals, and dormancy induction may represent a homeostatic neuroprotective mechanism active during pathological insults involving excitotoxicity. Interestingly, dormancy reversal may also be induced during Hebbian plasticity. Here, details of synaptic dormancy, recent insights into the molecular signaling cascades involved, and potential clinical and mechanistic implications of this form of synaptic plasticity are described.
Keywords: presynaptic terminals, synaptic transmission, neuronal plasticity, homeostasis, long-term potentiation, synaptic vesicles
Introduction
Neurons transmit electrical signals as the basis of information processing in the nervous system. Chemical synapses mediate much of the communication between neurons. Synapses pivotally influence the flow of information throughout the neural network and are primary sites of malleability that may underlie behavioral alterations and memory formation. Modulation of synaptic strength takes many forms. One form includes mature, functioning synapses rendered dormant, but the mechanisms underlying this are poorly understood. These so-called “silent” synapses are deficient either in neurotransmitter release or in postsynaptic receptors (Fig. 1). Here we will focus on presynaptically silent synapses, as postsynaptically silent synapses have been discussed extensively elsewhere (Kerchner and Nicoll 2008). Presynaptically silent synapses are identified throughout the literature also as “dormant,” “mute,” “non-functional,” or “inactive” presynaptic terminals. They are characterized by terminals that fail to release transmitter in response to strong calcium influx and are found in a variety of species and neurotransmitter systems. Dormant presynapses are distinct from weakly transmitting terminals because they are release-incompetent even after accounting for the normal heterogeneity of vesicle release probability at presynaptic terminals. Dormancy, therefore, represents a qualitative change in release competence. It remains unclear how the absence of vesicle fusion at strategic synaptic locations alters signaling within a network, but this phenomenon may play important roles in information processing and in pathology.
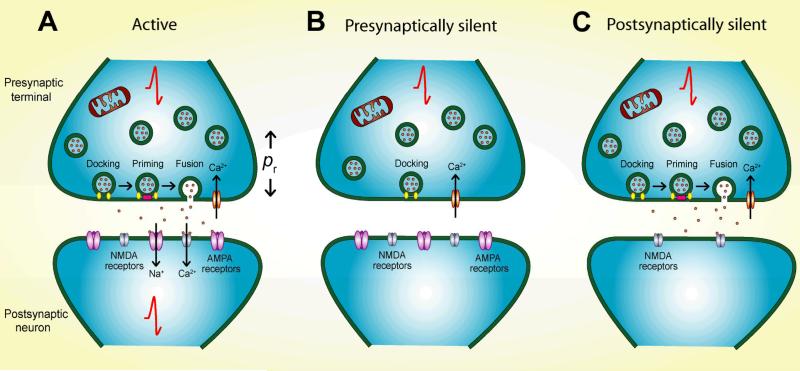
Figure 1.
Categories of silent synapses. A. Active synapses consist of presynaptic terminals with functional vesicle docking, priming, and release upon calcium influx, all powered primarily by mitochondrial ATP production. The probability of vesicle release (pr) is modulated without altering the qualitative release competence of the terminal. Neurotransmitter released from active presynaptic terminals binds to postsynaptic receptors and causes a postsynaptic response. At glutamate synapses, for example, glutamate released via presynaptic vesicle fusion will bind to AMPA receptors, allowing net cation influx that directly depolarizes the target cell and relieves voltage-dependent magnesium block of NMDA receptors (not depicted). Depolarizing effects of activated AMPA and NMDA receptors contribute to action potential generation. B. In presynaptically silent synapses, vesicle docking is intact, but priming and fusion are impaired, even with strong depolarization and calcium influx that overcomes low vesicle release probability. Without transmitter release, there is no postsynaptic response. C. Postsynaptically silent synapses maintain active presynaptic terminals, but the postsynaptic membrane is missing receptors necessary to generate a response. At glutamate synapses, AMPA receptors are absent, leaving NMDA receptors unable to overcome voltage-dependent block.
Evidence for dormancy
Early evidence for presynaptic dormancy came from studies using quantal analysis to calculate the number of release sites for neurotransmitter. By analyzing the underlying statistical structure of transmitter release, quantal analysis describes the size of postsynaptic current due to fusion of a single vesicle (quantal size, q), the number of functional release sites (n), and the probability that an action potential will cause vesicle fusion (probability of vesicle release, p) (reviewed in Redman 1990). These calculations allow for inferences about the function of a synaptic connection. Using quantal analysis in multiple systems, including crayfish and rodent neurons, studies have demonstrated that quantal n is smaller than the number of physical synaptic connections between neurons (Neale, Nelson, Macdonald, Christian, and Bowers 1983; Wojtowicz, Smith, and Atwood 1991). In other words, there are typically some nonfunctional synaptic connections present.
Strong evidence for a presynaptic locus of dormancy came from studies of the Mauthner cell, which functions in an auditory escape response pathway in goldfish. Membrane potential changes are reliably transmitted from stimulated presynaptic fibers to the lateral dendrite of the Mauthner cell through electrotonic coupling via gap junctions. Some presynaptic fibers, however, fail to transmit information through parallel chemical synapses (Faber, Lin, and Korn 1991). The maintained amplitude of the electrotonic potential argues against action potential conduction failure as an explanation for synaptic inefficacy between some neuron pairs. A small minority of the chemical synapses, however, can be activated by loading the presynaptic neuron with a cesium-containing solution (Faber, Lin, and Korn 1991). This manipulation blocks potassium channels, which broadens the action potential and provides prolonged depolarization to the presynaptic terminal. The ability to awaken the synapse with a presynaptic manipulation suggests a presynaptic deficit but a full complement of postsynaptic receptors. Although the awakened terminals could simply have low vesicle release probability, the synapses that could not be awakened may represent truly dormant terminals. This discovery prompted studies of the induction and expression mechanisms for silent synaptic connections.
More recently, fluorescence visualization of presynaptic function has allowed the direct study of presynaptic muting without ambiguities introduced by calculating n from statistical models. Styryl dyes like FM1-43 (Molecular Probes) partition into extracellularly-exposed membrane to reveal recycling vesicles. The number of active terminals, labeled with FM1-43 staining, is smaller than the total number of synaptic varicosities in dissociated hippocampal neurons (Ma, Zablow, Kandel, and Siegelbaum 1999), supporting the findings from previous studies that not all structurally-defined synapses are active. Dormant presynaptic terminals are presently defined as those terminals that label positively for synaptic proteins but not with styryl dyes during strong stimulation that should empty all release-competent vesicles. For example, synapses from cultured neurons that immunolabel for synaptophysin, Piccolo, GABAA receptors, or vesicular glutamate transporter 1 but not with FM1-43 are stereotypic examples of inactive presynaptic terminals (Altrock and others 2003; Kannenberg, Sieghart, and Reuter 1999; Moulder and others 2004; Fig. 2). Typically, these terminals have normal ultrastructure, including vesicle arrangement, and normal postsynaptic receptor function (Moulder, Jiang, Taylor, Olney, and Mennerick 2006; Moulder and others 2004).
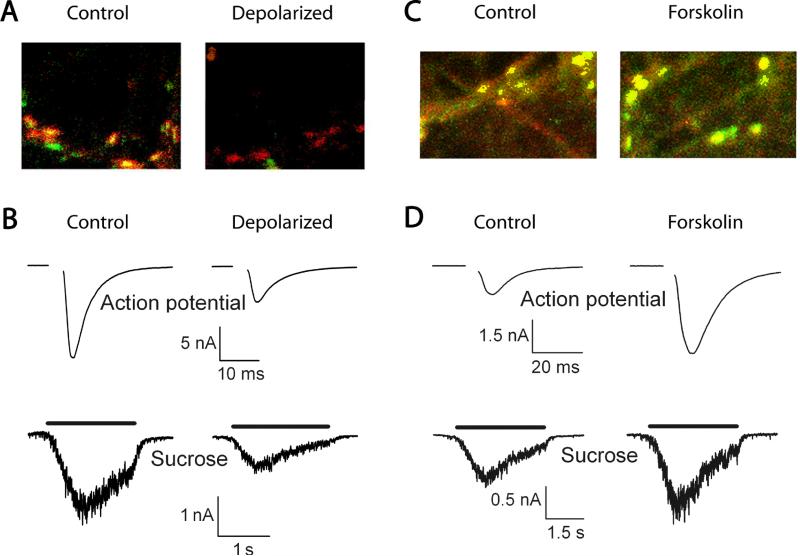
Figure 2.
Malleability in the number of dormant presynaptic terminals. A. The percentage of active glutamate terminals is measured using FM1-43 dye uptake (green) and its co-localization with a presynaptic marker (vesicular glutamate transporter 1 or vGluT-1; red); dormant terminals are, therefore, represented by red puncta without any overlying green. Activity also modulates the number of dormant terminals. For example, co-localization occurs more often in control than in depolarized (4 hr 30 mM KCl) cultured hippocampal neurons. This suggests that fewer glutamatergic presynaptic terminals are competent to recycle vesicles after depolarization. Modified with permission from Crawford, Chang, Hyrc, and Mennerick 2011. B. The percentage of active terminals correlates with the size of the readily releasable pool of vesicles. Excitatory postsynaptic currents (EPSCs) measured in autaptic cultured hippocampal neurons are depressed after prolonged (16 hr) depolarization with 30 mM KCl. Top: EPSCs were elicited after action potential stimulation, which are probabilistically dependent on calcium influx into the presynaptic terminal. Bottom: EPSCs were elicited by application of hypertonic sucrose, which causes calcium-independent fusion of all release-ready vesicles. Because both types of EPSCs are depressed after depolarization, this suggests that the size of the readily releasable pool of vesicles is decreased rather than the probability of vesicle release. C. The percentage of glutamatergic (red) terminals in cultured hippocampal neurons that take up the dye FM1-43 (green) increases after 4 hr 50 μM forskolin treatment, which increases adenylyl cyclase activity and, therefore, cAMP production. This suggests that more terminals are release-competent after increased cAMP signaling. Modified with permission from Moulder and others 2008. D. EPSCs in autaptic hippocampal neurons are increased after 4 hr 50 μM forskolin application. Because both calcium-dependent action-potential evoked EPSCs and calcium-independent sucrose-evoked EPSCs are increased after forskolin, this suggests that the readily releasable pool is increased rather than the probability of vesicle release. Modified with permission from Moulder and others 2008.
An increase in silent presynaptic terminals is registered in electrophysiological measures of excitatory postsynaptic currents as a decrease in the size of a neuron's readily releasable pool of vesicles rather than a decrease in the vesicle release probability (Moulder and others 2004; Fig. 2). Dormant presynaptic terminals cannot be forced to release vesicles by strong stimulation or by secretagogues that elicit calcium-independent vesicle fusion. These findings suggest that dormant presynaptic terminals are structurally mature and intact but have a deficit in vesicle fusion that renders the synapse unable to function. Some evidence suggests that this deficit is at the level of vesicle priming (Jiang and others 2010; Moulder, Jiang, Taylor, Olney, and Mennerick 2006), the biochemical maturation of release competence after vesicle docking but prior to calcium-dependent fusion. Thus, altered presynaptic priming protein function may induce dormancy.
Inducing dormancy
Silent presynaptic terminals are present in the absence of any manipulations, but the mechanisms controlling dormancy can be examined by manipulating the number of dormant terminals. In cultured hippocampal neurons, prolonged strong depolarization increases dormancy selectively in glutamatergic terminals (Fig. 2A, B), and this is slowly reversible (Moulder and others 2004). Weaker depolarization and increased spiking over several days also increase dormancy in a tetrodotoxin-sensitive manner (Moulder, Jiang, Taylor, Olney, and Mennerick 2006). Hypoxic depolarization also induces dormancy (Hogins, Crawford, Jiang, and Mennerick 2011). Dormancy does not simply represent arrested synaptic development because the dormant terminals identified after depolarization come from a previously active population (Moulder and others 2004). One major outstanding unknown in the literature has been the pathway leading from depolarization to silent presynaptic terminals.
Recently, inhibitory G-protein signaling was implicated in the induction of dormancy. Activation of CB1 cannabinoid receptors, which are G-protein-coupled receptors (GPCRs) linked to inhibitory G-proteins in hippocampal neurons, mutes GABAergic terminals in hippocampal slices (Losonczy, Biro, and Nusser 2004). Pertussis toxin, which inhibits Gi/o signaling, prevents depolarization-induced silencing in cultured glutamatergic neurons (Crawford, Chang, Hyrc, and Mennerick 2011). Muting of glutamatergic synapses by depolarization, however, has not been linked to a particular GPCR. Ionotropic and metabotropic glutamate receptors, CB1 cannabinoid receptors, A1 adenosine receptors, and GABAB receptors have all been pharmacologically excluded from an important role in depolarization-induced muting (Crawford, Chang, Hyrc, and Mennerick 2011). Prolonged stimulation of A1 or GABAB receptors, however, induces dormancy (Crawford, Chang, Hyrc, and Mennerick 2011), supporting the hypothesis that inhibitory G-proteins activate the induction cascade for dormancy. This begs the question of whether baseline dormancy is maintained by a constant but low level of GPCR activation, otherwise known as receptor “tone.” The source of the G-protein signal during rest or depolarization in glutamatergic neurons—including the activated receptor, ligand, and cell type from which the signal originates—remains unknown.
G-proteins have many downstream targets. Prior studies have found that reduced cAMP signaling increases the number of dormant presynaptic terminals (Moulder and others 2008). This suggests that dormancy can be modulated through alterations in cAMP levels, implicating adenylyl cyclase signaling as the downstream target of G-proteins in dormancy induction. Because a major target of cAMP is protein kinase A (PKA), PKA substrates may modulate presynaptic vesicle release during dormancy induction (Fig. 3). One PKA substrate that is also involved in vesicle priming is the presynaptic protein Rim1 (Calakos, Schoch, Sudhof, and Malenka 2004; Lonart and others 2003). Rim1 levels, and levels of an associated priming protein Munc13-1, are decreased in cultured hippocampal neurons after induction of depolarization-induced dormancy (Jiang and others 2010). This is consistent with prior literature that suggests loss of Munc13-1 increases presynaptic muting (Augustin, Rosenmund, Sudhof, and Brose 1999). Overexpression of Rim1 prevents depolarization from increasing presynaptic dormancy (Jiang and others 2010), strengthening the evidence that the levels of priming proteins at the presynaptic terminal are vital for determining dormancy status. Although Rim1 and Munc13-1 are the only proteins found to be degraded during depolarization-induced dormancy at glutamatergic terminals (Jiang and others 2010), genetic loss of other presynaptic proteins, like bassoon, also increases dormancy (Altrock and others 2003). Presynaptic proteins involved in vesicle coordination and release, therefore, appear to modulate dormancy through altered levels or function.
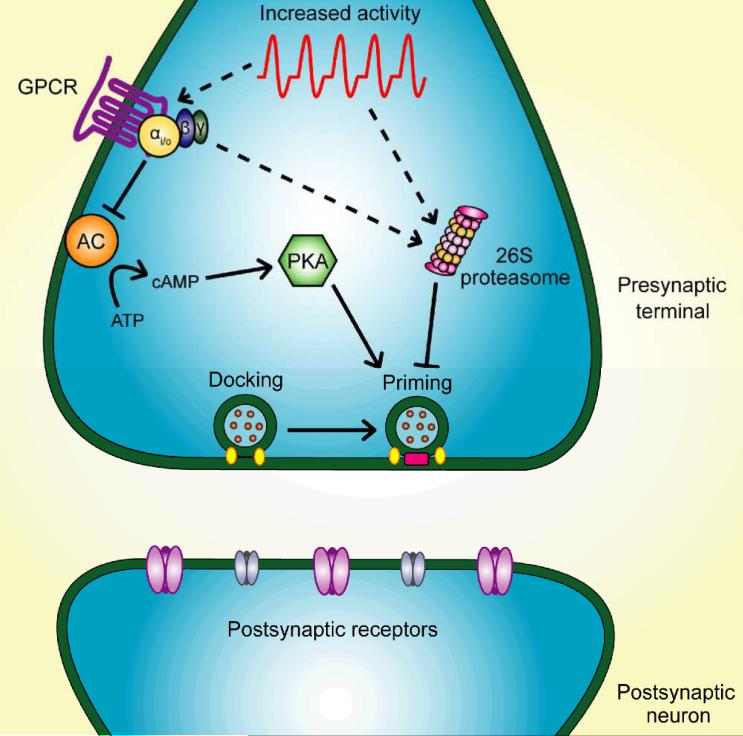
Figure 3.
Signaling cascades participating in presynaptic dormancy induction. Prolonged strong depolarization or increased action potential firing induces presynaptic dormancy through activation of inhibitory G-proteins and through activation of the ubiquitin-proteasome system. Depolarization increases proteasome activity through unknown mechanisms. Both depolarization- and G-protein-coupled receptor (GPCR) agonist-induced silencing require proteasome activity. Dormancy is also induced via reduced cAMP signaling, so inhibitory actions of the Gα subunit on adenylyl cyclase (AC) likely reduce cAMP and protein kinase A (PKA) signaling during silencing induction. PKA phosphorylates presynaptic priming proteins like Rim1, a modification that may render Rim1 resistant to proteasome degradation; therefore, less Rim1 phosphorylation is expected after depolarization. Increased proteasome activity, combined with a vulnerable presynaptic protein population, may then lead to priming protein degradation. This model provides a plausible mechanism for priming protein level reduction and dormancy induction by depolarization. Postsynaptic protein levels are unaltered by induction of presynaptic dormancy.
The ubiquitin-proteasome system, a protein tagging and degradation pathway, is in a unique position to manipulate presynaptic dormancy. Increased degradation could decrease presynaptic protein levels and, therefore, cause dormancy in protein-deficient terminals. In fact depolarization-, GPCR agonist-, and hypoxia-induced silencing of presynaptic terminals are all prevented by pharmacological proteasome inhibition (Crawford, Chang, Hyrc, and Mennerick 2011; Hogins, Crawford, Jiang, and Mennerick 2011; Jiang and others 2010). Together, these studies suggest that depolarization activates inhibitory G-proteins to reduce cAMP and upregulate proteasome-dependent degradation of proteins vital for vesicle fusion at the presynaptic terminal (Fig. 3).
Reducing dormancy
Depolarization leads to dormancy, but neuronal inactivity reduces dormancy. Thus, activity levels appear to set the percentage of release-competent terminals. The percentage of active glutamate terminals in primary hippocampal cultures increases after 6-10 days of tetrodotoxin treatment to block action potentials (Moulder, Jiang, Taylor, Olney, and Mennerick 2006). Unsilencing occurs within 4 hr when dormancy-inducing depolarization is removed from neurons (Moulder and others 2004). This unsilencing accounts for a return to baseline synaptic functionality. Together, these studies suggest that presynaptic silencing is a compensatory, homeostatic adaptation to altered neuronal activity.
Signaling cascades for both silencing and unsilencing of presynaptic terminals appear to utilize cAMP. As described earlier, decreased cAMP signaling increases dormancy. In turn, increased cAMP signaling reduces dormancy (Fig. 4). Sp-cAMPS, a phosphodiesterase-resistant cAMP analog that activates downstream protein kinases like PKA, increases the number of functional release sites (Bolshakov, Golan, Kandel, and Siegelbaum 1997; Kohara, Ogura, Akagawa, and Yamaguchi 2001; Ma, Zablow, Kandel, and Siegelbaum 1999). This same effect is induced by forskolin, which increases adenylyl cyclase activity (Kohara, Ogura, Akagawa, and Yamaguchi 2001; Moulder and others 2008; Fig. 2C, D). Recovery from depolarization-induced silencing of glutamate terminals depends on adenylyl cyclase 8 and PKA signaling, suggesting that cAMP signaling is important for this type of unsilencing as well (Moulder and others 2008). Interestingly, Sp-cAMPS-induced increases in the number of functional terminals are blocked with protein synthesis inhibitors (Ma, Zablow, Kandel, and Siegelbaum 1999), and the presynaptic protein synapsin has been implicated in a PKA-dependent form of presynaptic awakening (Cousin and Evans 2011). The dependence of unsilencing on protein synthesis mirrors the evidence that protein degradation induces dormancy and strengthens the hypothesis that presynaptic protein levels or function are important for modulating dormancy status of presynaptic terminals.
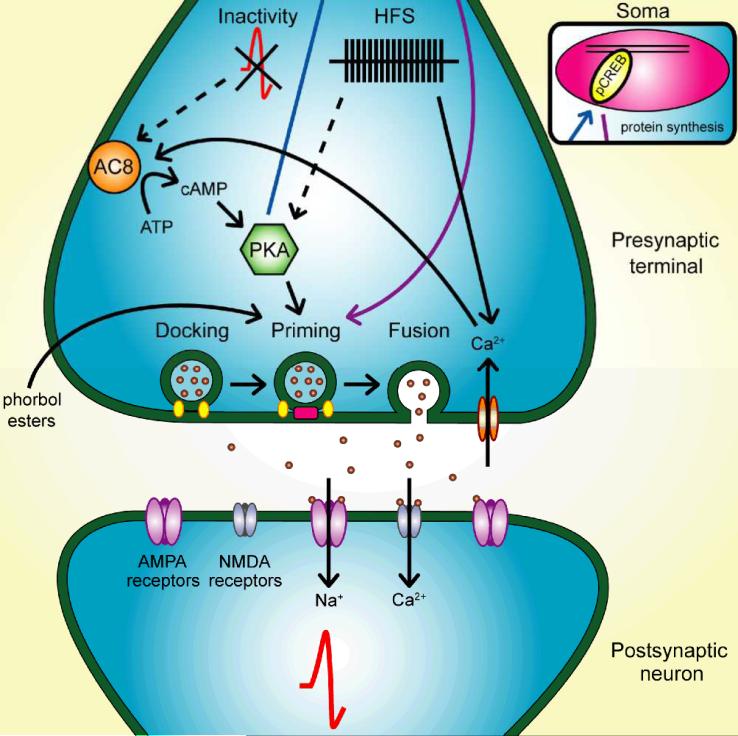
Figure 4.
Signaling cascades participating in presynaptic dormancy reduction. Under some conditions, inactivity and increased cAMP activate previously dormant terminals. Calcium-dependent adenylyl cyclase VIII (AC8) knockout prevents recovery of active terminals when elevated neuronal activity is removed, so AC8 may be the cAMP source responsible for this form of unsilencing. In other contexts multiple bursts of high frequency stimulation (HFS), which often causes strong calcium influx and long-term potentiation, lead to presynaptic awakening. Both activity reduction and HFS require protein kinase A (PKA) signaling for presynaptic activation, but how ostensibly opposite changes in activity both recruit PKA in different experimental contexts remains unknown. Once PKA is activated, phosphorylation events may slow priming protein degradation. Additionally, PKA phosphorylates and thereby activates nuclear transcription factors (blue arrow and inset) like cAMP response element-binding protein (CREB), and this may increase synthesis of presynaptic proteins vital for vesicle priming and release (purple arrow and inset). A third pathway to unsilencing is via phorbol esters, which enhance function of the priming protein Munc13-1. Under this model of dormancy reduction, postsynaptic responses are restored once the presynaptic terminal regains the ability to release neurotransmitter.
Although dormancy is modulated homeostatically in some contexts, it may also be modulated in a Hebbian fashion. Hebbian plasticity refers to reinforcing forms of synaptic plasticity like long-term potentiation (LTP), where patterned stimulation of a synaptic connection increases synaptic efficacy. Some stimuli that induce LTP also unsilence presynaptic terminals (Fig. 4). Although the role of the presynaptic terminal in LTP induction is still debated in the literature (Kerchner and Nicoll 2008; Voronin and Cherubini 2004), there are a few studies that clearly show an increase in the number of active presynaptic terminals after LTP has been induced. For example, one study showed that Sp-cAMPS, known to reduce dormancy, potentiates synapses and increases quantal n in rodent hippocampal slices (Bolshakov, Golan, Kandel, and Siegelbaum 1997). Another study used multiple bursts of high frequency stimulation in cultured dentate granule neurons to induce a form of PKA-dependent LTP (Tong, Malenka, and Nicoll 1996). Using a slowly reversible NMDA receptor antagonist, MK801, to block postsynaptic receptors at all active synapses, this study showed that new active release sites appear after LTP induction (Tong, Malenka, and Nicoll 1996). PKA is also important for activation of presynaptic terminals in immature cultured hippocampal neurons after repetitive depolarization challenges, although the possibility of enhanced synaptic maturation rather than awakening of established terminals is not ruled out (Yao, Qi, and Chen 2006). Another study in mature cultured hippocampal neurons showed that glutamate-induced synaptic potentiation increases the number of terminals that take up the dye FM1-43 (Ninan and Arancio 2004). Hebbian unsilencing of presynaptic terminals is also induced by serotonin in Aplysia sensory neurons prior to subsequent synaptogenesis (Kim and others 2003). Dormancy modulation, therefore, may be important for some Hebbian forms of synaptic plasticity, and the direction of cAMP change may critically determine whether silencing or unsilencing results from a particular stimulus.
Phorbol esters, which are analogues of diacylglycerol, also potentiate synaptic function. Among other presynaptic effects, phorbol esters unsilence presynaptic terminals within two minutes application to cultured hippocampal neurons (Chang, Jiang, Moulder, and Mennerick 2010). This form of unsilencing is much faster than the cAMP-dependent forms of unsilencing described previously. Phorbol esters upregulate protein kinase C signaling, but they also act directly on Munc13-1 (Betz and others 1998). Although PKA-induced unsilencing may involve protein synthesis, phorbol ester-induced unsilencing is likely too rapid to require protein synthesis. Instead, phorbol esters appear to alter Munc13-1 function, probably via translocation to the plasma membrane (Betz and others 1998). Altogether, these findings suggest that there are multiple signaling cascades that cause presynaptic unsilencing (Fig. 4). These cascades alter synaptic function on very different time scales, but they likely converge on presynaptic proteins responsible for vesicle maturation or fusion.
Implications of dormancy
Because dormant presynaptic terminals are widespread and modulated by a variety of mechanisms, dormancy status may represent an important facet of synaptic malleability. It is currently unknown how dormancy levels affect network function and what role this may play during neuropathology. Why should some synapses exist but not function? What benefit or difference in function is gained from dormancy over a more classical presynaptic change, like acute, reversible GPCR-mediated depression of calcium influx and the corresponding decreased vesicle release probability? An intriguing possibility is that dormancy is an activity-dependent mechanism for modulating connectivity between neurons. This would add another dimension to neural computation since dormancy provides a digital signal that is either “on” or “off,” similar to synaptogenesis and synaptic pruning but without the same resource requirements of these mechanisms. Having multiple methods for modulating synaptic connectivity likely increases flexibility of neural computation in the system.
It is also interesting that dormancy can be adaptive or reinforcing, depending on the context and the direction of cAMP change. Dormancy of glutamate terminals often increases with prolonged elevated neuronal activity but decreases with inactivity. This counteractive change in excitatory neurotransmitter release could potentially maintain signaling within an optimal range, one of the hypothesized roles of homeostatic synaptic plasticity (Pozo and Goda 2010). For example, induction of dormancy occurs during pathological insults, leading to reduced glutamate release and enhanced neuronal survival (Hogins, Crawford, Jiang, and Mennerick 2011). In this context, all-or-none muting seems well-tailored to self-defense; other forms of presynaptic depression through reduced calcium influx might quickly be overwhelmed by hypoxic or ischemic depolarizing insults. Dormancy can also be decreased rather than increased after stimulation, however, leading to potentiated synapses. Some of these Hebbian forms of unsilencing utilize cAMP signaling, which, like LTP, has been linked to memory processing in multiple systems (Alberini, Ghirardi, Huang, Nguyen, and Kandel 1995). So in addition to its potential homeostatic role, modulation of dormancy may also play a role in Hebbian plasticity and memory. Therapeutic exploitation of the signaling cascades increasing or decreasing dormancy could have valuable clinical implications.
Dormancy may also be more efficient than ostensibly similar forms of synaptic plasticity. Modulation of dormancy achieves similar changes in neuronal connectivity as synaptogenesis and synaptic pruning but appears to work on a faster time scale (minutes to hours rather than hours to days). This likely preserves physical resources the cell would otherwise require for large-scale structural alterations and allows the neuron to respond more quickly to perturbations to the system. Additionally, it is important to note that presynaptic function requires a large amount of energy, as evidenced by the nearly inevitable presence of mitochondria in presynaptic terminals. If the system requires a dormant synaptic connection, it would arguably waste less energy to preserve postsynaptic function and shut down presynaptic vesicle cycling than to preserve transmitter release onto a non-receptive postsynaptic membrane for the duration of the dormancy period. Although it is unclear whether unsilencing a presynaptic terminal would require more energy than unsilencing a postsynaptic terminal, maintaining presynaptically silent synapses may be more energy efficient than maintaining postsynaptically silent synapses. It is still unclear how the relatively fast digital changes in information flow created by dormancy alter neural computation, but it is evident that presynaptically silent terminals are a potentially economical way to introduce this dimension into the system.
Concluding remarks
Presynaptic dormancy exists in many systems and can be modulated by neuronal activity. Although the mechanisms responsible for muting of presynaptic neurotransmitter release are becoming clearer, plenty of questions remain. What role does dormancy play in neural computation? How are dormancy levels modulated throughout development in different neurotransmitter systems? Does the induction signal act only on the presynaptic neuron, or is communication with other cells necessary? Are there presynaptic terminals that are more prone to dormancy because of their synaptic location, local signaling molecules, or neurotransmitter class? How does modulation of dormancy change learning, cognition, behavior, or disease? Synaptic function and plasticity are vital for proper functioning of the nervous system, so further understanding of presynaptic dormancy will likely illuminate mechanisms of healthy neural function as well as therapeutic targets in disease.
Acknowledgments
Financial support: The authors are supported by National Institutes of Health grants NS066611 (D.C.C.) and MH078823 (S.M.).
References
- Alberini CM, Ghirardi M, Huang YY, Nguyen PV, Kandel ER. A molecular switch for the consolidation of long-term memory: cAMP-inducible gene expression. Ann N Y Acad Sci. 1995;758:261–86. doi: 10.1111/j.1749-6632.1995.tb24833.x. [DOI] [PubMed] [Google Scholar]
- Altrock WD, tom Dieck S, Sokolov M, Meyer AC, Sigler A, Brakebusch C. Functional inactivation of a fraction of excitatory synapses in mice deficient for the active zone protein bassoon. Neuron. 2003;37(5):787–800. doi: 10.1016/s0896-6273(03)00088-6. others. [DOI] [PubMed] [Google Scholar]
- Augustin I, Rosenmund C, Sudhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400(6743):457–61. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- Betz A, Ashery U, Rickmann M, Augustin I, Neher E, Sudhof TC. Munc13-1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron. 1998;21(1):123–36. doi: 10.1016/s0896-6273(00)80520-6. others. [DOI] [PubMed] [Google Scholar]
- Bolshakov VY, Golan H, Kandel ER, Siegelbaum SA. Recruitment of new sites of synaptic transmission during the cAMP-dependent late phase of LTP at CA3-CA1 synapses in the hippocampus. Neuron. 1997;19(3):635–51. doi: 10.1016/s0896-6273(00)80377-3. [DOI] [PubMed] [Google Scholar]
- Calakos N, Schoch S, Sudhof TC, Malenka RC. Multiple roles for the active zone protein RIM1alpha in late stages of neurotransmitter release. Neuron. 2004;42(6):889–96. doi: 10.1016/j.neuron.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Chang CY, Jiang X, Moulder KL, Mennerick S. Rapid activation of dormant presynaptic terminals by phorbol esters. J Neurosci. 2010;30(30):10048–60. doi: 10.1523/JNEUROSCI.1159-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin MA, Evans GJ. Activation of silent and weak synapses by cAMP-dependent protein kinase in cultured cerebellar granule neurons. J Physiol. 2011;589(Pt 8):1943–55. doi: 10.1113/jphysiol.2010.200477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DC, Chang CY, Hyrc KL, Mennerick S. Calcium-independent inhibitory G-protein signaling induces persistent presynaptic muting of hippocampal synapses. J Neurosci. 2011;31(3):979–91. doi: 10.1523/JNEUROSCI.4960-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber DS, Lin JW, Korn H. Silent synaptic connections and their modifiability. Annals of the New York Academy of Sciences. 1991;627:151–164. doi: 10.1111/j.1749-6632.1991.tb25920.x. [DOI] [PubMed] [Google Scholar]
- Hogins J, Crawford DC, Jiang X, Mennerick S. Presynaptic silencing is an endogenous neuroprotectant during excitotoxic insults. Neurobiol Dis. 2011;43(2):516–25. doi: 10.1016/j.nbd.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Litkowski PE, Taylor AA, Lin Y, Snider BJ, Moulder KL. A role for the ubiquitinproteasome system in activity-dependent presynaptic silencing. J Neurosci. 2010;30(5):1798–809. doi: 10.1523/JNEUROSCI.4965-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannenberg K, Sieghart W, Reuter H. Clusters of GABAA receptors on cultured hippocampal cells correlate only partially with functional synapses. Eur J Neurosci. 1999;11(4):1256–64. doi: 10.1046/j.1460-9568.1999.00533.x. [DOI] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9(11):813–25. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Udo H, Li HL, Youn TY, Chen M, Kandel ER. Presynaptic activation of silent synapses and growth of new synapses contribute to intermediate and long-term facilitation in Aplysia. Neuron. 2003;40(1):151–65. doi: 10.1016/s0896-6273(03)00595-6. others. [DOI] [PubMed] [Google Scholar]
- Kohara K, Ogura A, Akagawa K, Yamaguchi K. Increase in number of functional release sites by cyclic AMP-dependent protein kinase in cultured neurons isolated from hippocampal dentate gyrus. Neurosci Res. 2001;41(1):79–88. doi: 10.1016/s0168-0102(01)00267-x. [DOI] [PubMed] [Google Scholar]
- Lonart G, Schoch S, Kaeser PS, Larkin CJ, Sudhof TC, Linden DJ. Phosphorylation of RIM1alpha by PKA triggers presynaptic long-term potentiation at cerebellar parallel fiber synapses. Cell. 2003;115(1):49–60. doi: 10.1016/s0092-8674(03)00727-x. [DOI] [PubMed] [Google Scholar]
- Losonczy A, Biro AA, Nusser Z. Persistently active cannabinoid receptors mute a subpopulation of hippocampal interneurons. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(5):1362–1367. doi: 10.1073/pnas.0304752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Zablow L, Kandel ER, Siegelbaum SA. Cyclic AMP induces functional presynaptic boutons in hippocampal CA3-CA1 neuronal cultures. Nature Neuroscience. 1999;2(1):24–30. doi: 10.1038/4525. [DOI] [PubMed] [Google Scholar]
- Moulder KL, Jiang X, Chang C, Taylor AA, Benz AM, Conti AC. A specific role for Ca2+-dependent adenylyl cyclases in recovery from adaptive presynaptic silencing. Journal of Neuroscience. 2008;28(20):5159–68. doi: 10.1523/JNEUROSCI.5317-07.2008. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder KL, Jiang X, Taylor AA, Olney JW, Mennerick S. Physiological activity depresses synaptic function through an effect on vesicle priming. J Neurosci. 2006;26(24):6618–26. doi: 10.1523/JNEUROSCI.5498-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder KL, Meeks JP, Shute AA, Hamilton CK, de Erausquin G, Mennerick S. Plastic elimination of functional glutamate release sites by depolarization. Neuron. 2004;42(3):423–35. doi: 10.1016/s0896-6273(04)00184-9. [DOI] [PubMed] [Google Scholar]
- Neale EA, Nelson PG, Macdonald RL, Christian CN, Bowers LM. Synaptic interactions between mammalian central neurons in cell culture. III. Morphophysiological correlates of quantal synaptic transmission. J Neurophysiol. 1983;49(6):1459–68. doi: 10.1152/jn.1983.49.6.1459. [DOI] [PubMed] [Google Scholar]
- Ninan I, Arancio O. Presynaptic CaMKII is necessary for synaptic plasticity in cultured hippocampal neurons. Neuron. 2004;42(1):129–41. doi: 10.1016/s0896-6273(04)00143-6. [DOI] [PubMed] [Google Scholar]
- Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66(3):337–51. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman S. Quantal analysis of synaptic potentials in neurons of the central nervous system. Physiol Rev. 1990;70(1):165–98. doi: 10.1152/physrev.1990.70.1.165. [DOI] [PubMed] [Google Scholar]
- Tong G, Malenka RC, Nicoll RA. Long-term potentiation in cultures of single hippocampal granule cells: a presynaptic form of plasticity. Neuron. 1996;16(6):1147–57. doi: 10.1016/s0896-6273(00)80141-5. [DOI] [PubMed] [Google Scholar]
- Voronin LL, Cherubini E. ‘Deaf, mute and whispering’ silent synapses: their role in synaptic plasticity. J Physiol. 2004;557(Pt 1):3–12. doi: 10.1113/jphysiol.2003.058966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtowicz JM, Smith BR, Atwood HL. Activity-dependent recruitment of silent synapses. Ann N Y Acad Sci. 1991;627:169–79. doi: 10.1111/j.1749-6632.1991.tb25922.x. [DOI] [PubMed] [Google Scholar]
- Yao J, Qi J, Chen G. Actin-dependent activation of presynaptic silent synapses contributes to long-term synaptic plasticity in developing hippocampal neurons. J Neurosci. 2006;26(31):8137–47. doi: 10.1523/JNEUROSCI.1183-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]