Abstract
Given the deleterious consequences associated with chronic stress, individual differences in stress susceptibility can have important fitness implications. These differences may be explained in part by dominance status because high rank is typically associated with decreased aggression and improved nutrition. Here, we examined the relationship between dominance and social stress in lactating chimpanzees, Pan troglodytes schweinfurthii, at Gombe National Park, Tanzania. We did so by pairing daily demographic and behavioural data with faecal glucocorticoid metabolite (FGM) concentrations collected over 37 months. While there was no main effect of rank, interesting differences emerged by adult subgroup size and adult sex ratio (males/females). We found that differences in FGM concentrations between high- and low-ranking females were most pronounced as adult subgroup size and sex ratio increased. Low-ranking females had higher FGM concentrations in larger subgroups and in subgroups biased towards adult males; we observed no comparable change in FGM concentrations amongst high-ranking females. Because low-ranking females were the recipient of significantly more male aggression relative to females of high rank, these patterns may be driven by psychosocial stress in low-ranking females. There was no significant change in diet quality across subgroup sizes; this finding suggests that nutritional stressors were not driving differences in female FGM concentrations. Being susceptible to social stress has important fitness implications as it may constrain low-ranking females from ‘choosing’ optimal subgroups to take advantage of food resources and/or for the socialization of their offspring.
Keywords: chimpanzee, Gombe National Park, lactation, Pan troglodytes schweinfurthii, rank status, stress
Although an animal’s physiological stress response can be adaptive because it mobilizes energy reserves to cope with immediate demands, chronic exposure to elevated stress hormones can have deleterious consequences, including immunosuppression, muscle wasting and reduced reproduction (reviewed in Sapolsky, 2002). Identifying factors promoting individual differences in susceptibility to stress can thus have important implications for understanding the proximate causes underlying variation in health, survivorship and reproductive success.
Numerous studies have demonstrated that rates of aggressive interactions and diet quality are correlated with glucocorticoid concentrations in wild animals (reviewed in Creel, Dantzer, Goymann, & Rubenstein, 2013). Because high rank typically confers reduced rates of received aggression and priority of access to food (reviewed in Clutton-Brock, 1988; Ellis, 1995; Sapolsky, 2001), early studies of rank and physiological stress responses were based on the hypothesis that low rank is stressful. However, subsequent research, particularly on males, has demonstrated a more equivocal relationship. Although some species show a negative correlation between rank and glucocorticoid concentrations (e.g. Faulkes & Abbott, 1997), others are characterized by a positive correlation (e.g. Carlson et al., 2004; Cavigelli, Dubovick, Levash, Jolly, & Pitts, 2003; Creel & Sands, 2003; Holekamp & Smale, 1998). Primary factors contributing to this variability across species in the relationship between rank and glucocorticoid concentrations include the stability of the social hierarchy and the manner in which high rank is attained and maintained (reviewed in: Creel et al., 2013; Sapolsky, 2005).
Fission–fusion systems provide a unique opportunity to investigate rank effects on stress physiology. In fission–fusion societies, fluidity in subgroup size is a strategy by which individuals respond to immediate constraints and opportunities. The general consensus is that time spent alone or in small subgroups minimizes intragroup competition for food when foraging resources are scarce (e.g. reviewed in Aureli et al., 2008). Although subordinates can thus reduce the likelihood of aggression by avoiding dominants, they may, as a result, be relegated to lower-quality food patches, miss opportunities for cooperative infant care, or be more vulnerable to predation (Krause & Ruxton, 2002; Murray, Eberly, & Pusey, 2006; Murray, Heintz, Lonsdorf, Parr, & Santymire, 2007). Analysing individual behaviours and stressors in the context of fission–fusion dynamics can thus reveal the strategies used by animals to balance their social and ecological environments.
Chimpanzees, Pan troglodytes, live in multimale, multifemale communities characterized by fission–fusion social organization in which subgroup composition within a permanent community changes frequently over time (Goodall, 1986; Nishida, 1968). Subgroup size is positively correlated with food supply (Goodall, 1986; Isabirye-Basuta, 1988; Mitani, Watts, & Lwanga, 2002; White & Wrangham, 1988; Wrangham, 1977), which suggests that taking advantage of temporally abundant food resources can impose costs associated with maintaining close proximity to others (i.e. nutritional benefits may be offset by aggression and anxiety). Theory predicts that these costs may be particularly pronounced among low-ranking individuals (reviewed in Creel et al., 2013). However, whether individuals of varying rank within a community differentially respond to the costs and benefits of grouping is poorly established.
In this study, we examined the relationship between rank and social stress in lactating East African chimpanzees, Pan troglodytes schweinfurthii. Dominance hierarchies in female chimpanzees are stable over time, and high rank status confers fitness benefits, including improved infant survival, faster-maturing daughters and shorter interbirth intervals (Pusey, Williams, & Goodall, 1997). These benefits are thought to be the result of differential access to food resources because a female in better condition can invest more energy in reproduction and offspring care (Pusey et al., 1997; Emery Thompson & Wrangham, 2008).
We focus our analyses on lactating females primarily because lactation is the most energetically demanding reproductive phase (Clutton-Brock, Albon, & Guinness, 1989; Gittleman & Thompson, 1988; Hanwell & Peaker, 1977) and is a critical component of female reproductive success (e.g. Lee, 1996; chimpanzees: Emery Thompson, 2013; Emery Thompson, Muller, & Wrangham, 2012). Furthermore, by focusing exclusively on lactation, we minimized complications of stress associated with sexual coercion (Muller, Kahlenberg, Emery Thompson, & Wrangham, 2007) and avoided known variation in cortisol concentrations across reproductive states in chimpanzees (Emery Thompson, Muller, Kahlenberg, & Wrangham, 2010).
Despite the importance of off-setting the costs of offspring care (Dufour & Sauther, 2002; Gittleman & Thompson, 1988; Pond, 1977), lactating females may be constrained from capitalizing on food resources if doing so is costly in terms of increased aggression received from conspecifics. In chimpanzees, mothers with dependent offspring are typically less gregarious, particularly with males (Otali & Gilchrist, 2006), than other female chimpanzees (Goodall, 1986; Murray, Lonsdorf, Eberly, & Pusey, 2009; Wrangham & Smuts, 1980). This may be due to the vulnerability of young to aggressive attacks (‘infant safety hypothesis’: Otali & Gilchrist, 2006) and/or the costs of travelling with an infant: travel distance and subgroup size are positively correlated, and mothers with dependent offspring may not be able to keep pace with larger subgroups (Wrangham, 2000). In either case, evidence suggests that lactating mothers face unique social and ecological trade-offs relative to other females, yet little is known about how female rank status influences this trade-off.
Our study addresses two main objectives. First, we examined social stress in lactating chimpanzees by determining the effects of rank, adult subgroup size and adult subgroup sex ratio on faecal glucocorticoid metabolite (FGM) concentrations. Given the documented fitness benefits of rank in female chimpanzees, we predicted that low-ranking females would be more susceptible to social stress than high-ranking females. Second, we analysed how two possible sources of stress in lactating chimpanzees vary with rank and subgroup size: rates of received aggression and diet quality. Chimpanzees are ripe fruit specialists (Goodall, 1986), and the percentage of fruit in the diet indicates quality such that diets higher in fruit are considered better quality relative to diets lower in fruit (e.g. Chapman, Wrangham, & Chapman, 1995; Emery Thompson & Wrangham, 2008). We predicted that high-ranking females would have lower rates of received aggression and higher diet quality relative to low-ranking females, and that these differences would be most pronounced in larger subgroups, which typically form in response to fruit availability but foster greater opportunities for conflict (i.e. more potential aggressors). These two variables (rate of received aggression and diet quality) provide insight into the costs and benefits of grouping, and whether socioecological trade-offs are experienced equitably by individuals of varying rank.
METHODS
Tanzanian field assistants and M. Heintz collected demographic and behavioural data on chimpanzees at Gombe National Park, Tanzania, as a part of a long-term research project. Faecal samples were collected and processed as part of a broader study addressing the effects maternal stress on offspring health and development. Each observation day, researchers followed one lactating female and collected data on (1) subgroup composition every 5–15 min and (2) the female’s activity every 1 min (using instantaneous scan samples: Altmann, 1974). Observation began when a female descended from her sleeping nest or was first located, and continued (at maximum) until the female ascended into a sleeping site in the afternoon. Mean ± SE duration of observation days included in analyses was 8.6±0.25 h , and mean duration between consecutive observation days was 75±10.3 days.
Aggression received by the female was recorded ad libitium on observation days. For a 37-month period (1 January 2009–31 July 2012), researchers followed the mother again the next day to collect faecal samples for hormone quantification (see below). Because circulating glucocorticoid metabolites manifest in faeces after 12–24 h in chimpanzees (Murray et al., 2013), this 2-day pairing of observational data with physiological samples allowed us to link FGM levels to demographic and behavioural data with finer temporal resolution than in previous studies.
We categorized females as lactating from the birth of an infant until the earliest of the following: infant’s death, the conception of the female’s next infant, or the infant’s fourth birthday (infant chimpanzees are typically not fully weaned until 4–5 years: Clark, 1977; Pusey, 1983; van de Rijt-Plooij & Plooij, 1987). Long-term behavioural observations of immigrant females indicate that females are well integrated into their new community after they have given birth; spatial site fidelity and permanent residence occur after a female gives birth (Williams, Pusey, Carlis, Farm, & Goodall, 2002). Because all females included in this study were either natal to the study community (64.3%; N = 9), or they conceived after immigration (35.7%, N = 5), immigration status was not a variable tested in our analyses.
Female Rank Status
We categorized females as either ‘high-ranking’ or ‘low-ranking’ based upon their position in the female dominance hierarchy. The dominance hierarchy was calculated from the directionality of female–female pant-grunts observed during the study period. Pant-grunt vocalizations are formal indicators of subordination in chimpanzees (Bygott, 1979). All pant-grunts from the long-term data (for details, see Wilson, 2012) and student projects for our study period were consolidated and provided to us by the Jane Goodall Institute Research Center at Duke University (Durham, NC, U.S.A.). We used SocProg 2.4 (Whitehead, 2009) to calculate the modified David’s score (de Vries, Stevens, & Vervaecke, 2006). Only pant-grunts between females who were adults (≥ 12 years old) within 1 month of the interaction were included. To limit complications of third-party involvement in dominance assessment, we only included dyadic pant-grunts in our calculations. These criteria resulted in a sample size of 99 pant-grunts. For analyses, ‘high-ranking’ females (N = 5) were categorized as individuals with a modified David’s score greater than 0.5 SD above the mean modified David’s score; all other ranked females (N = 9) were considered ‘low-ranking’. This follows the precedent set in other studies of ranking female chimpanzees categorically (e.g. Murray, 2007; Pusey et al., 1997; Wrangham, Clark, & Isabirye-Basuta, 1992), and specifically for using two categories for analyses that focus on the physiological benefits experienced by high-ranking females (Emery Thompson et al., 2010).
Predictors of Stress: Subgroup Demographics, Rates of Received Aggression and Diet Quality
We calculated mean daily subgroup size as the average number of adults (≥ 12 years old) present in each subgroup composition taken over the course of the day; mean daily subgroup sex ratio was calculated as the average sex ratio (number of adult males/number of adult females) of each subgroup composition taken over the course of the day. We calculated daily aggression rate as the number of aggressive interactions directed towards the focal mother divided by the total hours of observation; aggression rates were calculated separately for male versus female aggressors. Finally, diet quality was determined as the percentage of fruit in the diet. We calculated this as the number of 1 min scans when the individual was eating fruit divided by the total number of minutes that the focal was feeding on an identified food item that day; this value was multiplied by 100 to express fruit as a percentage of the female’s daily diet. This measure of diet quality follows the precedent of other studies (e.g. Chapman et al., 1995; Emery Thompson & Wrangham, 2008) and is correlated with the percentage of time spent consuming fruit for full-day follows (focal female was observed ≥ 10 h; Spearman’s rS = −0.504, N = 35, P = 0.002).
Faecal Sample Collection and Preparation
Faecal samples were collected opportunistically during observation times as soon as feasible following defecation and stored in sealed, labelled bags. Staff stored the samples in a −13 °C freezer immediately upon return to the field station after completing a follow. For days on which more than one faecal sample was collected for a focal female (N = 20 days), we selected one sample at random for inclusion in our analyses. Faecal samples were processed at our field laboratory, stored as dry extracts and reconstituted for quantification via enzyme immunoassay at Lincoln Park Zoo (Chicago, IL, U.S.A.; Murray et al., 2013). Briefly, 5.0 ml of 90% ethanol was added to approximately 0.5 g of homogenized faeces. Samples were capped, shaken for 30 s and rotated on a low-energy rotator (Barnstead International, Model 400110, DuBuque, IA, U.S.A.) for 2 h. Samples were centrifuged for 20 min to pellet the faeces. The samples’ ethanol (now containing the faecal hormone metabolites) was poured into clean testtubes. Then, 1 ml of extracts were placed in to 12 × 75 mm tubes and allowed to evaporate in a pelican case with reusable desiccant (Eva-dry, Westchase, FL, U.S.A.). Dried extracts were capped and shipped to Lincoln Park Zoo for analyses. Samples were reconstituted in 1 ml of phosphate buffered saline (PBS; 0.2 M NaH2PO4, 0.2 M Na2HPO4, NaCl), sonicated for 20 min and stored in the freezer until analysed using a cortisol enzyme immunoassay (EIA). Cortisol antiserum (R4866) and HRP (provided by C. Munro, University of California-Davis, CA, U.S.A.) were used at dilutions of 1:8500 and 1:20000, respectively (Loeding, Thomas, Bernier, & Santymire, 2011). Cross-reactivities of cortisol R4866 antibody are reported as: cortisol 100%, prednisone 6.3%, corticosterone 0.7%, 21-deoxycorticosterone 0.5%, progesterone 0.2%, pregnenolone 0.1%, androstenedione 0.1%, dehydroisoandrosterone-3-sulfate 0.1%, oestradiol-17β 0.1%, oestriol 0.1%, cholesterol 0.1%, prednisolone 9.9%, cortisone 5.0%, deoxycorticosterone 0.3%, 11-desoxycortisol 0.2%, 17α-hydroxyprogesterone 0.2%, 17α-hydroxypregnenolone 0.1%, testosterone 0.1%, dehydroepiandrosterone 0.1%, aldosterone 0.1%, oestrone 0.1% and spironolactone 0.1% (Young et al., 2004). All samples were run in duplicate. Assay sensitivity was 1.95 pg/well and intra- and interassay coefficients of variation were less than 10%. This cortisol EIA was previously biochemically validated for chimpanzees by demonstrating parallelism between the binding inhibition curves of faecal extract dilutions and the cortisol standard and significant recovery (> 90%) of exogenous cortisol added to faecal extracts (Murray et al., 2013). An adrenocorticotrophic hormone (ACTH) challenge has also been used to physiologically validate the use of this EIA for chimpanzees (Murray et al., 2013).
Ethical Note
All project protocols complied with regulations in Tanzania and were approved by Tanzanian National Parks, Tanzania Wildlife Research Institute and Tanzanian Commission for Science and Technology, and adhered to the ASAB/ABS Guidelines for the Use of Animals in Research.
Statistical Analyses
We constructed linear mixed models (LMMs) to predict log-transformed FGM concentrations. LMMs extend general linear models by inclusion of random effects. In our first model, the predictor variables included rank category (high and low ranking) and average daily adult subgroup size. We also included an interaction term to test the relationship between these variables. Similarly, in our second model, we tested rank category, average daily adult sex ratio and an interaction term for rank and sex ratio. Given previous reports of higher urinary cortisol concentrations in older individuals (Emery Thompson et al., 2010), we tested female age in a third model. In all LMMs, offspring age on the day prior to sample collection was entered as a predictor variable to account for potential variability in FGM concentrations throughout the lactation period. We also entered categorical time of day (AM vs PM) of faecal sample collection as a predictor variable to account for diurnal variability in FGM concentrations (Murray et al., 2013). Lastly, chimpanzee identity was entered as a random variable to allow for potential individual differences between chimpanzees. We evaluated the effect of categorical rank and categorical subgroup size (<5 adults and ≥ 5 adults: see below for justification on this cut-off criterion) on received aggression rate, percentage of fruit in diet and FGM concentrations using a series of Mann–Whitney U tests to test for (1) overall differences between ranks and (2) differences within ranks between subgroup sizes. We used this nonparametric test because aggression rate were not normally distributed and therefore differences in aggression rates by rank and subgroup size could not be tested using LMMs. For consistency, we performed the same test on percentage of fruit in diet. For FGM concentrations, this Mann–Whitney U test provided additional insight beyond the LMM (described above) into differences in hormone levels by rank and subgroup size. To account for repeated sampling of individual females, we calculated mean values for each female when testing for overall differences between ranks and mean values for each female in each categorical subgroup size when testing within ranks for differences between subgroup sizes. Finally, we tested the direct effect of received aggression rate and percentage of fruit in diet on log-transformed mean daily FGM concentrations separately for high- and low-ranking females with LMMs controlling for offspring age, categorical time of day and female identity. Only data from days on which a focal female was observed for at least 3 h were included in our analyses. To avoid biases in the percentage of fruit in diet due to inadequate observation of food consumption, dietary analyses were further limited to days on which the focal female was observed feeding for at least 2 h. All analyses were performed in SPSS 20.0 with a significance value of 0.05.
RESULTS
We collected 102 faecal samples (from 14 lactating females) for which FGM concentrations were partnered with behavioural data from the day prior. Eighty-four of these faecal samples met the additional criterion of being partnered with behavioural observations that captured adequate foraging data for inclusion in diet analyses. On sample days, the mean ± SE daily subgroup size was 6.5±0.60 and the mean daily sex ratio was 0.54± 0.055. Mean ± SE number of faecal samples collected per female was 7±1.3 (range 1–15).
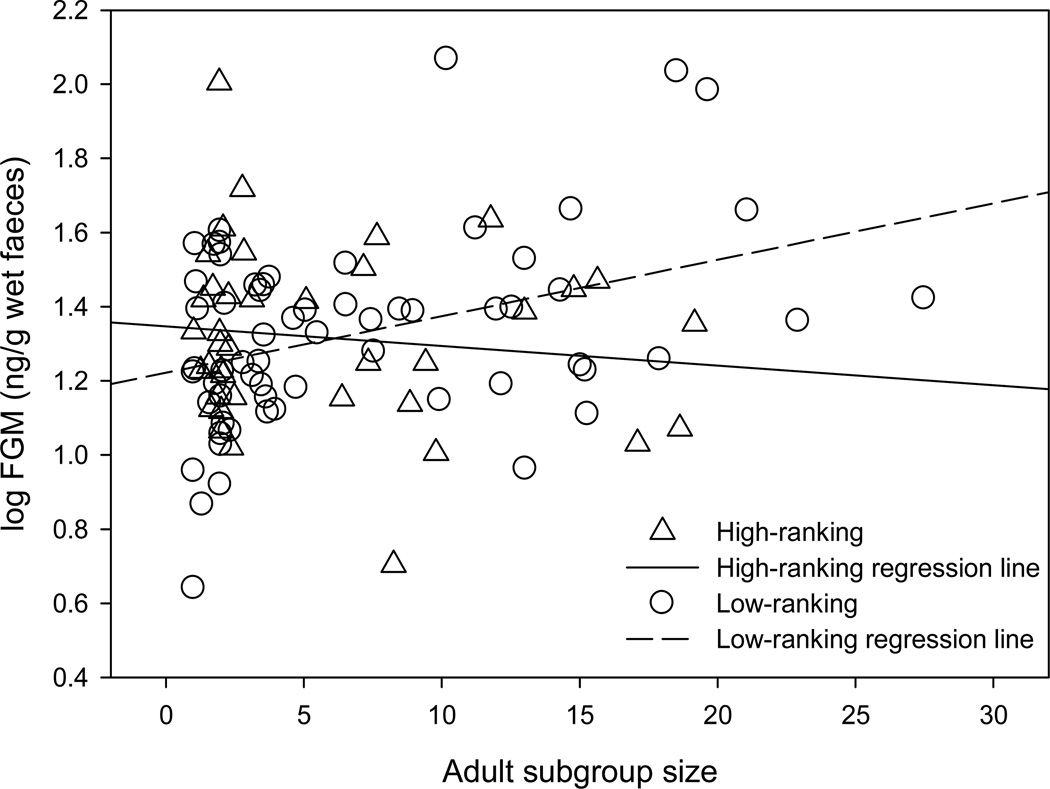
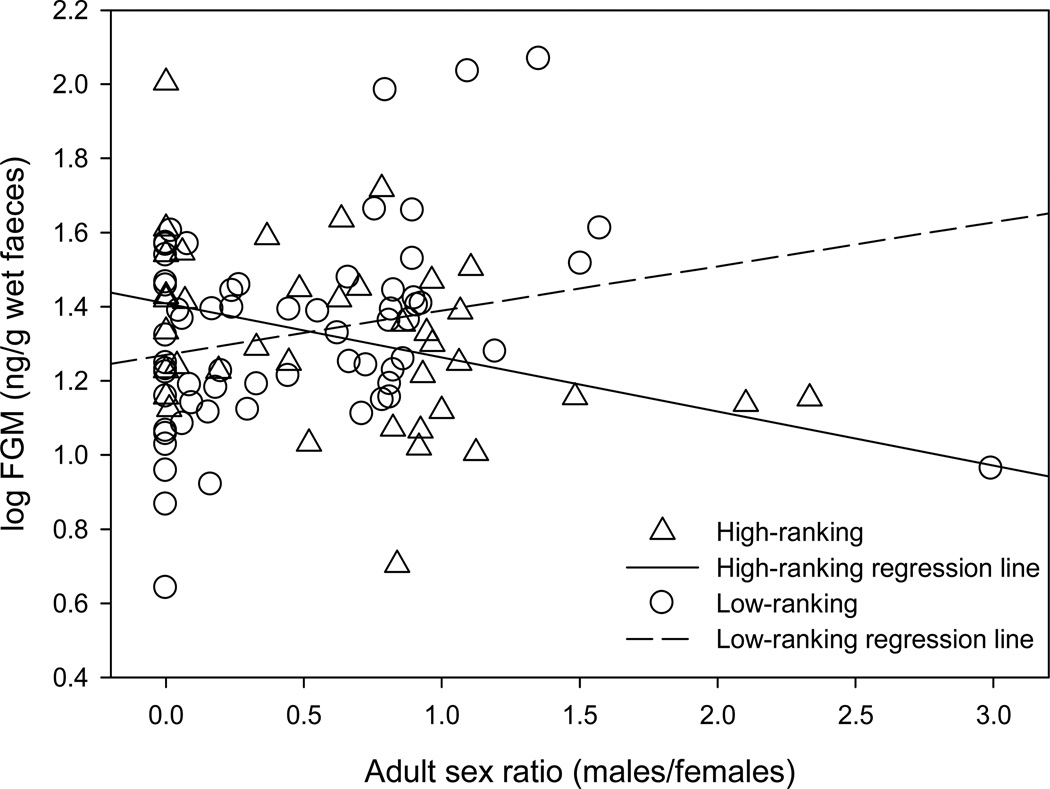
FGM concentrations were negatively predicted (LMM: F1, 95.953 = 5.049, P = 0.027) by the interaction of female rank and adult subgroup size; neither female rank, adult subgroup size, time of day, nor offspring age was a significant predictor (Table 1). The difference between high- and low-ranking females was more pronounced as subgroup size increased (Fig. 1). The intercept of the regression lines for high- and low-ranking females (5.14 adults) was used to classify small and large subgroups in subsequent analyses: small subgroups were defined as <5 adults and large subgroups were defined as ≥5 adults. Similarly, FGM concentrations were negatively predicted (LMM: F1, 57.585 = 5.174, P = 0.027) by the interaction of female rank and adult subgroup sex ratio; neither female rank, subgroup sex ratio, time of day, nor offspring age was a significant predictor (Table 2). Differences between high- and low-ranking females were more pronounced as the sex ratio became increasingly male biased (Fig. 2). Female age was not a predictor (LMM: F1, 4.169 = 0.044, P = 0.843) of FGM concentrations in a model controlling for time of sample collection (F1, 97.188 = 0.017, P = 0.896) and offspring age (F1, 21.157 = 2.963, P = 0.100).
Table 1.
Effect of categorical rank (high- and low-ranking), adult subgroup size, infant age (days), time of sample collection (AM vs PM), and an interaction term for rank by subgroup size on log-transformed faecal glucocorticoid metabolite (FGM) concentrations in female chimpanzees
| Variable | Estimate | Numerator df |
Denominator df |
F | P |
|---|---|---|---|---|---|
| Dependent variable: log FGM | |||||
| Intercept | 1.2877 | 1 | 20.336 | 533.745 | <0.001 |
| Rank (high vs low) | 0.1282 a | 1 | 15.734 | 2.846 | 0.111 |
| Adult subgroup size | 0.0140 | 1 | 95.964 | 0.835 | 0.363 |
| Time of faecal collection (AM vs PM) | 0.0057 b | 1 | 95.979 | 0.008 | 0.929 |
| Offspring age (days) | −0.0363 | 1 | 20.855 | 3.046 | 0.096 |
| Rank*adult subgroup size | −0.0198 c | 1 | 95.953 | 5.049 | 0.027 |
Statistically significant results appear in bold.
For high-rank;
For AM samples;
For high-rank*adult subgroup size.
Figure 1.
Effect of adult subgroup size on log-transformed faecal glucocorticoid metabolite (FGM) concentrations for high- and low-ranking lactating chimpanzees.
Table 2.
Effect of categorical rank (high- and low-ranking), adult subgroup sex ratio (number of adult males/number of adult females), infant age (days), time of sample collection (AM vs PM), and an interaction term for rank by sex ratio on log-transformed faecal glucocorticoid metabolite (FGM) concentrations in female chimpanzees
| Variable | Estimate | Numerator df |
Denominator df |
F | P |
|---|---|---|---|---|---|
| Dependent variable: log FGM | |||||
| Intercept | 1.3089 | 1 | 10.627 | 503.927 | <0.001 |
| Rank (high vs low) | 0.1239 a | 1 | 6.922 | 2.479 | 0.160 |
| Adult sex ratio | 0.1025 | 1 | 38.775 | 0.037 | 0.848 |
| Time of faecal collection (AM vs PM) | 0.0224 b | 1 | 95.999 | 0.121 | 0.728 |
| Offspring age (days) | −0.0293 | 1 | 17.856 | 1.740 | 0.204 |
| Rank*adult sex ratio | −0.2229 c | 1 | 57.585 | 5.174 | 0.027 |
Statistically significant results appear in bold.
For high-rank;
For AM samples;
For high-rank*adult subgroup size.
Figure 2.
Effect of adult subgroup sex ratio (number of adult males/number of adult females) on log-transformed faecal glucocorticoid metabolite (FGM) concentrations for high- and low-ranking lactating chimpanzees.
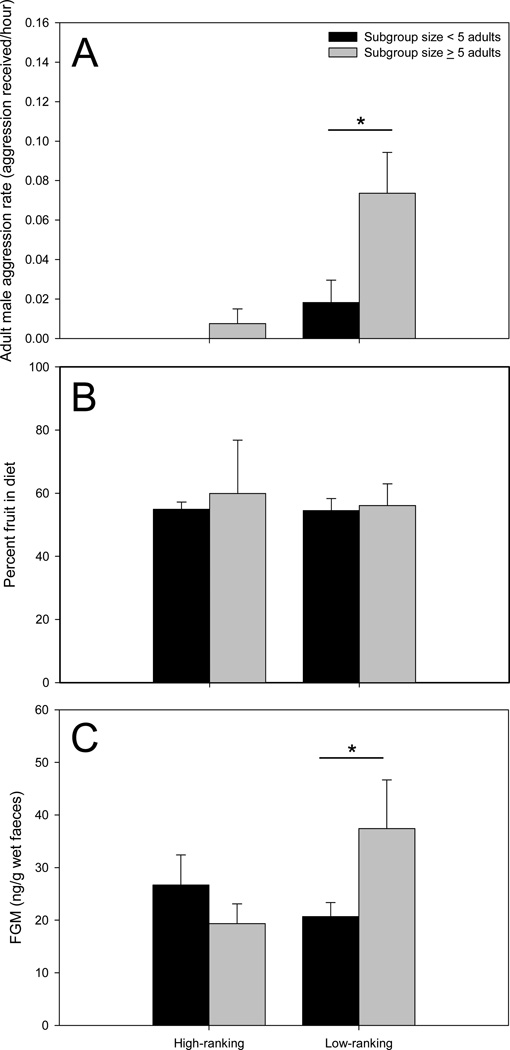
For both high- and low-ranking females, aggression received from adult females was rare: only two instances of female–female aggression were observed during the 865.4 h of focal observation. Because of the paucity of data, subsequent analyses focused exclusively on aggression from adult males (N = 23 instances). Rates of aggressive interactions with adult males was lower for high-ranking females than for low-ranking females (two-tailed Mann–Whitney U test: U = 2.5, Nhigh-ranking = 4, Nlow-ranking= 10, P = 0.008). Only low-ranking females experienced an increase (two-tailed Mann–Whitney U test: U = 13.0, Nsmall subgroup = 8, Nlarge subgroup = 9, P = 0.027) in aggression rates in large subgroups; there was no change (U = 4.5, Nsmall subgroup = 3, Nlarge subgroup = 4, P = 0.629) in aggression rates between large and small subgroups for high-ranking females (Fig. 3a). Rates of aggression were not a direct predictor (LMM: F1, 33.043 = 0.837, P = 0.367) of FGM concentrations for high-ranking females in a model controlling for time of sample collection (F1, 33.885 = 1.428, P = 0.240) and offspring age (F1, 6.412 = 3.825, P = 0.095). Similarly, rates of aggression were not a direct predictor (LMM: F1, 58.543 = 2.723, P = 0.104) of FGM concentrations for low-ranking females in a model controlling for time of sample collection (F1, 58.987= 2.343, P = 0.131) and offspring age (F1, 11.255 = 0.867, P = 0.371).
Figure 3.
Differences between categorical rank (high- and low-ranking) and categorical subgroup size (< 5 adults and ≥ 5 adults) on (a) mean rates of aggression received from adult males, (b) mean percentage of fruit in diet and (c) faecal glucocorticoid metabolite (FGM) concentrations in female chimpanzees. Asterisk indicates statistically significant differences.
High- and low-ranking females did not differ (two-tailed Mann–Whitney U test: U = 13.0, Nhigh-ranking = 4, Nlow-ranking = 10, P = 0.374) in the percentage of fruit in their diet. Percentage of fruit in diet did not vary by subgroup size for high-ranking females (U = 3.0, Nsmall subgroup = 3, Nlarge subgroup = 4, P = 0.400) or low-ranking females (U = 31.0, Nsmall subgroup = 8, Nlarge subgroup = 9, P = 0.673; Fig. 3b). Percentage of fruit in diet was not a direct predictor (LMM: F1, 26.604 = 0.065, P = 0.801) of FGM concentrations for high-ranking females in a model controlling for time of sample collection (F1, 26.676 = 1.291, P = 0.266) and offspring age (F1, 13.050 = 1.641, P = 0.222). Similarly, percentage fruit in diet was not a direct predictor (LMM: F1, 47.326 = 0.037, P = 0.849) of FGM concentrations for low-ranking females in a model controlling for time of sample collection (F1, 47.248= 0.791, P = 0.378) and offspring age (F1, 7.355 = 1.008, P = 0.347).
High- and low-ranking females did not differ in overall FGM concentrations (two-tailed Mann–Whitney U test: U = 15.0, Nhigh-ranking = 4, Nlow-ranking = 10, P = 0.539), but FGM concentrations for low-ranking females were higher in large subgroups than in small subgroups (U = 12.0, Nsmall subgroup = 8, Nlarge subgroup = 9,P = 0.021); FGM concentration did not vary by subgroup size for high-ranking females (U = 4.0, Nsmall subgroup = 3, Nlarge subgroup = 4, P = 0.629; Fig. 3c).
DISCUSSION
We found that differences in FGM concentrations between high- and low-ranking females were most pronounced as adult subgroup size and adult sex ratio (males/females) increased: compared to high-ranking females, low-ranking females had higher FGM concentrations in larger subgroups and in subgroups biased towards adult males. While previous studies have reported higher urinary cortisol concentrations in older females (Emery Thompson et al., 2010) and a positive correlation between female rank and age (Goodall, 1986; Murray et al., 2006; Nishida, 1989; Pusey et al., 1997), there was no direct relationship between FGM concentration and age in this study. One plausible explanation is that the ages (mean ± SE: 22.9±2.09 years; range 13.1–38.7; N = 14) of females included in this study were constrained relative to the age range of females analysed by Emery Thompson et al. (2010). Status patterns reported herein therefore are interpreted as rank effects independent of age.
Low-ranking females received more male aggression relative to high-ranking females in general, with the highest rates experienced in large subgroups. This suggests that negative social interactions may drive the FGM concentrations of low-ranking females to increase with subgroup size. Previous research has documented male aggression against females with dependent offspring (reviewed in: Arcadi & Wrangham, 1999; Muller et al., 2007), and the risk of aggressive encounters is considered a major factor influencing decreased sociality in lactating females (Otali & Gilchrist, 2006). We are aware of no prior analyses investigating biases in male aggression rates towards lower-ranking females, however.
One possible explanation for this pattern is that males preferentially form close social bonds with high-ranking females as in other primate species (e.g. Palombit, Seyfarth, & Cheney, 1997), and rank-biased aggression is a reflection of males ensuring the well-being of their preferred mating partners. Findings by Muller, Emery Thompson and Wrangham (2006) that male chimpanzees prefer mating with older females offers preliminary support for this hypothesis, given that female rank and age are highly correlated in this species (Goodall, 1986; Pusey et al., 1997; Murray et al., 2006; Nishida, 1989). Our result demonstrating that FGM concentrations in low-ranking females increase as the subgroup sex ratio becomes increasingly male-biased is also consistent with this pattern. Note, however, that we found no significant correlation between aggression received and FGM concentration. Emery Thompson et al. (2010) reported a similar pattern in lactating females in the Kanyawara chimpanzee community at Kibale National Park, Uganda, when urinary cortisol and aggression data were assessed at a broad, monthly resolution. Together, these results strongly suggest that low-ranking females may have an anticipatory stress response as subgroup size increases or becomes biased towards males.
Overall diet quality did not differ between high- and low-ranking females, and females in both rank categories experienced the same diet quality when in different subgroup sizes. Previous studies detecting rank differences in diet quality in this species did not consider reproductive state (Murray, Mane, & Pusey, 2007; Wittig & Boesch, 2003). The extent to which diet quality differs between high- and low-ranking females that are either cycling or pregnant warrants further investigation.
The lack of a relationship between FGM concentrations and diet quality in females is in marked contrast to patterns observed in other primate species, which highlight the importance of nutritional factors influencing stress in females (e.g. Cavigelli, 1999; Gesquiere et al., 2008; but see Cheney & Seyfarth, 2009). One plausible reason for this discrepancy may be differences in social organization. Unlike females in species characterized by stability in social group size and composition, it is possible for females to avoid competitive interactions with conspecifics during periods of food scarcity in fission–fusion social systems. Consequently, foraging alone or in small subgroups is itself an important coping strategy for periods of low food abundance uniquely available to females in fission–fusion societies (reviewed in Aureli et al., 2008). Nevertheless, lactating chimpanzees may benefit from the social flexibility to join subgroups at abundant food resources in order to meet the nutritional demands of milk production and offspring care. This is consistent with studies demonstrating that lactating chimpanzees have higher-quality diets relative to females that are neither lactating nor pregnant (Murray et al., 2009). A critical effect of rank status may be that joining subgroups carries a physiological cost for low-ranking females that is not experienced by high-ranking females.
Previous research on female chimpanzees provides interesting comparisons to our results. Emery Thompson et al. (2010) found that lactating individuals in the Kanyawara community of Kibale National Park, Uganda, had higher urinary cortisol levels during months of low fruit consumption relative to months of high fruit consumption. Although aggression from males led to higher urinary cortisol levels among oestrous females relative to lactating and pregnant females, there was no significant relationship between aggression received from males and urinary cortisol levels among lactating females. Rather, urinary cortisol levels among lactating females in Kanyawara were predicted by overall rates of female–female aggression within the community (regardless of whether the focal individual was the direct recipient of aggression); aggression received from females did not predict urinary cortisol levels. The authors also found that low dominance rank was associated with increased urinary cortisol levels, particularly in lactating (as opposed to pregnant or cycling) females. A possible explanation for this difference is that females in Kanyawara are more nutritionally constrained than females in other populations, including Gombe. This argument is consistent with the results that interbirth intervals are longer in Kanywara relative to those experienced by chimpanzees in Gombe (Emery Thompson, 2013; Knott, 2001; Potts, 2013), and emphasizes the importance of evaluating predictors of stress in a range of social and ecological conditions. Additionally, because Emery Thompson et al. (2010) assessed urinary cortisol reactivity using monthly averages, immediate (e.g. daily) changes in resource abundance and subgrouping patterns may be masked, whereas our daily assessments provide insight into a more direct relationship between hormone concentrations and socioecological conditions. Comparisons between Emery Thompson et al.’s findings and ours therefore highlight the importance of analysing physiological responses across multiple temporal scales.
Conclusion
While previous work has reported that lactating chimpanzees are less social than nonlactating females (Goodall, 1986; Murray et al., 2009; Otali & Gilchrist, 2006; Wrangham & Smuts, 1980), our results suggest important nuances in the physiological trade-offs of sociality based on rank; low-ranking females, but not high-ranking females, exhibited increased FGM concentrations in larger subgroups. This response to increased subgroup size among low-ranking females can have important fitness implications as it may constrain low-ranking females from ‘choosing’ optimal subgroups to take advantage of food abundances and/or opportunities for socializing their offspring. Furthermore, these findings contribute to our understanding of rank effects on female fitness in chimpanzees by highlighting the importance of social factors, in addition to the previously reported importance of nutritional factors (Pusey et al., 1997), as a benefit of high rank status.
Highlights.
We examined the relationship between dominance rank and social stress in wild lactating chimpanzees.
Rank differences in faecal glucocorticoid metabolite (FGM) concentration (low > high) increased with subgroup size.
Rank differences in FGM concentration (low > high) increased in male-biased subgroups.
These patterns may be driven by psychosocial stress in low-ranking females.
Our results suggest that FGM concentration in lactating females is not nutritionally driven.
Acknowledgments
We thank Tanzania National Parks, the Tanzania Wildlife Research Institute and the Tanzanian Council for Science and Technology for granting us permission to work in Gombe National Park. We also thank the Jane Goodall Institute for supporting long-term chimpanzee research at Gombe National Park and the Gombe Stream Research Centre staff for maintaining data collection. The Jane Goodall Institute Research Center at Duke University (supported by grants from the National Science Foundation (LTREB-1052693), the National Institutes of Health (R01 AI058715) and Duke University) provided pant-grunt data that was consolidated from the long-term data and graduate student projects. Physiological data collection and processing were supported by the National Institutes of Health (5R00HD057992) and the Davee Center for Epidemiology and Endocrinology (Lincoln Park Zoo, Chicago, IL, U.S.A.). Collection, digitization and analysis of behavioural data were supported by grants from the National Institutes of Health (5R00HD057992) and the Leo S. Guthman Foundation, and grants to M. Heintz (NSFGRF, Leakey Foundation, Wenner-Grenn Foundation and Field Museum African Council). Finally, we thank V. Fiorentino and K. Anderson for data management, J. Drutz, A. Mustapha, A. Schmitt and E. Schwartz for data entry, and A. Pusey, I. Gilby, M. Stanton and V. Fiorentino for invaluable discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altmann J. Observational study of behavior: sampling methods. Behaviour. 1974;49:227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Arcadi AC, Wrangham RW. Infanticide in chimpanzees: review of cases and a new within-group observation from Kanyawara study groups in Kibale National Park. Primates. 1999;40:337–351. [Google Scholar]
- Aureli F, Schaffner CM, Boesch C, Bearder SK, Call J, Chapman CA, Connor R, van Schaik CP. Fission–fusion dynamics: new research frameworks. Current Anthropology. 2008;49:627–654. [Google Scholar]
- Bygott JD. Agonistic behaviour, dominance and social structure in wild chimpanzees of the Gombe National Park. In: Hamburg DA, McCown ER, editors. The Great Apes. Menlo Park: Benjamin/Cummings; 1979. pp. 405–427. [Google Scholar]
- Carlson AA, Young AJ, Russell AF, Bennett NC, McNeilly AS, Clutton-Brock T. Hormonal correlates of dominance in meerkats (Suricata suricatta) Hormones and Behavior. 2004;46:141–150. doi: 10.1016/j.yhbeh.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Cavigelli SA. Behavioural patterns associated with faecal cortisol levels in free-ranging female ring-tailed lemurs, Lemur catta. Animal Behaviour. 1999;57:935–944. doi: 10.1006/anbe.1998.1054. [DOI] [PubMed] [Google Scholar]
- Cavigelli SA, Dubovick T, Levash W, Jolly A, Pitts A. Female dominance status and fecal corticoids in a cooperative breeder with low reproductive skew: Ring-tailed lemurs (Lemur catta) Hormones and Behavior. 2003;43:166–179. doi: 10.1016/s0018-506x(02)00031-4. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Wrangham RW, Chapman LJ. Ecological constraints on group size: An analysis of spider monkey and chimpanzee subgroups. Behavioral Ecology and Sociobiology. 1995;36:59–70. [Google Scholar]
- Cheney DL, Seyfarth RM. Stress and coping mechanisms in female primates. Advances in the Study of Behavior. 2009;39:1–44. [Google Scholar]
- Clark CB. A preliminary report on weaning among chimpanzees of the Gombe National Park, Tanzania. In: Chevalier-Skolnikoff S, Poirier F, editors. Primate bio-social development: Biological, social and ecological determinants. New York, NY, USA: Garland Press; 1977. pp. 235–260. [Google Scholar]
- Clutton-Brock TH. Reproductive success. In: Clutton-Brock TH, editor. Reproductive success: Studies of individual variation in contrasting breeding systems. Chicago: University of Chicago Press; 1988. pp. 472–486. [Google Scholar]
- Clutton-Brock TH, Albon SD, Guinness FE. Fitness costs of gestation and lactation in wild mammals. Nature. 1989;337:260–262. doi: 10.1038/337260a0. [DOI] [PubMed] [Google Scholar]
- Creel S, Dantzer B, Goymann W, Rubenstein DR. The ecology of stress: effects of the social environment. Functional Ecology. 2013;27:66–80. [Google Scholar]
- Creel S, Sands JL. Is social stress a consequence of subordination or a cost of dominance? In: de Waal F, Tyack P, editors. Animal Social Complexity. Cambridge, MA, U.S.A.: Harvard University Press; 2003. pp. 153–179. [Google Scholar]
- de Vries H, Stevens JMG, Vervaecke H. Measuring and testing the steepness of dominance hierarchies. Animal Behaviour. 2006;71:585–592. [Google Scholar]
- Dufour DL, Sauther ML. Comparative and evolutionary dimensions of the energetics of human pregnancy and lactation. American Journal of Human Biology. 2002;14:584–602. doi: 10.1002/ajhb.10071. [DOI] [PubMed] [Google Scholar]
- Ellis L. Dominance and reproductive success among nonhuman animals: a cross-species comparison. Ethology and Sociobiology. 1995;16:257–333. [Google Scholar]
- Emery Thompson M. Reproductive ecology of female chimpanzees. American Journal of Primatology. 2013;75:222–237. doi: 10.1002/ajp.22084. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M, Muller MN, Kahlenberg SM, Wrangham RW. Dynamics of social and energetic stress in wild female chimpanzees. Hormones and Behavior. 2010;58:440–449. doi: 10.1016/j.yhbeh.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery Thompson M, Muller MN, Wrangham RW. The energetic of lactation and the return to fecundity in wild chimpanzees. Behavioral Ecology. 2012;23:1234–1241. [Google Scholar]
- Emery Thompson M, Wrangham RW. Diet and reproductive function in wild female chimpanzees (Pan troglodytes schweinfurthii) at Kibale National Park, Uganda. American Journal of Physical Anthropology. 2008;135:171–181. doi: 10.1002/ajpa.20718. [DOI] [PubMed] [Google Scholar]
- Faulkes CG, Abbott DH. The physiology of a reproductive dictatorship: Regulation of male and female reproduction by a single breeding female in colonies of naked mole-rats. In: Solomon NG, French JA, editors. Cooperative Breeding in Mammals. Cambridge, UK: Cambridge University Press; 1997. pp. 302–334. [Google Scholar]
- Goodall J. The chimpanzees of Gombe. Cambridge, MA, USA: Harvard University Press; 1986. [Google Scholar]
- Gesquiere LR, Khan M, Shek L, Wango TL, Wango EO, Alberts SC, Altmann J. Coping with a challenging environment: Effects of seasonal variability and reproductive status on glucocorticoid concentrations of female baboons (Papio cynocephalus) Hormones and Behavior. 2008;54:410–416. doi: 10.1016/j.yhbeh.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittleman JL, Thompson SD. Energy allocation in mammalian reproduction. American Zoology. 1988;28:863–875. [Google Scholar]
- Hanwell A, Peaker M. Physiological effects of lactation on the mother. Symposia of the Zoological Society of London. 1977;41:297–311. [Google Scholar]
- Holekamp KE, Smale L. Dispersal status influences hormones and behavior in the male spotted hyena. Hormones and Behavior. 1998;33:205–216. doi: 10.1006/hbeh.1998.1450. [DOI] [PubMed] [Google Scholar]
- Isabirye-Basuta G. Food competition among individuals in a free-ranging chimpanzee community in Kibale Forest, Uganda. Behaviour. 1988;105:135–147. [Google Scholar]
- Knott C. Female reproductive ecology of the apes. In: Ellison PT, editor. Reproductive ecology and human evolution. New York, NY, USA: Aldine de Gruyter; 2001. pp. 429–463. [Google Scholar]
- Krause J, Ruxton GD. Living in groups. Oxford, UK: Oxford University Press; 2002. [Google Scholar]
- Lee PC. The meanings of weaning: growth, lactation, and life history. Evolutionary Anthropology. 1996;5:87–98. [Google Scholar]
- Loeding E, Thomas J, Bernier D, Santymire R. Using fecal hormonal and behavioral analyses to evaluate the introduction of two sable antelope at Lincoln Park Zoo. Journal of Applied Animal Welfare Science. 2011;14:220–246. doi: 10.1080/10888705.2011.576968. [DOI] [PubMed] [Google Scholar]
- Mitani JC, Watts DP, Lwanga JS. Ecological and social correlates of chimpanzee party size and composition. In: Boesch C, Hohmann G, Marchant LF, editors. Behavioural diversity in chimpanzees and bonobos. Cambridge, UK: Cambridge University Press; 2002. pp. 102–111. [Google Scholar]
- Muller MN, Emery Thompson M, Wrangham RW. Male chimpanzees prefer mating with old females. Current Biology. 2006;16:2234–2238. doi: 10.1016/j.cub.2006.09.042. [DOI] [PubMed] [Google Scholar]
- Muller MN, Kahlenberg SM, Emery Thompson M, Wrangham RW. Male coercion and the costs of promiscuous mating for female chimpanzees. Proceedings of the Royal Society B. 2007;274:1009–1014. doi: 10.1098/rspb.2006.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CM, Eberly LE, Pusey AE. Foraging strategies as a function of season and rank among wild female chimpanzees (Pan troglodytes) Behavioral Ecology. 2006;17:1020–1028. [Google Scholar]
- Murray CM, Heintz MR, Lonsdorf EV, Parr LA, Santymire RM. Validation of a field technique and characterization of fecal glucocorticoid metabolite analysis in wild chimpanzees (Pan troglodytes) American Journal of Primatology. 2013;75:57–64. doi: 10.1002/ajp.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CM, Lonsdorf EV, Eberly LE, Pusey AE. Reproductive energetics in free-living female chimpanzees (Pan troglodytes schweinfurthii) Behavioral Ecology. 2009;20:1211–1216. [Google Scholar]
- Murray CM, Mane SV, Pusey AE. Dominance rank influences female space use in wild chimpanzees: Towards an ideal despotic distribution. Animal Behaviour. 2007;74:1795–1804. [Google Scholar]
- Nishida T. The social group of wild chimpanzees in the Mahale Mountains. Primates. 1968;9:167–224. [Google Scholar]
- Nishida T. Social interactions between resident and immigrant female chimpanzees. In: Heltne PG, Marquardt LF, editors. Understanding chimpanzees. Cambridge, MA, USA: Harvard University Press; 1989. pp. 68–89. [Google Scholar]
- Otali E, Gilchrist JS. Why chimpanzee (Pan troglodytes schweinfurthii) mothers are less gregarious than nonmothers and males: The infant safety hypothesis. Behavioral Ecology and Sociobiology. 2006;59:561–570. [Google Scholar]
- Palombit RA, Seyfarth RM, Cheney DL. The adaptive value of ‘friendships’ to female baboons: Experimental and observational evidence. Animal Behaviour. 1997;54:599–614. doi: 10.1006/anbe.1996.0457. [DOI] [PubMed] [Google Scholar]
- Pond C. The significance of lactation in the evolution of mammals. Evolution. 1977;31:177–199. doi: 10.1111/j.1558-5646.1977.tb00995.x. [DOI] [PubMed] [Google Scholar]
- Potts KB. Nutritional ecology and reproductive output in female chimpanzees (Pan troglodytes): Variation among and within populations. In: Clancy KBH, Hinde K, Rutherford JN, editors. Building babies: Primate development in proximate and ultimate perspective. New York, NY, USA: Springer; 2013. pp. 83–100. [Google Scholar]
- Pusey AE. Mother–offspring relationships in chimpanzees after weaning. Animal Behaviour. 1983;31:363–377. [Google Scholar]
- Pusey AE, Williams J, Goodall J. The influence of dominance rank on reproductive success of female chimpanzees. Science. 1997;277:828–831. doi: 10.1126/science.277.5327.828. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Physiological and pathophysiological implications of social stress in mammals. In: McEwen BS, Goodman JM, editors. Handbook of physiology. Section 7, Vol. 4. Coping with the environment: Neural and endocrine mechanisms. Oxford, UK: Oxford University Press; 2001. pp. 517–528. [Google Scholar]
- Sapolsky RM. Endocrinology of the stress-response. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral endocrinology. Cambridge, MA, USA: MIT Press; 2002. pp. 409–450. [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- van de Rijt-Plooij HHC, Plooij FX. Growing independence, conflict and learning in mother-infant relations in free-ranging chimpanzees. Behaviour. 1987;101:1–86. [Google Scholar]
- White FJ, Wrangham RW. Feeding competition and patch size in the chimpanzee species Pan paniscus and Pan troglodytes . Behaviour. 1988;105:148–164. [Google Scholar]
- Whitehead H. SOCPROG programs: analysing animal social structures. Behavioral Ecology and Sociobiology. 2009;63:765–778. [Google Scholar]
- Williams JM, Pusey AE, Carlis JV, Farm BP, Goodall J. Female competition and male territorial behaviour influence female chimpanzees’ ranging patterns. Animal Behavour. 2002;63:347–360. [Google Scholar]
- Wittig RM, Boesch C. Food competition and linear dominance hierarchy among female chimpanzees of the Taï National Park. International Journal of Primatology. 2003;24:847–867. [Google Scholar]
- Wilson ML. Long-term studies of the chimpanzees of Gombe National Park, Tanzania. In: Kappeler PM, Watts DP, editors. Long-term field studies of primates. Heidelberg, Germany: Springer-Verlag; 2012. pp. 357–384. [Google Scholar]
- Wrangham RW. Behaviour of feeding chimpanzees in the Gombe National Park, Tanzania. In: Clutton-Brock TH, editor. Primate ecology. London, UK: Academic Press; 1977. pp. 502–538. [Google Scholar]
- Wrangham RW. Why are male chimpanzees more gregarious than mothers? A scramble competition hypothesis. In: Kappeler PM, editor. Primate males: Causes and consequences of variation in group composition. Cambridge, UK: Cambridge University Press; 2000. pp. 248–258. [Google Scholar]
- Wrangham RW, Clark AP, Isabirye-Basuta G. Female social relationships and social organization of Kibale Forest chimpanzees. In: Nishida T, McGrew WC, Marler P, Pickford M, de Waal F, editors. Topics in primatology: Human origins. Tokyo, Japan: University of Tokyo Press; 1992. pp. 81–98. [Google Scholar]
- Wrangham RW, Smuts BB. Sex differences in the behavioural ecology of chimpanzees in the Gombe National Park, Tanzania. Journal of Reproductive Fertility. 1980;28(Suppl.):13–31. [PubMed] [Google Scholar]
- Young KM, Walker SL, Lanthier C, Waddell WT, Monfort SL, Brown JL. Noninvasive monitoring of adrenocortical activity in carnivores by fecal glucocorticoid analyses. General and Comparative Endocrinology. 2004;137:148–165. doi: 10.1016/j.ygcen.2004.02.016. [DOI] [PubMed] [Google Scholar]