Abstract
Recent advances in understanding the cellular and molecular basis of psychiatric illnesses have shed light on the important role played by trophic factors in modulating functional parameters associated with disease causality and drug action. Disease mechanisms are now thought to involve multiple cell types, including neurons and endothelial cells. These functionally distinct but interactively coupled cell types engage in cellular cross talk via shared and common signaling molecules. Dysregulation in their cellular signaling pathways influences brain function and alters behavioral performance. Multifunctional trophic factors such as VEGF and EPO that possess both neurotrophic and angiogenic actions are of particular interest due to their ability to rescue structural and plasticity deficits in neurons and vasculature. Obtaining insight into the behavioral, cellular and molecular actions of multi-functional trophic factors has the potential to open new and transformative therapeutic approaches.
Keywords: Vascular endothelial growth factors, Erythropoietin, Trophic factor signaling, Neurovascular
Introduction
A substantial body of scientific evidence drawn from clinical, pre-clinical and basic research has demonstrated that neuronal atrophy and cell death are involved in psychiatric illnesses. The importance of neuronal plasticity and cellular resilience has been particularly highlighted in depression studies. Examination of the cellular response to stress has documented a reduction in BDNF levels and dysregulation of neurotrophic signaling, which could be reversed by antidepressant administration. These findings led to the formulation of the neurotrophic factor hypothesis of depression and antidepressant action [1]. Gene expression studies aimed at examining the potential involvement of other classes of trophic factors in antidepressant action revealed several additional trophic molecules and suggested that both vascular and neuronal factors are likely to be involved [2]. Subsequent studies validated the role played by multifunctional growth factors such as vascular endothelial growth factors (VEGF-A through D), erythropoietin (EPO) and basic fibroblast growth factor (bFGF) have both angiogenic and neurotrophic effects.
A clinical observation that has been known for at least 3 decades is that a bi-directional relationship exists between vascular disease and depression [3–5]. Depression is an independent risk factor for the occurrence of cerebrovascular and cardiovascular events, and conversely, vascular disease elevates rates of depression [6, 7]. In fact, depression is the predominant psychiatric disorder associated with cerebrovascular diseases [8]. A vascular hypothesis of depression that has helped guide clinical research is based primarily on the high incidence of cerebrovascular lesions observed in imaging studies of late-life depressed patients [9]. However, recent clinical imaging studies have reported impaired cerebrovascular perfusion in depressed middle-age patients, which was normalized by antidepressant treatment [10, 11]. This suggests that the association between vascular dysfunction and depression could be mechanistically coupled. Interestingly, stress-based rodent behavioral models of depression have shown a reduction in hippocampal microvessel density, providing additional support for a link between depression and the cerebral vasculature [12, 13]. The potential involvement of neuronal and vascular deficits in depression points toward vascular growth factors as candidate molecules worthy of investigation. This review will focus on the role of VEGF-A (the prototypic vascular growth factor) and EPO in depression and treatment response.
Role of vascular growth factors in brain function
Vascular growth factors serve key roles in the brain by influencing both neuronal and vascular function. VEGF is expressed at high levels and performs critical functions during development, guiding neuronal migration, blood vessel growth and branching [14]. Loss of VEGF in the developing brain impairs vascularization and causes neuronal apoptosis, hippocampal atrophy and microcephaly [15]. Transgenic mice with reduced levels of VEGF have reduced brain blood circulation, which could predispose them to neurodegenerative defects as a result of deficits in oxygen and metabolic support [16]. In addition to indirectly influencing neurons via its vascular actions, VEGF also has direct neuronal effects, enhancing survival and neurite outgrowth in cultured neurons and elevating neurogenesis when administered intracerebroventricularly (ICV) [17–21].
Conditional deletion of EPO in the brain decreases neurogenesis, particularly in the subventricular zone, resulting in smaller brain size [22]. These mice also exhibited atrophy of the choroid plexus, which constitutes the major blood-CSF barrier and is also the site where CSF and several neuroprotective polypeptides are produced. Lack of EPO receptor signaling also affects brain development by elevating apoptosis and limiting proliferation of neural progenitor cells [23]. In contrast, systemic delivery of EPO increases neurogenesis in the hippocampal subgranular zone [24, 25]. The mitogenic actions of EPO and VEGF on endothelial cells and neurons are indispensable for brain growth, development and function. Dysregulation in their level of expression adversely affects brain function and homeostasis.
Trophic factor-mediated neuroprotection
In addition to their mitogenic effects on neural cells, trophic factors possess neuroprotective actions that preserve neuronal function by opposing the harmful effects of cellular insults. Both VEGF and EPO are strongly induced by hypoxia because of the presence of transcription factor-binding sites for the hypoxia inducible factor (HIF) in their gene promoter regions [26, 27]. Both factors exhibit robust protection against neuronal damage associated with hypoxia and ischemia. Systemic administration of EPO has been shown to exhibit anti-apoptotic activity in protecting neurons from cell death after cerebral ischemia [28]. In vitro studies indicate that EPO is also protective against neuronal death arising from glutamate toxicity [29]. The release of excitotoxins, NMDA-induced apoptosis and free radical damage caused by proinflammatory cytokines was effectively blocked by pretreatment with EPO in cellular models of neurodegeneration [30]. The EPO receptor (EPOR) is expressed in adult rat dopaminergic neurons [31], and viral overexpression of EPO reverses the degeneration of dopamine neurons in rodent models of Parkinson’s disease [32, 33].
Endogenous VEGF induced by epileptic seizures was shown to be protective against neuronal loss in the hippocampus as selective blockade of VEGF signaling by infusion of a soluble receptor abolished neuroprotection with a two-fold increase in cell death [34]. A mild preconditioning exposure to hypoxia, which induces VEGF, is neuroprotective against subsequent ischemic insults, while also elevating neurogenesis and producing antidepressant-like effects [35]. VEGF plays a central role in mediating the neuroprotective mechanism of hypoxic preconditioning via its signaling actions [36]. An acute neuroprotective effect of VEGF accompanied by improvement in neurological parameters, reduction in infarct size and elevated angiogenesis was noted after cerebral ischemia [37]. The timing of VEGF administration significantly influences its efficacy in ischemia models, as early post-ischemic (within 1 h) delivery increases BBB leakage because of its inherent vascular permeabilization property, whereas later (48 h) administration reduces neuronal deficits and improves functional recovery [38].
In examining the neuroprotective activities of VEGF and EPO in conditions of oxygen and glucose deprivation, it can be noted that there is considerable overlap in their actions. This functional similarity could be mediated by signaling crosstalk between these trophic factors and integration of signaling at tumor necrosis factor receptor I (TNFRI), which sensitizes injured neurons and enables them to efficiently utilize EPO and VEGF for survival and restoration of function [39]. The potency of these molecules to protect and rescue neuronal function could be due to their ability to simultaneously influence both neurons and endothelial cells.
Integrating the neurovascular unit in neuropsychiatric disorders
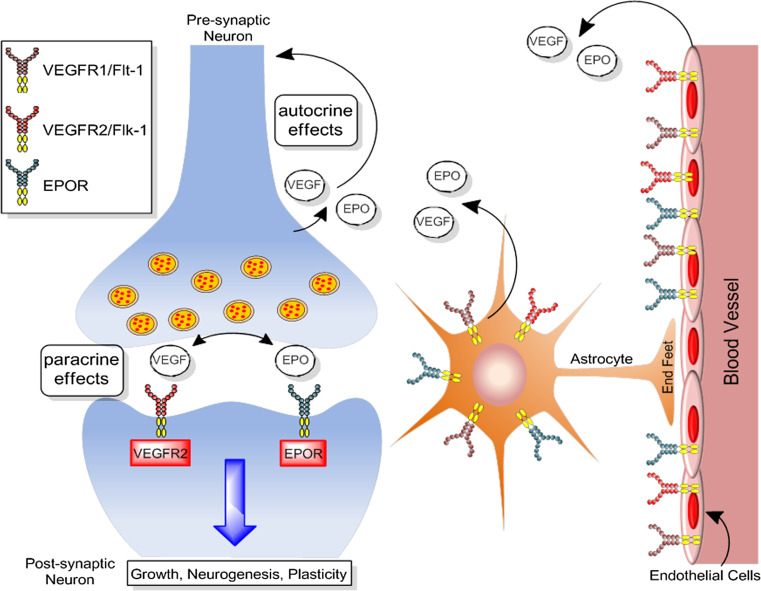
There has been a tendency in the investigation of neuropsychiatric disorders to focus primarily on neurons and pay little attention to the other cell types in the brain. It is important to recognize that neurons do not function in a network consisting only of other neurons but are intricately linked in a dynamic neurovascular network with other cell types including endothelial cells, astrocytes and perivascular cells (Fig. 1). The mammalian brain is highly vascularized, and neurons are dependent on the cerebral vasculature for oxygen and metabolic and trophic support. Therefore, even transient disruptions in brain blood flow adversely impact brain function. Furthermore, recent developments in the etiology of neuropsychiatric illnesses, such as Alzheimer’s disease, where mechanistic insight into vascular involvement is being obtained [40], and the failure of neuron centric approaches to treat stroke [41] indicate that it is necessary to develop a neurovascular framework to improve our understanding of neuropsychiatric disorders and develop efficacious therapies. With accumulating evidence suggesting that vascular function is impaired in depression [10, 42], it will be beneficial to consider therapeutic approaches that can promote both neuronal and vascular health.
Fig. 1.
Model of the neurovascular niche. Neurons, vasculature and astrocytes exist in a close anatomical and functional relationship that governs critical aspects of brain function and modulates blood flow in response to metabolic demands. Communication between these cell types occurs by the action of signaling molecules that facilitate cross talk and adaptive cellular regulation
Expression and regulation of EPO and VEGF in the neurovascular unit
Neurons, vasculature and astrocytes exist in a close anatomical and functional relationship that governs critical aspects of brain function and modulate blood flow in response to metabolic demands (Fig. 1). Communication between these cell types occurs by the action of signaling molecules that facilitate crosstalk and adaptive cellular regulation. VEGF and EPO are expressed in the three major cell types, which comprise the neurovascular unit, and it is therefore useful to examine cell-specific activity and regulation.
Neuronal
The expression of EPO and EPOR mRNA was shown in mouse brain, including specific binding sites of radiolabeled EPO [43]. EPO mRNA was detected in the hippocampus, amygdala and cortex of biopsied human brain tissue from epilepsy patients and several regions of monkey brain [44]. Neuronal expression of EPO was demonstrated using single-cell PCR in dissociated mouse cortical neurons and was elevated after systemic administration of cobalt chloride or hypoxia via a mechanism that includes transcriptional activation of hypoxia-inducible factor 1 (HIF-1) [45]. Examining EPO at the transcript level has been suggested to be more conclusive than immunohistochemical analysis because of concerns about the specificity of some commercial antibodies [45]. EPOR is expressed at higher levels in neuronal progenitors than mature neurons. Expression in neural cells, even at low levels, appears to have protective functions as cells that lack EPOR are vulnerable to hypoxia and glutamate toxicity [46]. During development, EPO facilitates the generation of neuronal progenitors from neural stem cells by functioning as an autocrine–paracrine factor [47].
Vascular endothelial growth factor is also highly expressed in developing and mature CNS tissue [48, 49]. Neuronal VEGF plays a key role in brain angiogenesis [50] and neuronal patterning during development [51]. At birth, VEGF production from neurons switches to astrocytes causing neuronal VEGF to be reduced to very low levels in the mature neurons once angiogenesis has ceased [52]. However, VEGF expression is strongly upregulated in both neurons and glia following hypoxic and excitotoxic events [53, 54]. VEGF binds to three main subtypes of receptor tyrosine kinases—fetal liver kinase (Flk-1 or VEGFR-2), fms-like tyrosine kinase 1 (Flt-1, VEGFR-1) and fms-like tyrosine kinase 4 (Flt-4 or VEGFR-3)—as well as to a family of co-receptors called neuropilins (NRP). In adult brain, Flt-1 is predominately expressed by endothelial cells and astrocytes, whereas Flk-1 is expressed by mature neurons, neuronal progenitors and endothelial cells [55]. Interestingly, Flk-1 is more highly expressed among neuronal progenitors than mature neurons, highlighting the important role of VEGF/Flk-1 signaling in regulating neurogenesis [21, 37, 56, 57]. Of the multiple VEGF receptors found in CNS tissue, Flk-1 appears to mediate almost all of the known cellular responses to VEGF. However, expression of the VEGF receptor Flt-1, which is not normally observed in mature neurons, can be strongly upregulated after CNS ischemia, suggesting a role in brain injury [58–60].
Endothelial
It is well known that VEGF exerts potent effects on the survival and proliferation of endothelial cells, and it is the predominant cellular function that has been extensively investigated (Fig. 1). Although the endothelial actions of EPO are not as widely studied as VEGF, the angiogenic potential of EPO has been reported to be similar [61]. However, it is likely that they differ in their mechanism of action as EPO lacks the vascular permeabilization property of VEGF. Two forms of EPOR, the membrane spanning receptor and an intron-containing soluble form, were observed in rat and mouse endothelial cells, eliciting dose-dependent mitogenic actions upon treatment with EPO [62]. The endothelial and angiogenic functions of EPO can also provide an indirect neuroprotective effect by inducing the secretion of trophic factors from the vasculature [57]. EPO is known to cause vasodilation of capillaries by elevating endothelial nitric oxide synthase (eNOS) and production of nitric oxide [63]. It is interesting to note that the EPO-induced elevation of nitric oxide requires interaction between heterodimeric EPOR and beta-common receptor with the VEGFR-2 receptor, indicating interactive coupling of EPO–VEGF signal transduction in endothelial cells [64].
In endothelial cells, binding of VEGF triggers rapid phosphorylation of VEGFR-2, which in turn allows the receptor to be associated with various effector molecules, including phosphatidylinositol 3-kinase (PI3K)–Akt, Raf–MAPK and phospholipase Cγ-protein kinase C (PLCγ–PKC). Important endothelial functions, including proliferation (via activation of the Raf–MAPK signaling cascade), survival (via activation of PI3K–Akt) and vasopermeability and angiogenesis (via PLCγ–PKC), have been shown to be regulated through VEGF/Flk-1 signaling [65]. Although the effects of EPO stimulation in endothelial cells have not been well described, in neurons, EPOR has been shown to transduce signaling via the Jak 2 and NF-κB pathways [23, 30]. However, the induction of other neurotrophic factors, such as BDNF, GDNF and neuritin by EPOR [66, 67], is likely to involve the MEK–Erk and PI3K–Akt signaling cascades [66].
Astrocytic
The strongest induction of EPO and VEGF has been observed in astrocytes following exposure to hypoxic conditions [68]. Cultured astrocytes exposed to low oxygen levels (1 %) exhibit a 100-fold elevation in EPO [44]. Breathing a gas mixture that contained 8 % oxygen (20 % is normoxic) elevated EPO levels in monkey brain threefold, and this increased to 20-fold when exposed to 0.1 % carbon monoxide, demonstrating that the degree of EPO induction in astrocytes is dependent on the severity of hypoxia [44, 69]. This is similar to the hypoxia-induced elevation of VEGF that is acutely dependent on oxygen levels. VEGF contributes to new blood vessel formation at 10 % oxygen, but at levels below 8 %, blood vessels become leaky, suggesting thresholds for beneficial and detrimental effects [70]. The high levels of inducible EPO expression in astrocytes have been suggested to serve paracrine functions by acting on neurons and protecting against neuronal damage [69, 71]. VEGF and EPO can also act on astrocytes in an autocrine manner to enhance astrocyte proliferation and also promote maturation of immature oligodendrocytes (Fig. 1) [51, 72]. Moreover, expression of VEGF receptors Flt-1 and Flk-1 is strongly expressed in the astroglial endfeet that closely surround the nearby endothelium. Astroglia treated with EPO were protected against apoptotic cell death caused by exposure to cellular stressors [73]. EPO prevents astrocytes from swelling-induced injury during conditions of brain edema by regulating water permeability via the aquaporin 4 water channel [74].
Role of VEGF and EPO in depression and stress responses
Increasing evidence suggests that vascular dysfunction plays a critical role in the etiology of depression [75–78]. For example, decreased cerebral blood flow and metabolism in the hippocampus and prefrontal cortices are frequently observed in patients with depression [79–83]. Moreover, decreased levels of circulating bone-marrow derived endothelial progenitor cells have been reported in patients with depression compared to healthy controls [84, 85]. Treatment with antidepressants can influence endothelial function [86], and several drugs used to manage vascular disease (e.g., calcium-channel blockers, statins, angiotensin-converting enzyme inhibitors) were able to reduce depressive symptoms in preclinical studies [87–91]. Because VEGF and EPO act as key signaling molecules in the CNS, being involved in neuroprotection, neuronal survival and synaptic plasticity, altered expression and/or function of these vascular factors could contribute to the cellular and morphological changes observed in animal models of depression and in patients with depression. Below we will summarize the clinical literature highlighting the relationship between VEGF and EPO-signaling in depressive illness and antidepressant action.
VEGF in depression
Over the last few years, the relationship between VEGF and stress-related disorders, including depression, in clinical populations, has been extensively examined. For example, VEGF mRNA expression in peripheral leukocytes was found to be elevated in patients with depression compared to healthy controls, and this difference was normalized after 8-weeks of antidepressant treatment and was associated with clinical improvement [92]. In addition, increased serum levels of VEGF have been reported in depressed patients with comorbid borderline personality disorder [93] and bipolar disorder [94]. On the contrary, other groups have noted significant decreases in peripheral VEGF/Flk1 levels with depression [85, 95–97], and a recent study showed higher plasma VEGF levels in 16 major depressive disorder patients who were in partial or full remission compared to controls [98]. Moreover, preclinical studies (described below) have typically observed decreases in brain VEGF and Flk-1 expression with exposure to chronic stress.
Although it is not clear what might account for the divergence across these studies, clinical factors such as such as age, gender, treatment history, depressive episodes (recurrent vs. acute), comorbidity with other health conditions (e.g., heart disease) and small patient group sizes could be important contributors. Another possibility is that there may be differences between blood and brain levels of VEGF in patients with depression. Indeed, recent data indicate that while serum VEGF levels remain unchanged, VEGF levels in the hippocampus and frontal cortex were significantly lower in a genetic rat model of depression [99]. Nonetheless, further work is necessary to develop the diagnostic and prognostic value of peripheral VEGF as a biomarker for clinical depression and antidepressant efficacy.
EPO in depression
Erythropoietin has been tested in several human imaging studies for its ability to modulate brain function and behavior. A single high dose of EPO reduced neuronal response to fear 1 week after administration in healthy volunteers, without evoking any erythropoietic alterations [100]. A specific reduction in blood oxygen level-dependent (BOLD) signal change in response to fearful stimuli was noted in the cortex of the EPO group in comparison to placebo treatment. A short-term effect of improved mood was reported in the first 3 days following EPO administration along with increased neural and cognitive processing of facial expressions [101]. Interestingly, the acute effects of EPO included heightened recognition of happiness and fear in a manner comparable to that of serotonin-reuptake inhibitors and similarly reversed after 1 week [101–103]. In a double-blind study comprising 19 acutely depressed patients, EPO was found to reduce left amygdala-hippocampal response to fearful stimuli [104]. This could reflect an ability to reverse negative emotional bias in this group of patients. Additional studies in larger clinical groups and later testing time points with conventional rating scales are awaited as phase II trials are ongoing.
Regulation of VEGF and EPO by stress and antidepressant treatments
Vascular endothelial growth factors and Flk-1 expression in the hippocampus and frontal cortex are lower with exposure to chronic stress or stress hormones [105–107]. In one study, Bergstrom and colleagues [107] showed a significant reduction of VEGF mRNA in the ventral hippocampus—a region with strong input to the amygdala and prefrontal cortex—following exposure to chronic mild stress. Interestingly, this decrease in VEGF expression only occurred in stress-sensitive rats; a subpopulation of stress-resistant rats did not show a similar reduction in VEGF after chronic mild stress. These findings suggest that VEGF signaling may play an important role in stress adaptability. Consistent with this view, corticosteroids are well-known inhibitors of angiogenesis, and previous work has shown that chronically stressed mice have decreased capillary density in the hippocampus [12]. Given the importance of VEGF in regulating neurogenesis and angiogenesis in the adult brain [21, 108], there has been interest in determining whether stress-induced changes in VEGF contribute to the decreases in hippocampal neurogenesis seen in depression. Indeed, stress-induced reductions in adult hippocampal cell proliferation are more pronounced near blood vessels than in areas not covered by blood vessels [105], indicating that VEGF is a key factor for promoting neurogenesis that is secreted from the vascular niche [108]. For further discussion on the effects of stress on VEGF and neurogenesis, the reader is directed to [109].
Early microarray experiments from our laboratory identified VEGF as a possible target of antidepressant action. We showed that electroconvulsive seizure (ECS), which is one the most effective options for the treatment of refractory depression, resulted in a rapid and robust upregulation of VEGF expression in the rat dentate gyrus [2]. Extending this early work, multiple antidepressants, including selective serotonin reuptake inhibitors, noradrenalin-serotonin reuptake inhibitors and tricyclic antidepressants, have now been shown to increase VEGF mRNA and protein expression in rat hippocampus [2, 110–112]. The induction of VEGF by chemical antidepressants requires chronic administration (~2 weeks), whereas ECS produces rapid changes in VEGF expression along a much shorter time frame (~48 h). This differential profile of VEGF induction closely overlaps with the onset of clinical effects associated with these treatments. Interestingly, chronic treatment with fluoxetine upregulated VEGF mRNA expression in neuronal and endothelial cells, but not in astrocytes, suggesting that antidepressant treatment might preferentially stimulate VEGF production and possibly release from different cell sources [105]. Other treatments known to produce an antidepressant response, such as exercise and sleep deprivation, also increase VEGF expression [113, 114]. Interestingly, a recent genetic study examining a functional polymorphism of the VEGF gene (2578 C/A) revealed that this allele was more frequent in patients with treatment-resistant depression than in healthy controls or a population of treatment-responsive patients [115]. These findings underscore the importance of VEGF as a common downstream target of antidepressant action and highlight this growth factor as an important mediator in the therapeutic response of antidepressant treatment.
While EPO has been shown to possess antidepressant-like activity in rodent models and clinical studies, regulation by chemical antidepressants is yet to be examined. However, robust induction of EPO gene expression was observed specifically in the rat dentate gyrus after ECS [67]. This is similar to the induction pattern of VEGF after ECS [2]. Clearly, further work is necessary to identify the full role of EPO in the therapeutic action of antidepressant treatments.
Vascular growth factors in pre-clinical behavioral models of depression and cognition
Studies of VEGF in models of depression and cognition
Vascular endothelial growth factor induces antidepressant and anxiolytic effects in various animal models. For example, mice overexpressing VEGF in forebrain neurons show reduced immobility in the forced swim test and make a greater number of open arm entries in the elevated plus maze [116]. In addition, ICV infusions of VEGF also mimic the action of antidepressant drugs in several behavioral tests, such as the forced swim test, learned helplessness and novelty-suppressed feeding tests [110]. In the forced swim test, VEGF increased swimming, but not climbing behavior. This is noteworthy given that swimming behavior in this test is influenced by SSRI antidepressants [117]. Interestingly, 5-HT1a receptor antagonists block both the increase in VEGF and the behavioral effects induced by the SSRI fluoxetine in chronically stressed rats, indicating that VEGF may exert its antidepressant actions through modulation of the serotonergic system [112]. Finally, central administration of VEGFR-2 (Flk-1) inhibitors (SU5416, SU1498) has revealed that the behavioral and neurogenic effect of chemical antidepressants and ECS appears to require VEGF/Flk-1 signaling [110, 112, 118]. However, there is some question regarding the specificity of the applied Flk-1 inhibitors since these compounds can exert effects on other growth factor such as FGF-2 and BDNF.
These findings provide important information regarding the role of VEGF in the behavioral and cellular actions of antidepressants. However, one major challenge of pharmacological studies is that they are complicated by issues of specificity, short ligand half-life, solubility, accessibility of the ligand to target tissues and side effects; problems that are compounded when chronic, rather than acute effects, need to be investigated. One strategy to circumvent many of the problems inherent in behavioral pharmacology is to use transgenic or knockout approaches. This powerful approach permits the study of single gene function in the nervous system and offers a high degree of molecular specificity over pharmacological blocking agents to probe important brain-behavior relationships. Through utilizing a conditional gene knockout strategy to achieve inactivation of the VEGF gene in neurons of the murine forebrain, we are currently examining the contribution of neuronal VEGF in depressive behavior and antidepressant response.
The effects of VEGF are not limited to depressive behavior. For example, hippocampal VEGF levels are increased with water maze training [119], and administration of VEGFR-2 inhibitors directly into the hippocampus following spatial training impairs long-term memory [120]. These findings raise the possibility that VEGF may play an important role in processes related to neuronal plasticity and behavior. Indeed, field recording studies in hippocampal slices revealed that direct application of recombinant human VEGF165 prior to high-frequency stimulation increases long-term potentiation [121]. Consistent with the idea that VEGF promotes synaptic plasticity and boosts memory performance, previous work has shown that overexpression of VEGF through either AAV-mediated gene transfer in the rodent hippocampus or globally in the forebrain of transgenic mice increases associative learning and spatial memory tasks [122, 123]. Finally, an elegant study by Licht and colleagues [124] recently showed that VEGF can modulate neuronal plasticity in the hippocampus and improve learning through a process that was independent of changes in either vascular perfusion or neurogenesis. These findings underscore the diverse pleiotropic actions of VEGF on brain function, although the exact receptors mediating these diverse effects have not been characterized.
Studies of EPO in models of depression and cognition
The antidepressant-like effects of EPO and non-erythropoietic variants have been tested in rodent behavioral models. Three days of EPO administration yielded a positive effect in the widely used forced swim model of antidepressant activity [67]. The reduction in immobility time was mainly due to increased swimming rather than climbing behavior, similar to the actions of VEGF in this test (see above), and to SSRI antidepressants [117]. Administration of EPO for 4 days decreased novelty-induced hypophagia in mice, measured as a reduction in the latency to approach the food source in the novel cage environment [67]. The effect was comparable to that obtained by chronic treatment (3 weeks) with SSRI antidepressants [125]. A biochemically modified (carbamoylated) form of EPO (CEPO) that is devoid of erythropoietic activity [126] reduced despair behavior in the tail suspension test, indicating that the antidepressant effects of EPO are independent of hematopoietic properties [25].
In addition to antidepressant-like efficacy, EPO has been successfully tested in models of neurocognition. Both EPO and CEPO improved performance in the spatial and object recognition tasks [25]. These effects are likely to be mediated by modulation of hippocampal plasticity and long-term potentiation [127]. The memory effects are selective and not accompanied by increases in spontaneous activity, exploratory behavior or motor performance [127]. Although EPO was dosed for 3 weeks and elevated hematocrit in the Adamcio study, the cognitive effects are not likely to be associated with erythropoiesis as the behavioral improvement persisted for an additional 3 weeks after the last EPO dose, a time point when the elevated hematocrit would have normalized. Conclusive evidence that the cognitive-enhancing effects are unrelated to hematopoiesis was provided by the use of a short 11-amino acid peptide that was derived from a portion of the three-dimensional structure of EPO. This non-erythropoietic peptide increased performance in the novel object recognition task with equivalent efficacy to the drug galantamine despite a plasma half-life of only a few minutes [128]. The behavioral actions of EPO and other EPO-like molecules and derivatives suggest that vascular trophic factors are worthy of further investigation as candidate molecules for treatment of psychiatric disorders.
Therapeutic potential
The ability to alter signaling in multiple brain cell types and produce trophic effects that lead to modulation of behavior in the setting of neuropsychiatric disorders qualifies vascular growth factors as potentially unique therapeutic agents with a novel mechanism of action. However, an important parameter that has to be addressed for CNS use is the issue of transport across the blood-brain barrier (BBB). While biologics are advancing as attractive candidates for drug development because of their specificity of action, resulting in higher rates of FDA approval than small molecules, their utility in treating CNS diseases can be challenging. Systemically administered EPO has been shown to traverse the BBB via a potential receptor-mediated translocation [129]. The efficacy of systemic administration in eliciting cellular and behavior effects is reflected by substantial literature of EPO use (over 350 papers) for CNS cytoprotective and neurotrophic activity. Nevertheless, the efficiency of CNS transport is far below that of small molecules, and hence large doses are needed to produce appreciable efficacy. This results in higher cost of treatment and can eventually limit applicability in the field. Strategies to address this challenge include producing recombinant molecules in alternate hosts such as plants, bacteria [130, 131] and chemical synthesis of peptides [128] with similar activity.
Structure-based design of biomimetics
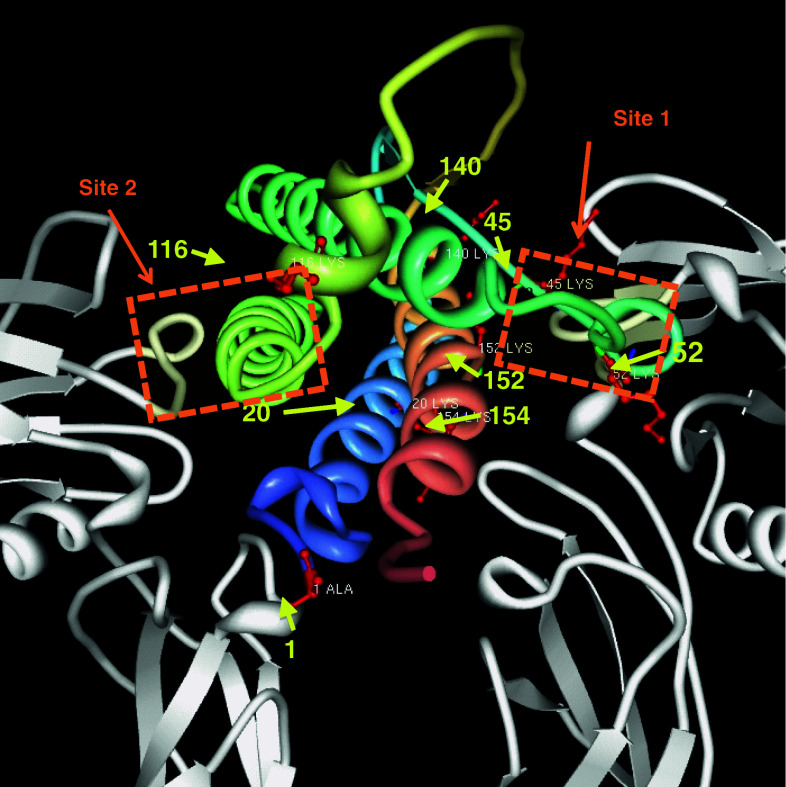
The availability of high-resolution crystallographic structural information [132–134] and receptor affinity data [135] has enabled modeling approaches to design molecules that activate receptor-mediated cell signaling (Fig. 2). Initial studies were performed using phage display methodology to identify peptides that exhibited strong EPO mimetic actions [136]. Interestingly, these peptides did not correspond to the primary sequence of EPO and yet produced the entire spectrum of biological activity with regard to erythropoiesis. A short peptide derived from surface-simulation analysis and composed of adjacent amino acids that represent the aqueous face of helix B, one of the four (A–D) helices of EPO, reproduced the neuroprotective actions of EPO both in vitro and in vivo, without any effect on erythropoiesis [128].
Fig. 2.
Crystal structure of EPO and EPOR (1EER, protein data bank). EPOR dimer is colored gray, and helices of EPO are shown multi-colored and bound to EPOR. The two active sites, site 1 and site 2, are indicated by orange boxes. Potential sites of carbamylation are indicated by red atoms, yellow arrows and residue number of the amino acid sequence
The success of this approach paved the way for the synthesis of additional biomimetic peptides. A non-erythropoietic tetrameric peptide corresponding to the C helix of EPO and the low-affinity site of EPOR produced neurite outgrowth in cultured neurons, effectively crossed the BBB and reduced kainic acid-induced toxicity in the brain [137]. Precisely how short peptides are able to activate signaling via receptor binding is currently unclear. The authors tested monomer, dimer and tetramer versions of the peptide and found that only the tetramer acquired an aqueous solution conformation that resembled EPO’s C-helix. It is useful to note that although the neurotrophic effects were comparable to EPO and required the EPO receptor, they were obtained only at doses that were 103 higher than full-length EPO [137]. The same group also generated another tetrameric, non-erythropoietic peptide toward the high-affinity receptor site using publicly available x-ray crytallography structural information [138]. This peptide had twofold lower affinity for EPOR than recombinant EPO, which could be due to partial coverage (428 Å) of the total intermolecular contact area (920 Å) of the high-affinity binding site. The fact that peptide agonists of the EPOR remain a useful avenue for CNS drug development is most likely due to their short plasma residence time, which precludes hematological consequences.
Downstream signaling molecules as drug candidates
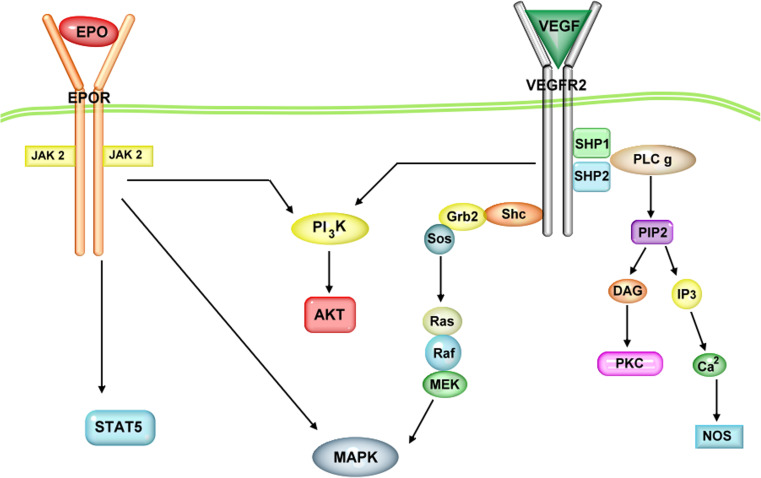
Cellular signal transduction modulated by EPO starts with binding to the membrane-bound receptor, dimerization and activation of the Janus protein tyrosine kinase 2 (Jak2) (Fig. 3). Jak2 then phosphorylates multiple tyrosine residues in the cytoplasmic region of EPOR [139]. This causes phosphorylation and activation of the transcription factor, signal transducer and activator of transcription 5 (STAT5), which subsequently translocates into the nucleus and binds to specific promoter elements to initiate transcription of target genes. The Jak-STAT pathway is considered the canonical EPO signaling cascade; however, it is not activated by carbamylated EPO, which lacks erythropoietic activity [126] but retains the neurogenic and angiogenic properties of EPO [140]. In addition to the Jak-STAT pathway, EPO can also signal via the PI3kinase–Akt and MAPK–ERK pathways [141]. In a similar manner, VEGF-signaling also promotes activation of the PI3-kinase–Akt and MAPK–ERK pathways [65]. Activation of the MAPK and Akt pathways is well known to mediate important trophic effects related to synaptic plasticity, neuronal survival/protection and neurogenesis [20, 21, 37, 121, 142, 143]. However, the precise involvement of these signaling pathways in the behavioral actions of EPO and VEGF is yet to be elucidated and is an interesting and important field of investigation. Dissecting these pathways and identifying the particular signaling molecules that contribute to functional output in cellular and behavioral assays can provide key targets for drug development and also help reduce undesirable side effects.
Fig. 3.
Schematic of EPO and VEGF receptor signaling pathways. Intracellular cascades are shown activated by EPO and VEGF binding to EPO receptor (EPOR) and VEGF receptor 2 (VEGFR-2), respectively. Janus kinase 2 (JAK 2), signal transducer and activator of transcription 5 (STAT 5), phosphatidylinositol 3-kinase (PI3K), protein kinase B (AKT/PKB), mitogen activated protein kinase (MAPK), Src homology 2 domain containing transforming protein (Shc), growth factor receptor- bound protein 2 (Grb2), sons of sevenless (Sos), rat sarcoma GTPase (Ras), rapidly accelerated fibrosarcoma kinase (Raf), mitogen-activated protein kinase kinase (MEK), Src homology region 2 domain containing phosphatase-1, 2 (SHP1, SHP 2), phosphoinositide phospholipase C (PLC), phosphatidylinositol 4,5-biphosphate (PIP2), inositol triphosphate (IP3), diacylglycerol (DAG), protein kinase C (PKC) and nitric oxide synthase (NOS)
Summary and conclusions
The neuronal and vascular actions of growth factors such as VEGF and EPO are intricately intertwined to the extent that a new term “angioneurin” was coined to highlight their dual functionality [144]. The robust induction of these molecules in the brain in response to insults such as stroke or hypoxia and their ability to provide robust protective effects against cellular damage reveal that they are key endogenous components of homeostasis and survival strategies employed by the mammalian brain. The fact that these growth factors act on multiple cell types suggests that simultaneously exerting trophic actions on neuronal and vascular cells could provide superior efficacy in producing regenerative effects. Substantial evidence accruing from clinical and pre-clinical studies indicates that cellular atrophy is an important element in the pathophysiology of neuropsychiatric illnesses. The ability to reverse cellular and behavioral deficits by trophic factor administration reinforces support for testing this class of growth factors, their derivatives and biomimetics as novel therapeutic compounds for the treatment of psychiatric and neurodegenerative diseases. However, it will be critical to address the erythopoietic activity of EPO and the vascular permeability effect of VEGF as they can have detrimental hematological and BBB weakening consequences. Interestingly, another member of the VEGF family, VEGF-B, also possesses neurogenic and neuroprotective properties but does appear to exert vasopermeability effects [145]. However, whether VEGF-B expression and/or signaling are adversely affected in neuropsychiatric illness or upregulated by treatments will require further investigation. In conclusion, advancing our understanding of vascular, glial and neuronal mechanisms of action can enable us to develop safe angioneurin-based treatment strategies that maximize the clinical benefit of vascular trophic factors and preclude adverse effects when applied for the treatment of neuropsychiatric or neurological disorders.
Acknowledgments
N.M.F. is a James Hudson Brown-Alexander Brown Coxe postdoctoral research fellow and was supported by a fellowship provided by the Canadian Institutes of Health Research. This work is supported by US Public Health Service grants MH45481 (R.S.D.) and 2 P01 MH25642 (R.S.D.) and the Connecticut Mental Health Center (R.S.D.).
References
- 1.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54(7):597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 2.Newton SS, Collier EF, Hunsberger J, Adams D, Terwilliger R, Selvanayagam E, Duman RS. Gene profile of electroconvulsive seizures: induction of neurotrophic and angiogenic factors. J Neurosci. 2003;23(34):10841–10851. doi: 10.1523/JNEUROSCI.23-34-10841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55(7):580–592. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- 4.Carney RM, Freedland KE. Depression, mortality, and medical morbidity in patients with coronary heart disease. Biol Psychiatry. 2003;54(3):241–247. doi: 10.1016/s0006-3223(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 5.Plante GE. Depression and cardiovascular disease:a reciprocal relationship. Metabolism. 2005;54(5 Suppl 1):45–48. doi: 10.1016/j.metabol.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Salaycik KJ, Kelly-Hayes M, Beiser A, Nguyen AH, Brady SM, Kase CS, Wolf PA. Depressive symptoms and risk of stroke: the Framingham study. Stroke. 2007;38(1):16–21. doi: 10.1161/01.STR.0000251695.39877.ca. [DOI] [PubMed] [Google Scholar]
- 7.Teper E, O’Brien JT. Vascular factors and depression. Int J Geriatr Psychiatry. 2008;23(10):993–1000. doi: 10.1002/gps.2020. [DOI] [PubMed] [Google Scholar]
- 8.Ghoge H, Sharma S, Sonawalla S, Parikh R. Cerebrovascular diseases and depression. Curr Psychiatry Rep. 2003;5(3):231–238. doi: 10.1007/s11920-003-0048-7. [DOI] [PubMed] [Google Scholar]
- 9.Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54(10):915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 10.Vlassenko A, Sheline YI, Fischer K, Mintun MA. Cerebral perfusion response to successful treatment of depression with different serotoninergic agents. J Neuropsychiatry Clin Neurosci. 2004;16(3):360–363. doi: 10.1176/jnp.16.3.360. [DOI] [PubMed] [Google Scholar]
- 11.Kohn Y, Freedman N, Lester H, Krausz Y, Chisin R, Lerer B, Bonne O. 99mTc-HMPAO SPECT study of cerebral perfusion after treatment with medication and electroconvulsive therapy in major depression. J Nucl Med. 2007;48(8):1273–1278. doi: 10.2967/jnumed.106.039354. [DOI] [PubMed] [Google Scholar]
- 12.Czeh B, Abumaria N, Rygula R, Fuchs E. Quantitative changes in hippocampal microvasculature of chronically stressed rats: no effect of fluoxetine treatment. Hippocampus. 2010;20(1):174–185. doi: 10.1002/hipo.20599. [DOI] [PubMed] [Google Scholar]
- 13.Neigh GN, Owens MJ, Taylor WR, Nemeroff CB. Changes in the vascular area fraction of the hippocampus and amygdala are induced by prenatal dexamethasone and/or adult stress. J Cereb Blood Flow Metab. 2010;30(6):1100–1104. doi: 10.1038/jcbfm.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James JM, Gewolb C, Bautch VL. Neurovascular development uses VEGF-A signaling to regulate blood vessel ingression into the neural tube. Development. 2009;136(5):833–841. doi: 10.1242/dev.028845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raab S, Beck H, Gaumann A, Yuce A, Gerber HP, Plate K, Hammes HP, Ferrara N, Breier G. Impaired brain angiogenesis and neuronal apoptosis induced by conditional homozygous inactivation of vascular endothelial growth factor. Thromb Haemost. 2004;91(3):595–605. doi: 10.1160/TH03-09-0582. [DOI] [PubMed] [Google Scholar]
- 16.Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, Brusselmans K, Van Dorpe J, Hellings P, Gorselink M, Heymans S, Theilmeier G, Dewerchin M, Laudenbach V, Vermylen P, Raat H, Acker T, Vleminckx V, Van Den Bosch L, Cashman N, Fujisawa H, Drost MR, Sciot R, Bruyninckx F, Hicklin DJ, Ince C, Gressens P, Lupu F, Plate KH, Robberecht W, Herbert JM, Collen D, Carmeliet P. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28(2):131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- 17.Sondell M, Sundler F, Kanje M. Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk-1 receptor. Eur J Neurosci. 2000;12(12):4243–4254. doi: 10.1046/j.0953-816x.2000.01326.x. [DOI] [PubMed] [Google Scholar]
- 18.Khaibullina AA, Rosenstein JM, Krum JM. Vascular endothelial growth factor promotes neurite maturation in primary CNS neuronal cultures. Brain Res Dev Brain Res. 2004;148(1):59–68. doi: 10.1016/j.devbrainres.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 19.Jin K, Mao XO, Greenberg DA. Vascular endothelial growth factor stimulates neurite outgrowth from cerebral cortical neurons via Rho kinase signaling. J Neurobiol. 2006;66(3):236–242. doi: 10.1002/neu.20215. [DOI] [PubMed] [Google Scholar]
- 20.Fournier NM, Lee B, Banasr M, Elsayed M, Duman RS. Vascular endothelial growth factor regulates adult hippocampal cell proliferation through MEK/ERK- and PI3K/Akt-dependent signaling. Neuropharmacology. 2012;63(4):642–652. doi: 10.1016/j.neuropharm.2012.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 2002;99(18):11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai PT, Ohab JJ, Kertesz N, Groszer M, Matter C, Gao J, Liu X, Wu H, Carmichael ST. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J Neurosci. 2006;26(4):1269–1274. doi: 10.1523/JNEUROSCI.4480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu X, Shacka JJ, Eells JB, Suarez-Quian C, Przygodzki RM, Beleslin-Cokic B, Lin CS, Nikodem VM, Hempstead B, Flanders KC, Costantini F, Noguchi CT. Erythropoietin receptor signalling is required for normal brain development. Development. 2002;129(2):505–516. doi: 10.1242/dev.129.2.505. [DOI] [PubMed] [Google Scholar]
- 24.Ransome MI, Turnley AM. Systemically delivered erythropoietin transiently enhances adult hippocampal neurogenesis. J Neurochem. 2007;102(6):1953–1965. doi: 10.1111/j.1471-4159.2007.04684.x. [DOI] [PubMed] [Google Scholar]
- 25.Leconte C, Bihel E, Lepelletier FX, Bouet V, Saulnier R, Petit E, Boulouard M, Bernaudin M, Schumann-Bard P. Comparison of the effects of erythropoietin and its carbamylated derivative on behaviour and hippocampal neurogenesis in mice. Neuropharmacology. 2011;60(2–3):354–364. doi: 10.1016/j.neuropharm.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 26.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16(9):4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12(12):5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siren AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, Mennini T, Heumann R, Cerami A, Ehrenreich H, Ghezzi P. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA. 2001;98(7):4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morishita E, Masuda S, Nagao M, Yasuda Y, Sasaki R. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience. 1997;76(1):105–116. doi: 10.1016/s0306-4522(96)00306-5. [DOI] [PubMed] [Google Scholar]
- 30.Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412(6847):641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- 31.Csete M, Rodriguez L, Wilcox M, Chadalavada S. Erythropoietin receptor is expressed on adult rat dopaminergic neurons and erythropoietin is neurotrophic in cultured dopaminergic neuroblasts. Neurosci Lett. 2004;359(1–2):124–126. doi: 10.1016/j.neulet.2004.01.068. [DOI] [PubMed] [Google Scholar]
- 32.Puskovic V, Wolfe D, Wechuck J, Krisky D, Collins J, Glorioso JC, Fink DJ, Mata M. HSV-mediated delivery of erythropoietin restores dopaminergic function in MPTP-treated mice. Mol Ther. 2006;14(5):710–715. doi: 10.1016/j.ymthe.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Xue YQ, Ma BF, Zhao LR, Tatom JB, Li B, Jiang LX, Klein RL, Duan WM. AAV9-mediated erythropoietin gene delivery into the brain protects nigral dopaminergic neurons in a rat model of Parkinson’s disease. Gene Ther. 2010;17(1):83–94. doi: 10.1038/gt.2009.113. [DOI] [PubMed] [Google Scholar]
- 34.Nicoletti JN, Shah SK, McCloskey DP, Goodman JH, Elkady A, Atassi H, Hylton D, Rudge JS, Scharfman HE, Croll SD. Vascular endothelial growth factor is up-regulated after status epilepticus and protects against seizure-induced neuronal loss in hippocampus. Neuroscience. 2008;151(1):232–241. doi: 10.1061/j.neuroscience.2007.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu XH, Yan HC, Zhang J, Qu HD, Qiu XS, Chen L, Li SJ, Cao X, Bean JC, Chen LH, Qin XH, Liu JH, Bai XC, Mei L, Gao TM. Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant-like effects in adult rats. J Neurosci. 2010;30(38):12653–12663. doi: 10.1523/JNEUROSCI.6414-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wick A, Wick W, Waltenberger J, Weller M, Dichgans J, Schulz JB. Neuroprotection by hypoxic preconditioning requires sequential activation of vascular endothelial growth factor receptor and Akt. J Neurosci. 2002;22(15):6401–6407. doi: 10.1523/JNEUROSCI.22-15-06401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111(12):1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106(7):829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taoufik E, Petit E, Divoux D, Tseveleki V, Mengozzi M, Roberts ML, Valable S, Ghezzi P, Quackenbush J, Brines M, Cerami A, Probert L. TNF receptor I sensitizes neurons to erythropoietin- and VEGF-mediated neuroprotection after ischemic and excitotoxic injury. Proc Natl Acad Sci USA. 2008;105(16):6185–6190. doi: 10.1073/pnas.0801447105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009;118(1):103–113. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67(2):181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barnes DE, Yaffe K, Byers AL, McCormick M, Schaefer C, Whitmer RA. Midlife vs late-life depressive symptoms and risk of dementia: differential effects for alzheimer disease and vascular dementia. Arch Gen Psychiatry. 2012;69(5):493–498. doi: 10.1001/archgenpsychiatry.2011.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Digicaylioglu M, Bichet S, Marti HH, Wenger RH, Rivas LA, Bauer C, Gassmann M. Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Proc Natl Acad Sci USA. 1995;92(9):3717–3720. doi: 10.1073/pnas.92.9.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marti HH, Wenger RH, Rivas LA, Straumann U, Digicaylioglu M, Henn V, Yonekawa Y, Bauer C, Gassmann M. Erythropoietin gene expression in human, monkey and murine brain. Eur J Neurosci. 1996;8(4):666–676. doi: 10.1111/j.1460-9568.1996.tb01252.x. [DOI] [PubMed] [Google Scholar]
- 45.Marti HH, Bernaudin M, Petit E, Bauer C. Neuroprotection and angiogenesis: dual role of erythropoietin in brain ischemia. News Physiol Sci. 2000;15:225–229. doi: 10.1152/physiologyonline.2000.15.5.225. [DOI] [PubMed] [Google Scholar]
- 46.Chen ZY, Asavaritikrai P, Prchal JT, Noguchi CT. Endogenous erythropoietin signaling is required for normal neural progenitor cell proliferation. J Biol Chem. 2007;282(35):25875–25883. doi: 10.1074/jbc.M701988200. [DOI] [PubMed] [Google Scholar]
- 47.Shingo T, Sorokan ST, Shimazaki T, Weiss S. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci. 2001;21(24):9733–9743. doi: 10.1523/JNEUROSCI.21-24-09733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monacci WT, Merrill MJ, Oldfield EH. Expression of vascular permeability factor/vascular endothelial growth factor in normal rat tissues. Am J Physiol. 1993;264(4 Pt 1):C995–C1002. doi: 10.1152/ajpcell.1993.264.4.C995. [DOI] [PubMed] [Google Scholar]
- 49.Marti HH, Risau W. Systemic hypoxia changes the organ-specific distribution of vascular endothelial growth factor and its receptors. Proc Natl Acad Sci USA. 1998;95(26):15809–15814. doi: 10.1073/pnas.95.26.15809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Breier G, Albrecht U, Sterrer S, Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992;114(2):521–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- 51.Ruiz de Almodovar C, Lambrechts D, Mazzone M, Carmeliet P. Role and therapeutic potential of VEGF in the nervous system. Physiol Rev. 2009;89(2):607–648. doi: 10.1152/physrev.00031.2008. [DOI] [PubMed] [Google Scholar]
- 52.Ogunshola OO, Stewart WB, Mihalcik V, Solli T, Madri JA, Ment LR. Neuronal VEGF expression correlates with angiogenesis in postnatal developing rat brain. Brain Res Dev Brain Res. 2000;119(1):139–153. doi: 10.1016/s0165-3806(99)00125-x. [DOI] [PubMed] [Google Scholar]
- 53.Hayashi T, Abe K, Suzuki H, Itoyama Y. Rapid induction of vascular endothelial growth factor gene expression after transient middle cerebral artery occlusion in rats. Stroke. 1997;28(10):2039–2044. doi: 10.1161/01.str.28.10.2039. [DOI] [PubMed] [Google Scholar]
- 54.Madri JA. Modeling the neurovascular niche: implications for recovery from CNS injury. J Physiol Pharmacol. 2009;60(Suppl 4):95–104. [PubMed] [Google Scholar]
- 55.Yang SZ, Zhang LM, Huang YL, Sun FY. Distribution of Flk-1 and Flt-1 receptors in neonatal and adult rat brains. Anat Rec A Discov Mol Cell Evol Biol. 2003;274(1):851–856. doi: 10.1002/ar.a.10103. [DOI] [PubMed] [Google Scholar]
- 56.Newton SS, Duman RS. Regulation of neurogenesis and angiogenesis in depression. Curr Neurovasc Res. 2004;1(3):261–267. doi: 10.2174/1567202043362388. [DOI] [PubMed] [Google Scholar]
- 57.Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34(6):945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 58.Islamov RR, Chintalgattu V, Pak ES, Katwa LC, Murashov AK. Induction of VEGF and its Flt-1 receptor after sciatic nerve crush injury. Neuroreport. 2004;15(13):2117–2121. doi: 10.1097/00001756-200409150-00024. [DOI] [PubMed] [Google Scholar]
- 59.Choi JS, Kim HY, Cha JH, Choi JY, Chun MH, Lee MY. Upregulation of vascular endothelial growth factor receptors Flt-1 and Flk-1 in rat hippocampus after transient forebrain ischemia. J Neurotrauma. 2007;24(3):521–531. doi: 10.1089/neu.2006.0139. [DOI] [PubMed] [Google Scholar]
- 60.Jin K, Mao XO, Eshoo MW, Nagayama T, Minami M, Simon RP, Greenberg DA. Microarray analysis of hippocampal gene expression in global cerebral ischemia. Ann Neurol. 2001;50(1):93–103. doi: 10.1002/ana.1073. [DOI] [PubMed] [Google Scholar]
- 61.Jaquet K, Krause K, Tawakol-Khodai M, Geidel S, Kuck KH. Erythropoietin and VEGF exhibit equal angiogenic potential. Microvasc Res. 2002;64(2):326–333. doi: 10.1006/mvre.2002.2426. [DOI] [PubMed] [Google Scholar]
- 62.Yamaji R, Okada T, Moriya M, Naito M, Tsuruo T, Miyatake K, Nakano Y. Brain capillary endothelial cells express two forms of erythropoietin receptor mRNA. Eur J Biochem. 1996;239(2):494–500. doi: 10.1111/j.1432-1033.1996.0494u.x. [DOI] [PubMed] [Google Scholar]
- 63.Santhanam AV, Smith LA, Nath KA, Katusic ZS. In vivo stimulatory effect of erythropoietin on endothelial nitric oxide synthase in cerebral arteries. Am J Physiol Heart Circ Physiol. 2006;291(2):H781–H786. doi: 10.1152/ajpheart.00045.2006. [DOI] [PubMed] [Google Scholar]
- 64.Sautina L, Sautin Y, Beem E, Zhou Z, Schuler A, Brennan J, Zharikov SI, Diao Y, Bungert J, Segal MS. Induction of nitric oxide by erythropoietin is mediated by the beta common receptor and requires interaction with VEGF receptor 2. Blood. 2009;115(4):896–905. doi: 10.1182/blood-2009-04-216432. [DOI] [PubMed] [Google Scholar]
- 65.Matsumoto T, Claesson-Welsh L. VEGF receptor signal transduction. Sci STKE. 2001;2001(112):re21. doi: 10.1126/stke.2001.112.re21. [DOI] [PubMed] [Google Scholar]
- 66.Dzietko M, Felderhoff-Mueser U, Sifringer M, Krutz B, Bittigau P, Thor F, Heumann R, Buhrer C, Ikonomidou C, Hansen HH. Erythropoietin protects the developing brain against N-methyl-d-aspartate receptor antagonist neurotoxicity. Neurobiol Dis. 2004;15(2):177–187. doi: 10.1016/j.nbd.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 67.Girgenti MJ, Hunsberger J, Duman CH, Sathyanesan M, Terwilliger R, Newton SS. Erythropoietin induction by electroconvulsive seizure, gene regulation, and antidepressant-like behavioral effects. Biol Psychiatry. 2009;66(3):267–274. doi: 10.1016/j.biopsych.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 68.Chavez JC, Baranova O, Lin J, Pichiule P. The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. J Neurosci. 2006;26(37):9471–9481. doi: 10.1523/JNEUROSCI.2838-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Masuda S, Okano M, Yamagishi K, Nagao M, Ueda M, Sasaki R. A novel site of erythropoietin production. Oxygen-dependent production in cultured rat astrocytes. J Biol Chem. 1994;269(30):19488–19493. [PubMed] [Google Scholar]
- 70.Schoch HJ, Fischer S, Marti HH. Hypoxia-induced vascular endothelial growth factor expression causes vascular leakage in the brain. Brain. 2002;125(Pt 11):2549–2557. doi: 10.1093/brain/awf257. [DOI] [PubMed] [Google Scholar]
- 71.Ruscher K, Freyer D, Karsch M, Isaev N, Megow D, Sawitzki B, Priller J, Dirnagl U, Meisel A. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci. 2002;22(23):10291–10301. doi: 10.1523/JNEUROSCI.22-23-10291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sugawa M, Sakurai Y, Ishikawa-Ieda Y, Suzuki H, Asou H. Effects of erythropoietin on glial cell development; oligodendrocyte maturation and astrocyte proliferation. Neurosci Res. 2002;44(4):391–403. doi: 10.1016/s0168-0102(02)00161-x. [DOI] [PubMed] [Google Scholar]
- 73.Diaz Z, Assaraf MI, Miller WH, Jr, Schipper HM. Astroglial cytoprotection by erythropoietin pre-conditioning: implications for ischemic and degenerative CNS disorders. J Neurochem. 2005;93(2):392–402. doi: 10.1111/j.1471-4159.2005.03038.x. [DOI] [PubMed] [Google Scholar]
- 74.Gunnarson E, Song Y, Kowalewski JM, Brismar H, Brines M, Cerami A, Andersson U, Zelenina M, Aperia A. Erythropoietin modulation of astrocyte water permeability as a component of neuroprotection. Proc Natl Acad Sci USA. 2009;106(5):1602–1607. doi: 10.1073/pnas.0812708106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Broadley AJ, Korszun A, Jones CJ, Frenneaux MP. Arterial endothelial function is impaired in treated depression. Heart. 2002;88(5):521–523. doi: 10.1136/heart.88.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wagner JA, Tennen H, Mansoor GA, Abbott G. History of major depressive disorder and endothelial function in postmenopausal women. Psychosom Med. 2006;68(1):80–86. doi: 10.1097/01.psy.0000195868.68122.9e. [DOI] [PubMed] [Google Scholar]
- 77.Jansson L, Hellsten J, Tingstrom A. Region specific hypothalamic neuronal activation and endothelial cell proliferation in response to electroconvulsive seizures. Biol Psychiatry. 2006;60(8):874–881. doi: 10.1016/j.biopsych.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 78.Smith PJ, Blumenthal JA, Babyak MA, Hoffman BM, Doraiswamy PM, Waugh R, Hinderliter A, Sherwood A. Cerebrovascular risk factors, vascular disease, and neuropsychological outcomes in adults with major depression. Psychosom Med. 2007;69(6):578–586. doi: 10.1097/PSY.0b013e31812f7b8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saxena S, Brody AL, Ho ML, Alborzian S, Ho MK, Maidment KM, Huang SC, Wu HM, Au SC, Baxter LR., Jr Cerebral metabolism in major depression and obsessive-compulsive disorder occurring separately and concurrently. Biol Psychiatry. 2001;50(3):159–170. doi: 10.1016/s0006-3223(01)01123-4. [DOI] [PubMed] [Google Scholar]
- 80.Videbech P, Ravnkilde B, Pedersen AR, Egander A, Landbo B, Rasmussen NA, Andersen F, Stodkilde-Jorgensen H, Gjedde A, Rosenberg R. The Danish PET/depression project: PET findings in patients with major depression. Psychol Med. 2001;31(7):1147–1158. doi: 10.1017/s0033291701004469. [DOI] [PubMed] [Google Scholar]
- 81.Norbury R, Mannie Z, Cowen PJ. Imaging vulnerability for depression. Mol Psychiatry. 2011;16(11):1067–1068. doi: 10.1038/mp.2011.44. [DOI] [PubMed] [Google Scholar]
- 82.Savitz JB, Drevets WC. Imaging phenotypes of major depressive disorder: genetic correlates. Neuroscience. 2009;164(1):300–330. doi: 10.1016/j.neuroscience.2009.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61(2):198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 84.Dome P, Halmai Z, Dobos J, Lazary J, Gonda X, Kenessey I, Sallai T, Makkos Z, Faludi G. Investigation of circulating endothelial progenitor cells and angiogenic and inflammatory cytokines during recovery from an episode of major depression. J Affect Disord. 2012;136(3):1159–1163. doi: 10.1016/j.jad.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 85.Dome P, Teleki Z, Rihmer Z, Peter L, Dobos J, Kenessey I, Tovari J, Timar J, Paku S, Kovacs G, Dome B. Circulating endothelial progenitor cells and depression: a possible novel link between heart and soul. Mol Psychiatry. 2009;14(5):523–531. doi: 10.1038/sj.mp.4002138. [DOI] [PubMed] [Google Scholar]
- 86.d’Audiffret AC, Frisbee SJ, Stapleton PA, Goodwill AG, Isingrini E, Frisbee JC. Depressive behavior and vascular dysfunction: a link between clinical depression and vascular disease? J Appl Physiol. 2010;108(5):1041–1051. doi: 10.1152/japplphysiol.01440.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Giardina WJ, Ebert DM. Positive effects of captopril in the behavioral despair swim test. Biol Psychiatry. 1989;25(6):697–702. doi: 10.1016/0006-3223(89)90240-0. [DOI] [PubMed] [Google Scholar]
- 88.Gard PR, Mandy A, Sutcliffe MA. Evidence of a possible role of altered angiotensin function in the treatment, but not etiology, of depression. Biol Psychiatry. 1999;45(8):1030–1034. doi: 10.1016/s0006-3223(98)00101-2. [DOI] [PubMed] [Google Scholar]
- 89.Renshaw PF, Parsegian A, Yang CK, Novero A, Yoon SJ, Lyoo IK, Cohen BM, Carlezon WA., Jr Lovastatin potentiates the antidepressant efficacy of fluoxetine in rats. Pharmacol Biochem Behav. 2009;92(1):88–92. doi: 10.1016/j.pbb.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Srivastava SK, Nath C. The differential effects of calcium channel blockers in the behavioural despair test in mice. Pharmacol Res. 2000;42(4):293–297. doi: 10.1006/phrs.2000.0696. [DOI] [PubMed] [Google Scholar]
- 91.Nowak G, Paul IA, Popik P, Young A, Skolnick P. Ca2+ antagonists effect an antidepressant-like adaptation of the NMDA receptor complex. Eur J Pharmacol. 1993;247(1):101–102. doi: 10.1016/0922-4106(93)90144-x. [DOI] [PubMed] [Google Scholar]
- 92.Iga J, Ueno S, Yamauchi K, Numata S, Tayoshi-Shibuya S, Kinouchi S, Nakataki M, Song H, Hokoishi K, Tanabe H, Sano A, Ohmori T. Gene expression and association analysis of vascular endothelial growth factor in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(3):658–663. doi: 10.1016/j.pnpbp.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 93.Kahl KG, Bens S, Ziegler K, Rudolf S, Kordon A, Dibbelt L, Schweiger U. Angiogenic factors in patients with current major depressive disorder comorbid with borderline personality disorder. Psychoneuroendocrinology. 2009;34(3):353–357. doi: 10.1016/j.psyneuen.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 94.Lee BH, Kim YK. Increased plasma VEGF levels in major depressive or manic episodes in patients with mood disorders. J Affect Disord. 2012;136(1–2):181–184. doi: 10.1016/j.jad.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 95.Isung J, Mobarrez F, Nordstrom P, Asberg M, Jokinen J. Low plasma vascular endothelial growth factor (VEGF) associated with completed suicide. World J Biol Psychiatry. 2011 doi: 10.3109/15622975.2011.624549. [DOI] [PubMed] [Google Scholar]
- 96.Fowles ER, Murphey C, Ruiz RJ. Exploring relationships among psychosocial status, dietary quality, and measures of placental development during the first trimester in low-income women. Biol Res Nurs. 2011;13(1):70–79. doi: 10.1177/1099800410378733. [DOI] [PubMed] [Google Scholar]
- 97.Katsuura S, Kamezaki Y, Yamagishi N, Kuwano Y, Nishida K, Masuda K, Tanahashi T, Kawai T, Arisawa K, Rokutan K. Circulating vascular endothelial growth factor is independently and negatively associated with trait anxiety and depressive mood in healthy Japanese university students. Int J Psychophysiol. 2011;81(1):38–43. doi: 10.1016/j.ijpsycho.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 98.Takebayashi M, Hashimoto R, Hisaoka K, Tsuchioka M, Kunugi H. Plasma levels of vascular endothelial growth factor and fibroblast growth factor 2 in patients with major depressive disorders. J Neural Transm. 2010;117(9):1119–1122. doi: 10.1007/s00702-010-0452-1. [DOI] [PubMed] [Google Scholar]
- 99.Elfving B, Plougmann PH, Wegener G. Differential brain, but not serum VEGF levels in a genetic rat model of depression. Neurosci Lett. 2010;474(1):13–16. doi: 10.1016/j.neulet.2010.02.063. [DOI] [PubMed] [Google Scholar]
- 100.Miskowiak K, O’Sullivan U, Harmer CJ. Erythropoietin reduces neural and cognitive processing of fear in human models of antidepressant drug action. Biol Psychiatry. 2007;62(11):1244–1250. doi: 10.1016/j.biopsych.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 101.Miskowiak K, Inkster B, Selvaraj S, Wise R, Goodwin GM, Harmer CJ. Erythropoietin improves mood and modulates the cognitive and neural processing of emotion 3 days post administration. Neuropsychopharmacology. 2008;33(3):611–618. doi: 10.1038/sj.npp.1301439. [DOI] [PubMed] [Google Scholar]
- 102.Harmer CJ, Bhagwagar Z, Perrett DI, Vollm BA, Cowen PJ, Goodwin GM. Acute SSRI administration affects the processing of social cues in healthy volunteers. Neuropsychopharmacology. 2003;28(1):148–152. doi: 10.1038/sj.npp.1300004. [DOI] [PubMed] [Google Scholar]
- 103.Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry. 2006;59(9):816–820. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 104.Miskowiak KW, Favaron E, Hafizi S, Inkster B, Goodwin GM, Cowen PJ, Harmer CJ. Erythropoietin modulates neural and cognitive processing of emotional information in biomarker models of antidepressant drug action in depressed patients. Psychopharmacology. 2010;210(3):419–428. doi: 10.1007/s00213-010-1842-7. [DOI] [PubMed] [Google Scholar]
- 105.Heine VM, Zareno J, Maslam S, Joels M, Lucassen PJ. Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression. Eur J Neurosci. 2005;21(5):1304–1314. doi: 10.1111/j.1460-9568.2005.03951.x. [DOI] [PubMed] [Google Scholar]
- 106.Howell KR, Kutiyanawalla A, Pillai A. Long-term continuous corticosterone treatment decreases VEGF receptor-2 expression in frontal cortex. PLoS ONE. 2011;6(5):e20198. doi: 10.1371/journal.pone.0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bergstrom A, Jayatissa MN, Mork A, Wiborg O. Stress sensitivity and resilience in the chronic mild stress rat model of depression; an in situ hybridization study. Brain Res. 2008;1196:41–52. doi: 10.1016/j.brainres.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 108.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425(4):479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 109.Fournier NM, Duman RS. Role of vascular endothelial growth factor in adult hippocampal neurogenesis: implications for the pathophysiology and treatment of depression. Behav Brain Res. 2011 doi: 10.1016/j.bbr.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci USA. 2007;104(11):4647–4652. doi: 10.1073/pnas.0610282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee JS, Jang DJ, Lee N, Ko HG, Kim H, Kim YS, Kim B, Son J, Kim SH, Chung H, Lee MY, Kim WR, Sun W, Zhuo M, Abel T, Kaang BK, Son H. Induction of neuronal vascular endothelial growth factor expression by cAMP in the dentate gyrus of the hippocampus is required for antidepressant-like behaviors. J Neurosci. 2009;29(26):8493–8505. doi: 10.1523/JNEUROSCI.1321-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Greene J, Banasr M, Lee B, Warner-Schmidt J, Duman RS. Vascular endothelial growth factor signaling is required for the behavioral actions of antidepressant treatment: pharmacological and cellular characterization. Neuropsychopharmacology. 2009;34(11):2459–2468. doi: 10.1038/npp.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kiuchi T, Lee H, Mikami T. Regular exercise cures depression-like behavior via VEGF-Flk-1 signaling in chronically stressed mice. Neuroscience. 2012;207:208–217. doi: 10.1016/j.neuroscience.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 114.Ibrahim L, Duncan W, Luckenbaugh DA, Yuan P, Machado-Vieira R, Zarate CA., Jr Rapid antidepressant changes with sleep deprivation in major depressive disorder are associated with changes in vascular endothelial growth factor (VEGF): a pilot study. Brain Res Bull. 2011;86(1–2):129–133. doi: 10.1016/j.brainresbull.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Viikki M, Anttila S, Kampman O, Illi A, Huuhka M, Setala-Soikkeli E, Mononen N, Lehtimaki T, Leinonen E. Vascular endothelial growth factor (VEGF) polymorphism is associated with treatment resistant depression. Neurosci Lett. 2010;477(3):105–108. doi: 10.1016/j.neulet.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 116.Udo H, Yoshida Y, Kino T, Ohnuki K, Mizunoya W, Mukuda T, Sugiyama H. Enhanced adult neurogenesis and angiogenesis and altered affective behaviors in mice overexpressing vascular endothelial growth factor 120. J Neurosci. 2008;28(53):14522–14536. doi: 10.1523/JNEUROSCI.3673-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8(6–7):523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 118.Segi-Nishida E, Warner-Schmidt JL, Duman RS. Electroconvulsive seizure and VEGF increase the proliferation of neural stem-like cells in rat hippocampus. Proc Natl Acad Sci USA. 2008;105(32):11352–11357. doi: 10.1073/pnas.0710858105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Oh DH, Kim BW, Choi M, Lee G, Choi JS, Hyeon S. Changes in vascular endothelial growth factor (VEGF) induced by the Morris water maze task. Mol Cells. 2012;33(3):295–300. doi: 10.1007/s10059-012-2254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pati S, Orsi SA, Moore AN, Dash PK. Intra-hippocampal administration of the VEGF receptor blocker PTK787/ZK222584 impairs long-term memory. Brain Res. 2009;1256:85–91. doi: 10.1016/j.brainres.2008.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim BW, Choi M, Kim YS, Park H, Lee HR, Yun CO, Kim EJ, Choi JS, Kim S, Rhim H, Kaang BK, Son H. Vascular endothelial growth factor (VEGF) signaling regulates hippocampal neurons by elevation of intracellular calcium and activation of calcium/calmodulin protein kinase II and mammalian target of rapamycin. Cell Signal. 2008;20(4):714–725. doi: 10.1016/j.cellsig.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 122.Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36(8):827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- 123.Plaschke K, Staub J, Ernst E, Marti HH. VEGF overexpression improves mice cognitive abilities after unilateral common carotid artery occlusion. Exp Neurol. 2008;214(2):285–292. doi: 10.1016/j.expneurol.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 124.Licht T, Goshen I, Avital A, Kreisel T, Zubedat S, Eavri R, Segal M, Yirmiya R, Keshet E. Reversible modulations of neuronal plasticity by VEGF. Proc Natl Acad Sci USA. 2011 doi: 10.1073/pnas.1007640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29(7):1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- 126.Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, Savino C, Bianchi M, Nielsen J, Gerwien J, Kallunki P, Larsen AK, Helboe L, Christensen S, Pedersen LO, Nielsen M, Torup L, Sager T, Sfacteria A, Erbayraktar S, Erbayraktar Z, Gokmen N, Yilmaz O, Cerami-Hand C, Xie QW, Coleman T, Cerami A, Brines M. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305(5681):239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- 127.Adamcio B, Sargin D, Stradomska A, Medrihan L, Gertler C, Theis F, Zhang M, Muller M, Hassouna I, Hannke K, Sperling S, Radyushkin K, El-Kordi A, Schulze L, Ronnenberg A, Wolf F, Brose N, Rhee JS, Zhang W, Ehrenreich H. Erythropoietin enhances hippocampal long-term potentiation and memory. BMC Biol. 2008;6:37. doi: 10.1186/1741-7007-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brines M, Patel NS, Villa P, Brines C, Mennini T, De Paola M, Erbayraktar Z, Erbayraktar S, Sepodes B, Thiemermann C, Ghezzi P, Yamin M, Hand CC, Xie QW, Coleman T, Cerami A. Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin. Proc Natl Acad Sci USA. 2008;105(31):10925–10930. doi: 10.1073/pnas.0805594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA. 2000;97(19):10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Conley AJ, Mohib K, Jevnikar AM, Brandle JE. Plant recombinant erythropoietin attenuates inflammatory kidney cell injury. Plant Biotechnol J. 2009;7(2):183–199. doi: 10.1111/j.1467-7652.2008.00389.x. [DOI] [PubMed] [Google Scholar]
- 131.Wang YJ, Liu YD, Chen J, Hao SJ, Hu T, Ma GH, Su ZG. Efficient preparation and PEGylation of recombinant human non-glycosylated erythropoietin expressed as inclusion body in E. coli . Int J Pharm. 2010;386(1–2):156–164. doi: 10.1016/j.ijpharm.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 132.Johnson DL, Middleton SA, McMahon F, Barbone FP, Kroon D, Tsao E, Lee WH, Mulcahy LS, Jolliffe LK. Refolding, purification, and characterization of human erythropoietin binding protein produced in Escherichia coli . Protein Expr Purif. 1996;7(1):104–113. doi: 10.1006/prep.1996.0014. [DOI] [PubMed] [Google Scholar]
- 133.Livnah O, Stura EA, Johnson DL, Middleton SA, Mulcahy LS, Wrighton NC, Dower WJ, Jolliffe LK, Wilson IA. Functional mimicry of a protein hormone by a peptide agonist: the EPO receptor complex at 2.8 Å. Science. 1996;273(5274):464–471. doi: 10.1126/science.273.5274.464. [DOI] [PubMed] [Google Scholar]
- 134.Syed RS, Reid SW, Li C, Cheetham JC, Aoki KH, Liu B, Zhan H, Osslund TD, Chirino AJ, Zhang J, Finer-Moore J, Elliott S, Sitney K, Katz BA, Matthews DJ, Wendoloski JJ, Egrie J, Stroud RM. Efficiency of signalling through cytokine receptors depends critically on receptor orientation. Nature. 1998;395(6701):511–516. doi: 10.1038/26773. [DOI] [PubMed] [Google Scholar]
- 135.Philo JS, Aoki KH, Arakawa T, Narhi LO, Wen J. Dimerization of the extracellular domain of the erythropoietin (EPO) receptor by EPO: one high-affinity and one low-affinity interaction. Biochemistry. 1996;35(5):1681–1691. doi: 10.1021/bi9524272. [DOI] [PubMed] [Google Scholar]
- 136.Wrighton NC, Farrell FX, Chang R, Kashyap AK, Barbone FP, Mulcahy LS, Johnson DL, Barrett RW, Jolliffe LK, Dower WJ. Small peptides as potent mimetics of the protein hormone erythropoietin. Science. 1996;273(5274):458–464. doi: 10.1126/science.273.5274.458. [DOI] [PubMed] [Google Scholar]
- 137.Pankratova S, Kiryushko D, Sonn K, Soroka V, Kohler LB, Rathje M, Gu B, Gotfryd K, Clausen O, Zharkovsky A, Bock E, Berezin V. Neuroprotective properties of a novel, non-haematopoietic agonist of the erythropoietin receptor. Brain. 2010;133(Pt 8):2281–2294. doi: 10.1093/brain/awq101. [DOI] [PubMed] [Google Scholar]
- 138.Pankratova S, Gu B, Kiryushko D, Korshunova I, Kohler LB, Rathje M, Bock E, Berezin V. A new agonist of the erythropoietin receptor, Epobis, induces neurite outgrowth and promotes neuronal survival. J Neurochem. 2012;121(6):915–923. doi: 10.1111/j.1471-4159.2012.07751.x. [DOI] [PubMed] [Google Scholar]
- 139.Constantinescu SN, Ghaffari S, Lodish HF. The erythropoietin receptor: structure, activation and intracellular signal transduction. Trends Endocrinol Metab. 1999;10(1):18–23. doi: 10.1016/s1043-2760(98)00101-5. [DOI] [PubMed] [Google Scholar]
- 140.Mahmood A, Lu D, Qu C, Goussev A, Zhang ZG, Lu C, Chopp M. Treatment of traumatic brain injury in rats with erythropoietin and carbamylated erythropoietin. J Neurosurg. 2007;107(2):392–397. doi: 10.3171/JNS-07/08/0392. [DOI] [PubMed] [Google Scholar]
- 141.Chong ZZ, Kang JQ, Maiese K. Hematopoietic factor erythropoietin fosters neuroprotection through novel signal transduction cascades. J Cereb Blood Flow Metab. 2002;22(5):503–514. doi: 10.1097/00004647-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 142.Ogunshola OO, Antic A, Donoghue MJ, Fan SY, Kim H, Stewart WB, Madri JA, Ment LR. Paracrine and autocrine functions of neuronal vascular endothelial growth factor (VEGF) in the central nervous system. J Biol Chem. 2002;277(13):11410–11415. doi: 10.1074/jbc.M111085200. [DOI] [PubMed] [Google Scholar]
- 143.Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci. 1999;19(14):5731–5740. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zacchigna S, Lambrechts D, Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nat Rev Neurosci. 2008;9(3):169–181. doi: 10.1038/nrn2336. [DOI] [PubMed] [Google Scholar]
- 145.Sun Y, Jin K, Childs JT, Xie L, Mao XO, Greenberg DA. Vascular endothelial growth factor-B (VEGFB) stimulates neurogenesis: evidence from knockout mice and growth factor administration. Dev Biol. 2006;289(2):329–335. doi: 10.1016/j.ydbio.2005.10.016. [DOI] [PubMed] [Google Scholar]