Abstract
Traumatic brain injury (TBI) is a significant health issue that often causes enduring cognitive deficits, in particular memory dys-function. The hippocampus, a structure crucial in learning and memory, is frequently damaged during TBI. Since long-term potentiation (LTP) is the leading cellular model underlying learning and memory, this study was undertaken to examine how injury affects area CA1 LTP in mice using lateral fluid percussion injury (FPI). Brain slices derived from FPI animals demonstrated an inability to induce LTP in area CA1 7 days postinjury. However, area CA1 long-term depression could be induced in neurons 7 days postinjury, demonstrating that some forms of synaptic plasticity can still be elicited. Using a multidisciplined approach, potential mechanisms underlying the inability to induce and maintain area CA1 LTP were investigated. This study demonstrates that injury leads to significantly smaller N-methyl-d-aspartate potentials and glutamate-induced excitatory currents, increased dendritic spine size, and decreased expression of α-calcium calmodulin kinase II. These findings may underlie the injury-induced lack of LTP and thus, contribute to cognitive impairments often associated with TBI. Furthermore, these results provide attractive sites for potential therapeutic intervention directed toward alleviating the devastating consequences of human TBI.
Keywords: NMDA receptor, α-calmodulin kinase II (α-CaMKII), dendrite spine size, cognition, limbic function
INTRODUCTION
Traumatic brain injury (TBI) is a common neurological disorder that affects an estimated 5.3 million Americans (Report of the 1998 Consensus Conference on Neuroblastoma Screening, 1999) and often results in life-long cognitive deficits. Even mild TBI damages hippocampal anatomy (Kotapka et al., 1991, 1992; Lowenstein et al., 1992; Grady et al., 2003), leading to a reduction in information processing (Mathias et al., 2004) because of altered physiological function (Toth et al., 1997; Santhakumar et al., 2000, 2001; Witgen et al., 2005), motor deficits (Nimmo et al., 2004), and learning and memory impairment (Barth et al., 1983; Bennett-Levy, 1984; Lyeth et al., 1990; McAllister, 1992).
Long-term potentiation (LTP) is currently our best physiological correlate of learning and memory and has been studied in the rat with several different brain injury models demonstrating a consistent inability to induce area CA1 LTP following brain injury (Reeves et al., 1995, 1997; D'Ambrosio et al., 1998; Sick et al., 1998; Sanders et al., 2000). The complement of LTP is long-term depression (LTD), which is a decrease in synaptic efficacy following low frequency stimulation (Bear and Abraham, 1996). LTD is unaffected by fluid percussion injury (FPI) (D'Ambrosio et al., 1998). Here, we initially corroborate these data in the mouse, which allows for the potential future use of transgenic models. However, the primary goal of this study was to examine the possible mechanism(s) underlying the inability to induce area CA1 LTP effect after FPI. Using a multi-faceted approach including electrophysiological, anatomical, and molecular biological methods, we show that injury causes alterations in N-methyl-d-aspartate (NMDA) and AMPA receptor function, dendritic spine size, and α-calcium calmodulin kinase II (α-CaMKII) protein expression.
MATERIALS AND METHODS
Animals
Experiments were performed on 5- to 7-week old, 20–25 g C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME). All experimental procedures and protocols for animal studies were approved by the Children's Hospital of Philadelphia Institutional Animal Care and Use Committee in accordance with international guidelines on the ethical use of animals. Experiments were designed to minimize the number of animals required and those used were cared for, handled and medicated to minimize suffering (National Research Council, 1996).
FPI
FPI was conducted as previously described by Witgen et al. (2005). Briefly, on the first day, a craniectomy was performed over the right parietal area making sure that the dura was not breeched. On the second day, a 20-ms pulse (ranging from 1.4 to 2.1 atm) of saline was delivered to the brain resulting in a mild to moderate injury. Sham animals underwent all of the FPI procedures with the exception of the fluid pulse. All experiments were undertaken in animals that were allowed to recover for 7 days after sham surgery or FPI.
Tissue Preparation
The brain was dissected rapidly and placed in ice-cold artificial cerebral spinal fluid (aCSF) (in mM: 126 NaCl, 3.2 KCl, 1.25 NaHPO4, 26 NaHCO3, 10 glucose, 2 MgCl2, and 2 CaCl2; 95% O2/5% CO2) for 2 min. The ipsilateral hippocampal formation was identified and removed en bloc. The hippocampus was then sliced perpendicular to its long axis using a Brinkman mechanical tissue chopper (Brinkman Instruments, Westbury, NY) in 300-μm increments.
Field Potential Recording
The Schaffer collateral afferent pathway was stimulated and field excitatory postsynaptic potentials (fEPSPs) were recorded in stratum radiatum of area CA1. Mini input/output (I/O) curves from 10 to 100 V were conducted and the stimulation that produced half maximal response was used (60 V) yielding fEPSPs of ~1 mV with overlapping ranges among groups (sham: 0.5–1.3 mV, FPI: 0.4–1.2 mV, P > 0.05). N-methyl-d-aspartate (NMDA) potentials were isolated in modified aCSF containing 1 mM MgCl2, 4 mM CaCl2, and 6 μM CNQX. GABAA-mediated inhibition was blocked by the addition of bicuculline methiodide (BMI, 30 μM) to the super-fusing aCSF. Paired pulse stimulation was accomplished by delivery of two pulses (duration 15 ms, stimulation 60% of maximal response) separated by 50 ms and quantified by dividing the amplitude of the second pulse by the amplitude of the first pulse.
LTP and LTD Quantification
Baseline field potentials were recorded every 30 s for 30 min. LTP was induced by tetanic stimulation, consisting of either two trains of conditioning stimulation (1 s at 100 Hz) at 30-s intervals (high-frequency stimulation (HFS)) or theta burst stimulation (TBS), at the test pulse intensity consisting of 5 × 100 Hz bursts (5 dipahsic pulses per burst, 200-ms interburst interval). LTD was induced by low frequency stimulation of 1 Hz for 10 min. Responses were subsequently followed for 60 min at 30-s intervals.
The slope of the fEPSP was measured (see Witgen et al. 2005 for specifics) using Powerlab 5 (ADInstruments, Colorado Springs, CO) and expressed as percent change from baseline values (the mean of the last 10 points prior to HFS or TBS). LTP or LTD was quantified as the mean of last 10 values 60 min post-tetanus. Data were presented as the percent change in the fEPSP slope relative to pretetanus baseline values.
Whole-Cell Recording of Glutamate Evoked Currents
Brain slices were prepared as previously reported (Witgen et al., 2005). Whole-cell voltage-clamp recordings were conducted at room temperature from visually identified area CA1 pyramidal neurons using infrared differential interference contrast video microscopy (Stuart et al., 1993; Cohen et al., 2000). Cells were voltage clamped at –40 mV, which is 20 mV more positive than the empirically determined excitatory postsynaptic current (EPSC) reversal potential of –60 mV to remove the voltage-dependent blockade of NMDA receptors by extracellular magnesium (Mayer et al., 1984). Signals were recorded and amplified with Axopatch 200 (Axon instruments, Foster city, CA), filtered at 2 kHz, digitized and stored on a PC microcomputer for off line analysis. Electrodes were pulled on a two stage puller (Sutter instruments, Novato, CA) to a resistance between 7 and 10 MΩ when filled with an internal solution composed of 130 mM CsMeSO4, 10 mM KCl, 10 mM HEPES, 2 mM MgSO4, 0.5 mM EGTA, 3 mM ATP; pH 7.4 (KOH). Excitatory currents were evoked by focal application of glutamate using a Picospritzer II (100 μM, 50-ms duration, 40 psi, General Valve Corporation, Fairfield, NJ). Recordings were conducted in a modified aCSF containing tetrodotoxin (TTX; 0.4 μM, Sigma, St. Louis, MO) to block action-potential-mediated events, BMI (30 μM, Sigma) to block GABAA inhibition, CGP 55845 (5 μM, Tocris, Ellisville, MO) to block GABAB inhibition, and Glycine (10 μM, Sigma), a cofactor for the activation of the NMDA receptor. Subsequently, to isolate NMDA-mediated currents, 6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX, 6 μM, Sigma) was added to block AMPAergic component of the response.
Sub Regional Dissection of Area CA1
Seven days after injury, the ipsilateral (injured) hippocampi from sham and injured animals were harvested and sectioned using a Brinkman tissue chopper. Each section was then placed flat on the dissecting surface so that the pyramidal cell layer, hippocampal fissure, supra and infra granular blades of the dentate gyrus (DG) were clearly visible. CA1 and DG were microdissected and tissue was then placed into lysis buffer containing protease inhibitors cocktail tablets (Roche, Indianapolis, IN). Regionally dissected tissue was pooled from five animals for both sham and FPI populations.
Western Blot
Western blots were performed using standard laboratory techniques. In brief, tissue samples (50 μg) were boiled in gel loading buffer and then run on a 12% SDS polyacrylamide gel and subsequently transferred to PVDF membrane at (Bio Rad, Hercules, CA) 24 V overnight at 4°C. The membrane was first incubated with a rabbit polyclonal antibody against α-calcium calmodulin kinase II (α-CaMKII; 1:1,000 Chemicon, Temecula, CA) at 4°C overnight followed by a secondary antibody (1:3,000) and detected with enhanced chemiluminescence (Pierce, Rockford, IL). Membranes were subsequently stripped and reprobed using a rabbit polyclonal β-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA) to confirm proper protein loading. Densitometry analysis of blots with UN-SCAN-IT gel-scanning software (Silk Scientific, Orem, UT) was used to quantify and statistically compare protein expression between sample sets.
Dendritic Size Measurement
Intracellular loading: Seven days post sham surgery or injury animals were transcardiacally perfused with 10 ml of saline, followed by 50 ml of a fixative containing 10% neutral formalin and 0.125% glutaraldehyde. Brains were removed and 250-μm thick coronal sections were cut using a Leica VT 1000S vibratome (Leica Microsystems, Bannockburn, IL). Sections were mounted on nitrocellulose filter paper (Whatman, England, UK) and immersed in phosphate-buffered saline (0.1 M, pH 7.4). Neurons were loaded with 5% lucifer yellow (LY; Molecular Probes, Eugene, OR) by intracellular iontophoretic injection (Radley et al., 2004), under DC current (10 nA Master 8, A.M.P.I., Israel). Loaded sections were mounted and cover-slipped with PermaFluor (Thermo Shandon, Pittsburgh, PA).
LY-loaded CA1 pyramidal neurons were observed with a fluorescent microscope. To minimize sampling error, ending segments by the second order of apical dendrites were selected and used in the present study. For basal dendrites, fourth order segments were selected. Confocal images of LY-labeled dendrites with spines were acquired from the bifurcating nodes, using Olympus Fluoview Microsystem, with steps of 0.2 μm along the Z-axis under 100× oil objective. Stacking three-dimentional images of the selected dendritic spine segments were merged onto an X–Y plane. For each animal, 3–5 apical or basal dendritic segments were used. The merged images were then opened in a Neurolucida program. The whole length of the dendritic segments was traced and the contours of all the spine heads along the dendritic segments were drawn. Tracings were then opened with Neuroexplorer software and the length of dendritic segments, size (μm2 in area) of every spine, and total spine number were analyzed. The average size of spines, percentage of spines with different size, and spine density (as a function of total number of spines to the length of their parent dendrite) were compared between sham and injured groups.
Statistics
All data are reported as mean ± standard error of mean (SEM). Statistical comparisons were made using two-tailed unpaired Student's t-test (P < 0.05).
RESULTS
FPI inhibits Area CA1 LTP Induction
Previous studies in the rat demonstrated an inability to induce area CA1 LTP at varying time points after injury (D'Ambrosio et al., 1998; Sick et al., 1998; Sanders et al., 2000). First, we confirmed that FPI also resulted in a lack of area CA1 LTP in the mouse. LTP was induced by high frequency tetanic stimulation (HFS) similar to that utilized in previous studies (D'Ambrosio et al., 1998; Sick et al., 1998; Sanders et al., 2000) in three experimental groups: naïve (no surgery), sham (surgery, no injury), and injured (surgery and injury). HFS resulted in significant potentiation with a percent change of fEPSP slope from baseline of 51.4 ± 4.2% in slices from sham (n = 5; Fig. 1A) and 57.0 ± 3.7% in naive (n = 4; Fig. 1C) animals. In accordance with previous data in the rats, HFS yielded no significant potentiation (0.6 ± 1.3%, n = 7; Fig. 1A) in brain slices of FPI mice 7 days following injury. To examine whether altering the tetanic protocol might lead to LTP, we attempted to use a more physiologically relevant protocol i.e., TBS. However, TBS like HFS did not induce LTP in slices of injured animals (1.9 ± 4.2%) but led to 52.8 ± 22.0% change in fEPSP slope from baseline in slices from sham animals (Fig. 1C). Thus, varying the LTP inducing stimulation protocol does not rescue LTP after brain injury.
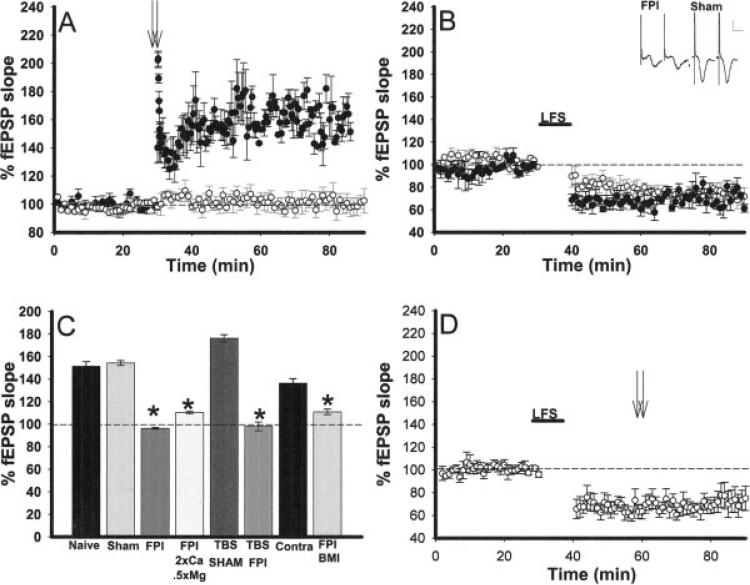
FIGURE 1.
Inability to induce area CA1 LTP but not LTD following lateral FPI in mice. A: High frequency tetanic stimulation (HFS, 2 × 100 Hz trains) denoted by arrows resulted in facilitation of the evoked field excitatory postsynaptic potential (fEPSP) in hippocampal slices from sham (filled circles, n = 4) but not in slices from brain injured (open circles, n = 7) mice. B: Low frequency stimulation (LFS, 1 Hz, 10 min) resulted in LTD of the fEPSP in slices derived from both injured (open circles, n = 4) and sham (filled circles, n = 4) animals. Inset: Paired pulse facilitation is observed in slices from FPI and sham mice (n = 3 FPI, n = 6 sham P > 0.05). Scale bar: 0.5 mV, 5 ms. C: Histogram demonstrating that LTP can be induced in slices from sham and naïve animals using either the standard HFS or theta burst stimulation (TBS); however, with both protocols, LTP cannot be induced in slices from FPI animals. LTP can be induced and maintained in area CA1 in the hippocampus contralateral to injury (n = 4), but ipsilateral area CA1 LTP cannot be rescued by varying the stimulation protocol, altering extracellular Ca2+ and Mg2+ concentrations (n = 4), or bath application of BMI (n = 4). All FPI treatment groups were significantly different from the sham slices exhibiting LTP (P < 0.05, denoted by *). Furthermore, no rescue was observed when statistically comparing BMI and altered aCSF treatment groups to the FPI LTP treatment group (P > 0.05). D: LTD induced by a LFS (denoted by LFS and a line) in slices from FPI animals does not make subsequent LTP induction by HFS (2 × 100 Hz trains, denoted by arrows) possible. Responses were followed for an additional 30 min post-tetanic stimulation, no potentiation (even a return to baseline) was observed (n = 6).
To determine whether FPI alters synaptic plasticity physically further from the site of impact, we attempted to induce LTP in area CA1 in the contralateral hippocampus. Interestingly, as opposed to the ipsilateral hippocampus, the contralateral side exhibited LTP 7 days following injury (Fig. 1C) at a level not significantly different from that recorded in slices derived from sham animals (P > 0.05). Therefore, all subsequent experiments were conducted in ipsilateral area CA1. LTD, the converse of LTP, has been shown to be unaffected by FPI in rats (D'Ambrosio et al., 1998). We confirmed these data by demonstrating that low frequency tetanic stimulation (LFS) resulted in LTD in area CA1 in slices from FPI animals substantiating that not all forms of synaptic plasticity are impaired by brain injury. LTD generated in slices from FPI mice was not significantly different from that induced in slices from sham animals (Fig. 1B). Furthermore, LTD induced in slices from sham and FPI animals was NMDA-dependent as it could not be induced in the presence of APV (n = 3 for each sham and FPI. Data not shown).
Lack of PTP Following FPI
To ascertain the underlying mechanisms for impaired LTP induction, we initially examined the finding that in slices from our FPI animals, as well as other injury models (Sick et al., 1998; Li et al., 2005), post-tetanic potentiation (PTP), an initial component of LTP, was not observed. PTP is thought to be due to residual presynaptic calcium buildup resulting in increased transmitter release (Del Castillo and Katz, 1954; Martin and Pilar, 1964; Barrett and Stevens, 1972; Hirst et al., 1981). Residual calcium build up is also known to cause another short-term plasticity, paired pulse facilitation (Zucker and Regehr, 2002). This is measured with a paradigm of identical stimulus pulses that are separated by less than 100 ms. Facilitation, or an increase, in the second response is indicative of residual calcium build up (Hess and Kuhnt, 1992; Zucker and Regehr, 2002). Therefore to determine whether injury affects presynaptic mechanisms, we measured the synaptic response to a pair of stimuli separated by a 50-ms interval and found that both FPI and sham slices exhibited similar paired pulse facilitation (109.6% ± 4.1, n = 3, 109.5% ± 0.9, n = 6, inset Fig. 1B).
FPI alters NMDA Receptor Function
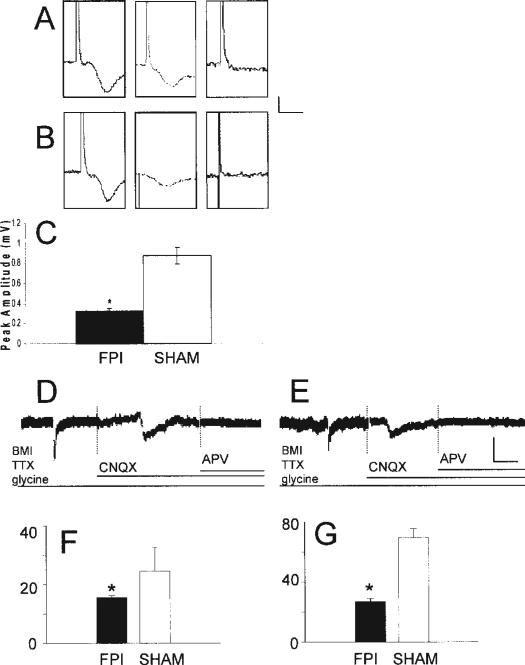
Synaptic plasticity requires a balance between inhibition and excitation (Liu, 2004). Previous studies from our laboratory showed that 7 days following FPI there is a downward amplitude shift in the area CA1 input/output (I/O) curve, demonstrating hypoexcitability due to augmented inhibition (Witgen et al., 2005). Thus, our initial hypothesis postulated that increased inhibition was causing the inability to induce area CA1 LTP. When inhibition was blocked by the addition of BMI (30 μM) to the aCSF, area CA1 LTP was still not induced in slices from FPI mice (Fig. 1C) disproving the hypothesis that overwhelming inhibition alone was the causal mechanism underlying this dysfunction. Therefore, the next logical step was then to examine possible alterations in excitation. NMDA receptor activation is crucial for LTP induction (Bliss and Collingridge, 1993; Nicoll and Malenka, 1995; Malenka and Nicoll, 1999). Thus, using extracellular recording techniques, we compared isolated NMDA potentials in slices from sham and FPI animals (Fig. 2). To ensure that comparable afferent fibers were being stimulated in slices from sham and injured animals, all experiments began with equal sized AMPA-mediated potentials. NMDA potentials were then isolated by perfusion of slices with a modified aCSF with a decreased magnesium concentration, CNQX (6 μM), to block AMPA-mediated activity and BMI (30 μM) to eliminate GABAA-mediated inhibition (Cohen and Abraham, 1996). NMDA potential amplitude recorded in slices from injured animals (Figs. 2B,C) was significantly smaller compared to the NMDA amplitude recorded in slices obtained from sham animals (Figs. 2A,C, P < 0.05). None of the NMDA potentials were contaminated with population spikes and all were confirmed to be mediated by NMDA by total blockade upon application of 10 μM 2-amino-5-phosphonopentanoic acid (APV) at the end of the experiment (Figs. 2A,B). To further assess excitatory receptor dysfunction, whole-cell voltage-clamp recordings were performed in visually identified pyramidal neurons in the presence of tetrodotoxin (TTX) and BMI. Focal application of glutamate resulted in an excitatory current recorded in slices from sham (Fig. 2D, left panel) and FPI (Fig. 2E, left panel) mice. The NMDA current was then isolated by switching to a modified aCSF with CNQX (6 μM) in addition to TTX and BMI (Figs. 2D,E, middle panels). At the end of each experiment, the perfusing solution was switched once again to an aCSF containing APV (50 μM) in addition to the aforementioned antagonists (Figs. 2D,E, right panels) ensuring that the current was NMDA-mediated. Glutamate-evoked currents recorded from neurons in slices from FPI animals showed a 45.3% reduction in the peak amplitude compared to those from sham animals (P < 0.05, n = 4). The peak amplitude of the isolated NMDA-mediated current in slices from injured animals was 15.6 ± 0.7 pA (n = 5), significantly smaller than that recorded in slices from sham mice (24.6 ± 8.1 pA, n = 4, Fig. 2G). By subtracting the NMDA-mediated current from the total glutamate-evoked current, the presumed AMPAergic component can be calculated and it also showed a significant reduction in the peak amplitude of slices from FPI animals (sham = 69.5 ± 5.8 pA, n = 4; FPI = 26.9 ± 1.8 pA, n = 4, Fig. 2H, P < 0.05).
FIGURE 2.
Injury diminishes isolated NMDA receptor potentials and EPSCs following injury. A (sham), B (FPI): Trace of fEPSP, isolated NMDA potential, recording after addition of APV to ensure the waveform is NMDA dependent. Scale bar: 0.5 mV, 10 ms. C: Histogram showing the quantification of the reduction in the peak amplitude of the NMDA potential recorded in slices from FPI mice compared to sham animals (n = 11 and 7 for FPI and sham, respectively, P < 0.05, denoted by *). D (sham), E (FPI): Glutamatergic currents were recorded by focal application of glutamate (100 μM, 50 ms, 40 psi), the NMDA component was isolated with the addition of CNQX to the bath, and at the end of each experiment, APV was perfused to ensure the current is NMDA-dependent. Scale bar: 15 pA, 100 ms. F: The histogram shows FPI causes a significant reduction in the peak amplitude of the NMDA current. G: The histogram is created by subtracting the isolated NMDA-mediated current from the entire glutamate-evoked current to determine the AMPA-mediated current. This shows FPI also causes a significant reduction in the peak amplitude of the AMPA currents. *denotes significance, P < 0.05.
Since the data described above demonstrates that the NMDA receptor is compromised, we hypothesized that altering the ionic composition of the superfusing aCSF to increase calcium flux via compromised NMDA receptors might allow for LTP induction. Therefore, we performed a series of LTP experiments using a modified aCSF with half of the normal concentration of Mg2+ and twice the concentration of Ca2+. However, the modified aCSF did not restore LTP in the FPI slices (Fig. 1C).
The data suggesting that LTD can be induced in slices from injured animals shows that plasticity is possible in mice that have undergone FPI. Thus, one possible hypothesis is that the mechanisms to induce LTP are not altered rather the injury results in saturation of LTP. To investigate this hypothesis, slices from injured animals were first depotentiated (using the LFS protocol used to induce LTD) and then subsequently tetanized with HFS to attempt to induce LTP. Potentiation or a return to baseline was not observed in slices from FPI animals (n = 6, Fig. 1D). This further suggests that regardless of the level of synaptic activity that is used (normal or depressed) the mechanism(s) necessary for long-term synaptic facilitation have been altered by injury.
Dendritic Spine Anatomy
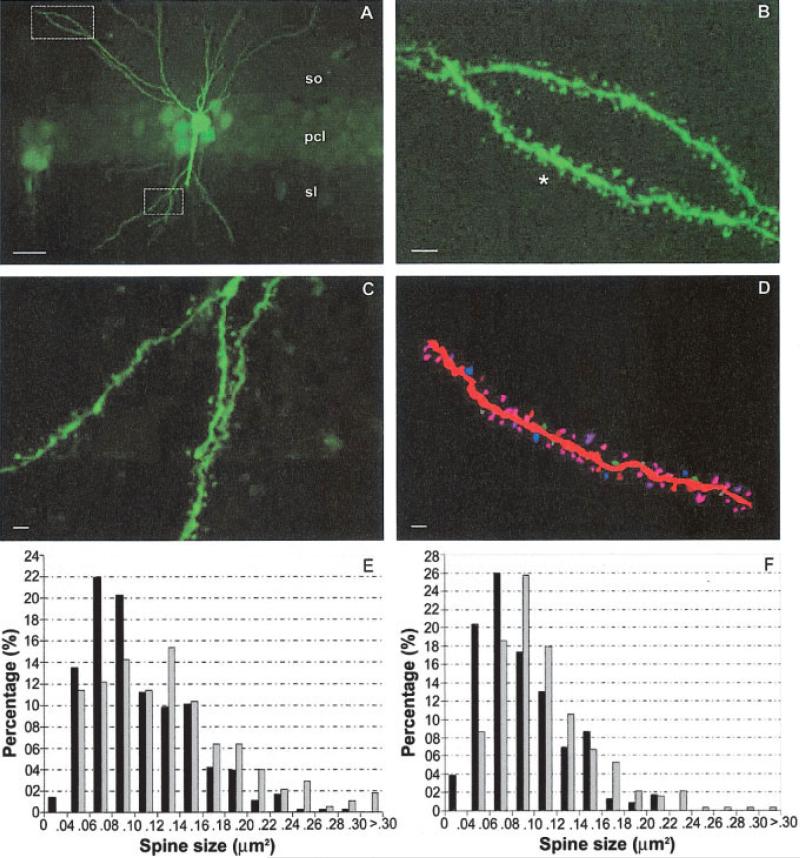
The anatomical substrate for LTP induction and expression is the dendritic spine (Leuner et al., 2003; Segal, 2005), which is highly enriched in α-CaMKII (Erondu and Kennedy, 1985). To assess whether injury alters spine anatomy, apical and basal dendritic spines of Lucifer yellow-loaded CA1 pyramidal neurons (see Materials and Methods for details) from injured and sham animals were visualized (Figs. 3A–D) and quantified. Following FPI, the mean apical dendritic spine size (μm2) significantly increases from 0.106 ± 0.05 to 0.131 ± 0.06, which is a 23.1% increase (P < 0.05). Likewise, the mean basal dendritic spine size (μm2) also significantly increases from 0.089 ± 0.38 to 0.109 ± 0.05, which is a 22.7% increase (P < 0.05, Fig. 3F). When plotting the number of apical and basal dendritic spines of each size following injury, there is a trend for a right-ward shift to larger spine sizes (Figs. 3E,F, respectively). Although there is a significant increase in both apical and basal mean spine size, there was no significant change in spine density between sham and injured groups, either in apical (1.51 ± 0.22 spine per μm vs. 1.60 ± 0.37, P > 0.05) or basal dendrites (1.81 ± 0.4 vs. 1.60 ± 0.44, P > 0.05). Changes in dendritic spine size become important, since the level of calcium required to trigger calcium-mediated biochemical cascades is kept to a minimum by the small spine size (Malenka and Nicoll, 1999; Gazzaley et al., 2002), thus even small increases in spine size will have serious ramifications on the threshold level of calcium and eventually LTP induction.
FIGURE 3.
Increased dendritic spine size in slices from injured mice. A: CA1 pyramidal neuron and its spiny dendrites from an FPI-injured mouse loaded with Lucifer yellow. pcl, pyramidal cell layer; sl, stratum lucidum; so, stratum oriens. Scale bar: 10 μm. B: Higher magnification of area highlighted by box in section A showing fourth order basal dendrite segment spines. Scale bar: 1 μm. C: Higher magnification of area highlighted by box in section A showing second order apical dendrite segment spines. Scale bar: 1 μm. D: Neurolucida drawing of the spiny segment shown in B (noted by asterisk *). Scale bar: 1 μm. E: Comparison of the distribution of dendritic spine size of sham (black) and FPI (gray) apical (E) and basal (F) CA1 pyramidal cells. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
α-CaMKII Protein Expression
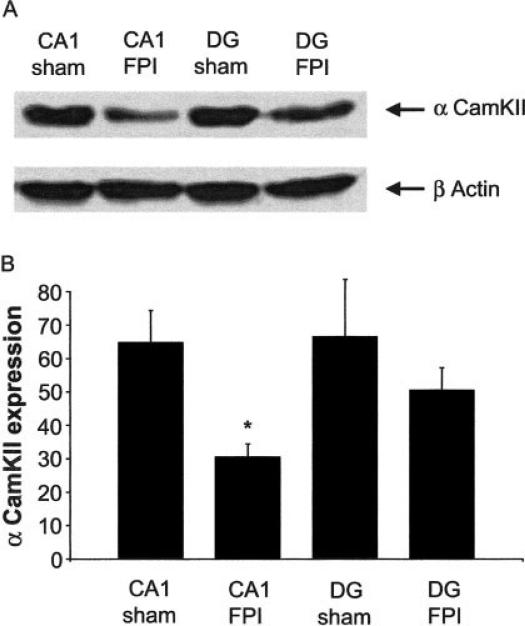
Through the use of inhibitors and genetic deletion studies it has been demonstrated that α-CaMKII is critical for LTP induction (Malenka et al., 1989). Hence, a functional decrease in α-CaMKII after injury could contribute to the inability to induce LTP. Western blot analysis demonstrated a significant decrease (52.6% ± 7.3, n = 3, three groups of pooled tissue, each from five FPI and sham animals, respectively) in the alpha form of CaMKII protein expression in regionally dissected area CA1 tissue from injured animals. In contrast, α-CaMKII expression was not significantly altered in DG from injured animals, demonstrating regional specificity of decreased α-CaMKII expression to area CA1 (Fig. 4).
FIGURE 4.
Decreased α-CaMKII expression following FPI. A: Western blot analysis demonstrating a decrease in α-CaMKII protein expression from tissue regionally dissected from area CA1 (n = 3, each independent sample from tissue pooled from five sham or FPI mice). The blot was reprobed with β-actin to ensure proper loading of the samples. B: Histogram depicting a significant reduction of α-CaMKII protein expression in tissue from FPI mice, which is specific to area CA1.
DISCUSSION
We found that in the mouse, FPI causes an inability to induce area CA1 LTP; however, it does not affect the induction or maintenance of area CA1 LTD. These results are consistent with previous studies in the rat. More importantly, this study extends these findings to provide a basis for the underlying mechanisms of this dysfunction. To this end, the inability to induce and maintain area CA1 LTP may be due to multi-focal alterations in impaired NMDA and AMPA receptor function, increased dendritic spine size, and decreased α-CaMKII protein expression.
LTP induction and maintenance is a complex manifestation involving many important and critical steps. Brain injury itself is a complicated response that could be inhibiting LTP at any of the inducing steps. To originally confirm that it is FPI that is causing the lack of LTP, the induction protocol and methodology was examined. First, to be sure that the tetanic stimulation protocol is simply not producing optimal stimulation for potentiation, several different HFS and TBS paradigms were examined by varying the number of trains, stimulation time length, and interval length. All paradigms, regardless of severity, induced LTP in slices from sham mice but did not elicit LTP in slices from injured mice. In addition, we established that the basal responses from FPI and sham animals were approximately equivalent prior to LTP, thus the inability to induce LTP was not an artifact of decreased synaptic activation observed in slices from injured mice. Scrutinizing the methodology and protocols ensured that it is injury-mediated alterations and not a methodological anomaly causing the lack of LTP.
Our results together with other studies indicate a loss of post-tetanic potentiation (PTP) along with the injury-induced inability to induce LTP (Sandkuhler and Liu, 1998; Sick et al., 1998 ; Li et al., 2005). As mentioned in the Results section, PTP is thought to be due to an increase in quantal content because of residual calcium build up. Interestingly, injury did not alter paired-pulse facilitation in slices from FPI animals compared to values recorded in slices from sham animals. Additionally, a comparable number of presynaptic axons are stimulated as fiber volley amplitude is not significantly different in slices from sham and FPI animals (data not shown). These findings are relatively consistent with data from Reeves et al. (2000) that show 7 days after FPI, there are not significant differences in the fiber volley amplitude. This study further demonstrated that 7 days postinjury, paired-pulse responses in injured rats were only elevated at the 50-ms interpulse interval, which was used in our study. Methodological concerns might underlie this discrepancy as Reeves et al. (2000) used rats and recorded at 34°C, whereas our study used mice and recorded at room temperature. We hypothesize that the observed lack of PTP 7 days after injury is most likely NMDA-dependent mediated postsynaptically due to the slow sampling rate during the LTP experiments (Sastry et al., 1986; Hanse and Gustafsson, 1992; Malenka and Nicoll, 1993) and due to diminished NMDA receptor function. Further experiments investigating putative injury-induced presynaptic alterations are currently being undertaken.
Activation of the NMDA receptor is a critical component in the induction of LTP (Madison et al., 1991; Malenka and Nicoll, 1993). By isolating NMDA potentials and NMDA-mediated currents, we found an injury-induced decrease in both the NMDA potential and current amplitude, indicating that the NMDA receptor is compromised. This provides an initial site for the inability to induce LTP after FPI. Furthermore, our data corresponds with molecular biology data from Osteen et al. (2004) that shows a reduction in the NR2A and NR2B subunits of the NMDA receptor (Osteen et al., 2004). Current reports suggest that both the NR2A and NR2B subunits play a role in LTP (Wong et al., 2004), however the NR2A subunit is not obligatory for LTP (Weitlauf et al., 2005). To attempt to offset the injury-induced alteration in NMDA receptor function, LTP experiments were conducted in modified aCSF favoring calcium influx by doubling the Ca2+ concentration and halving the Mg2+ concentration. However, this manipulation did not rescue LTP. These data raise three possible hypotheses. First, the compromised NMDA receptor function is not the only causal mechanism for the lack of LTP. Secondly, that changing the ionic composition of the aCSF is not sufficient to offset NMDA receptor dysfunction. Finally, by measuring glutamate-evoked currents, we found that not only was the NMDA component diminished after injury, but when subtracting this component from the total excitatory current, we found a significant decrease in the presumed AMPA-mediated current. The diminished function may result in insufficient membrane depolarization during the tetanus that would relieve external Mg2+ block, allowing NMDA receptor activation and Ca2+ influx leading to LTP induction. Even though NMDA potentials and currents are diminished after injury, it is still feasible that the injury itself causes a saturation-like phenomenon of the evoked excitatory synaptic response. This was tested and subsequently disproved by first depotentiating area CA1 synapses by LFS and then tetanizing the same synapses with HFS. If injury was causing a saturation-like phenomenon, then some degree of facilitation in the depotentiated synapses should occur. Not only was no facilitation observed but even a return to pre-LFS baseline did not occur demonstrating instead that synapses remained depressed. This provides further evidence that the mechanism(s) to induce synaptic facilitation is altered after injury.
The dendritic spine is the anatomical substrate for LTP induction and expression (Leuner et al., 2003; Segal, 2005). Therefore, alterations in spine morphology could provide mechanisms for modifying synaptic strength (Patel et al., 1988; O'Malley et al., 2000; Leuner et al., 2003; Neigh et al., 2004). We report here for the first time that FPI leads to an increase in apical and basal dendritic spine size. The increase in spine size is likely due to severance of axons caused by diffuse axonal damage (Povlishock et al., 1992). Previous reports suggest that a decrease in axonal input will lead to the compensatory mechanism of enlarged dendritic spines (Kirov and Harris, 1999; Fiala et al., 2002). The diffusional equilibration of calcium across the spine varies according to spine morphology (Sabatini et al., 2002), thus injury-induced increased spine size may lead to subthreshold calcium levels within the spine causing an inability to induce LTP. Another hypothesis is that an increased postsynaptic membrane area means there is more capacitance, thus more current is needed to charge the membrane to the necessary depolarized level. As stated above, diminished NMDA receptor function may not be sufficient to depolarize this increased area. Since, it is known that synaptic activation can produce rapid input-specific changes in dendritic structure (Harris and Stevens, 1989; Maletic-Savatic et al., 1999), a final supposition is that injury leads to a compensatory spine growth reaction. If TBI results in chemical processes that mimic those of LTP, thus acting to saturate excitatory transmission (Sick et al., 1998), this increased synaptic strength would be accompanied by increased spine shape. In this case, it's possible that instead of inducing LTD in the FPI slices, we are actually observing depotentiation. However, we find this unlikely since FPI results in area CA1 decreased excitability (Reeves et al., 1997; Witgen et al., 2005). If all the synapses were enhanced by FPI-like-LTP, then a decrease in regional excitability should not occur.
Activation of a-CaMKII and several other protein kinases (PKA, MAPK, and Src) have been proven to be important to induction and maintenance of LTP (Lledo et al., 1995; Malenka and Nicoll, 1999). Our studies focused on α-CaMKII because the evidence supporting its critical role in LTP is much stronger than for the other kinases (Malenka et al., 1989). Evaluation of the α-CaMKII protein expression using Western blot analysis reveals decreased expression 7 days following injury. These data support the hypothesis that FPI is disrupting the second messenger cascade critical for LTP induction. Altered α-CaMKII expression and spine morphology can both result in changes in excitatory receptor anchoring and positioning. Thus, the underlying causal mechanism for injury-induced inability to generate LTP can be due to altering dendritic spine signal transduction dynamics.
In summary, this study reveals that LTP can not be induced in the ipsilateral hippocampus in mice 7 days following lateral FPI. Our findings present three novel injury-induced multi focal alterations contributing to this dysfunction: (1) diminished NMDA and AMPA receptor function, (2) an increase in dendrite spine size, and (3) a reduction in α-CaMKII expression that culminate in a synaptic plasticity dysfunction. Notably, this is not a transient increase due to injury-induced brain swelling, since it is observed 7 days after injury. The inability to induce and maintain LTP following injury might contribute to cognitive impairments following injury and our results provide promising sites for the development of therapeutic intervention to improve the outcome of TBI patients.
Acknowledgments
We thank Drs. E.J. Oh, B.J. Pfister, and W.C. Abraham for constructive suggestions on previous drafts of the manuscript.
Grant sponsor: NIH/NINDS; Grant number: NS45975 (ASC).
REFERENCES
- Barrett EF, Stevens CF. Quantal independence and uniformity of presynaptic release kinetics at the frog neuromuscular junction. J Physiol. 1972;227:665–689. doi: 10.1113/jphysiol.1972.sp010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth JT, Macciocchi SN, Giordani B, Rimel R, Jane JA, Boll TJ. Neuropsychological sequelae of minor head injury. Neuro-surgery. 1983;13:529–533. doi: 10.1227/00006123-198311000-00008. [DOI] [PubMed] [Google Scholar]
- Bear MF, Abraham WC. Long-term depression in hippocampus. Annu Rev Neurosci. 1996;19:437–462. doi: 10.1146/annurev.ne.19.030196.002253. [DOI] [PubMed] [Google Scholar]
- Bennett-Levy JM. Long-term effects of severe closed head injury on memory: Evidence from a consecutive series of young adults. Acta Neurol Scand. 1984;70:285–298. doi: 10.1111/j.1600-0404.1984.tb00826.x. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Abraham WC. Facilitation of long-term potentiation by prior activation of metabotropic glutamate receptors. J Neurophysiol. 1996;76:953–962. doi: 10.1152/jn.1996.76.2.953. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Lin DD, Coulter DA. Protracted postnatal development of inhibitory synaptic transmission in rat hippocampal area CA1 neurons. J Neurophysiol. 2000;84:2465–2476. doi: 10.1152/jn.2000.84.5.2465. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio R, Maris DO, Grady MS, Winn HR, Janigro D. Selective loss of hippocampal long-term potentiation, but not depression, following fluid percussion injury. Brain Res. 1998;786:64–79. doi: 10.1016/s0006-8993(97)01412-1. [DOI] [PubMed] [Google Scholar]
- Del Castillo J, Katz B. Action, and spontaneous release, of acetylcholine at an inexcitable nerve-muscle junction. J Physiol. 1954;126:27P. [PubMed] [Google Scholar]
- Erondu N, Kennedy M. Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J Neurosci. 1985;5:3270–3277. doi: 10.1523/JNEUROSCI.05-12-03270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala JC, Spacek J, Harris KM. Dendritic spine pathology: Cause or consequence of neurological disorders? Brain Res Brain Res Rev. 2002;39:29–54. doi: 10.1016/s0165-0173(02)00158-3. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Kay S, Benson DL. Dendritic spine plasticity in hippocampus. Neuroscience. 2002;111:853–862. doi: 10.1016/s0306-4522(02)00021-0. [DOI] [PubMed] [Google Scholar]
- Grady MS, Charleston JS, Maris D, Witgen BM, Lifshitz J. Neuronal and glial cell number in the hippocampus after experimental traumatic brain injury: Analysis by stereological estimation. J Neurotrauma. 2003;20:929–941. doi: 10.1089/089771503770195786. [DOI] [PubMed] [Google Scholar]
- Hanse E, Gustafsson B. Postsynaptic, but not presynaptic, activity controls the early time course of long-term potentiation in the dentate gyrus. J Neurosci. 1992;12:3226–3240. doi: 10.1523/JNEUROSCI.12-08-03226.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Stevens JK. Dendritic spines of CA1 pyramidal cells in the rat hippocampus: Serial electron microscopy with reference to their biophysical characteristics. J Neurosci. 1989;9:2982–2997. doi: 10.1523/JNEUROSCI.09-08-02982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess G, Kuhnt U. Presynaptic calcium transients evoked by paired-pulse stimulation in the hippocampal slice. Neuroreport. 1992;3:361–364. doi: 10.1097/00001756-199204000-00018. [DOI] [PubMed] [Google Scholar]
- Hirst GD, Redman SJ, Wong K. Post-tetanic potentiation and facilitation of synaptic potentials evoked in cat spinal motoneur-ones. J Physiol. 1981;321:97–109. doi: 10.1113/jphysiol.1981.sp013973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov SA, Harris KM. Dendrites are more spiny on mature hippocampal neurons when synapses are inactivated. Nat Neurosci. 1999;2:878–883. doi: 10.1038/13178. [DOI] [PubMed] [Google Scholar]
- Kotapka MJ, Gennarelli TA, Graham DI, Adams JH, Thibault LE, Ross DT, Ford I. Selective vulnerability of hippocampal neurons in acceleration-induced experimental head injury. J Neuro-trauma. 1991;8:247–258. doi: 10.1089/neu.1991.8.247. [DOI] [PubMed] [Google Scholar]
- Kotapka MJ, Graham DI, Adams JH, Gennarelli TA. Hippo-campal pathology in fatal non-missile human head injury. Acta Neuropathol (Berl) 1992;83:530–534. doi: 10.1007/BF00310031. [DOI] [PubMed] [Google Scholar]
- Leuner B, Falduto J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. J Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Maier DL, Cross B, Doherty JJ, Christian EP. Fimbriafornix lesions compromise the induction of long-term potentiation at the Schaffer collateral-CA1 synapse in the rat in vivo. J Neurophysiol. 2005;93:3001–3006. doi: 10.1152/jn.00546.2004. [DOI] [PubMed] [Google Scholar]
- Liu G. Local structural balance and functional interaction of excitatory and inhibitory synapses in hippocampal dendrites. Nat Neurosci. 2004;7:373–379. doi: 10.1038/nn1206. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Hjelmstad GO, Mukherji S, Soderling TR, Malenka RC, Nicoll RA. Calcium/calmodulin-dependent kinase II and long-term potentiation enhance synaptic transmission by the same mechanism. Proc Natl Acad Sci USA. 1995;92:11175–11179. doi: 10.1073/pnas.92.24.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein DH, Thomas MJ, Smith DH, McIntosh TK. Selective vulnerability of dentate hilar neurons following traumatic brain injury: A potential mechanistic link between head trauma and disorders of the hippocampus. J Neurosci. 1992;12:4846–4853. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyeth BG, Jenkins LW, Hamm RJ, Dixon CE, Phillips LL, Clifton GL, Young HF, Hayes RL. Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res. 1990;526:249–258. doi: 10.1016/0006-8993(90)91229-a. [DOI] [PubMed] [Google Scholar]
- Madison DV, Malenka RC, Nicoll RA. Mechanisms underlying long-term potentiation of synaptic transmission. Annu Rev Neurosci. 1991;14:379–397. doi: 10.1146/annurev.ne.14.030191.002115. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. NMDA-receptor-dependent synaptic plasticity: Multiple forms and mechanisms. Trends Neurosci. 1993;16:521–527. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation—a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Kauer JA, Perkel DJ, Mauk MD, Kelly PT, Nicoll RA, Waxham MN. An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature. 1989;340:554–557. doi: 10.1038/340554a0. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- Martin AR, Pilar G. Presynaptic and post-synaptic events during post-tetanic potentiation and facilitation in the avian ciliary ganglion. J Physiol. 1964;175:17–30. doi: 10.1113/jphysiol.1964.sp007500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias JL, Beall JA, Bigler ED. Neuropsychological and information processing deficits following mild traumatic brain injury. J Int Neuropsychol Soc. 2004;10:286–297. doi: 10.1017/S1355617704102117. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- McAllister TW. Neuropsychiatric sequelae of head injuries. Psychiatr Clin North Am. 1992;15:395–413. [PubMed] [Google Scholar]
- Neigh GN, Glasper ER, Kofler J, Traystman RJ, Mervis RF, Bachstetter A, DeVries AC. Cardiac arrest with cardiopulmonary resuscitation reduces dendritic spine density in CA1 pyramidal cells and selectively alters acquisition of spatial memory. Eur J Neurosci. 2004;20:1865–1872. doi: 10.1111/j.1460-9568.2004.03649.x. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature. 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- Nimmo AJ, Cernak I, Heath DL, Hu X, Bennett CJ, Vink R. Neurogenic inflammation is associated with development of edema and functional deficits following traumatic brain injury in rats. Neuropeptides. 2004;38:40–47. doi: 10.1016/j.npep.2003.12.003. [DOI] [PubMed] [Google Scholar]
- O'Malley A, O'Connell C, Murphy KJ, Regan CM. Transient spine density increases in the mid-molecular layer of hippocampal dentate gyrus accompany consolidation of a spatial learning task in the rodent. Neuroscience. 2000;99:229–232. doi: 10.1016/s0306-4522(00)00182-2. [DOI] [PubMed] [Google Scholar]
- Osteen CL, Giza CC, Hovda DA. Injury-induced alterations in N-methyl-D-aspartate receptor subunit composition contribute to prolonged 45calcium accumulation following lateral fluid percussion. Neuroscience. 2004;128:305–322. doi: 10.1016/j.neuroscience.2004.06.034. [DOI] [PubMed] [Google Scholar]
- Patel SN, Rose SP, Stewart MG. Training induced dendritic spine density changes are specifically related to memory formation processes in the chick, Gallus domesticus. Brain Res. 1988;463:168–173. doi: 10.1016/0006-8993(88)90542-2. [DOI] [PubMed] [Google Scholar]
- Povlishock JT, Erb DE, Astruc J. Axonal response to traumatic brain injury: Reactive axonal change, deafferentation, and neuro-plasticity. J Neurotrauma. 1992;9(Suppl 1):S189–200. [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial pre-frontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Reeves TM, Lyeth BG, Povlishock JT. Long-term potentiation deficits and excitability changes following traumatic brain injury. Exp Brain Res. 1995;106:248–256. doi: 10.1007/BF00241120. [DOI] [PubMed] [Google Scholar]
- Reeves TM, Lyeth BG, Phillips LL, Hamm RJ, Povlishock JT. The effects of traumatic brain injury on inhibition in the hippo-campus and dentate gyrus. Brain Res. 1997;757:119–132. doi: 10.1016/s0006-8993(97)00170-4. [DOI] [PubMed] [Google Scholar]
- Reeves TM, Kao CQ, Phillips LL, Bullock MR, Povlishock JT. Presynaptic excitability changes following traumatic brain injury in the rat. J Neurosci Res. 2000;60:370–379. doi: 10.1002/(SICI)1097-4547(20000501)60:3<370::AID-JNR12>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Report of the 1998 consensus conference on neuroblastoma screening. Med Pediatr Oncol. 1999;33:357–359. [PubMed] [Google Scholar]
- Sabatini BL, Oertner TG, Svoboda K. The life cycle of Ca(2+) ions in dendritic spines. Neuron. 2002;33:439–452. doi: 10.1016/s0896-6273(02)00573-1. [DOI] [PubMed] [Google Scholar]
- Sanders MJ, Sick TJ, Perez-Pinzon MA, Dietrich WD, Green EJ. Chronic failure in the maintenance of long-term potentiation following fluid percussion injury in the rat. Brain Res. 2000;861:69–76. doi: 10.1016/s0006-8993(00)01986-7. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J, Liu X. Induction of long term potentiation at spinal synapses by noxious stimulation or nerve injury. Eur J Neurosci. 1998;10:2476–2480. doi: 10.1046/j.1460-9568.1998.00278.x. [DOI] [PubMed] [Google Scholar]
- Santhakumar V, Bender R, Frotscher M, Ross ST, Hollrigel GS, Toth Z, Soltesz I. Granule cell hyperexcitability in the early post-traumatic rat dentate gyrus: The ‘irritable mossy cell’ hypothesis. J Physiol. 2000;524(Part 1):117–134. doi: 10.1111/j.1469-7793.2000.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar V, Ratzliff AD, Jeng J, Toth Z, Soltesz I. Long-term hyperexcitability in the hippocampus after experimental head trauma. Ann Neurol. 2001;50:708–717. doi: 10.1002/ana.1230. [DOI] [PubMed] [Google Scholar]
- Sastry BR, Goh JW, Auyeung A. Associative induction of post-tetanic and long-term potentiation in CA1 neurons of rat hippo-campus. Science. 1986;232:988–990. doi: 10.1126/science.3010459. [DOI] [PubMed] [Google Scholar]
- Segal M. Dendritic spines and long-term plasticity. Nat Rev Neurosci. 2005;6:277–284. doi: 10.1038/nrn1649. [DOI] [PubMed] [Google Scholar]
- Sick TJ, Perez-Pinzon MA, Feng ZZ. Impaired expression of long-term potentiation in hippocampal slices 4 and 48 h following mild fluid-percussion brain injury in vivo. Brain Res. 1998;785:287–292. doi: 10.1016/s0006-8993(97)01418-2. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Dodt HU, Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflugers Arch. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Toth Z, Hollrigel GS, Gorcs T, Soltesz I. Instantaneous perturbation of dentate interneuronal networks by a pressure wave-transient delivered to the neocortex. J Neurosci. 1997;17:8106–8117. doi: 10.1523/JNEUROSCI.17-21-08106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitlauf C, Honse Y, Auberson YP, Mishina M, Lovinger DM, Winder DG. Activation of NR2A-containing NMDA receptors is not obligatory for NMDA receptor-dependent long-term potentiation. J Neurosci. 2005;25:8386–8390. doi: 10.1523/JNEUROSCI.2388-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witgen BM, Lifshitz J, Smith ML, Schwarzbach E, Liang SL, Grady MS, Cohen AS. Regional hippocampal alteration associated with cognitive deficit following experimental brain injury: A systems, network and cellular evaluation. Neuroscience. 2005;133:1–15. doi: 10.1016/j.neuroscience.2005.01.052. [DOI] [PubMed] [Google Scholar]
- Wong TP, Liu L, Sheng M, Wang YT. Response to comment on “role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity.”. Science. 2004;305:1912. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]