Abstract
Sleep disorders are highly prevalent in patients with traumatic brain injury (TBI) and can significantly impair cognitive rehabilitation. No proven therapies exist to mitigate the neurocognitive consequences of TBI. We show that mild brain injury in mice causes a persistent inability to maintain wakefulness and decreases orexin neuron activation during wakefulness. We gave mice a dietary supplement of branched-chain amino acids (BCAAs), precursors for de novo glutamate synthesis in the brain. BCAA therapy reinstated activation of orexin neurons and improved wake deficits in mice with mild brain injury. Our data suggest that dietary BCAA intervention, acting in part through orexin, can ameliorate injury-induced sleep disturbances and may facilitate cognitive rehabilitation after brain injury.
INTRODUCTION
Recent data from the U.S. Centers for Disease Control estimate that traumatic brain injury (TBI) afflicts nearly 2 million people in the nation annually and is a major cause of disability in all age groups. Sleep disorders are highly prevalent in patients with TBI (1, 2). In fact, sleep disturbances have been reported in up to 72% of patients with TBI (including mild TBI) up to 3 years after injury (3, 4). TBI patients with sleep disturbances have longer inpatient hospital stays, a higher cost of rehabilitation, and a higher rate of functional disability (5, 6). Moreover, sleep disruption impairs attention and memory formation and exacerbates cognitive deficits in TBI (7–9). Seventy-five percent of reported TBI cases are mild in nature (that is, concussion), but even concussion can have chronic neurological sequelae, including cognitive, motor, and sleep problems (10, 11).
Thus far, most studies on animal models of TBI have focused on either primary histological outcomes or cognitive and motor deficits in the acute setting after injury (12). To date, no animal models have rigorously described activity or sleep-wake patterns in the chronic setting after brain injury. Even more pressing, there are no proven therapies to mitigate or prevent the neurocognitive and neurobehavioral consequences of TBI (13). Therefore, there is an imminent need to better understand chronic sleep disturbances in brain injury and to identify new therapeutic options.
We applied a variety of established activity and behavioral state assays to define sleep-wake disturbances in an animal model of mild TBI using fluid percussion injury (FPI) in mice. To accomplish this, we first monitored long-term activity using a previously validated locomotor beam break assay (14). To further investigate changes in activity profiles, we next performed electroencephalography (EEG)/electromyography (EMG) recordings in freely behaving mice, which allowed assessment of non–rapid eye movement (NREM) sleep, rapid eye movement (REM) sleep, and wake states, as well as power spectral analyses. To investigate the mechanisms underlying injury-induced sleep disturbances, we initially focused on the neuropeptide orexin (also known as hypocretin), which is involved in maintaining wakefulness (15). To test the hypothesis that mild TBI causes dysfunction of the orexin system, we examined orexin neuron activation in response to a period of sustained wakefulness. We next hypothesized that dietary branched-chain amino acid (BCAA) supplementation could alleviate injury-induced deficits in wakefulness. We have previously shown that BCAAs restore network excitability in the hippocampus after mild TBI (16, 17). Dietary amino acids have been shown to act directly on orexin neurons to modulate membrane excitability (18). We gave mice BCAA supplementation in the drinking water, which improved both orexin neuron activation and injury-induced sleep disturbances, indicating that BCAAs can restore wakefulness, at least in part, by activating orexinergic neurons.
RESULTS
TBI causes subtle yet persistent alterations in activity over 30 days
A widely accepted, commonly used mouse model of mild TBI is the lateral FPI model (19, 20). This experimental method provides a highly reproducible, closed head injury that recapitulates many key features of human TBI including memory deficits, gliosis, and electro-physiological perturbation (19, 20).
Mice were randomized to either FPI or sham surgery and subjected to extensive locomotor activity monitoring from 3 to 34 days. The experimental timeline denoting the predetermined time blocks is shown in Fig. 1A, and consists of the immediate postoperative (days 0 to 4), acute (days 5 to 14), subacute (days 15 to 24), and chronic (days 25 to 34) periods after injury or sham surgery. Activity patterns were analyzed for each of the time blocks. We applied our previously established algorithm in which 40 s of continuous inactivity was shown to be highly predictive of sleep; therefore, an inactive bout was counted as sleep only when mice remained still for greater than 40 continuous seconds (14).
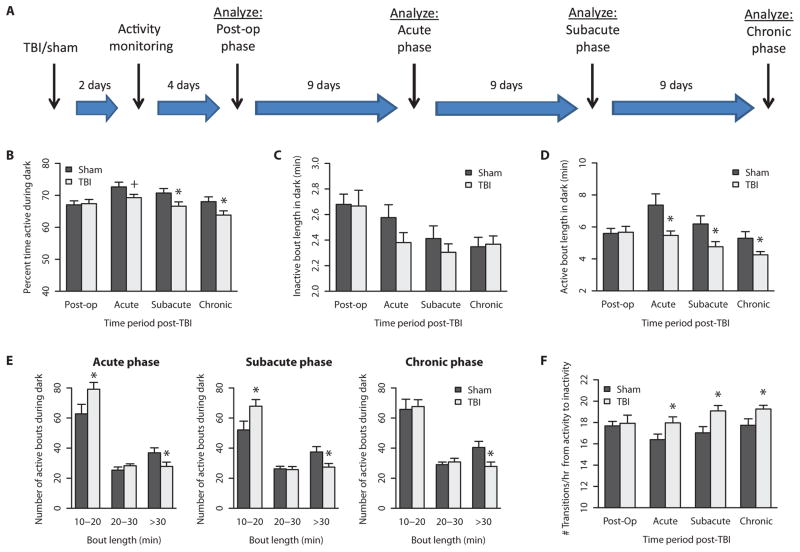
Fig. 1. Mild TBI decreased overall activity and fragmented activity patterns during dark phase.
(A) Activity monitoring timeline. Activity patterns were binned into post-op, acute, subacute, and chronic time points after injury (n = 18) or sham (n = 12) surgery. (B) The percent time spent active during the dark phase was decreased across acute, subacute, and chronic periods after TBI. (C) The average length of each inactivity bout did not significantly differ between groups. (D) However, the average length of each activity bout was significantly decreased after TBI across the three time points. (E) Active bouts longer than 30 min were particularly affected after TBI across all three phases. (F) The number of transitions from active to inactive bouts was significantly increased after TBI across the three time points. +P < 0.1, *P < 0.05, Student’s two-tailed independent t tests. See table S1 for detailed statistics.
During the dark phase (7:00 p.m. to 7:00 a.m.), when mice are typically more active, injured mice were significantly less active compared to sham controls during the subacute and chronic phases (Fig. 1B; t = 2.122 and 2.079, respectively; P < 0.05; see table S1 for detailed statistics). The average length of time spent continuously inactive, or the inactive bout length, did not significantly differ between groups (Fig. 1C and table S1). However, the average length of time spent continuously active, or active bout length, was significantly shorter in TBI mice compared to controls (Fig. 1D; t = 2.508, 2.352, and 2.216; P < 0.05; table S1). This suggests that mild TBI decreases total activity, that is, wakefulness, by causing shorter bouts of continuous activity.
TBI and sham mice did not differ in total amount of activity or in average active/inactive bout lengths during the light phase (7:00 a.m. to 7:00 p.m.), when mice are typically less active (fig. S1, A to C; see table S1 for detailed statistics).
To further dissect the nature of the shortened activity bouts after TBI, we examined the frequency of bouts of varying duration, ranging from 10 to 20 min, 20 to 30 min, and greater than 30 min in length during the dark phase. Whereas the vast majority of bout durations are less than 10 min in length for both groups of mice, TBI mice have significantly more bouts in the range of 10 to 20 min and significantly fewer bouts greater than 30 min in length compared to sham controls, throughout the acute, subacute, and chronic time blocks (Fig. 1E; t = −2.085 and −2.140 for 10- to 20-min bouts, and t = 2.077, 2.344, and 2.533 for >30-min bouts, respectively; P < 0.05; see table S1 for detailed statistics). TBI and sham mice did not show the same magnitude of activity differences during the light phase, compared to the dark phase, but the pattern of decreased and shortened activity bouts was still evident in brain-injured mice (fig. S1D and table S1). The total number of transitions between continuous active and inactive bouts was significantly increased after TBI across the three time blocks (Fig. 1F; t = −2.044, −2.662, −2.179; P < 0.05; see table S1 for detailed statistics). This indicates that there is marked fragmentation of activity bouts in injured mice.
To investigate whether diurnal rhythms were affected in TBI mice, we plotted actograms over the entirety of the 30 days of activity monitoring period. Sample actograms are shown in fig. S2. Gross diurnal activity rhythms were intact in both sham and TBI mice, consistent with the phenotype of an intact circadian clock. This suggests that TBI affects activity mechanisms downstream of the clock.
Together, the locomotor activity monitoring data demonstrate that a single episode of mild injury causes persistent alterations in activity lasting at least 30 days. Given that our previously described algorithm shows that locomotor activity is highly predictive of behavioral state, these data suggest that there may be injury-induced alterations in sleep and wakefulness (14). In particular, brain-injured mice show greater differences during the dark phase, when mice are typically more awake; therefore, we hypothesized that there would be specific deficits in the ability to sustain wakefulness.
TBI causes an inability to sustain wakefulness
To investigate the behavioral mechanisms underlying injury-induced decreases in activity, we implanted the mice with chronic indwelling EEG/EMG electrodes. Behavioral states were analyzed over a 5-day period after an initial 7-day recovery period from surgery (Fig. 2A). Because FPI causes changes in brain network excitability, which are normalized by administration of dietary BCAAs, we performed EEG/EMG recordings on a separate cohort of injured mice on BCAA therapy (16). We hypothesized that deficits in sleep-wake network excitability would be ameliorated by this dietary supplement in the drinking water.
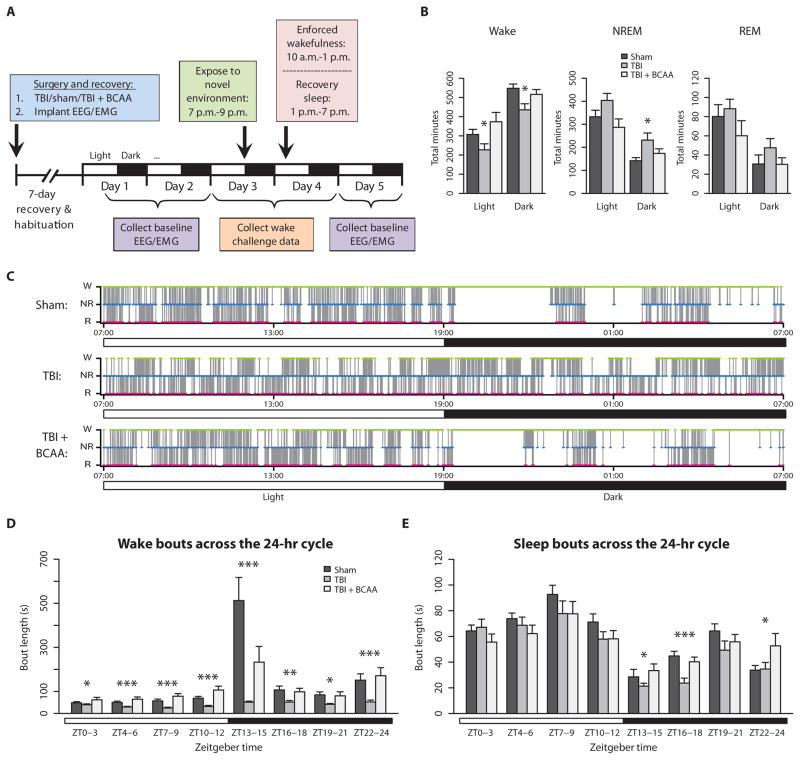
Fig. 2. Baseline EEG recording over 24 hours showed alterations in wake/sleep patterns, which were ameliorated by BCAA treatment.
(A) Experimental timeline for EEG/EMG recording. (B) TBI mice (n = 6) spent less time awake and more time in NREM sleep compared to sham mice (n = 7) and TBI + BCAA mice (n = 6). (C) Representative hypnograms from sham, TBI, and TBI + BCAA mice. Wake, green; NREM, blue; and REM, red. Note the absence of the long wake period (green) at 7:00 p.m. (lights off) in the TBI mouse, which was restored in the TBI + BCAA mouse. (D) Distribution of wake bout length over the circadian cycle showed that TBI significantly shortened wake bouts throughout the light and dark phases, and the normal diurnal fluctuation in wake bout length was abolished. (E) Distribution of sleep (NREM + REM) bout length over the circadian cycle showed significantly shorter sleep bouts in TBI mice during the dark phase. ZT0–3 = 7:00 a.m. to 10:00 a.m., ZT13–15 = 7:00 p.m. to 10:00 p.m., and so forth. *P < 0.05, **P < 0.01, ***P < 0.001, one-way analysis of variance (ANOVA) with Dunnett’s post hoc test. See table S2 for detailed statistics.
Baseline recordings consisted of 24-hour periods from 7:00 a.m. to 7:00 a.m. the next day. During baseline conditions, TBI mice spent significantly less time awake over both the light and dark phases, and more time in NREM sleep during the dark phase compared to sham controls (Fig. 2B; F = 3.653, 4.692, and 4.596, respectively; P < 0.05; see table S2 for detailed statistics). TBI mice treated with BCAAs showed a partial reversal of changes in wake and NREM states. These results suggest that the decrease in activity measured by locomotor monitoring is explained by reduced wakefulness and increased NREM sleep.
Hypnograms were calculated for each animal by plotting Wake, NREM, and REM stages consecutively epoch by epoch over a 24-hour period, beginning with lights on at 7:00 a.m. Naïve adult C57BL/6 wild-type mice typically showed long bouts of wakefulness during the dark phase, particularly at the start of lights off (21). Sample hypnograms from sham, TBI, and BCAA-treated TBI mice are shown in Fig. 2C. Sham mice maintained long wake bouts at 7:00 p.m. lights off, whereas TBI mice continued to have fragmented wake bouts. BCAA intervention reinstated these prolonged wake episodes.
Average continuous wake bout lengths were quantified over the 24-hour circadian cycle, subdivided into 3-hour bins beginning with lights on at 7:00 a.m. or Zeitgeber time (ZT) 0. TBI mice showed shorter wake bouts across both the light and dark phases compared to sham mice (Fig. 2D; see table S2 for detailed statistics). This was particularly marked in the early part of the lights off period. Injured mice were unable to achieve long bouts of wakefulness compared to sham control mice. BCAA therapy lengthened wake bouts throughout the 24-hour cycle, thereby improving normal maintenance of wakefulness, but did not completely restore the ability to sustain wakefulness to control levels (Fig. 2D).
Average continuous sleep bout lengths were also quantified over the 24-hour circadian cycle, subdivided into 3-hour bins beginning as above. TBI mice had significantly shorter continuous sleep bouts during the dark phase compared to sham mice, and this was partially restored with BCAA intervention, particularly during ZT16–18, or 10:00 p.m. to 1:00 a.m. (Fig. 2E; F = 11.29, P < 0.0001, TBI versus TBI + BCAA P < 0.001; see table S2 for detailed statistics).
Overall, these data indicate that TBI impairs the ability to sustain wakefulness and disrupts the normal diurnal fluctuation seen in wakefulness. The effect was most marked in the early part of the lights off period. BCAA therapy partially reinstated prolonged wake episodes.
TBI causes fragmentation of behavioral states, which is rescued by BCAA therapy
To investigate the transitions between behavioral states in brain injury, we quantified the number of sleep and wake bout switches over the light and dark phases. During the dark phase, the total number of wake-to-sleep transitions (sleep = NREM + REM) was significantly increased in TBI mice compared to sham mice, and BCAA intervention after TBI decreased the number of transitions back to sham control levels (Fig. 3A; F = 3.690, P < 0.05; see table S3 for detailed statistics).
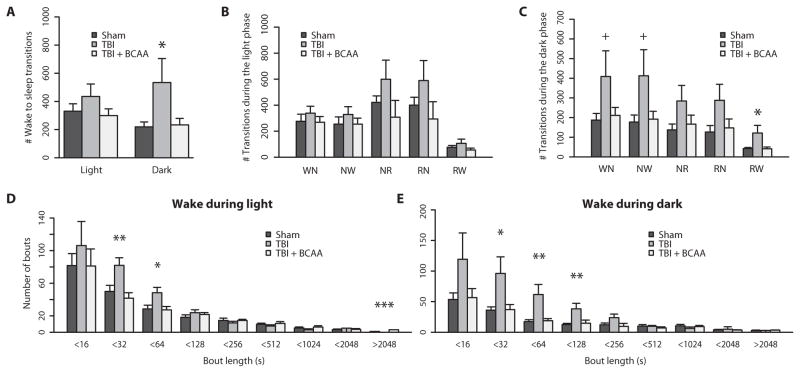
Fig. 3. TBI causes behavioral state instability, which is restored by BCAA therapy.
(A) TBI significantly increased the total number of wake to sleep (NREM + REM) transitions during the dark or active phase. (B) Transitions subcategorized as Wake to NREM (WN), NREM to Wake (NW), NREM to REM (NR), REM to NREM (RN), and REM to Wake (RW) during the light phase. (C) Transition subcategories during the dark phase. TBI mice had more transitions to and from Wake (WN, NW, RW), and this was ameliorated by BCAA therapy. Also, group differences were more robust in the dark phase compared to the light phase. (D and E) TBI mice had significantly more short wake bouts compared to sham mice, and BCAA therapy restored the distribution of long wake bouts. Group differences in wake bout lengths were more robust in the dark phase compared to the light phase. +P < 0.1, *P < 0.05, one-way ANOVA with Dunnett’s post hoc test. See table S3 for detailed statistics.
Transition types were categorized into possible combinations between Wake, NREM, and REM stages and subdivided by light and dark phases. TBI mice had more Wake to NREM, NREM to Wake, and REM to Wake transitions in comparison to sham controls as well as in comparison to TBI mice treated with a BCAA intervention during the dark phase (Fig. 3C and table S3). There were no significant differences between groups for transition subcategories during the light phase, when mice typically spend more time sleeping (Fig. 3B and table S3). This indicates that the transitions that specifically involve the wake state (that is, Wake to NREM, NREM to Wake, and REM to Wake) are most susceptible to injury.
To further characterize the nature of wake transitions, we plotted a histogram of varying wake bout durations (Fig. 3, D and E). During both the light and dark phases, TBI mice had more bouts of shorter duration. In particular, during the dark phase, TBI mice had significantly more bouts of 16 to 31, 32 to 63, and 64 to 127 s in length compared to sham control mice and TBI mice on BCAA therapy (F = 5.135, 7.782, and 6.615; P < 0.05, P < 0.01, and P < 0.01 for respective bout length bins; detailed statistics in table S3).
Bouts were also analyzed in NREM and REM sleep during the light and dark phases (fig. S3, A to D). Whereas the most pronounced group differences were during the wake state, there was a similar, consistent pattern of shorter bouts after injury during NREM and REM states, indicating some degree of sleep fragmentation (fig. S3; detailed statistics in table S3).
Together, these data highlight the severity of fragmentation during wakefulness and, to a lesser degree, also during sleep, induced by mild TBI. Behavioral state fragmentation and excessive daytime sleepiness are phenomena frequently cited in sleep disorders such as narcolepsy and posttraumatic hypersomnia (3, 22–24).
TBI alters the power spectral density, which is mitigated by BCAA therapy
Power spectral analysis is a widely accepted method used for quantification of EEG signals. The power spectral density reflects the distribution of signal power [calculated by fast Fourier transform (FFT) of the polygraphic signal] plotted over specific frequency bins.
Baseline polygraphic signals were analyzed for power spectra in various frequency bands for the different conditions (Fig. 4, figs. S4 and S5, and statistics in tables S4 to S6). Wake spectra for TBI mice were significantly lower at the theta frequency range, in particular at 8 to 9 Hz, compared to sham control mice (Fig. 4A; F = 3.58 and 3.485; P < 0.05 and P < 0.05 for 8 and 9 Hz, respectively; see detailed statistics in table S4). This was also found in NREM and REM spectra for TBI mice (Fig. 4, B and C; F = 6.802 and 7.482; P < 0.01 and P < 0.01 for NREM 8 Hz and NREM 9 Hz, respectively; F = 11.43 and 6.457; P < 0.0001 and P < 0.01 for REM 8 Hz and REM 9 Hz, respectively; table S4). Theta power was restored by BCAA therapy for spectra in the NREM state (table S4).
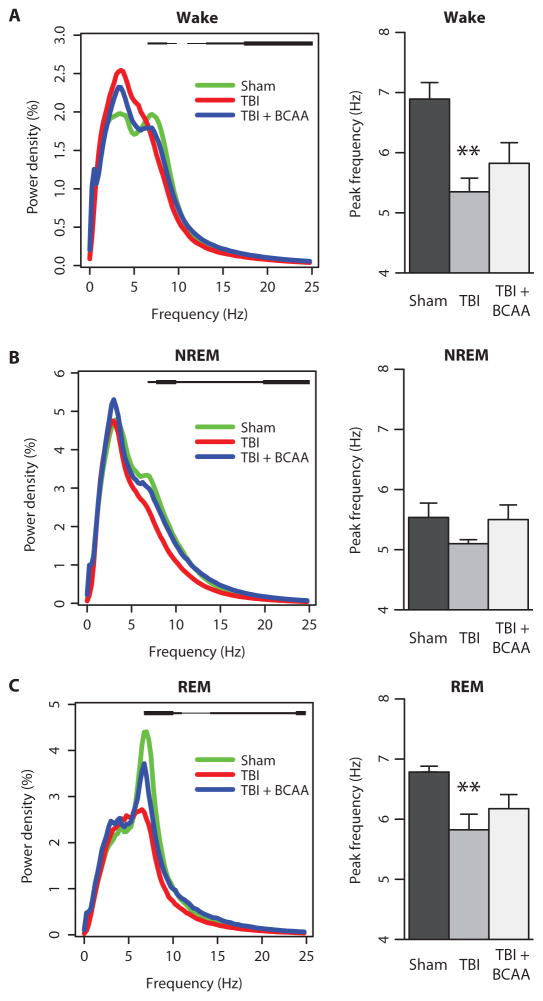
Fig. 4. TBI alters baseline power spectra and theta peak frequency.
(A) Wake power spectra. Statistical group differences denoted as a black bar above the power spectral curves from 7 to 24 Hz. Note the loss of the theta peak (5 to 8 Hz) after TBI. (B) NREM power spectra showing a similar flattening of the theta peak after TBI. Group differences are denoted as a black bar above the power spectral curves from 7 to 24 Hz. (C) REM power spectra showing the decreased theta peak after TBI. Group differences are denoted as a black bar above the power spectral curves ranging from 7 to 24 Hz. Green, sham; red, TBI; blue, TBI + BCAA. Thin black bar, P < 0.10; medium black bar, P < 0.05; thick black bar, P < 0.01. **P < 0.01, one-way ANOVA with Dunnett’s post hoc test. See table S4 for detailed statistics.
Power spectra were further categorized by sleep stage and light/dark phases, which highlight group differences in particular for wake spectra during the dark and NREM/REM spectra during the light (fig. S4; statistics in table S5).
Because we found robust group differences in theta power, we further evaluated this effect by calculating the theta peak frequency for each behavioral state. Theta peak frequency was derived as the frequency value between 5 and 8 Hz with the maximum power density. In both wake and REM states, theta peak frequency for TBI mice was significantly slower compared to that for sham control mice (Fig. 4, A and C; F = 8.139 and 6.928; P < 0.001 and P < 0.01 for wake and REM, respectively; full statistics in table S4). BCAA therapy increased theta peak frequency, albeit not to control levels.
Power density for specific frequency bands was calculated during each behavioral state (fig. S5). Group differences were noted in alpha, beta, and gamma power densities, with injured mice showing a decrease in all three compared to sham mice, with partial improvement in some bands with BCAA therapy (see detailed statistics in table S6).
Together, the power spectral analyses demonstrated that mild TBI produced persistent alterations in EEG rhythms. In particular, injury reduced theta power and shifted the theta peak to slower frequencies. This phenotype was in part ameliorated with BCAA intervention.
TBI causes wake deficits during situations challenging wakefulness, and these are partially restored by BCAA therapy
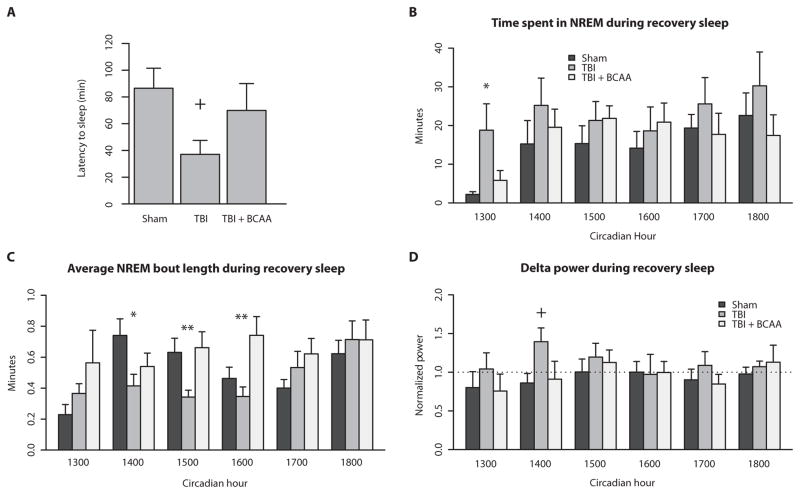
Next, we sought to determine whether injury affects behavioral state in situations that challenge the arousal system. Mice were exposed to a novel environment at the start of the dark phase. The latency to first sleep episode was calculated for the 2-hour behavioral test (Fig. 5A). TBI mice had a shorter latency to sleep compared to sham mice, and this shorter latency was partially restored by BCAA intervention (F = 2.805, P = 0.0923, sham versus TBI P = 0.05; one-way ANOVA with Dunnett’s post hoc test).
Fig. 5. TBI mice exhibit a higher sleep pressure during situations challenging wakefulness.
(A) After being placed in a novel environment, TBI mice showed a shorter latency to the first sleep episode, which was reinstated with BCAA therapy. (B) After a relatively short 3-hour period of sleep deprivation, TBI mice spent more time in NREM sleep during the first hour of the recovery period. BCAA therapy decreased NREM sleep back to sham levels. (C) Despite greater NREM sleep time, average NREM bout length was significantly shorter in TBI compared to sham mice, indicating sleep fragmentation. BCAA therapy lengthened the average NREM bout particularly in hours 3 and 4 of recovery sleep. (D) Delta power, a proxy for sleep pressure, was increased in TBI mice during hour 2 of recovery sleep compared to sham and TBI + BCAA mice. +P < 0.10, *P < 0.05, **P < 0.01, one-way ANOVA with Dunnett’s post hoc test. See table S7 for detailed statistics.
The second wake challenge consisted of examining EEG response to a 3-hour period of enforced wakefulness using sleep deprivation by gentle handling. We performed the wake challenge from 10:00 a.m. to 1:00 p.m., which is a period of high sleep pressure in mice. The 3-hour period was chosen as an abbreviated length of time during which naïve mice typically do not show much sleep rebound, so as to maximize effect size (25). Data from the next 6 hours from 1:00 to 7:00 p.m., or “recovery sleep,” were analyzed for NREM time, NREM bout length, and NREM delta power (Fig. 5, C to D; see table S7 for detailed statistics).
TBI mice spent significantly more time in NREM sleep in the first hour immediately after sleep deprivation compared to sham controls and mice receiving BCAA therapy (Fig. 5B; F = 5.571; P < 0.05; detailed statistics in table S7). Despite having more NREM sleep, TBI mice had significantly shorter NREM bout lengths during circadian hours 1400, 1500, and 1600, indicating the presence of sleep fragmentation (Fig. 5C; F = 3.279, 6.34, and 5.729; P < 0.05, P < 0.001, and P < 0.01, respectively; table S7). Delta power during NREM sleep is widely accepted to be an accurate proxy for sleep pressure after a period of sleep deprivation (25, 26). TBI mice showed more delta power in the second hour during recovery sleep compared to sham and TBI mice treated with BCAAs (Fig. 5D).
Together, these data indicate that mild brain injury imparts an increased pressure to sleep after situations of enforced arousal. However, despite increased sleep pressure, TBI mice were still unable to sustain long NREM sleep bouts. BCAA intervention improved and prolonged wakefulness after mild sleep deprivation.
TBI causes decreased orexin neuron activation, which is rescued by BCAA therapy
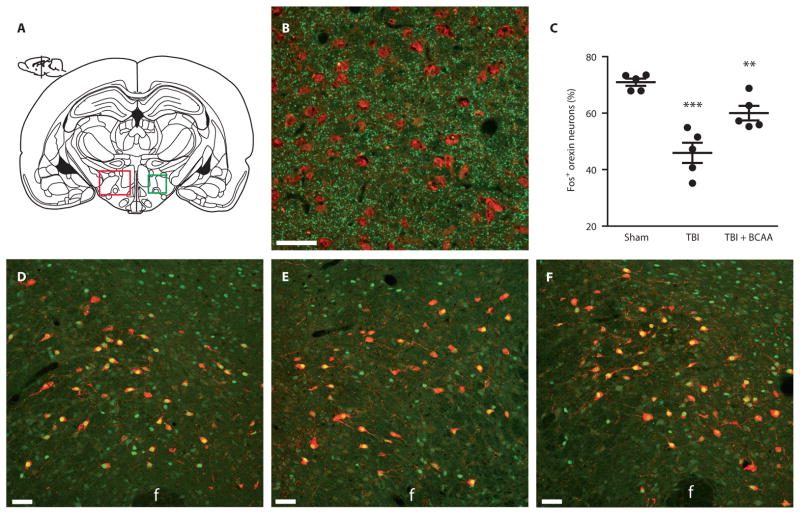
The phenotype of daytime sleepiness and state instability resembled the phenotype caused by orexin dysfunction (21). We therefore hypothesized that orexin dysfunction was potentially contributing to the phenotype of injury-induced sleep disturbances. To test this hypothesis, we examined orexin neuron activation in response to a 3-hour period of enforced wakefulness from 10:00 a.m. to 1:00 p.m.—the same paradigm used in the wake challenge test described above. Mice were at 4 weeks after TBI or sham surgery, and a third group of mice received BCAA dietary supplementation for 4 weeks before sacrifice. We measured orexin neural activation by the presence of the immediate early marker c-Fos protein in the lateral hypothalamus, the region containing orexin neurons along with glutamatergic terminals (Fig. 6, A and B) (27). Compared to the sham and TBI + BCAA groups, TBI mice had significantly fewer activated orexin neurons (Fig. 6C; F = 22.47, P < 0.0001; sham versus TBI P < 0.001, TBI versus TBI + BCAA P < 0.01; one-way ANOVA with Dunnett’s post hoc test). Representative photomicrographs showing orexin and c-Fos colocalization are shown for sham (Fig. 6D), TBI (Fig. 6E), and TBI + BCAA (Fig. 6F) mice. Total orexin neuron numbers were not significantly different between groups, indicating that injury primarily affects orexin physiology rather than gross cell loss per se (fig. S6).
Fig. 6. Orexin neuron activation after 3 hours of enforced wakefulness was decreased after TBI, and was restored with BCAA therapy.
(A) Schematic coronal section of a mouse brain showing the lateral hypothalamus (LH, red box), the region where orexin neurons reside and where orexin cells were counted. The green box represents the area depicted in photomicrographs in (D) to (F). (B) Photomicrograph of the lateral hypothalamus showing presynaptic glutamate vesicles (VGLUT1 immuno-labeling, green) in close proximity to orexin cell bodies (orexin-A immuno-labeling, red) at ×40 magnification. (C) Quantification of orexin neurons expressing c-Fos, a marker of neural activation, after the 3-hour wake challenge. There was decreased orexin activation after TBI compared to sham mice, which was restored with BCAA therapy (F = 22.47, P < 0.0001; TBI versus sham P < 0.001, TBI versus TBI + BCAA P < 0.01; one-way ANOVA followed by Dunnett’s post hoc test; n = 5 per group). (D to F) Representative photomicrographs of the lateral hypothalamus from sham, TBI, and TBI + BCAA mice showing orexin (red) colocalization with c-Fos (green) at ×20 magnification. F, fornix. Scale bars, 50 μm. **P < 0.01, ***P < 0.001.
DISCUSSION
Sleep disturbances, including excessive daytime sleepiness and sleep maintenance insomnia, have been reported as a persistent disabling consequence of mild TBI as part of post-concussive syndrome (3, 4, 24). Early interventions for sleep problems would not only improve quality of life but might also facilitate cognitive and neurobehavioral recovery after brain injury (5, 6). Our data established a mouse model of chronic sleep/wake disturbances after mild TBI, namely, a persistent inability to maintain wakefulness. Here, we extensively characterized locomotor activity and EEG-based behavioral states after a single mild nonpenetrating head injury in mice. We then examined one potential candidate wake-promoting system and demonstrated that orexin neuron activation was decreased in response to a wake stimulus. These data suggest that brain injury alters orexin network excitability, similar to what has been previously described for hippocampal networks after brain injury (16).
No therapies currently exist to treat the neurobehavioral sequelae of TBI. Given that our data show injury-induced deficits in orexin neuron activation, an intervention that could restore orexin network excitability, thereby potentially restoring wakefulness, would be very useful. Indeed, treatment with dietary BCAAs rescued sleep/wake disturbances, including increasing wake time, consolidating sleep and wake bouts, and raising arousal levels to those seen in control mice during situations of heightened wakefulness. BCAA therapy achieved this effect by restoring EEG power spectral peaks, in particular theta power, and reinstating activation of orexin neurons during wakefulness.
In our model, we found that a single mild brain injury caused persistent alterations in locomotor activity patterns, notably for over 4 weeks after injury. This prolonged time course of disease was consistent with sleep and fatigue symptoms reported in the human condition (4, 24). The persistence of symptoms suggested a secondary cascade of effects after injury that chronically altered brain physiology.
A single mild brain injury also caused profound deficits in brain EEG at 2 weeks after injury. Injured mice showed deficits primarily in the dark phase, when mice are typically most active. During the dark phase, injured mice slept more and were unable to sustain long bouts of wakefulness. They had more fragmented behavioral states and lost the normal diurnal fluctuation in wake bout length. This was consistent with seemingly paradoxical complaints of daytime sleepiness and nighttime insomnia frequently reported in both TBI and narcolepsy patients (1, 3, 24, 28). In addition, injured mice were unable to sustain wakefulness in situations requiring increased arousal, such as exposure to a novel environment, and showed increased sleep pressure after these short periods of enforced wakefulness. These data suggest that injured mice had less so-called wake reserve, a concept perhaps similar to “cognitive reserve” in aging and neurodegenerative disease (29). It is possible that the widely reported symptoms of fatigue and inattention described after concussion could, in fact, reflect the same mechanisms underlying decreased wake reserve (30).
After injury, there was an overall shift in EEG power density to slower frequencies during the wake state, in particular for theta power. Slowing of theta peak frequency has been implicated in aging and hippocampal-dependent cognitive deficits (31, 32). The same left shift in power spectrum seen in patients with Alzheimer’s disease is thought to reflect dysfunctional connections among cortical areas (33, 34). Similarities in EEG pathology may reflect shared neuropathological mechanisms between TBI and Alzheimer’s disease. Overall nonspecific EEG slowing during the wake phase could also reflect more “local sleep” or microsleep episodes within epochs that are otherwise scored as wake (35).
Because of similarities in phenotype to human narcolepsy, and human TBI studies implicating dysfunction of the orexin system, we initially chose to investigate alterations in the expression of the neuropeptide orexin (36). Although there are several other candidate wake-promoting regions of the brain (that is, ventral periaqueductal gray, locus coeruleus, basal forebrain, and tuberomammillary nucleus), their roles have not yet been clearly demonstrated in TBI. However, the upstream position of orexin as the master regulator of several other wake-promoting systems makes it likely that other wake systems will be affected after brain injury (36). In support of orexin’s involvement, several human studies have reported deficits in orexin after TBI. One small study of four patients who died 7 to 42 days after severe TBI showed a 27% reduction in the number of orexin neurons compared to non-TBI controls (37). Measured levels of CSF orexin were low in 95% of 44 patients within the first 4 days of moderate to severe TBI (38). We also recently showed that controlled cortical impact acutely decreased brain orexin levels in mice, as measured by intracerebral microdialysis within the first 3 days of injury (39). Here, as well as in our previous study, the total number of orexin neurons was unchanged after injury, in contrast to the findings in the small human study, although this may be explained by differences between mild/moderate and severe TBI (37, 39). Our data indicate that mild/moderate brain injury primarily affects orexin physiology rather than gross cell loss, at least in the immediate weeks after injury.
Although the exact mechanisms of injury-induced wake dysfunction are unknown, it is clear that BCAA intervention restores many aspects of wakefulness, including underlying deficits in EEG oscillations and orexin neuron activation. We have previously shown that BCAA therapy restores network excitability and ameliorates hippocampal-dependent cognitive deficits in a mouse model of mild TBI, possibly by restoring pools of releasable vesicular glutamate and γ-aminobutyric acid (GABA) (16). Indeed, glutamate inputs to orexin neurons regulate wakefulness, and glutamatergic interneurons have been suggested to play a role in a positive feedback recruitment of orexin on orexin neurons (40). However, measurements of glutamate and GABA in injured brain have not been able to distinguish synaptic pools from the much larger, independent metabolic pools of glutamate and GABA (16). Another possible mechanism is that dietary amino acids have been shown to directly affect orexin neuron membrane excitability; this macronutrient sensing mechanism is thought to explain the role of orexin in appetite (18).
BCAAs are essential amino acids and cannot be synthesized de novo, and therefore must be acquired through diet. The three BCAAs (L-leucine, L-valine, and L-isoleucine) are key amino acids involved in de novo glutamate synthesis. About 50% of brain glutamate and 40% of releasable synaptic glutamate contain BCAA-derived nitrogen (41, 42). In a small number of human studies, plasma BCAA concentrations were decreased in mild and severe TBI, and one follow-up study found that intravenous BCAA therapy in severe TBI yielded improvement in disability outcomes (43–45). Dietary BCAA administration has been studied extensively in healthy people and in disease states over many decades (46). Patients with a variety of disorders (including liver cirrhosis, bipolar disorder, and spinocerebellar degeneration) have been treated with BCAA therapy for up to 2 years without adverse effects (47). Overall, BCAA therapy is well tolerated and associated with minimal to no side effects, and therefore is a potentially viable therapy for treating sleep/wake disturbances associated with mild TBI.
Our preclinical mouse model offers a unique opportunity to study the mechanisms underlying sleep disturbances after mild TBI. Our data indicate that injury-induced deficits in orexin neuron activation likely contribute to the persistent inability to maintain wakefulness, and that BCAA dietary intervention represents a promising therapy for treating sleep/wake disturbances after TBI.
MATERIALS AND METHODS
Animals
All experiments were performed on 5- to 7-week-old, 20- to 25-g, male C57BL/J6 mice (The Jackson Laboratory). The animals were housed in an insulated and soundproof recording room that was maintained at an ambient temperature of 23 ± 1°C with a relative humidity of 25 ± 5% and that was on an automatically controlled 12-hour light/12-hour dark cycle (lights on at 7:00 a.m., illumination intensity ≈100 lux). The animals had free access to food and water. Every effort was made to minimize the number of animals used and any pain and discomfort experienced by the subjects. Animal experiments were performed in accordance with the guidelines published in the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and were approved by the University of Pennsylvania and Children’s Hospital of Philadelphia Animal Care and Use Committee in accordance with international guidelines on the ethical use of animals.
Study design
The overall objective of the study was to compare activity, sleep-wake, and EEG measurements between brain-injured, sham, and injured animals on BCAA intervention. Mice were randomized after surgery to one of the three experimental groups, and data analysis was performed blinded to experimental group. Power analyses were performed on our study of continuous response variables from independent control and experimental subjects with one control per experimental subject. In EEG pilot studies, the response within each subject group was normally distributed with an SD of about 15%. If the true difference in the experimental and control means is about 30%, we calculated the need to study at least five subjects per group to be able to reject the null hypothesis that the population means of the experimental and control groups are equal with a probability (power) of 0.8. The type I error probability associated with this test of this null hypothesis is 0.05.
Fluid percussion brain injury
Mice were divided into two groups: TBI (surgery and FPI) and sham (surgery alone). The fluid percussion brain injury (FPI) protocol was carried out over 2 days as previously described (16, 20). On the first day, the animal was anesthetized with a combination of ketamine (100 mg/kg) and xylazine (10 mg/kg) and placed in a mouse stereo-tactic frame (Stoelting). The scalp was incised and reflected. The following was conducted under ×0.7 to ×3.5 magnification: A craniectomy was performed with a trephine (3-mm outer diameter) over the right parietal area between bregma and lambda, just medial to the sagittal suture and lateral to the lateral cranial ridge. The dura remained intact throughout the craniotomy procedure. A rigid Luer-loc needle hub (3-mm inside diameter) was secured to the skull over the opening with Loctite adhesive and subsequently with cyanoacrylate plus dental acrylic. The skull sutures were sealed with the cyanoacrylate during this process to ensure that the fluid bolus from the injury remained within the cranial cavity. The Luer-loc needle hub was filled with isotonic sterile saline, and the hub was capped. The mouse was then placed on a heating pad and returned to the home cage once ambulatory. On the second day, the animal was briefly placed under isoflurane anesthesia (500 ml/min) via a nose cone, and respiration was visually monitored. When the animal was breathing once per 2 s, the nose cone was removed, the cap over the hub was also removed, and dural integrity was visually confirmed. The hub was topped off with isotonic sterile saline, and a 32-cm section of high-pressure tubing extending from the FPI device was attached to the Luer-loc fitting of the hub (Department of Biomedical Engineering, Virginia Commonwealth University, Richmond, VA). The animal was then placed on its left side and observed. Once normal breathing resumed and just as the animal regained its toe pinch withdrawal reflex, a 20-ms pulse of saline onto the dura was delivered. A pressure gauge attached to an oscilloscope was used to ensure delivered pressures between 1.4 and 2.1 atm, which have been previously shown to generate a mild brain injury. Immediately after injury, the hub was removed from the skull, and the animal was placed in a supine position. The animal was then reanesthetized with isoflurane for scalp closure. Sham animals received all of the above, with the exception of the fluid pulse. The animal was returned to a heating pad until ambulatory and then returned to the home cage.
Assessment of activity and inactivity: Infrared beam breaks
The activity monitoring timeline is detailed in Fig. 1A. Activity/inactivity was determined with the AccuScan monitoring system (Omnitech Electronics Inc.) and Fusion 4.0 software collection system. After FPI or sham surgery, mice were individually housed in their home cages with the AccuScan monitoring system for 30 consecutive days (n = 18 FPI, n = 12 sham). The AccuScan system consists of infrared beams that are 1 inch apart on the horizontal plane, providing a high-resolution grid covering the cage bottom. Data acquisition software provides counts of beam breaks by the mouse in 10-s epochs. The mouse was considered inactive if there were no beam breaks in four consecutive 10-s epochs, in accordance with an algorithm developed previously in our laboratory to estimate sleep and wakefulness (14).
EEG/EMG assessment of sleep and wakefulness
To assess sleep and wakefulness, mice were surgically implanted with EEG/EMG electrodes as previously described, with slight modifications for the craniotomy from FPI (14). Briefly, animals were anesthetized by injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). The skull was exposed, and three small holes were prepared for placement of three silver ball EEG electrodes—two frontal and one left parietal (anteroposterior, +1.0 mm; mediolateral, +1.5 mm from bregma, and anteroposterior, −2.0 mm; mediolateral, −2.0 mm)—according to the atlas of Paxinos and Franklin (48). Two insulated stainless steel EMG electrodes bared at the tips were buried on the surface of dorsal neck muscles. All leads from the electrodes were connected to a plastic socket connector (Plastics One), which was fixed to the skull with dental acrylate. After surgery, animals were allowed to recover for 5 days before any studies were performed. EEG and EMG signals were amplified with the Neurodata amplifier system (model M15, Astro-Med Inc.). Signals were amplified (20,000×) and conditioned with neuroamplifiers/filters (model 15A94, Grass). Settings for EEG signals were a low cut frequency (−6 dB) of 0.1 Hz and a high cut frequency (−6 dB) of 100 Hz. Recordings were digitized at 256 samples per second per channel. All data were acquired using Grass Gamma software (Natus).
BCAA administration
Two days after FPI procedure, a subset of mice was randomly assigned to receive either BCAA-supplemented water or untreated tap water (control). BCAA supplementation consisted of a combination of L-leucine, L-isoleucine, and L-valine at 100 mM each as previously described (obtained individually from Sigma-Aldrich) (16). The amount of drinking water remaining in the bottle was measured each day, and fresh BCAA or control water was replaced each week. Mice drank on average 3 to 5 ml of solution per day, and it has been previously shown that BCAAs do not affect body weight (16).
Recording timeline and behavior testing
The EEG/EMG recording timeline is shown in Fig. 2. Mice were connected to lightweight recording cables in individual cages (n = 7 sham, n = 6 TBI, n = 6 TBI + BCAA). Sleep recordings were initiated after 24 hours of acclimation to the cables. Ability to move freely within the entire cage and stand on hindlimbs to explore the top of the cage was confirmed in all mice studied. Baseline sleep was recorded on the first, second, and fifth days to ensure stable sleep/wake activity across days. At the start of the dark phase (7:00 p.m.) on recording day 3, mice were exposed to a novel environment without direct handling by adding new bedding and a nestlet to their home cages. EEG/EMG signals were recorded and analyzed from 7:00 p.m. to 9:00 p.m. for response to a novel environment. On recording day 4, mice were sleep-deprived using gentle handling for 3 hours, from 10:00 a.m. to 1:00 p.m., which is a time of heightened sleep. Gentle handling was accomplished by providing the mice with novel materials (bedding, nestlets, pieces of paper towels, aluminum foil, and saran wrap) and occasionally stroking the mice with a soft paintbrush as previously described (49). During this enforced wakefulness, wake was electrographically confirmed during the entire 3-hour period. Subsequently, recovery sleep for the next 6 hours was recorded and analyzed for wake probability and delta power as described in the next section.
Data analysis: Baseline EEG/EMG scoring
Polygraphic records were scored offline by an experienced scorer for NREM and REM sleep and wakefulness (W) in 4-s epochs across a 24-hour baseline (12-hour light-dark cycle from 7:00 a.m. to 7:00 p.m.). Data collected using the Grass Gamma software were converted to European Data Format and automatically scored using the SleepSign analysis package according to standard criteria (Kissei Comtec). Automatically scored epochs were then visually inspected for artifact and manually removed (<0.5% of all epochs). As a final step, defined sleep-wake stages were examined and corrected if necessary. Over a baseline period of 24 hours consisting of exactly 21,600 epochs per mouse, we quantified total minutes of Wake, NREM, and REM sleep, the average length of each sleep and wake bout, and the characteristics of the transitions within and between sleep and wake states (as seen in Figs. 4 and 5 and fig. S2).
Data analysis: Baseline power spectral analysis
Scored polygraphic signals underwent FFT using SleepSign for power spectral analysis of each 0.25-Hz frequency bin from 0 to 128 Hz in total for each sleep stage (NREM, REM, and Wake). Baseline zeroing and Hanning window corrections were applied to each epoch before FFT as part of the SleepSign standard algorithm. The data were then aggregated using R (version 2.15.2) for power density within particular EEG frequency bands: delta (1 to 4 Hz), theta (5 to 8 Hz), alpha (9 to 13 Hz), beta (14 to 30 Hz), total gamma (31 to 128 Hz), slow gamma (35 to 55 Hz), and total power (1 to 128 Hz) (51). The EEG power density for each epoch was normalized by the total power averaged from all epochs and summed within each frequency bin for each sleep stage. Epochs containing artifacts were eliminated from power spectral analysis. Theta peak frequency was calculated by identifying the frequency between 5 and 8 Hz, which had the greatest power density for each individual mouse, and then averaging this value within groups.
Data analysis: Novel environment
Polygraphic records over the analysis period for a novel environment were extracted from 7:00 p.m. to 9:00 p.m. on recording day 3, and scored in the same manner as detailed above. The latency to the first sleep period, defined as three consecutive NREM or REM epochs in any combination, was quantified for each mouse.
Data analysis: Recovery sleep
Polygraphic signals were extracted from the recovery sleep period occurring immediately after a 3-hour period of enforced wakefulness, from 1:00 p.m. to 7:00 p.m., on recording day 4. The epochs were scored as above and analyzed for total NREM time and average NREM bout length. Delta power was calculated by quantifying the average NREM power for each hour immediately after sleep deprivation from 1:00 p.m. to 7:00 p.m., and then normalized by dividing by the baseline NREM delta power for each individual mouse. The baseline NREM delta power was calculated by averaging values over the last 4 hours of lights on (3:00 p.m. to 7:00 p.m.) during baseline day 3, where values are presumably at their zenith (50).
Immunohistochemistry
Immunohistochemistry was used to characterize the waking c-Fos response in orexin neurons, an immediate early gene marker of recent neural activation. Mice were perfused with 4% paraformaldehyde, and brains were postfixed and then cryopreserved in sucrose. Cryostat sections were collected at 40-μm thickness in a 1:6 series. Sections containing the lateral hypothalamus underwent double-labeling for orexin and c-Fos as previously described (49). Briefly, sections were incubated with goat polyclonal anti–orexin-A antibody (Santa Cruz Biotechnology) at 1:1000 concentration and rabbit polyclonal anti–c-Fos antibody (Calbiochem) at 1:6000 concentration. Orexin-A was visualized with an anti-goat Alexa Fluor 594 (red) secondary antibody at 1:200, and c-Fos was visualized with an anti-rabbit Alexa Fluor 488 (green) secondary antibody at 1:200 (Molecular Probes). For vesicular glutamate transporter 1 (VGLUT1) and orexin-A double labeling, the protocol was the same with the exception of using rat polyclonal anti-VGLUT1 antibody (Synaptic Systems) at 1:2000 concentration. Sections were mounted onto microscope slides and analyzed for cell counting with an epifluorescent/fluorescence microscope (Leica) at ×10 and ×40 magnifications as previously described (49).
Cell counting
All immunopositive orexin-A neurons with visible nuclei in four rostral-caudal sections across bilateral nuclei for each mouse were analyzed (n = 5 mice per group). Cells were scored as c-Fos–positive if c-Fos labeling in the nucleus was more intense than background using ImageJ analysis software (NIH) as previously described (49). Two scorers, one of whom was blinded to conditions, independently counted orexin neuron numbers and c-Fos labeling with >90% agreement. For each mouse, more than 150 neurons per region were examined.
Statistical procedures
Statistical calculations and analyses were performed using R (version 2.15.2) (51). Where appropriate, all data were analyzed using either two-tailed Student’s t test or one-way ANOVA followed by Dunnett’s post hoc test if the F values reached statistical significance. Statistical significance was defined at the P < 0.05 confidence level when comparing different treatment groups. All data are presented as group means ± SEM. Detailed statistics for all figures are presented in tables S1 to S7.
Supplementary Material
Acknowledgments
We thank D. Raizen, A. Seghal, D. Holtzman, S. Voglmaier, S. Barmada, and J. Gerstner for their insightful comments in reviewing the manuscript.
Funding: Supported by NIH T32 HL007713 and University of Pennsylvania Department of Medicine/Measey Research Fellowship to M.M.L., NIH R01HL111725-01A1 to A.I.P., and NIH R01NS069629 and R01HD059288 to A.S.C.
Footnotes
www.sciencetranslationalmedicine.org/cgi/content/full/5/215/215ra173/DC1
Fig. S1. Activity patterns during the light phase.
Fig. S2. Sample double-plotted actograms over the 30-day activity monitoring period show intact diurnal rhythms in TBI mice.
Fig. S3. Distribution of short to long bouts of NREM and REM sleep.
Fig. S4. TBI alters baseline power spectra.
Fig. S5. TBI alters power density in specific frequency bands.
Fig. S6. Orexin neuron cell counts.
Table S1. Detailed statistics for Fig. 1 and fig. S1.
Table S2. Detailed statistics for Fig. 2.
Table S3. Detailed statistics for Fig. 3 and fig. S3.
Table S4. Detailed statistics for Fig. 4.
Table S5. Detailed statistics for fig. S4.
Competing interests: A.S.C. and the Children’s Hospital of Philadelphia hold a provisional patent for the use of BCAAs as a therapeutic intervention for traumatic brain injury: U.S. Provisional Patent Application Nos. 61/883,526 and 61/812,352, filed under the title “Compositions and methods for the treatment of brain injury.”
Data and materials availability: Relevant data and materials will be made available upon request.
Author contributions: M.M.L. designed, performed, and analyzed all experiments and wrote the manuscript. J.E. and L.Z. assisted with animal surgeries. G.X. and J.Z. assisted with immunohistochemistry. R.G. and J.L. assisted with collection of sleep recording data. J.R. performed microscope image analysis. N.N.K. implemented data postprocessing analysis and statistical calculations. A.I.P. and A.S.C. provided funding and expertise and co-edited the manuscript.
REFERENCES AND NOTES
- 1.Castriotta RJ, Murthy JN. Sleep disorders in patients with traumatic brain injury: A review. CNS Drugs. 2011;25:175–185. doi: 10.2165/11584870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Castriotta RJ, Wilde MC, Lai JM, Atanasov S, Masel BE, Kuna ST. Prevalence and consequences of sleep disorders in traumatic brain injury. J Clin Sleep Med. 2007;3:349–356. [PMC free article] [PubMed] [Google Scholar]
- 3.Baumann CR, Werth E, Stocker R, Ludwig S, Bassetti CL. Sleep-wake disturbances 6 months after traumatic brain injury: A prospective study. Brain. 2007;130:1873–1883. doi: 10.1093/brain/awm109. [DOI] [PubMed] [Google Scholar]
- 4.Kempf J, Werth E, Kaiser PR, Bassetti CL, Baumann CR. Sleep-wake disturbances 3 years after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2010;81:1402–1405. doi: 10.1136/jnnp.2009.201913. [DOI] [PubMed] [Google Scholar]
- 5.Makley MJ, English JB, Drubach DA, Kreuz AJ, Celnik PA, Tarwater PM. Prevalence of sleep disturbance in closed head injury patients in a rehabilitation unit. Neurorehabil Neural Repair. 2008;22:341–347. doi: 10.1177/1545968308315598. [DOI] [PubMed] [Google Scholar]
- 6.Makley MJ, Johnson-Greene L, Tarwater PM, Kreuz AJ, Spiro J, Rao V, Celnik PA. Return of memory and sleep efficiency following moderate to severe closed head injury. Neurorehabil Neural Repair. 2009;23:320–326. doi: 10.1177/1545968308325268. [DOI] [PubMed] [Google Scholar]
- 7.McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci. 2003;23:9687–9695. doi: 10.1523/JNEUROSCI.23-29-09687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruskin DN, Liu C, Dunn KE, Bazan NG, LaHoste GJ. Sleep deprivation impairs hippocampus-mediated contextual learning but not amygdala-mediated cued learning in rats. Eur J Neurosci. 2004;19:3121–3124. doi: 10.1111/j.0953-816X.2004.03426.x. [DOI] [PubMed] [Google Scholar]
- 9.Bloomfield IL, Espie CA, Evans JJ. Do sleep difficulties exacerbate deficits in sustained attention following traumatic brain injury? J Int Neuropsychol Soc. 2010;16:17–25. doi: 10.1017/S1355617709990798. [DOI] [PubMed] [Google Scholar]
- 10.McCrea M, Kelly JP, Randolph C, Cisler R, Berger L. Immediate neurocognitive effects of concussion. Neurosurgery. 2002;50:1032–1040. doi: 10.1097/00006123-200205000-00017. discussion 1040–1042. [DOI] [PubMed] [Google Scholar]
- 11.Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train. 2001;36:228–235. [PMC free article] [PubMed] [Google Scholar]
- 12.Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013;14:128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castriotta RJ, Atanasov S, Wilde MC, Masel BE, Lai JM, Kuna ST. Treatment of sleep disorders after traumatic brain injury. J Clin Sleep Med. 2009;5:137–144. [PMC free article] [PubMed] [Google Scholar]
- 14.Pack AI, Galante RJ, Maislin G, Cater J, Metaxas D, Lu S, Zhang L, Von Smith R, Kay T, Lian J, Svenson K, Peters LL. Novel method for high-throughput phenotyping of sleep in mice. Physiol Genomics. 2007;28:232–238. doi: 10.1152/physiolgenomics.00139.2006. [DOI] [PubMed] [Google Scholar]
- 15.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 16.Cole JT, Mitala CM, Kundu S, Verma A, Elkind JA, Nissim I, Cohen AS. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc Natl Acad Sci USA. 2010;107:366–371. doi: 10.1073/pnas.0910280107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witgen BM, Lifshitz J, Smith ML, Schwarzbach E, Liang SL, Grady MS, Cohen AS. Regional hippocampal alteration associated with cognitive deficit following experimental brain injury: A systems, network and cellular evaluation. Neuroscience. 2005;133:1–15. doi: 10.1016/j.neuroscience.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 18.Karnani MM, Apergis-Schoute J, Adamantidis A, Jensen LT, de Lecea L, Fugger L, Burdakov D. Activation of central orexin/hypocretin neurons by dietary amino acids. Neuron. 2012;72:616–629. doi: 10.1016/j.neuron.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 19.Dixon CE, Lyeth BG, Povlishock JT, Findling RL, Hamm RJ, Marmarou A, Young HF, Hayes RL. A fluid percussion model of experimental brain injury in the rat. J Neurosurg. 1987;67:110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- 20.McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soares H, Faden AL. Traumatic brain injury in the rat: Characterization of a lateral fluid-percussion model. Neuroscience. 1989;28:233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- 21.Sakurai T. The neural circuit of orexin (hypocretin): Maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 22.Guilleminault C, Yuen KM, Gulevich MG, Karadeniz D, Leger D, Philip P. Hypersomnia after head-neck trauma: A medicolegal dilemma. Neurology. 2000;54:653–659. doi: 10.1212/wnl.54.3.653. [DOI] [PubMed] [Google Scholar]
- 23.Rao V, Spiro J, Vaishnavi S, Rastogi P, Mielke M, Noll K, Cornwell E, Schretlen D, Makley M. Prevalence and types of sleep disturbances acutely after traumatic brain injury. Brain Inj. 2008;22:381–386. doi: 10.1080/02699050801935260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verma A, Anand V, Verma NP. Sleep disorders in chronic traumatic brain injury. J Clin Sleep Med. 2007;3:357–362. [PMC free article] [PubMed] [Google Scholar]
- 25.Franken P, Tobler I, Borbély AA. Sleep homeostasis in the rat: Simulation of the time course of EEG slow-wave activity. Neurosci Lett. 1991;130:141–144. doi: 10.1016/0304-3940(91)90382-4. [DOI] [PubMed] [Google Scholar]
- 26.Franken P, Dijk DJ, Tobler I, Borbély AA. Sleep deprivation in rats: Effects on EEG power spectra, vigilance states, and cortical temperature. Am J Physiol. 1991;261:R198–R208. doi: 10.1152/ajpregu.1991.261.1.R198. [DOI] [PubMed] [Google Scholar]
- 27.Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: Involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- 28.Harada M, Minami R, Hattori E, Nakamura K, Kabashima K. Sleep in brain-damaged patients. An all night sleep study of 105 cases. Kumamoto Med J. 1976;29:110–127. [PubMed] [Google Scholar]
- 29.Alexander GE, Furey ML, Grady CL, Pietrini P, Brady DR, Mentis MJ, Schapiro MB. Association of premorbid intellectual function with cerebral metabolism in Alzheimer’s disease: Implications for the cognitive reserve hypothesis. Am J Psychiatry. 1997;154:165–172. doi: 10.1176/ajp.154.2.165. [DOI] [PubMed] [Google Scholar]
- 30.Dockree PM, Kelly SP, Roche RA, Hogan MJ, Reilly RB, Robertson IH. Behavioural and physiological impairments of sustained attention after traumatic brain injury. Brain Res Cogn Brain Res. 2004;20:403–414. doi: 10.1016/j.cogbrainres.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 31.Colas D, Cespuglio R, Sarda N. Sleep wake profile and EEG spectral power in young or old senescence accelerated mice. Neurobiol Aging. 2005;26:265–273. doi: 10.1016/j.neurobiolaging.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Perouansky M, Rau V, Ford T, Oh SI, Perkins M, Eger EI, II, Pearce RA. Slowing of the hippocampal θ rhythm correlates with anesthetic-induced amnesia. Anesthesiology. 2010;113:1299–1309. doi: 10.1097/ALN.0b013e3181f90ccc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moretti DV, Frisoni GB, Binetti G, Zanetti O. Anatomical substrate and scalp EEG markers are correlated in subjects with cognitive impairment and Alzheimer’s disease. Front Psychiatry. 2011;1:152. doi: 10.3389/fpsyt.2010.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeong J. EEG dynamics in patients with Alzheimer’s disease. Clin Neurophysiol. 2004;115:1490–1505. doi: 10.1016/j.clinph.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Vyazovskiy VV, Olcese U, Hanlon EC, Nir Y, Cirelli C, Tononi G. Local sleep in awake rats. Nature. 2011;472:443–447. doi: 10.1038/nature10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 37.Baumann CR, Bassetti CL, Valko PO, Haybaeck J, Keller M, Clark E, Stocker R, Tolnay M, Scammell TE. Loss of hypocretin (orexin) neurons with traumatic brain injury. Ann Neurol. 2009;66:555–559. doi: 10.1002/ana.21836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baumann CR, Stocker R, Imhof HG, Trentz O, Hersberger M, Mignot E, Bassetti CL. Hypocretin-1 (orexin A) deficiency in acute traumatic brain injury. Neurology. 2005;65:147–149. doi: 10.1212/01.wnl.0000167605.02541.f2. [DOI] [PubMed] [Google Scholar]
- 39.Willie JT, Lim MM, Bennett RE, Azarion AA, Schwetye KE, Brody DL. Controlled cortical impact traumatic brain injury acutely disrupts wakefulness and extracellular orexin dynamics as determined by intracerebral microdialysis in mice. J Neurotrauma. 2012;29:1908–1921. doi: 10.1089/neu.2012.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Acuna-Goycolea C, Li Y, Van Den Pol AN. Group III metabotropic glutamate receptors maintain tonic inhibition of excitatory synaptic input to hypocretin/orexin neurons. J Neurosci. 2004;24:3013–3022. doi: 10.1523/JNEUROSCI.5416-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yudkoff M. Brain metabolism of branched-chain amino acids. Glia. 1997;21:92–98. doi: 10.1002/(sici)1098-1136(199709)21:1<92::aid-glia10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 42.Sakai R, Cohen DM, Henry JF, Burrin DG, Reeds PJ. Leucine-nitrogen metabolism in the brain of conscious rats: Its role as a nitrogen carrier in glutamate synthesis in glial and neuronal metabolic compartments. J Neurochem. 2004;88:612–622. doi: 10.1111/j.1471-4159.2004.02179.x. [DOI] [PubMed] [Google Scholar]
- 43.Jeter CB, Hergenroeder GW, Ward NH, III, Moore AN, Dash PK. Human mild traumatic brain injury decreases circulating branched-chain amino acids and their metabolite levels. J Neurotrauma. 2013;30:671–679. doi: 10.1089/neu.2012.2491. [DOI] [PubMed] [Google Scholar]
- 44.Vuille-Dit-Bille RN, Ha-Huy R, Stover JF. Changes in plasma phenylalanine, isoleucine, leucine, and valine are associated with significant changes in intracranial pressure and jugular venous oxygen saturation in patients with severe traumatic brain injury. Amino Acids. 2012;43:1287–1296. doi: 10.1007/s00726-011-1202-x. [DOI] [PubMed] [Google Scholar]
- 45.Aquilani R, Iadarola P, Contardi A, Boselli M, Verri M, Pastoris O, Boschi F, Arcidiaco P, Viglio S. Branched-chain amino acids enhance the cognitive recovery of patients with severe traumatic brain injury. Arch Phys Med Rehabil. 2005;86:1729–1735. doi: 10.1016/j.apmr.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 46.Fernstrom JD. Branched-chain amino acids and brain function. J Nutr. 2005;135:1539S–1546S. doi: 10.1093/jn/135.6.1539S. [DOI] [PubMed] [Google Scholar]
- 47.Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, Kato M, Nakamura T, Higuchi K, Nishiguchi S, Kumada H. Long-Term Survival Study Group, Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol. 2005;3:705–713. doi: 10.1016/s1542-3565(05)00017-0. [DOI] [PubMed] [Google Scholar]
- 48.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2. Gulf Professional Publishing; San Diego, CA: 2004. [Google Scholar]
- 49.Naidoo N, Zhu J, Zhu Y, Fenik P, Lian J, Galante R, Veasey S. Endoplasmic reticulum stress in wake-active neurons progresses with aging. Aging Cell. 2011;10:640–649. doi: 10.1111/j.1474-9726.2011.00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franken P, Chollet D, Tafti M. The homeostatic regulation of sleep need is under genetic control. J Neurosci. 2001;21:2610–2621. doi: 10.1523/JNEUROSCI.21-08-02610.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.R. Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.