Abstract
The primate orbitofrontal cortex (OFC) is often treated as a single entity, but architectonic and connectional neuroanatomy indicates that it has distinguishable parts. Nevertheless, few studies have attempted to dissociate the functions of its subregions. Here we review findings from recent neuropsychological and neurophysiological studies that do so. The lateral OFC seems to be important for learning, representing and updating specific object–reward associations. Medial OFC seems to be important for value comparisons and choosing among objects on that basis. Rather than viewing this dissociation of function in terms of learning versus choosing, however, we suggest that it reflects the distinction between contrasts and comparisons: differences versus similarities. Making use of high-dimensional representations that arise from the convergence of several sensory modalities, the lateral OFC encodes contrasts among outcomes. The medial MFC reduces these contrasting representations of value to a single dimension, a common currency, in order to compare alternative choices.
Keywords: Orbitofrontal cortex, macaque, reward, reversal learning
INTRODUCTION
The last twenty years have seen a dramatic shift in our understanding of the functions of the orbitofrontal cortex (OFC). Two decades ago, the OFC was widely thought to function in the inhibitory control of behavior [1-3]. This idea derived from the deficits displayed by humans and other animals with OFC damage. They seemed to be unable to alter their actions or emotions in a flexible manner, especially when faced with negative feedback.
A different picture has emerged from contemporary neuroscience. For example, neurophysiological studies in monkeys and rats have shown that the activity of neurons within the OFC is modulated by stimuli and the specific outcomes associated with them [4-7]. Complementing these findings, studies of how OFC lesions affect behavior have shown that this area is critical for the ability to learn and to update stimulus–outcome associations in humans, monkeys and rats [8-12]. As a result, most neuroscientists accept the idea that the OFC plays a critical role in learning, representing and updating the relationship between stimuli and their specific outcomes [13, 14], although the older ideas about behavioral inhibition remain.
In addition to such stimulus–outcome associations, or perhaps through them, the OFC plays a key role in emotion, emotion regulation, decision-making and reinforcement learning. Given the importance of these functions, the intrinsic organization of the OFC is something we would like to understand in some detail. With rare exceptions [15], however, the precise parts of the OFC responsible for subserving its various functions have been largely ignored. This omission is surprising given the anatomical heterogeneity of the OFC, which is composed of distinct regions, each of which has different patterns of connections to other cortical and subcortical structures [16-18]. Compared to the OFC as a whole, relatively little is known about the specific functions of its anatomical subregions.
In this article, we review findings from recent studies that examined the functions of OFC's subregions, with a focus on experimental studies in primates. We will first describe the anatomy of these subregions in humans and monkeys. Next, drawing primarily from neuropsychological and neurophysiological studies in monkeys, we will review recent evidence regarding functional distinctions among the subregions of OFC. We will suggest that the more lateral parts of the OFC function in learning, representing and updating specific stimulus–reward associations by virtue of their connections to sensory and structures and the amygdala. By contrast, more medial parts of the OFC function in comparing the value of different options in order to make choices among them. Finally, we will discuss some unresolved issues.
Anatomical subdivisions of OFC
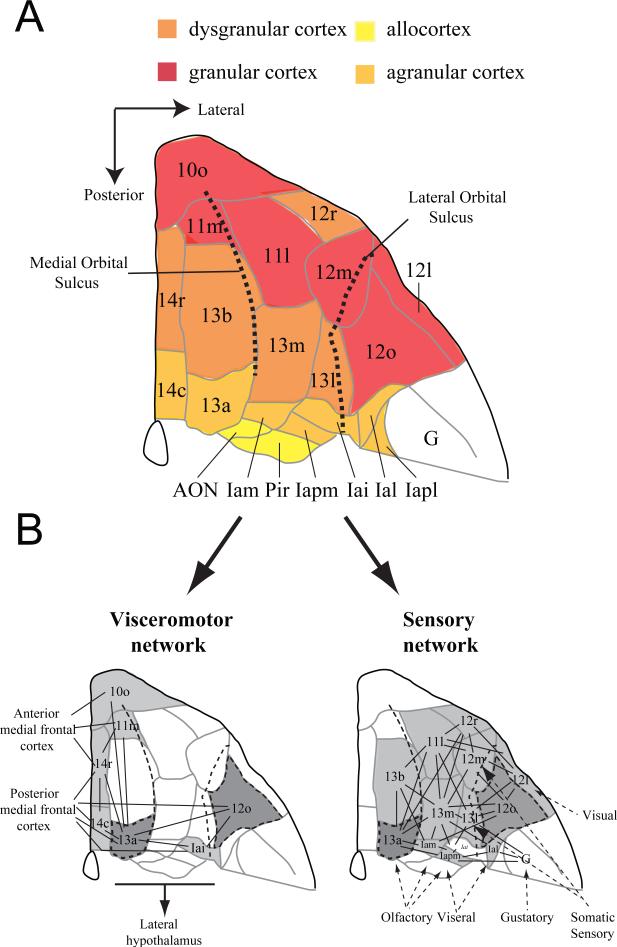
In the first half of the 20th century, neuroanatomists divided the frontal cortex of the macaque brain into areas on the basis of architectonic features. Published maps included those of Brodmann [17], Vogt and Vogt [19], Walker [16], and von Bonin and Bailey [20], among others. Walker's map has proven to be the most influential for studies of the OFC, mainly because contemporary neuroanatomists have adopted it with modifications [21-25]. Carmichael and Price [22], for example, recognized several subdivisions of Walker's areas 11, 13, 14 and 10 based on a combination of anatomical methods. They recognized the small agranular areas that make up the caudal portion of the OFC (Figure 1a), which Walker failed to mark on his maps.
Figure 1.
Anatomy of the macaque orbitofrontal cortex (OFC). A) Architectonic map of the orbital surface of the macaque brain based on Carmichael and Price's (1994) parcellation of the orbitofrontal cortex (adapted from Wise, 2008, with permission). Colored shading (red-yellow) has been added to indicate the different types of cortex present within each subdivision. The medial and lateral orbital sulci are marked by dotted lines. B) Sensory and visceromotor orbitofrontal networks described by Carmichael and Price (1996). Lines indicate connections between cortical areas and arrows indicate projections into or out of the OFC. Areas shaded in dark grey are intermediate, in that they share connections with both the sensory and visceromotor networks. Abbreviations: AON, anterior olfactory nucleus; G, gustatory cortex; Iapl, posterior lateral agranular insula cortex; Ial, lateral agranular insula cortex; Iai, inferior agranular insula cortex; Iapm, posterior medial agranular insula cortex; Iam, medial agranular insula cortex; Pir, Piriform cortex.
Orbital cortex is a descriptive term for the parts of the frontal cortex overlying the orbit in primates and for their presumptive homologues in other species. To say the least, its boundaries are indistinct in any species and often include small parts of the medial surface of the hemisphere in addition to the orbital surface. As an operational definition, we consider OFC of the macaque to extend from the fundus of the lateral orbital sulcus, laterally, to the fundus of the rostral sulcus, medially (Figure 1a). It extends from the rostral end of the medial orbital sulcus, rostrally, to the junction of the frontal lobe and temporal lobe, caudally. Accordingly, the OFC includes the small cortical fields that make up the rostral part of the insula, all the subdivisions of areas 11, 13 and 14, as well as the ventral part of what Carmichael and Price called area 10m (not illustrated). Our operational definition excludes area 10o, which we consider to be part of the frontal polar cortex, as well as area 12o, the part of area 12 that lies lateral to the lateral orbital sulcus (Figure 1a). Importantly, this definition of OFC is the one used most often in neuropsychological studies of macaque monkeys.
Most of the anatomical subdivisions of OFC identified by Carmichael and Price [22] fall into what they considered to be a lateral, sensory network, which is characterized by connections with gustatory, olfactory and visual cortex. In addition, however, the most medial portion of OFC falls into a medial, visceromotor network, which is characterized by connections with autonomic control nuclei in the hypothalamus and periaqueductal gray [18, 26]. A few orbital areas contribute fairly equally to both networks (Figure 1b). The section entitled Functional specialization within the OFC explains how these affiliations informed our experiments.
The dramatic balkanization of the OFC, to the trained anatomical eyes of Carmichael and Price, and their subsequent grouping of these areas into networks on the basis of connections, might seem like an exercise in splitting hairs. But we think that these subdivisions reflect something about the evolutionary history of the OFC. Preuss and Goldman-Rakic [24] were the first to show that primates have more OFC areas than do rats and other mammals. They concluded that primates share several caudal agranular areas with other mammals, but early primates evolved new granular areas that are located more rostrally (Figure 1a). Interestingly, most of the sensory inputs arrive in areas specific to primates, namely, the granular fields of the sensory network: areas 13m, 13l, 13b, 11m and 11l of Carmichael and Price. Areas 13 and 11 appear to be the earliest sites at which visual information about objects converges with olfactory, gustatory and visceral inputs. Object-related information comes from the perirhinal cortex to the granular OFC and the chemovisceral inputs are relayed to these areas via the agranular orbitofrontal cortex. Murray and colleagues [27] have proposed that this visual-chemovisceral convergence permits enhanced flexibility in evaluating and responding to rewards. The sight of a particular food, a fruit at a particular stage of ripeness, for example, might evoke its taste and smell, along with the pleasant sensations and positive affect that follow its consumption.
Macaque monkeys have most of the cytoarchitectonic subdivisions of frontal cortex observed in humans [28], including all of the subdivisions of areas 11 and 13. Thus, we can expect that the functional organization of the macaque brain will inform us about the functional organization of the human brain. Other cross-species comparisons are more difficult. One consequence of the elaboration of the frontal cortex in primates, compared to other mammalian orders, is that it imposes important limitations in cross-species comparisons. If, as we have said, the granular parts of the OFC evolved in primates, then these parts of the OFC cannot be studied in other mammals, including rodents [29]. The areas common to both primates and other mammals, such as the agranular parts of the insular cortex, can be studied in any mammal, but there is no guarantee that they maintained the same function in primates once new areas appeared.
Similar to the study of rodent OFC, research on the OFC of humans is not without its limitations. Although neuropsychological studies in humans with stroke, tumor excision or arterial rupture have yielded important findings regarding broad swaths of OFC, the brain damage resulting from these and other insults is not restricted to any of its subregions or, indeed, to OFC as defined here [30, 31]. Signal dropout, distortion and susceptibility artifacts from nearby air sinuses mean that functional imaging of the human OFC also has its challenges [32]. Of course, all neuroscience research has limitations, and neuropsychological research on monkeys has its share, as well. Yet in view of the difficulties just mentioned for OFC research in humans and rats, it seems to us that studies in macaque monkeys will be essential for elucidating the functions of the different parts of the primate OFC. One cannot study OFC subregions in rats that they do not have, and one cannot study the effects of well-defined lesions in humans because they do not occur commonly enough.
Functional specialization within the OFC
Evidence from object reversal learning
In a seminal paper, Butter (1969) reported that lesions of the entire orbital surface in macaques, including Walker's areas 11, 12, 13 and 14, produced “perseverative” type impairments in behavior. Butter used three tasks intended to assess monkey's ability to alter behavior in the face of changing reward contingencies: object reversal learning, spatial reversal learning, and response extinction. Macaques with damage to the entire orbital surface (i.e., OFC as defined here plus the part of area 12 on the orbital surface) exhibited deficits on object reversal learning and response extinction, but not spatial reversal learning tasks [1], suggesting that the OFC is critical for altering reward associations. The same study also revealed that ventral prefrontal cortex (Walker's area 12; labeled lateral OFC in his Figure 1a), was functionally distinct from areas 11, 13 and 14. Lesions of the ventral prefrontal cortex, including the parts of area 12 on both the orbital surface and inferior convexity, affected spatial as well as object reversal learning but not response extinction.
It is often overlooked, however, that Butter's study went further, and provided evidence for functional specialization within the OFC. Based on Walker's (1940) and von Bonin and Bailey's (1947) anatomical maps of the macaque frontal lobe Butter made lesions in the anterior and posterior portions of the OFC. His lesions did not correspond to precisely defined anatomical regions, but the anterior OFC lesions preferentially damaged Walker's area 11 and the more posterior lesions did more damage to Walker's area 13. Macaques with lesions of the anterior OFC were unimpaired on any of the three tasks. Animals with ablation of the posterior portion of the OFC, by contrast, exhibited an inability to extinguish responding of a previously rewarded response, similar to that seen in the group of macaques with lesions of the whole orbital surface. Despite this profound deficit, monkeys with posterior OFC lesions were unimpaired on spatial and object reversal tasks.
Butter found that the functions of OFC are distinct from those of the ventral prefrontal cortex (area 12), a result that has been replicated repeatedly [3, 33, 34]. Whereas lesions of ventral prefrontal cortex impair monkey's performance in the initial stages of object reversal learning, OFC lesions lead to a more pervasive deficit in this task, pointing to a key role in using reward outcomes to guide choices among objects [33-35]. Butter's findings on the different effects of anterior and posterior OFC lesions have not been replicated, however, despite their obvious significance. So while this early work had a profound and lasting influence over how we view the function of the OFC in contrast to that of the ventral prefrontal cortex (area 12), it had less impact on understanding the subregions of OFC.
A 2009 study by Kazama and Bachevalier was one of the first to tackle this problem recently. Their study probed one of the more puzzling results of Butter's earlier work. Despite the fact that lesions of the entire orbital surface led to deficits on object reversal learning, neither lesions of anterior OFC nor posterior OFC affected macaque's performance on this task [1]. It is important to appreciate that, in the decades since Butter's study, impairments in object reversal learning have been viewed as the hallmark of OFC dysfunction [2, 3, 9, 14, 36, 37]. To test whether Walker's areas 11 and 13, collectively, are necessary for altering object-reward associations, Kazama and Bachevalier made lesions that included both of these subregions. Macaques with these combined lesions were unimpaired on an object reversal learning task similar to the one used by Butter. The combined lesions were not without effect on other tasks; social behavior was altered and their ability to update specific object–reward associations was impaired relative to controls, as assessed by the devaluation task [38, 39]. Because lesions of the entire OFC (areas 11, 13, and 14) produced deficits on reversal learning, but lesions restricted to areas 11 and 13 did not, Kazama and Bachevalier concluded that medial parts of OFC, including Walker's area 14, must be the critical part of the OFC for mediating object reversal learning.
Our recent studies of OFC have revisited this problem. We reasoned that, given the anatomical connections of medial OFC, it was unlikely that this region would be critical for object reversal learning. Specifically, medial parts of OFC receive relatively few projections from sensory cortical areas, which would presumably be important for linking visual object information with reward outcomes. In contrast, lateral OFC, especially area 13 and the agranular insula cortex, receive much stronger inputs from sensory cortical areas [40, 41]. Accordingly, we prepared two groups of monkeys, one with excitotoxic lesions limited to areas 11 and 13, roughly corresponding to Carmichael and Price's [18] lateral sensory network, and another with excitotoxic lesions limited to areas 14 and 10m, corresponding to a portion of the medial, visceromotor network (Figures 1b, 2a). We said above that their anatomical analysis informed our neuropsychological studies, and the lesion placement is one of the ways it did so. The ability of these two groups of monkeys to perform object reversal learning was directly compared to unoperated controls. We were surprised to find that neither lesion affected macaque's performance on this task.
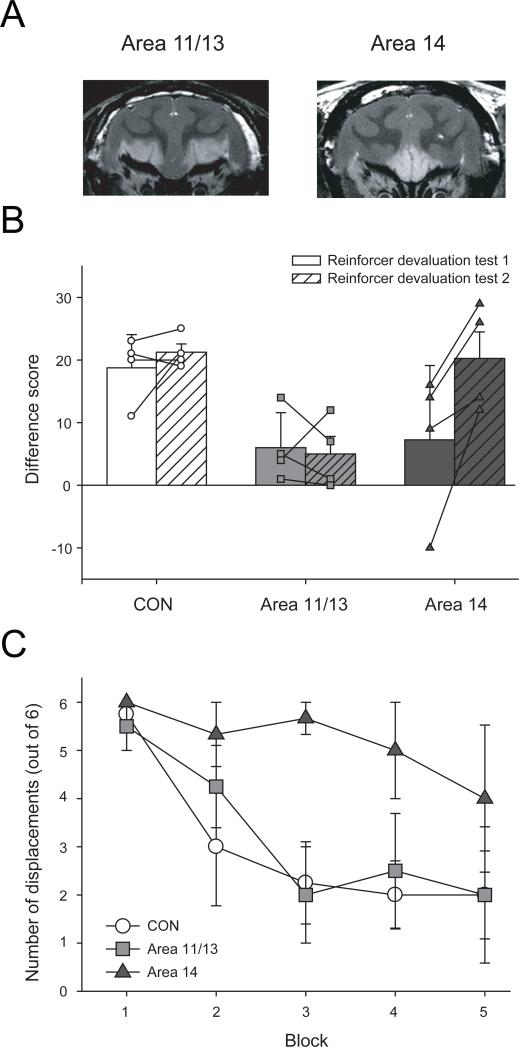
Figure 2.
The effect of lesions of lateral OFC (areas 11 and 13) or medial OFC (area 14) on reward-guided behavior. A) Images from T2-weighted MRI scans obtained from monkeys that had received injections of excitotoxin targeting either areas 11 and 13 (left) or area 14 (right). White hypersignal is associated with edema and likely reflects the extent of the lesion. B) Performance (mean ± SEM difference score) of monkeys on a reinforcer devaluation task. Two reinforcer devaluation tests are conducted approximately one month apart. Difference score provides a measure of adaptive behavior following selective satiety. The higher the difference score, the greater the shift in responses away from objects overlying the devalued food. The difference scores for individual monkeys in each group are represented by different symbols; unfilled circles CON group; light grey filled squares, area 11/13 group; dark grey filled triangles, area 14 group. C) Performance (mean ± SEM number of object displacements) of monkeys during the first extinction session of an object extinction task. Adapted from Rudebeck and Murray, 2008, with permission.
Our findings thus confirm the result reported by Kazama and Bachevalier, who showed that areas 11 and 13, together, are not essential for object reversal learning. However, our results extend their findings and lead to a different conclusion. Our results showed that areas 14 and 10m, collectively, are likewise not essential for performing the object reversal task. Taken together, the results suggest that all parts of the OFC must be damaged to disrupt object reversal learning. Such a conclusion would be hasty, however, because the pattern of results might reflect differences in lesion methods. Total OFC lesions have been made with ablation methods, but our partial lesions have used excitotoxins. This methodological difference could explain the null effect of partial lesions if one assumes that ablation methods damage axons passing near the OFC on the way to or from more anterior or medial parts of the prefrontal cortex. This issue is open to empirical investigation.
Evidence beyond object reversal learning
One of the weaknesses of the object reversal learning paradigm is that monkeys can apply a simple strategy to perform it. For example, they can adopt a win-stay/lose shift strategy. If they perform the task in this way, then they do not need to keep track of the history of previous choices or rewards, at least not beyond the immediately preceding trial, and they do not need to remember the specific outcome associated with particular objects. Improvements in behavioral methods are needed to overcome the confounded factors of strategies, choice–outcome associations, and object–outcome associations.
One task that has elucidated the function of OFC subregions is the reinforcer devaluation task [42]. In a standard version, monkeys learn that specific objects are paired with one of two different foods (food 1 and food 2). In series of test sessions, objects associated with food 1 and food 2 are presented together and the monkeys can choose between them to obtain the food they value most. Prior to some test sessions, monkeys are allowed to eat one of the two foods to satiety in order to devalue that food. In test sessions given after selective satiation, unoperated control monkeys rarely choose objects associated with the devalued food. Monkeys with complete lesions of OFC, by contrast, continue to select objects associated with the sated food [10]. Recently we have assessed the effects of subtotal OFC lesions on the devaluation task [43]. Monkeys with lesions of areas 11 and 13, but not those with lesions of area 14, were impaired in shifting their choices of objects to avoid the devalued food (Figure 2b). In addition, the magnitude of the impairment after the selective, excitotoxic lesions of areas 11 and 13 was roughly equivalent to that seen after complete aspiration lesions of OFC. Thus, areas 11 and 13 appear to be the critical site for updating the value of food rewards based on current biological needs.
These same groups of monkeys were also tested on an object extinction task. The task has two phases: acquisition and extinction. In the acquisition phase, monkeys learned to displace an object to obtain a food reward. Once monkeys were routinely displacing the object to earn food rewards, the extinction phase was initiated. During extinction, food reward was no longer available. We recorded the number of unrewarded object displacements, a measure that reflects how long it took for monkeys to stop responding to the unrewarded object.
The extinction task yielded the converse pattern of results from the reinforcer devaluation task. Monkeys with lesions of area 14, but not those with lesions of areas 11 and 13, were impaired on the extinction task, i.e. they were slow to stop responding to the previously rewarded object (Figure 2c). This deficit was milder than that seen after complete OFC lesions [44] indicating that other areas also contribute to this kind of learning. Alternatively, differences in lesion method may be a factor.
We also developed a new task to examine the transitivity of value judgments, which has been proposed as a key OFC function [45]. To test this idea, our monkeys were allowed to choose between objects that had been associated with different familiar foods, among which the monkeys had an ordered preference. After the monkeys had learned to discriminate the objects from each other and had also learned which food was associated with each object, they were allowed to choose between two objects associated with different foods that had never been paired together before. If the OFC is important for value-based transitivity, then monkeys with OFC lesions should have difficulty in choosing objects based on their food preferences. We found that monkeys with lesions of area 14 made slightly more choices that were inconsistent with their overall food preferences than did controls. Monkeys with lesions of the areas 11/13 did not differ from controls on this task [43].
The finding that macaques with lesions of lateral OFC were unable to update sensory-specific object–reward associations, as assessed by the devaluation task, fits well with the idea that the sensory network is “important for the assessment of foods” [46]. The inability of macaques with lesions of medial OFC to extinguish responding to a previously rewarded object at a normal rate may be related to altered visceral control mechanisms. The medial OFC is part of what Carmichael and Price called the visceromotor network, and a deficit in visceral or autonomic control might affect extinction behavior. Alternatively the deficit could be related to this area's proposed role in value comparison [43, 47], as explained later. The deficit on the value-based transitivity test is consistent with either idea. This test involves comparisons between objects, but if an object evokes a visceral or autonomic response that reflects the value of the associated food, and such ‘somatic markers’ influence monkeys’ choices, then both ideas can be combined. The same account could apply to the extinction test.
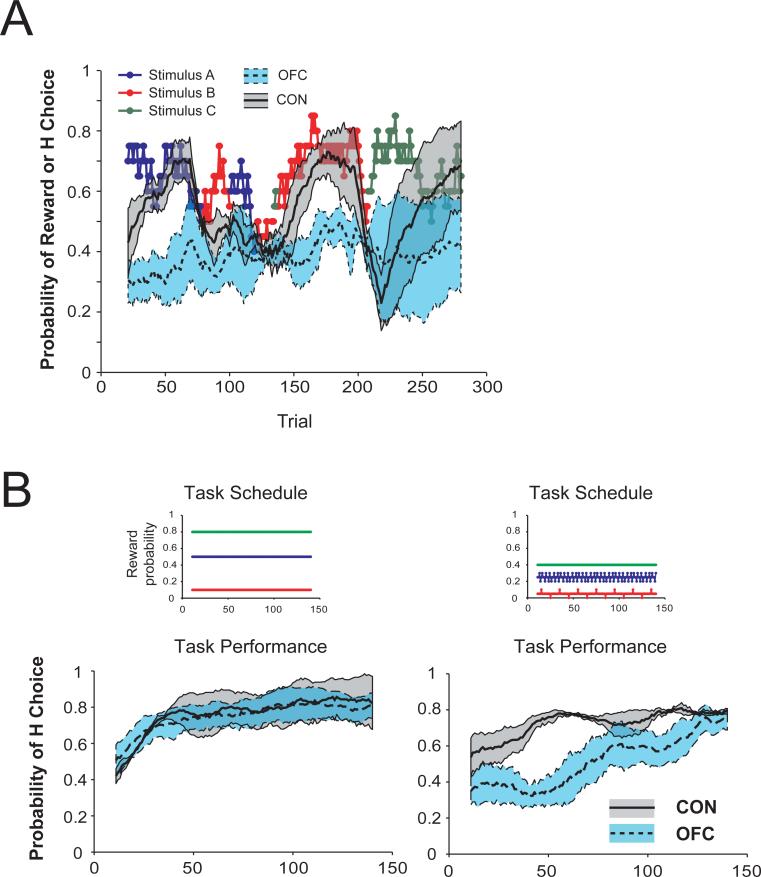
Another useful paradigm was recently developed by Rushworth and colleagues and was directly applied to the problem of dissociating the functions of OFC subregions [8, 47, 48]. Macaques performed a three-arm bandit task for food reward. On each trial of this task, monkeys chose among three different stimuli. Over the course of a session they could learn which of the three stimuli was associated with the highest probability of receiving a food reward. Unoperated control monkeys quickly learned the best stimulus–reward association (Figure 3a). By contrast, macaques with lesions largely restricted to areas 11 and 13 were markedly slower to learn this relationship [8, 48]. By analyzing the trial-by-trial choices of each monkey, it was shown that damage predominantly in but not limited to the more lateral parts of OFC (corresponding to areas 11 and 13) alters credit assignment, the process by which outcomes are assigned to the specific choices or actions that led to them [8].
Figure 3.
Lesions encompassing areas 11 and 13 disrupt credit assignment. A) Monkeys perform a three-arm bandit task with changing probabilities. Colored points represent the stimulus (stimulus A-C) associated with the highest probability of reward on a given trial. Curves show mean probability of selecting the stimulus associated with the highest probability of reward (H Choice) where the probability of reward associated with the three options changed within a session. Shaded regions represent the SEM for each group. OFC group: dashed line and blue shaded region; unoperated controls: solid line and grey shaded region. B) Mean probability of selecting the stimulus associated with the highest probability of reward (H choice) when the difference in probability of reward between options is large (left) and small (right). Shaded regions represent the SEM associated with each group. OFC group: dashed line and blue shaded region; unoperated controls: solid line and grey shaded region. Colored points in the Task Schedule above each graph show reward probabilities associated with each stimulus (stimulus A-C). A) adapted from Rudebeck et al. (2008) and B) adapted from Walton et al. (2010), with permission.
During learning, monkeys presumably build up a representation of the value of each of the available options based on the history of outcomes (rewards or non-rewards) received when that option was chosen. Only by correctly crediting an outcome to the particular stimulus chosen on that trial will a monkey be able to accurately represent the value of the available options. Walton et al. (2010) showed that monkeys with damage mainly affecting the lateral OFC areas 11 and 13 are unable to correctly assign outcomes to the choices that led to them; instead, the monkeys choose on the basis of an average of recent choices and outcomes. This deficit is especially prominent when the difference in value between the available options is small (Figure 3b).
The findings from the devaluation and three-arm bandit tasks are, in a sense, complementary. The results from the three-arm bandit task suggest that lateral parts of the OFC (areas 11 and 13) assign reward to particular objects or, alternatively, to a choice among objects. This experiment does not reveal anything about the current value of the reward in relation to the monkeys’ internal state. By contrast, the findings from the devaluation task show that the lateral OFC is important for assessing the current value of particular reward outcomes. Put simply, the three-arm bandit task shows that the lateral OFC learns that the choice of a given object is likely to yield a reward. In addition, the devaluation task shows that the lateral OFC learns the specific reward associated with an object and evaluates that reward in terms of the monkeys’ current state. Interestingly, despite impairments in credit assignment and value updating, monkeys with lateral OFC lesions are still able, eventually, to learn which of a number of available options is associated with the highest probability of reward [8] and to display distinct object and food preferences[43]. Theories of lateral OFC function will have to take into account not only the impairments but also the spared abilities.
It is possible that an inability to correctly assign distinct outcomes to specific stimuli or objects is the cause of the deficits on the devaluation task observed following damage to lateral OFC [38, 43]. On this view, the disruption of reinforcer devaluation effects would occur because the monkeys with lateral OFC lesions learn that some objects are generally associated with rewards, as opposed to learning the association between objects and the sensory properties of particular rewards. Without representations of specific object-reward associations it is impossible to update the value of the specific reward outcomes. An alternative possibility is that macaques are able to learn the associations between particular objects and the sensory properties of specific reward outcomes, but that they are unable to update or hold online the value of the rewards following selective satiation [49]. Thus we view the lesion effects on the devaluation task as adding to those on the three-arm bandit task. Future research will be needed to resolve this issue, including the possibility that the credit assignment and value updating functions are subserved by distinct parts of the lateral OFC.
A deficit in credit assignment is, by contrast, unable to explain the effects of lesions to areas 14 and 10m: the medial OFC as defined here. Noonan et al., (2010) revealed that, unlike lateral OFC (area 11 and 13), the medial OFC is important for appropriately contrasting the value of alternative choices. Similar to the aforementioned studies, monkeys performed a three-arm bandit task and had to learn which one of three stimuli was associated with the highest probability of receiving a food reward. Whereas monkeys with lesions of medial OFC were able to learn at a similar rate to controls, their ability to choose the most valuable option was affected by the value of the alternatives. A role for medial OFC in value comparison as opposed to value learning is consistent with reports from both humans and monkeys that lesions within medial OFC are associated with inconsistent choices between different options [43, 50].
Thus it appears that the function of medial OFC is distinct from that of lateral OFC. As we have argued, lateral OFC is important primarily for learning and flexibly updating stimulus-reward associations. In this summary statement, we use the term updating in two senses: updating the relationship between an object and reward probability when this relationship changes and updating the valuation of that reward based on a current motivational state. Indeed it may be that when making a decision, more lateral parts of OFC provide the current valuation of the available options to the medial OFC, where the values are compared before making a choice [43, 47]. The difference in function between the lateral and medial OFC could thus be described in terms of contrasts and comparisons. The lateral OFC learns about the contrasts among outcomes, in part by constructing high-dimensional representations of specific foods and fluids. The connectional anatomy reviewed above shows that the lateral OFC, but not the medial OFC, is in a good position to form such feature conjunctions because of its inputs from chemosensory and visual areas of sensory cortex. In contrast to lateral OFC's learning about contrasts, the medial OFC learns about comparisons, constructing a unidimensional representation of value amenable to making choices. This is the kind of value representation often called a common currency.
Evidence from neurophysiology
Early recording studies of OFC in nonhuman primates reported that single unit activity in this area responded to both rewarding and aversive events as well as stimuli that predicted them [7, 51]. More recently, investigators have shown that the activity of single cells in the OFC is correlated with numerous aspects of rewarding or aversive outcomes including the size of reward, probability of reward, the costs associated with a reward, the previous action associated with a reward, the relative or economic value of both offered and chosen outcomes and even hypothetical or unobtained rewards [5, 6, 52-58]. Researchers typically record the activity of neurons located between the lateral and medial orbital sulci. Thus, they are likely sampling from areas 11 and 13, but possibly also 10o, and 12o. Few studies of the properties of neurons within areas 14 or 10m have been reported.
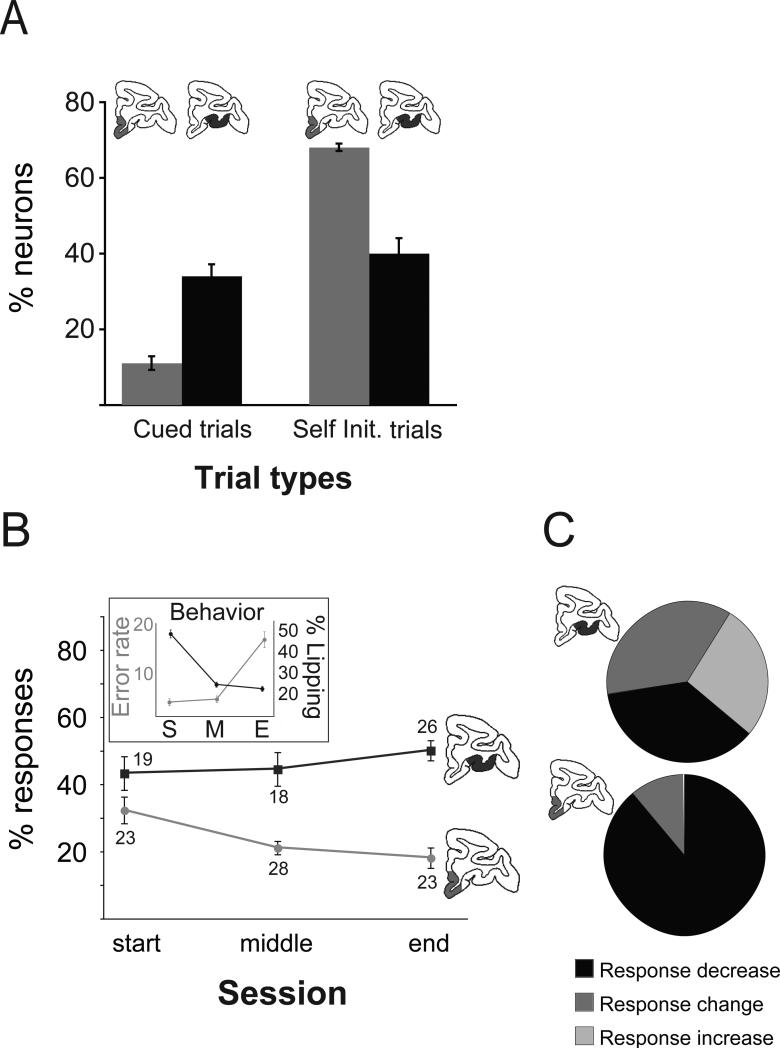
A distinction between the functional properties of neurons within the lateral and medial parts of the OFC has recently been reported. Bouret and Richmond (2010) recorded the activity of single neurons either within lateral OFC (areas 11/13) or medial OFC (areas 14 and 10m) together with adjacent parts of the medial frontal cortex (areas 25 and 32), while macaques performed two reward-guided tasks. Both tasks required the monkeys to hold and then release a bar to earn fluid reward. In one task monkeys were cued to hold and release in response to visual stimuli, whereas in the other they were free to initiate trials at their own pace. Although the activity of neurons in both parts of OFC was correlated with the amount of reward that the monkey would receive on a given trial, encoding of reward quantity was more prevalent in lateral OFC than in medial OFC when monkeys were performing the cued version of the task (Figure 4a). This result was reversed in the self-initiated task.
Figure 4.
Differential responses to reward in lateral and medial orbitofrontal cortex. A) The proportion of medial (light grey) and lateral (dark grey) OFC neurons that represent the quantity of reward in cued and self-initiated tasks. B) Neural and behavioral (error rate and lipping responses) responses over the course of a session where monkeys are shown cues associated with different quantities of reward. Behavioral and neural responses in medial OFC track the decreasing value of reward across the course of a session. C) The relative effect of satiation on the neuronal responses:increase (light gray), decrease (black) or non-specific change, obtained in populations of single neurons within lateral (top) and medial (bottom) OFC. The activity of neurons within medial OFC, but not lateral OFC, decreases following consumption of a large quantity of water. Adapted from Bouret and Richmond (2010), with permission.
Bouret and Richmond also analyzed how reward related activity changed across the course of a session, as monkeys became increasingly sated. Intriguingly, the activity of most neurons in lateral OFC was consistent across the course of a session, being well correlated with the amount of reward available on a given trial irrespective of whether the monkey was at the start or end of a session. By contrast, as the session progressed, fewer cells in medial OFC encoded the amount of reward, paralleling changes in behavior (Figure 4b, inset). These data suggest that the medial part of the OFC (and nearby parts of the medial frontal cortex) tracks the internal motivational state of the animal or the current value of available rewards. A control experiment demonstrated that this change in encoding was not related to fatigue or increased work demands. Part way through a cued testing session macaques were given a large quantity of water and were then put back onto the task. Following this manipulation there was little or no change in the responses of neurons in lateral OFC that encoded the amount of reward. Reward related responses of neurons in medial OFC, by contrast, were dramatically decreased (figure 4c).
The finding that activity of neurons in lateral OFC was not as correlated with the state of satiety, compared to medial OFC, seems to be at odds with our results. Lesions of lateral, and not medial, OFC disrupt the ability to update the value of outcomes following selective satiation [43]. But it is important to understand that lesion studies allow conclusions about whether a brain structure is necessary for a particular function, whereas neurophysiological studies reveal correlations of neuronal activity with behavior. The correlations thus revealed might, for example, simply monitor signals coming from other brain areas without playing any necessary role in the behavior underway. Thus there are several possible explanations for the apparent discrepancies, and we recognize that neurophysiological and neuropsychological data cannot be genuinely contradictory: merely interpreted differently.
While it may seem difficult to reconcile the conclusions of Bouret and Richmond with our own and Noonan el al.'s findings it is worth noting that Bouret and Richmond found that a substantial proportion of lateral OFC cells showed decrementing value coding after satiation (4c). So their result is not at all inconsistent with our findings. Furthermore, a previous report also documented satiety related alterations in neuronal firing within lateral OFC [59]. We also need to understand what factors in their task produced their results. Among the possibilities is attention. It may be the case that as monkeys become sated they will devote less attention to the stimuli that flash on and off during the task, paralleling decrements in task performance associated with satiety. These issues emphasize the need for future neurophysiological studies that include experimental control of attention as a variable distinct from motivation.
Balkanizing the OFC: Conclusions and future directions
Our review has highlighted differences in the function of the medial and lateral sectors of the OFC. That such medial versus lateral differences in function were found is in large part due to the perspective provided by neuroanatomists [16, 18]. The experimental findings we have discussed answer some questions and raise others. We have concentrated on the mediolateral dimension of OFC. Yet the differences in cytoarchitecture and connections between anterior and posterior parts of primate OFC are, if anything, more pronounced [22, 40, 41, 60]. A posterior to anterior hierarchical organization of reward encoding has been proposed within OFC, with more abstract or higher order aspects encoded in more anterior regions [15, 61]. Perhaps the learning and updating of specific object–reward associations depends on the interaction between the anteriorly situated area 11 and the posteriorly located area 13. Even more posteriorly, the agranular parts of the OFC, including the agranular insular cortex, have received scant attention. These more posterior portions of the OFC have dense interconnections with the amygdala and other parts of the limbic system, as well as inputs from primary sensory areas for taste, touch and smell [40, 41, 62, 63]. Given the idea that these posterior parts of the OFC are homologous in all mammals, examination of its specific functions has important implications about the ancestral functions of the OFC and its common functions in rats, monkeys and humans.
Although we recognize that many questions remain, we can provide some provisional answers to questions about what lateral and medial parts of OFC do. One way of looking at the results reviewed here is to view the lateral OFC as learning about, representing and updating the associations between objects and the value of specific outcomes, with the medial OFC viewed as contributing to choices among objects based on value comparisons. This distinction is, to a first approximation, one between learning and choosing. Another way to summarize these results places the two subregions with a more similar conceptual framework. According to this idea, both the orbital and medial OFC learn about, represent and update the associations between objects and value. Lateral OFC does so for contrasts among specific outcomes, whereas medial OFC does so for comparisons among outcomes in a common currency, i.e., a unidimensional representation of value. In the sense used here, contrasts deal with differences and comparisons deal with similarities, much like the distinction between discrimination and generalization commonly recognized in cognitive psychology.
ACKNOWLEDGEMENTS
This work was supported by the Intramural Research Program of the National Institute of Mental Health. We thank Steve Kennerley and Steve Wise for thoughtful comments on an earlier version of the manuscript.
Footnotes
CONFLICT OF INTEREST: None
REFERENCES
- 1.Butter CM. Perseveration in extinction and in discrimination reversal tasks following selective frontal ablations in Macaca mulatta. Physiol. and Behav. 1969;4:163–171. [Google Scholar]
- 2.Jones B, Mishkin M. Limbic lesions and the problem of stimulus--reinforcement associations. Exp. Neurol. 1972;36:362–377. doi: 10.1016/0014-4886(72)90030-1. [DOI] [PubMed] [Google Scholar]
- 3.Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 4.Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J. Neurosci. 1999;19:1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- 6.Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur. J. Neurosci. 2003;18:2069–2081. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- 7.Thorpe SJ, Rolls ET, Maddison S. The orbitofrontal cortex: neuronal activity in the behaving monkey. Exp. Brain Res. 1983;49:93–115. doi: 10.1007/BF00235545. [DOI] [PubMed] [Google Scholar]
- 8.Walton ME, et al. Separable learning systems in the macaque brain and the role of orbitofrontal cortex in contingent learning. Neuron. 2010;65:927–939. doi: 10.1016/j.neuron.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J. Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J. Neurosci. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. J. Neurosci. 1999;19:6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuchida A, Doll BB, Fellows LK. Beyond reversal: a critical role for human orbitofrontal cortex in flexible learning from probabilistic feedback. J. Neurosci. 2010;30:16868–16875. doi: 10.1523/JNEUROSCI.1958-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Ann. Rev. Neurosci. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- 14.Murray EA, O'Doherty JP, Schoenbaum G. What we know and do not know about the functions of the orbitofrontal cortex after 20 years of cross-species studies. J. Neurosci. 2007;27:8166–8169. doi: 10.1523/JNEUROSCI.1556-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog. Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Walker A. A cytoarchitectural study of the prefrontal area of the macaque monkey. J. Comp. Neurol. 1940;73:59–86. [Google Scholar]
- 17.Brodmann K. Vegleichende Lokalisationslehre der Grosshirnde. Barth; Leipzig: 1909. [Google Scholar]
- 18.Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J. Comp. Neurol. 1996;371:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 19.Vogt C, Vogt O. Allgemeinere Ergebnisse unserer Hirnforschung. J. Psychol. Neurol. 1919;25:292–398. [Google Scholar]
- 20.Von Bonin G, Bailey P. The Neocortex of Macaca Mulatta. University of Illinois Press; Urbana, Illinois: 1947. [Google Scholar]
- 21.Morecraft RJ, Geula C, Mesulam MM. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. J. Comp. Neurol. 1992;323:341–358. doi: 10.1002/cne.903230304. [DOI] [PubMed] [Google Scholar]
- 22.Carmichael ST, Price JL. Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. J. Comp. Neurol. 1994;346:366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]
- 23.Petrides M, Pandya DN. Comparative architectonic analysis of the human and macaque frontal cortex. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. Vol. 9. Elsevier Science Publishers; Amsterdam: 1994. pp. 17–58. [Google Scholar]
- 24.Preuss TM, Goldman-Rakic PS. Myelo- and cytoarchitecture of the granular frontal cortex and surrounding regions in the strepsirhine primate Galago and the anthropoid primate Macaca. J. Comp. Neurol. 1991;310:429–474. doi: 10.1002/cne.903100402. [DOI] [PubMed] [Google Scholar]
- 25.Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J. Comp. Neurol. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- 26.Ongur D, An X, Price JL. Prefrontal cortical projections to the hypothalamus in macaque monkeys. J. Comp. Neurol. 1998;401:480–505. [PubMed] [Google Scholar]
- 27.Murray EA, Wise SP, Rhodes SE. What can different brains do with reward? In: Gottfried JA, editor. Neurobiology of Sensation and Reward. CRC Press; 2011. [PubMed] [Google Scholar]
- 28.Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J. Comp. Neurol. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- 29.Wise SP. Forward frontal fields: phylogeny and fundamental function. Trends Neurosci. 2008;31:599–608. doi: 10.1016/j.tins.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- 31.Bechara A, et al. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 32.Cools R, et al. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J. Neurosci. 2002;22:4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp. Brain Res. 1970;11:376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- 34.Rygula R, et al. Differential contributions of the primate ventrolateral prefrontal and orbitofrontal cortex to serial reversal learning. J. Neurosci. 2010;30:14552–14559. doi: 10.1523/JNEUROSCI.2631-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudebeck PH, Murray EA. Amygdala and orbitofrontal cortex lesions differentially influence choices during object reversal learning. J. Neurosci. 2008;28:8338–8343. doi: 10.1523/JNEUROSCI.2272-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rolls ET, et al. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J. Neurol. Neurosurg. and Psych. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clark L, Cools R, Robbins TW. The neuropsychology of ventral prefrontal cortex: decision-making and reversal learning. Brain and Cognition. 2004;55:41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- 38.Machado CJ, Bachevalier J. The effects of selective amygdala, orbital frontal cortex or hippocampal formation lesions on reward assessment in nonhuman primates. Eur. J. Neurosci. 2007;25:2885–2904. doi: 10.1111/j.1460-9568.2007.05525.x. [DOI] [PubMed] [Google Scholar]
- 39.Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta). Behav. Neurosci. 2006;120:761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- 40.Carmichael ST, Price JL. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J. Comp. Neurol. 1995;363:642–664. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- 41.Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J. Comp. Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- 42.Malkova L, Gaffan D, Murray EA. Excitotoxic lesions of the amygdala fail to produce impairment in visual learning for auditory secondary reinforcement but interfere with reinforcer devaluation effects in rhesus monkeys. J. Neurosci. 1997;17:6011–6020. doi: 10.1523/JNEUROSCI.17-15-06011.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rudebeck PH, Murray EA. Dissociable effects of subtotal lesions within the Macaque orbital prefrontal cortex on reward-guided behavior. J. Neurosci. 2011;31:10569–10578. doi: 10.1523/JNEUROSCI.0091-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Izquierdo A, Murray EA. Opposing effects of amygdala and orbital prefrontal cortex lesions on the extinction of instrumental responding in macaque monkeys. Eur. J. Neurosci. 2005;22:2341–2346. doi: 10.1111/j.1460-9568.2005.04434.x. [DOI] [PubMed] [Google Scholar]
- 45.Padoa-Schioppa C, Assad JA. The representation of economic value in the orbitofrontal cortex is invariant for changes of menu. Nat. Neurosci. 2008;11:95–102. doi: 10.1038/nn2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saleem KS, Kondo H, Price JL. Complementary circuits connecting the orbital and medial prefrontal networks with the temporal, insular, and opercular cortex in the macaque monkey. J. Comp. Neurol. 2008;506:659–693. doi: 10.1002/cne.21577. [DOI] [PubMed] [Google Scholar]
- 47.Noonan MP, et al. Separate value comparison and learning mechanisms in macaque medial and lateral orbitofrontal cortex. Proc. Nat. Acad. Sci. U S A. 2010;107:20547–20552. doi: 10.1073/pnas.1012246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rudebeck PH, et al. Frontal cortex subregions play distinct roles in choices between actions and stimuli. J. Neurosci. 2008;28:13775–13785. doi: 10.1523/JNEUROSCI.3541-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pickens CL, et al. Orbitofrontal lesions impair use of cue-outcome associations in a devaluation task. Behav. Neurosci. 2005;119:317–322. doi: 10.1037/0735-7044.119.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fellows LK, Farah MJ. The role of ventromedial prefrontal cortex in decision making: judgment under uncertainty or judgment per se? Cereb. Cortex. 2007;17:2669–2674. doi: 10.1093/cercor/bhl176. [DOI] [PubMed] [Google Scholar]
- 51.Niki H, Sakai M, Kubota K. Delayed alternation performance and unit activity of the caudate head and medial orbitofrontal gyrus in the monkey. Brain Res. 1972;38:343–353. doi: 10.1016/0006-8993(72)90717-2. [DOI] [PubMed] [Google Scholar]
- 52.Kennerley SW, et al. Neurons in the frontal lobe encode the value of multiple decision variables. J. Cog. Neurosci. 2009;21:1162–1178. doi: 10.1162/jocn.2009.21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O'Neill M, Schultz W. Coding of reward risk by orbitofrontal neurons is mostly distinct from coding of reward value. Neuron. 2010;68:789–800. doi: 10.1016/j.neuron.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 54.Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441:223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roesch MR, Olson CR. Neuronal activity in primate orbitofrontal cortex reflects the value of time. J. Neurophys. 2005;94:2457–2471. doi: 10.1152/jn.00373.2005. [DOI] [PubMed] [Google Scholar]
- 56.Roesch MR, Olson CR. Neuronal activity related to reward value and motivation in primate frontal cortex. Science. 2004;304:307–310. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- 57.Tsujimoto S, Genovesio A, Wise SP. Monkey orbitofrontal cortex encodes response choices near feedback time. J. Neurosci. 2009;29:2569–2574. doi: 10.1523/JNEUROSCI.5777-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morrison SE, Salzman CD. The convergence of information about rewarding and aversive stimuli in single neurons. J. Neurosci. 2009;29:11471–11483. doi: 10.1523/JNEUROSCI.1815-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Critchley HD, Rolls ET. Hunger and satiety modify the responses of olfactory and visual neurons in the primate orbitofrontal cortex. J. Neurophys. 1996;75:1673–1686. doi: 10.1152/jn.1996.75.4.1673. [DOI] [PubMed] [Google Scholar]
- 60.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sescousse G, Redoute J, Dreher JC. The architecture of reward value coding in the human orbitofrontal cortex. J. Neurosci. 2010;30:13095–13104. doi: 10.1523/JNEUROSCI.3501-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mufson EJ, Mesulam MM. Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. J. Comp. Neurol. 1982;212:23–37. doi: 10.1002/cne.902120103. [DOI] [PubMed] [Google Scholar]
- 63.Mufson EJ, Mesulam MM, Pandya DN. Insular interconnections with the amygdala in the rhesus monkey. Neuroscience. 1981;6:1231–1248. doi: 10.1016/0306-4522(81)90184-6. [DOI] [PubMed] [Google Scholar]