Abstract
Whether cortical projection neurons (CPNs) are generated by multipotent or fate-restricted progenitors is not completely understood. In this issue of Neuron, Guo et al. (2013) provide evidence that mouse Fezf2-expressing radial glial cells are multipotent progenitors that sequentially generate all major CPN subtypes and glia.
Understanding how neuronal diversity is generated and how neural circuits form during development remains one of neuroscience’s greatest challenges, particularly in the cerebral cortex, due to its remarkable diversity of neuronal cell types and intricate synaptic circuitry.
Neuronal Diversity in the Cerebral Cortex
Cortical neurons can be broadly classified into two categories: glutamatergic excitatory projection neurons and GABAergic inhibitory interneurons. Cortical projection neurons (CPNs), also called pyramidal neurons, account for approximately 80% of all cortical neurons and serve as both the sole output from and the largest input system to the cortex. Interneurons, in contrast, represent approximately 20% of all cortical neurons and form local synaptic connections that play critical roles in shaping cortical network activity patterns.
Distinct subtypes of CPNs display different molecular and electrophysiological properties as well as patterns of synaptic connectivity (Greig et al., 2013; Kwan et al., 2012; Leone et al., 2008). These differences provide the basis for the functional divisions of the cerebral cortex into layers and areas. The more superficial or upper layers (L2–L4) contain CPNs that form synaptic connections with cerebral nuclei (i.e., claustrum, amygdala, and basal ganglia) and between cortical areas of either ipsilateral or contralateral hemisphere (intracerebral CPNs). In contrast, the majority of lower-layer (L5 and L6) CPNs project to subcerebral structures (i.e., diencephalon, brainstem, and spinal cord). Furthermore, within the same layer, the patterns of axonal projections of CPNs are distinct based on the cortical area. For example, L5 CPNs that form the corticospinal tract, the principal projection system controlling discrete voluntary movements, are located primarily in the motor-somesthetic cortex. The molecular and cellular mechanisms of how distinct subtypes of CPNs are generated and specified have been extensively investigated over the past several decades.
The Developmental and Evolutionary Origins of CPN Diversity
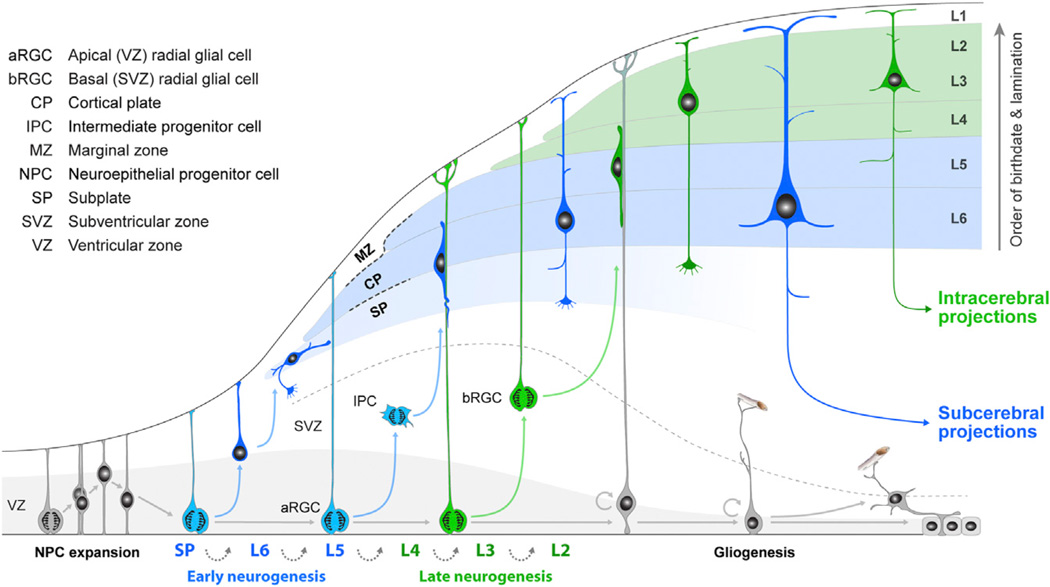
Pioneering birth-dating studies of cortical cells in mice (Angevine and Sidman, 1961) demonstrated that CPNs are generated over time by progenitor cells located within the ventricular zone (VZ) and sub-ventricular zone (SVZ) (Figure 1). Nascent neurons migrate outward toward the pial surface, where they form distinct layers in an “inside-first, outside-last” manner within the emerging cortical plate. CPNs that occupy the subplate (SP) and lower layers (L6 and L5) are generated first, followed by those in more superficial layers (L2–L4). Succeeding discovery that CPNs in the reeler mutant mouse are aligned in a practically inverted (“outside-in”) laminar fashion, while retaining their identity and connectivity patterns characteristic of their birthdates (Caviness and Sidman, 1973), further supported the concept that the birthdate of CPNs is intimately associated with their ultimate laminar position and function. These studies provided a framework for future studies of cortical progenitors and neuronal diversity and raised the question of whether neurons in different layers are generated by a common multipotent progenitor cell, separate fate-restricted progenitors, or a combination of both.
Figure 1. Schematic Illustration of the Development of Cortical Progenitor Cells and CPN Diversity.
Prior to the onset of cortical neurogenesis, neuroepithelial progenitor cells (NPCs) undergo a few rounds of symmetric division to expand the progenitor pool. NPCs then differentiate into early RGCs. Guo et al. (2013) provide evidence that early Fezf2-expressing RGCs (blue) are multipotent progenitors that sequentially generate all major CPN subtypes in both lower (mainly subcerebral CPNs; blue) and upper (exclusively intracerebral CPNs; green) layers, and subsequently glial cells. Adapted from Kwan et al. (2012).
A number of studies involving cell transplantation, retroviral lineage tracing, transgenic reporter mice, chimeric mice, or clonal cell cultures demonstrated that a single cortical progenitor can generate CPNs of multiple, and sometimes all, layers (Leone et al., 2008; Shen et al., 2006; Soriano et al., 1995; Tan and Breen, 1993; Walsh and Cepko, 1990). Subsequent studies demonstrated that the major class of embryonic cortical cells, the radial glial cells (RGCs), serves not only as guides for migrating neurons, as discovered decades earlier, but also as progenitor cells that give rise to CPNs directly or indirectly via SVZ intermediate progenitor cells (IPCs) (Kriegstein and Alvarez-Buylla, 2009). Cortical interneurons, on the other hand, follow substantially different developmental programs. Interneurons are generated by progenitor cells in the ventral forebrain and migrate tangentially to the cortex (Marín and Rubenstein, 2003). These studies provided evidence that cortical RGCs directly or indirectly generate CPNs in all layers through successive mitoses within the same lineage.
Interestingly, multiple lines of evidence supporting layer- or CPN subtype-restricted progenitors also emerged at the same time. For example, analyses of chimeric mice revealed that large clones of cortical cells were restricted to a subset of layers or were irregular in their columnar organization (see Kuan et al., 1997). In retroviral lineage-tracing studies, a mixture of complete and incomplete columns and widely dispersed distributions of cells were also reported (Walsh and Cepko, 1990). Furthermore, Harvey Karten’s hypothesis on the evolutionary origins of the cerebral cortex proposed that lower- and upper-layer CPNs have independent origins and that, in reptiles and birds, equivalent neuronal subtypes arise from different, spatially segregated progenitor pools (Karten, 2013). Together, the above observations and hypothesis supported an alternative model in which separate or fate-restricted progenitors exist for lower- and upper-layer CPNs.
Molecular Specification of Cortical Progenitor Cells and CPNs
Interestingly, CPNs often express layer-and subtype-specific genes, including many encoding transcription factors (TFs) involved in CPN specification and differentiation (Greig et al., 2013; Kwan et al., 2012; Leone et al., 2008). For example, FEZF2 (also known as FEZL or ZFP312) is enriched in L5 and, to a lesser extent SP and L6 CPNs but absent from the upper-layer (L2–L4) CPNs. Experimental studies have demonstrated that Fezf2 is necessary for the proper specification and subcerebral connectivity of lower-layer CPNs. In contrast, CUX2 and closely related CUX1 are enriched in upper-layer CPNs and play important roles in their dendritic arborization and synapse formation.
Moreover, both Cux2 and Fezf2 are expressed not only in subsets of CPNs, but also in cortical progenitors during neurogenesis. The fact that Fezf2+ progenitors and Cux2+ progenitors both exist in the cortical proliferation zone raises the question of whether it reflects an early diversification of fate-restricted progenitors, or progressive differences intrinsic to the progenitors within the same lineage. To address this question, Franco et al. (2012) and Guo et al. (2013) took the advantage of these two molecular markers and the CRE-LoxP recombination system to trace the descendants of the Cux2− and Fezf2-expressing progenitors, respectively. Both studies generated novel and valuable tools and provided new insights into cortical progenitor and CPN diversity. Interestingly, the two studies have also reached some different conclusions regarding the fate of CPN progenitors.
Franco et al. (2012) generated Cux2-Cre and Cux2-CreERT2 knockin mice and showed that the two CRE-mediated systems label RGCs in the early cortical VZ. Intriguingly, these Cux2+ RGCs gave rise only to upper-layer CPNs but not lower-layer subcerebral CPNs or macroglia (i.e., astrocytes and oligodendrocytes). This finding challenged the prevailing model that all CPNs are generated by a common progenitor cell through sequential mitoses and progressive restriction in their fate potential.
In this issue of Neuron, Guo et al. (2013) generated Fezf2-CreERT2 transgenic mice using a bacterial artificial chromosome to examine the lineage and fate of Fezf2-expressing progenitor cells. Guo et al. (2013) showed that in these mice, CRE is active upon tamoxifen administration in both early and late RGCs. With a series of elegant experiments, Guo et al. (2013) showed that Fezf2+ RGCs are multipotent progenitors that sequentially give rise to both lower- and upper-layer CPNs, as well as macroglia. Notably, the fraction of upper-layer CPNs significantly increases in the daughter cells of late Fezf2+ RGCs, indicating progressive restriction in the fate potential of these progenitors. Interestingly, previous studies showed that Fezf2 is highly enriched in the early VZ progenitors and their direct progenies, L6 and L5 neurons, but not expressed at appreciable levels in late VZ and SVZ progenitor cells, when upper-layer CPNs are generated (Greig et al., 2013; Kwan et al., 2012; Leone et al., 2008). Using the more sensitive CRE-reporter system, Guo et al. (2013) revealed that Fezf2 retains low levels of expression in the late progenitors designated to the production of upper-layer CPNs and subsequently, macroglia. This finding is thus consistent with the model of progressive restriction of cortical progenitor potency and the instructive role of Fezf2, when ectopically expressed, in directing late cortical progenitors as well as striatal progenitors to generate lower-layer CPN-like neurons (Greig et al., 2013; Kwan et al., 2012; Leone et al., 2008).
Furthermore, Guo et al. (2013) also traced Fezf2+ lineage at the clonal level. By analyzing the cell-type composition of individual clones in the Fezf2-CreERT2, Confetti reporter mouse, Guo et al. (2013) showed that a single Fezf2+ RGC in the early VZ gives rise to both lower- and upper-layer CPNs, as well as glia cells, whereas clones of late Fezf2+ RGCs consist of only upper-layer CPNs and glia cells. Together, these experiments convincingly showed that Fezf2-expressing RGCs are multipotent progenitors, rather than fate-restricted progenitors committed to generating lower-layer CPNs.
However, the observation that Fezf2+ RGCs also give rise to CUX2+ upper-layer CPNs is in contrast to the finding by Franco et al. (2012) that Cux2+ RGCs are early fate-restricted to generate upper-layer neurons. To explore this discrepancy, Guo et al. (2013) examined the identity of Cux2-Cre-labeled RGCs. Guo et al. (2013) found that, similar to the report by Franco et al. (2012), many VZ/SVZ cells labeled by the reporter protein under the control of the Cux2-Cre and Cux2-CreERT2 system express RGC marker PAX6 and IPC marker TBR2. However, they also found that nearly all cells in the VZ/SVZ expressing CUX2 protein during the same developmental period are migrating interneurons. This suggests that the CRE-reporter system might be more sensitive than immunostaining for detecting low levels of Cux2 expression. Interestingly, Guo et al., (2013) found that progenies of Cux2-Cre- and Cux2-CreERT2-labeled RGCs contain a considerable fraction of both lower-layer and upper-layer CPNs, as indicated by various layer-specific molecular markers associated with either subcerebral or intracerebral CPNs. This indicates that Cux2-Cre-labeled progenitors are multipotent progenitors and less likely to be fate restricted to generate upper-layer CPNs.
Why did the two studies with complementary approaches reach different conclusions regarding the diversity of cortical progenitor cells? One possible explanation suggested by Guo et al. (2013) is that, in the Cux2-Cre and Cux2-CreERT2 experiments by Franco et al. (2012), the CRE-mediated recombination could occur postmitotically in upper-layer CPNs, thus masking the fate potential of Cux2+ RGCs. In contrast, Guo et al. (2013) used a variety of reporter mice to observe recombination events that occurred in either progenitor or postmitotic cells. This allowed them to perform lineage-tracing experiments without the ambiguity caused by CRE-mediated recombination in postmitotic cells.
Some Remaining Key Questions
Although it is evident that Fezf2+ RGCs are multipotent progenitors and that the intrinsic downregulation of Fezf2 occurs progressively within this RGC lineage, several key questions remain. Considering that ectopically expressed Fezf2 will direct late cortical progenitors to generate lower-layer subcerebral CPN-like neurons (Greig et al., 2013; Kwan et al., 2012; Leone et al., 2008), an obvious question to ask is whether the downregulation of Fezf2 functionally contributes to the progressive restriction of RGC’s fate potential. Also, would this potential differential function of FEZF2 in early versus late RGCs be mediated by FEZF2-dose-dependent effectors? Finally, what are the molecular mechanisms that attenuate Fezf2 in the late RGCs and eventually prevent its expression by the upper-layer CPNs? Learning the answers to these questions will provide novel insights into the molecular mechanisms involved in the development and diversity of CPNs.
Another set of questions relates to the relationship between the early RGCs labeled by Cux2-Cre and Cux2-CreERT2 system and those labeled by Fezf2-CreERT2. As shown by Guo et al. (2013), Cux2 mRNA is expressed at a relatively low level at embryonic day (E) 10.5. In contrast, Fezf2 mRNA expression is observed in pallial neuroepithelial progenitor cells (NPCs) as early as E8.5, prior to their acquisition of radial glia characteristics and the onset of cortical neurogenesis (Greig et al., 2013; Kwan et al., 2012; Leone et al., 2008). Considering these differences in the timing of onset and extent of Fezf2 and Cux2 expression, it is possible that Cux2+ RGCs are derived from the earliest Fezf2+ progenitors. Furthermore, these early Cux2+ RGCs could also coexpress Fezf2. Fezf2-CreERT2 genetic temporal fate-mapping studies in conjunction with the analysis of Cux2 mRNA and protein expression at early embryonic developmental stage would probably provide answers to these questions.
Furthermore, the fact that both Fezf2+ and Cux2+ RGCs exhibit properties of multipotent progenitors does not completely eliminate the possibility that some fate-restricted progenitors also exist. As Guo et al. (2013) pointed out, the upper-layer fate-restricted RGCs cannot be identified by Cux2-Cre expression alone. In addition, they observed clones from early Fezf2+ RGCs that were composed of only macroglia, which would be consistent with the existence of early fate-restricted progenitors within the Fezf2+ RGC lineage. One potential approach to further evaluate the existence of fate-restricted cortical progenitors would be to investigate the fate potential of early Fezf2-negative RGCs, if they exist, as well as to compare their potency and molecular profile to those of Fezf2+ RGCs. Finally, these additional analyses of cortical progenitor heterogeneity may provide new insights into the evolutionary origins of cortical progenitors and CPN diversity and the presence of related cell types in the developing pallium of birds and reptiles.
REFERENCES
- Angevine JB, Jr, Sidman RL. Nature. 1961;192:766–768. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Jr, Sidman RL. J. Comp. Neurol. 1973;148:141–151. doi: 10.1002/cne.901480202. [DOI] [PubMed] [Google Scholar]
- Franco SJ, Gil-Sanz C, Martinez-Garay I, Espinosa A, Harkins-Perry SR, Ramos C, Müller U. Science. 2012;337:746–749. doi: 10.1126/science.1223616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig LC, Woodworth MB, Galazo MJ, Padmanabhan H, Macklis JD. Nat. Rev. Neurosci. 2013;14:755–769. doi: 10.1038/nrn3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Eckler MJ, McKenna WL, Mckinsey GL, Rubenstein JLR, Chen B. Neuron. 2013;80(this issue):1167–1174. doi: 10.1016/j.neuron.2013.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karten HJ. Curr. Biol. 2013;23:R12–R15. doi: 10.1016/j.cub.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. Annu. Rev. Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan C-Y, Elliott EA, Flavell RA, Rakic P. Proc. Natl. Acad. Sci. USA. 1997;94:3374–3379. doi: 10.1073/pnas.94.7.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Sestan N, Anton ES. Development. 2012;139:1535–1546. doi: 10.1242/dev.069963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone DP, Srinivasan K, Chen B, Alcamo E, McConnell SK. Curr. Opin. Neurobiol. 2008;18:28–35. doi: 10.1016/j.conb.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín O, Rubenstein JL. Annu. Rev. Neurosci. 2003;26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Dimos JT, Fasano CA, Phoenix TN, Lemischka IR, Ivanova NB, Stifani S, Morrisey EE, Temple S. Nat. Neurosci. 2006;9:743–751. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- Soriano E, Dumesnil N, Auladell C, Cohen-Tannoudji M, Sotelo C. Proc. Natl. Acad. Sci. USA. 1995;92:11676–11680. doi: 10.1073/pnas.92.25.11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S-S, Breen S. Nature. 1993;362:638–640. doi: 10.1038/362638a0. [DOI] [PubMed] [Google Scholar]
- Walsh C, Cepko CL. Experientia. 1990;46:940–947. doi: 10.1007/BF01939387. [DOI] [PubMed] [Google Scholar]