Abstract
Background
Crohn disease (CD) patients with elevated Granulocyte-Macrophage Colony-Stimulating Factor auto-antibodies (GM-CSF Ab) are more likely to develop stricturing behavior requiring surgery. Computed Tomography or Magnetic Resonance Enterography (CTE or MRE) may detect luminal narrowing (LN) prior to stricture development.
Objective
To determine whether CD patients with elevated GM-CSF Ab (≥ 1.6 mcg/mL) have a higher prevalence of LN and stricturing on CTE or MRE.
Methods
A single center, cross-sectional study of 153 pediatric CD patients and controls undergoing CTE or MRE. A novel scoring system evaluated for disease activity, presence of LN, stricture, intra-abdominal abscess, or fistulae Ouutcomes were compared with respect to antibody status using Fisher's exact test, logistic regression, and the unpaired t-test.
Results
GM-CSF Ab were elevated in CD patients (n=114) with a median (IQR) GM-CSF Ab level of 2.3 mcg/mL (0.5, 6.6) compared with healthy and disease controls, p=0.001. Ileal disease location was more common in CD patients with high GM-CSF Ab, p<0.001. Luminal narrowing increased from 39% in CD patients with low GM-CSF Ab to 71% in those with high levels (p=0.004). High GM-CSF Ab remained significantly associated with LN in a multivariate logistic model. Stricturing increased from 4% in CD patients with low GM-CSF Ab to 19% in those with high GM-CSF Ab (p=0.03).
Conclusions
Pediatric CD patients with high GM-CSF Ab levels have a higher prevalence of LN on CTE or MRE. Further study will be needed to determine whether medical therapy will reduce progression to stricturing behavior in these patients.
Keywords: Pediatric Crohn disease, Granulocyte-Macrophage Colony Stimulating Factor, cytokine auto-antibody, stricture, luminal narrowing, enterography, CT enterography, MR enterography, CTE, MRE
Introduction
The incidence of Inflammatory Bowel Disease (IBD) in children has doubled over the last two decades to 2.44 cases/100,000 people/year with most new cases being Crohn Disease (CD).1 Patterns of CD behavior vary by age and are classified as inflammatory, stricturing (narrowing and blockage of the intestine), internal penetrating (development of fistulae or abscesses), or both stricturing and penetrating.2-5 Patients diagnosed during the pediatric period frequently have more aggressive disease and up to 34% of these patients require surgery within 5 years of diagnosis.2,3,6,7
Granulocyte-Macrophage Colony-Stimulating Factor auto antibodies (GM-CSF Ab) have been identified as a new biomarker in CD which is stable over time and elevated levels have been shown to be an independent risk factor for complicated behavior and ileal disease location.8 First identified in patients with pulmonary alveolar proteinosis, GM-CSF Ab affects mucosal barrier function andanti-microbial sero-reactivity in mice and in humans.8-10 Patients with CD and high GM-CSF Ab typically have ileal disease location and exhibit rapid progression of disease marked by a two-fold higher risk of strictures, penetrating disease, and surgery.8 While GM-CSF Ab has the benefit of predicting disease behavior in the absence of other biomarkers, the positive predictive value for identifying patients likely to have complicated behavior at 1 year is 21% and 52% at 5 years and is not superior to other existing biomarkers.8 These data are based on defining stricturing by the less sensitive radiographic technique small bowel follow through (SBFT), or at the time of surgery after disease has progressed.8
While SBFT is still considered an acceptable choice for small bowel imaging, computed tomography enterography (CTE) has largely replaced SBFT as the radiologic gold standard for the identification of small bowel disease in adults due to sensitivities ranging from 83-95% compared with SBFT 32-65%.11-13 CTE also has the capability to detect extra-enteric disease, a feature lacking with SBFT or capsule endoscopy.11-14 At our own institution and in other centers, investigations focused on the radiation free alternative magnetic resonance enterography (MRE) have found comparable, although slightly lower sensitivities than CTE, but available evidence indicates that both modalities should be considered acceptable options for the management of pediatric CD.12,13,15,16 Both modalities have been shown to affect changes in medical management and physician's assessment of disease activity.17-19
Application of highly sensitive enterography techniques for high-risk CD patients may permit detection of early lesions prior to the development of complications when medications might still be altered. Specifically, patients at risk for stricture such as those with high GM-CSF Ab levels would be potential beneficiaries of the combination of sensitive CTE and MRE techniques and biomarkers for disease behavior, particularly since available information about disease progression was based on less sensitive SBFT or surgery after disease had already progressed. While the detection of active inflammation using CTE and MRE features such as mucosal hyper enhancement, bowel wall thickening, and mural stratification have been shown to correlate well with histologic inflammation, early prediction of lesions that may progress to stricture has proven difficult, and there is no standard mechanism to identify radiographic features associated with progression to more severe complications such as stricture.12,13,15-17,20-24 Luminal narrowing (LN), or narrowing of the intestinal lumen without pre-stenotic dilation or fecalization suggestive of true stricture, may sometimes be indicative of early or “low-grade stenosis” without signs of obstruction and has emerged as a potential feature which may predict future stricture sites and is observed with and without other signs of inflammation.16,22,25 Patients with luminal narrowing without signs of obstruction have been shown to be more responsive to medical therapy than patients with both luminal narrowing and pre-stenotic dilation indicative of fixed stricture.25 Further investigation into this particular lesion would help determine if it is present with greater frequency in patients at highest risk for development of stricture and if these lesions progress to true stricturing disease.
We hypothesized that patients with CD and elevated GM-CSF auto-antibodies would have a significant increase in the prevalence of LN visualized by CTE and MRE. In addition, we hypothesized that patients with elevated GM-CSF Ab would have a greater cumulative number of radiologic features of disease severity and active disease using a score based on previously validated indices of mucosal inflammation.
Methods
Patients
The Institutional Review Boards at Cincinnati Children's Hospital Medical Center (CCHMC) and the University of Cincinnati (UC) approved this study. Patients were eligible for inclusion if they fulfilled the following criteria: (1) age greater than five years, (2) signed parental permission from parents and/or child's assent if over age 11, (3) clinically indicated CTE or MRE. Exclusion criteria included children five years of age or younger, and there was no upper age limit for study inclusion. A total of 51 with CD had previously undergone CTE or MRE and had GM-CSF Ab testing available and were available for inclusion. An additional 120 patients were prospectively recruited at the time of CTE or MRE. Prospectively recruited patients were included regardless of diagnosis. Clinical, diagnostic, and demographic data were collected for all patients.
GM-CSF Ab
Patients underwent blood testing for GM-CSF Ab at the time of enterography if no previously collected sample was available. GM-CSF Ab was measured in serum using enzyme-linked immunosorbent assay (ELISA) at our facility. The intra-assay coefficient of variation (CV) for the test is 4.3%, while the inter-assay CV is 10.3%. Based upon our previous study of CD patients, risk for ileal disease location and stricturing/penetrating disease behavior increased for patients with active CD in whom the GM-CSF Ab level was greater than the previously reported pediatric CD median value of 1.6 mcg/mL, and this was used as the cut-off for high vs low.8
Enterography
Patients underwent clinically indicated CTE or MRE as dictated by their primary gastroenterologist. All enterographies were performed using standard imaging protocols. Patients were not sedated and were required to have nothing by mouth for four hours prior to the procedure. Besides this, they did not undergo any bowel preparation prior to arriving for imaging. Patients consumed 20 mL/kg of low-osmolality iodinated contrast material (VoLumen; Bracco Diagnostics Inc., Princeton, NJ) one hour before the procedure to a maximum dose of 1350 mL over 30 - 45 minutes and followed by 8 oz water. Patients undergoing CT examinations were given a 2ml/kg IV dose of contrast up to a maximum of 100mL (Opti-ray; Mallinckrodt, Inc., St. Louis, MO). The exam was then performed on a helical or volume CT scanner with 3.0mm slices and no slice overlap. The tube current (mA) and kVp were adjusted by patient size/weight. Reformatted coronal images were routinely available for review. For MRI exams, patients were given a 0.3 mg of glucagon subcutaneously before precontrast images were obtained. Subsequently a second 0.3 mg dose of glucagon was given by slow IV push was given and immediately followed by a 0.1 mmol/kg IV dose of gadolinium-based contrast material (Magnevist; Bayer Healthcare Pharmaceuticals, Wayne, NJ). All MR examinations were performed on a 1.5-T MR scanner with an 8-channel phased array body or cardiac coil. Standard MRE included a minimum of nine sequences including a dynamic cine peristalsis sequence.
Each CTE or MRE test was evaluated by two pediatric radiologists blinded to the subjects GM-CSF Ab value (readers 1 and 2). Features identified as primary or secondary outcomes were labeled as present or absent and included luminal narrowing (narrowing of the intestinal lumen only with no signs of obstruction), stricture (narrowing of the lumen with pre-stenotic dilation or fecalization), intra-abdominal abscess, phlegmon, and internal fistula (see Figure 1). The entire exam was reviewed to identify areas of luminal narrowing. Surgical findings were used to confirm stricturing and penetrating outcomes if available. In the case of discrepancy between the readers or no surgery, a consensus reading was used to determine these outcomes.
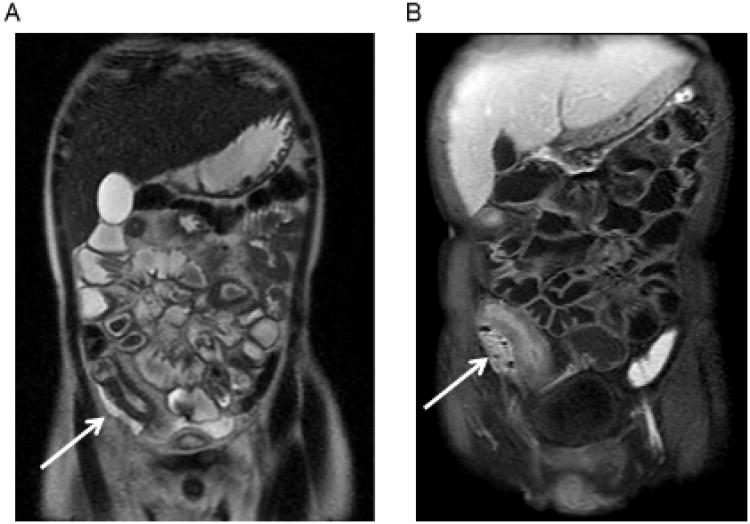
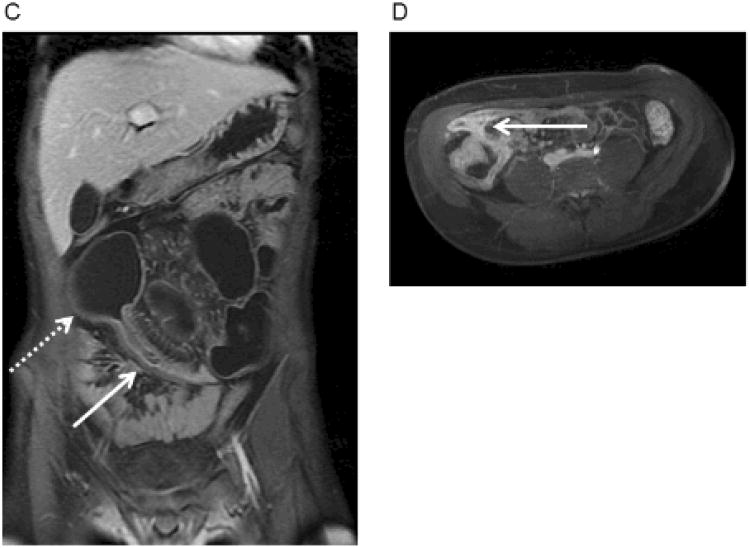
Figure 1.
Representative Images of Primary Outcomes. A) Coronal single shot fast spin echo T2 weighted MR enterography (MR-E) image demonstrates a loop of terminal ileum with wall thickening, luminal narrowing, and adjacent fluid within the right lower quadrant (arrow). B) Coronal post-contrast T1 weighted fat-saturated (FS) MR-E image demonstrates the same loop of inflamed terminal ileum with mural hyper enhancement and an adjacent enhancing phlegmon with foci of gas (arrow). There is also a small amount of fibrofatty proliferation. C) Coronal post-contrast T1 weighted FS MR-E image demonstrates a loop of mid-jejunum with mucosal hyper enhancement, mural stratification, luminal narrowing, and adjacent engorgement of the vasa recta (arrow) with proximal dilated segment (dashed arrow) indicating a stricture. D) Axial post-contrast T1 weighted FS MR-E image demonstrates an ileal to appendiceal tip fistula with mild bowel wall thickening (arrow). Note: Images 1A-C are from the same patient over the course of 11 months. Patient underwent two surgeries for jejunal, proximal ileal, and distal ileal stricture (with distal ileal abscess).
A score of disease extent or “Intestinal Severity Score” (ISS) was determined by totaling previously reported findings shown to correlate well with active inflammation as well as the number of intestinal segments affected.15-17,22,23 These features are shown in Figure, Supplemental Digital Content 1, http://links.lww.com/IBD/A186. All findings were recorded as “absent” or “present (scored “0” or “1”) with the exception of skip lesions which were scored from 0 to 3 (see Figure, Supplemental Digital Content1, http://links.lww.com/IBD/A186). Luminal narrowing, stricturing, and penetrating outcomes were not included in the ISS since these outcomes were evaluated separately. The ISS for readers 1 and 2 was averaged to derive the ISS for each subject. Inter-reader reliability was recently evaluated using MR-E at our institution and was found to be moderate with a kappa of 0.59.16 Extent and severity of disease activity were determined using the “Intestinal Severity Score” (ISS). Scores were determined by cumulative number of radiologic signs of inflammation and number of diseased segments affected. Scores were determined separately for each radiologist and averaged to determine the final result. Agreement between radiologists was determined using Pearson correlation coefficient..
Statistical analysis
Statistical analysis was performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC) and GraphPad PRISM version 5.03 (GraphPad Inc., San Diego, CA). Power analysis was performed using previously reported data that at least 30% of patients would develop complicated disease behavior after two years of disease, and that the rate of complicated disease behavior is at least 2.5 times higher for patients with high GM-CSF antibody.7,8 Under these assumptions, we predicted that a sample size of 130CD patients would have greater than 80% power with α=0.05 to detect a difference in stricturing between those with high and low GM-CSF Ab. Continuous variables were analyzed using the unpaired t-test and dichotomous variables using Fisher's exact test, ANOVA, or a nonparametric alternative. Multiple variable logistic regression was used to further evaluate the relationship between GM-CSF Ab levels (high or low using a 1.6 mcg/mL cut-off) and luminal narrowing while controlling for age, gender, duration of disease, and disease location.
Results
Clinical and Demographic Features
A total of 169 patients were enrolled in the study, but 14 did not complete either the CTE/MRE examination or GM-CSF Ab testing and were not considered for analysis. Two patients were prospectively enrolled at the time of enterography but had already undergone GM-CSF Ab testing and at least one CTE or MRE examination and were considered duplicates. Non-completers did not complete the radiology portion of the examination due to a change in clinical decision or patient preference. The remaining 153 patients included114 with CD and 39 healthy and disease controls. Patients without CD and conditions not typically associated with elevated GM-CSF Ab levels or small bowel disease were considered controls. Patients with chronic abdominal pain accounted for 56% of controls, while the remainder had either ulcerative colitis (UC, n=7), indeterminate colitis (IC, n=2), or “other diagnoses”, n=8. There was no significant difference in gender, age, or race between the CD patients or the healthy and disease controls (data not shown). MR-E examinations accounted for 74% of all examinations for patients with and without CD.
Serum GM-CSF Ab Concentration is Increased in CD Patients
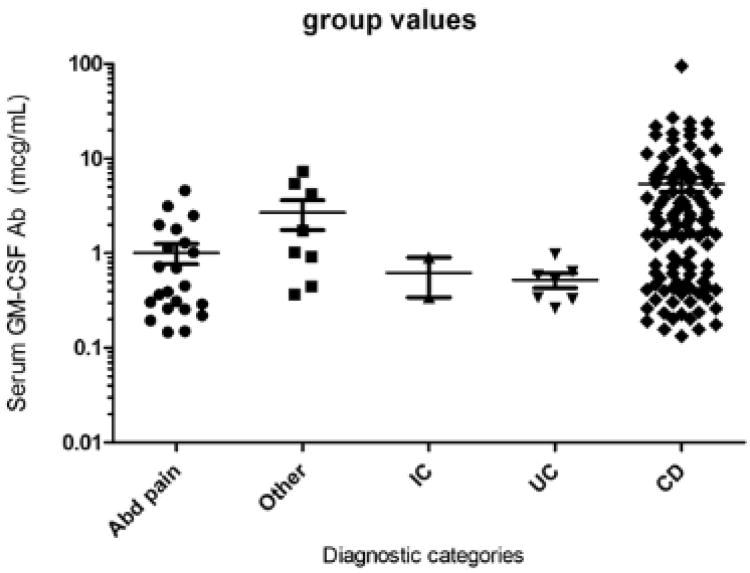
As expected, the median (IQR) GM-CSF Ab level for CD patients was significantly elevated at 2.3 mcg/mL (0.5, 6.6) compared with the non-CD median of 0.6mcg/mL (0.3, 1.3), p<0.001. GM-CSF Ab levels varied between diagnostic groups with the highest levels present in patients with CD (Figure 2). When groups were compared individually using the Kruskal-Wallis test, the CD median(IQR) of 2.3 mcg/mL (0.5, 6.6) was significantly higher than in patients with chronic abdominal pain which had a median (IQR) of 0.4 mcg/mL (0.6, 1.3), p<0.05. Comparisons between other diagnoses were not significant, likely due to low group numbers.
Figure 2.
Scatter plot of serum GM-CSF Ab Concentration by Disease Categories. Serum GM-CSF Ab concentration was measured by ELISA and is shown as the median(IQR). Differences between groups were tested with the Kruskal-Wallis test, **p<0.009. Among CD patients (n=114), the median (IQR) GM-CSF Ab was 2.3 mcg/mL (0.5, 6.6) and was significantly higher than that of the chronic abdominal pain patients (n=22) with a median (IQR) of 0.4 mcg/mL (0.6, 1.3), p<0.05. Note: Abd pain=abdominal pain, IC = Indeterminate Colitis, UC = Ulcerative Colitis.
GM-CSF Ab and Crohn Disease Characteristics
CD patients were examined with respect to GM-CSF Ab status. Antibody status was categorized as high or low based on our previous data showing increased risk of strictures and surgeries for patients with serum concentration ≥ 1.6mcg/mL.8 The mean(SD) age of the CD patients was 15 (6) years with a mean(SD) disease duration of 2.8 (3.2) years, neither of which differed by serum GM-CSF Ab concentration (Table 1). Patients diagnosed within two months of enrollment accounted for 30% (n=37) of the cohort. The mean(SD) follow-up period after CTE or MRE was equal to 9.4 (11) months and did not differ by serum GM-CSF Ab concentration. Similar to the entire cohort, MR-E accounted for 74% of enterography examinations for patients with CD and did not differ by antibody status (Table 2). Gender, race, and age were similar between groups.
Table 1. Clinical and Demographic Characteristics of Crohn Disease Patients.
| GM-CSF Ab Low (n=49) | GM-CSF Ab High (n=65) | |
|---|---|---|
| Age, years (SD) | 14.4 (7.8) | 15.4 (4.0) |
| Females, n (%) | 24 (49%) | 34 (52%) |
| Race | ||
| Cauc, (n,%) | 44 (90%) | 59 (91%) |
| AA, n(%) | 4 (8%) | 4 (6%) |
| MRE, n (%) | 35 (71%) | 49 (75%) |
| Disease duration, years (SD) | 2.8 (2.8) | 2.9 (3.6) |
| Duration if no previous surgery, years (SD) | 2.5 (2.7) | 2.0 (3.0) |
| Follow-up, months (SD) | 9 (10) | 10 (12) |
| Ileal location (L1,L3), n (%) | 27 (55%) | 60 (92%)** |
| Complex disease behavior | 10 (20%) | 29 (45%)* |
| B2(stricturing), n (%) | 3 (6%) | 16 (25%)* |
| B3 (penetrating), n (%) | 2 (4%) | 3 (5%) |
| B2B3 (both stricturing and penetrating), n (%) | 5 (10%) | 10 (15%) |
p-value <0.01,
p-value <0.001.
All other comparisons not significant with p-value >0.05. GM-CSF Ab High defined as ≥ 1.6mcg/mL.Cauc: Caucasian, AA: African-American, L1: ileum-only, L3: ileo-colonic location. Follow-up since enterography study.
Table 2. Logistic Regression for GM-CSF Ab and Luminal Narrowing.
| Odds Ratio (95th CI) | p-value | |
|---|---|---|
| Luminal narrowing | ||
| GM-CSF Ab high vs low | 2.88 (1.02-8.11) | 0.046 |
| Ileal/ileocolonic disease location (L1/L3 vs L2) | 2.22 (0.74-6.70) | 0.157 |
| Female vs male | 0.63 (0.23-1.66) | 0.348 |
| Age at procedure | 1.02 (0.89-1.18) | 0.771 |
| Duration of disease | 0.91 (0.76-1.09) | 0.287 |
GM-CSF Ab high defined as ≥ 1.6mcg/mL. Forced inclusion of gender, age, and duration of disease were performed for the model of luminal narrowing. L1: ileum-only, L2: colon-only, L3: ileo-colonic location.
Patients were classified according to the Paris Classification system for categorization of pediatric IBD phenotypes.5 Using the Paris age groupings by age at diagnosis, 70% of patients were categorized as A1b (age 10 to 17 at diagnosis) and the remainder were A1a or A2. There was no difference in age at diagnosis between the high and low GM-CSF Ab groups (data not shown). As expected, 92% of the GM-CSF Ab high patients exhibiting ileal disease location compared with 55% of those with lower GM-CSF Ab, p<0.001 (Table 1). Complicated disease behavior (Paris criteria B2, B3, or B2B3) was seen in 45% of high GM-CSF Ab patients, compared with 20% of the GM-CSF Ab low patients, p<0.01. When separated out by individual Paris criteria for behavior, there was no difference in prevalence of penetrating behavior or combination stricturing/penetrating behavior, but the prevalence of stricturing behavior alone was nearly four times higher in patients with high compared with low GM-CSF Ab, p<0.01 (Table 1).
Previous medication exposures were examined with respect to GM-CSF antibody status. Patients with both high and low GM-CSF Ab had high rates of exposure to corticosteroids, aminosalicylates, immuno modulators (methotrexate, 6-mercaptopurine, azathioprine), and anti-tumor necrosis factor (anti-TNF) agents such as infliximab, with 40-85% of patients having been exposed to each medication (data not shown). With the exception of oral corticosteroids, there was no difference in exposure to each medication class when patients were stratified by antibody status. Patients with high GM-CSF Ab were less likely to have been exposed to corticosteroids with 63% of patients having been exposed, compared with 85% of the low antibody group, p = 0.03. Neither methotrexate nor topical steroid use differed by GM-CSF antibody status, and both were used infrequently compared to other medications with only 14-16% of patients being exposed to either medication class (data not shown). There was no difference in medication exposure with respect to the primary outcome of luminal narrowing.
Serum GM-CSF Ab is Increased in CD Patients with LN or Stricturing on Enterography
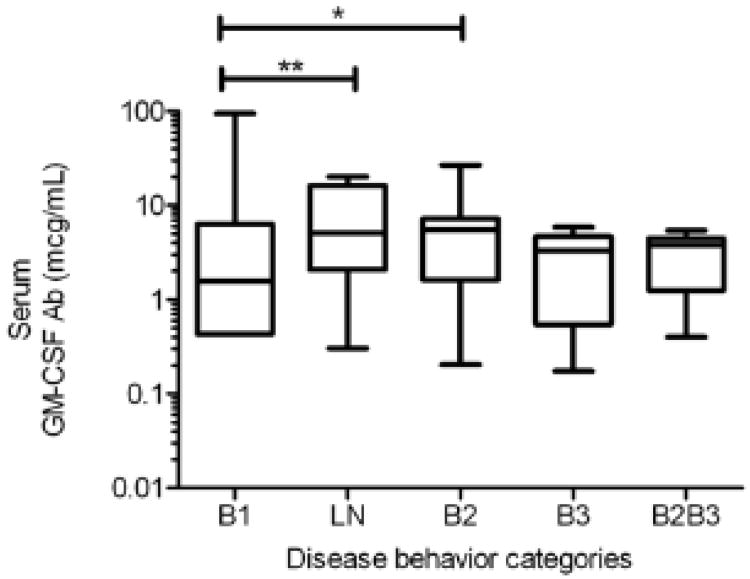
It was not known whether GM-CSF Ab would be elevated in CD patients prior to the onset of stricturing behavior. To test this, we compared the serum GM-CSF Ab concentration between CD patients with inflammatory behavior (B1) and either LN, stricturing, or internal penetrating behavior on the CTE or MRE exam. We found that the median (IQR) was significantly different when compared by Kruskal-Wallis, with differences between inflammatory and either LN or stricturing behaviors (see Figure 3). The median(IQR) serum GM-CSF Ab concentration did not differ between the CD patients with internal penetrating and inflammatory behavior, although this comparison was likely not adequately powered. The serum GM-CSF Ab concentration did not differ between CD patients with LN versus stricturing, demonstrating that GM-CSF Ab are elevated in CD patients with LN alone, prior to the development of stricturing.
Figure 3.
Box and Whisker plot of serum GM-CSF Ab Concentration by Disease Behavior Categories. Serum GM-CSF Ab concentration was measured by ELISA and is shown as the median(IQR). Differences between groups were tested with the Kruskal-Wallis test. The serum GM-CSF Ab concentration increased from 0.8 (0.4, 2.3) mcg/mL in CD patients with inflammatory behavior (B1), to 5.1 (2.1,16.3) mcg/mL in those with LN, and 5.5 (1.6,7.2) in those with stricturing behavior (B2). *p=0.03, **p=0.002. Note: Disease behavior categories defined using Paris Criteria. B1: Inflammatory only, LN: Inflammatory with luminal narrowing, B2: Stricturing only, B3: penetrating only, B2B3: Both stricturing and penetrating behavior
GM-CSF Ab and Overall Intestinal Disease Activity
The average ISS trended towards a higher value for CD patients with high GM-CSF Ab, with a mean(SD) of 6.2 (3.0) compared to a score of 4.9 (4.0) in those with low GM-CSF Ab, but this difference was not significant with p=0.07. When ISS scores were examined across diagnostic categories, there was a difference between groups with p<0.001. The ISS differed between CD patients and abdominal pain patients with a CD mean (SD) ISS of 5.6 (3.7) and abdominal pain with an ISS of 1.3 (1.8) and other diagnoses 1.4 (1.2), p<0.05. There was no statistical difference in the ISS between CD, UC, or IC. ISS features most commonly reported in abdominal pain patients included prominent lymph nodes, free fluid, and bowel wall thickening without other signs of inflammation. Radiologists were noted to have highly correlated ISS scores with a Pearson correlation coefficient of 0.81, p<0.001.
Patient symptoms collected at the time of CTE or MRE were classified according to the Short Pediatric Crohn's Disease Activity Index (Short PCDAI) for patients with CD.26 Patients with UC or IC were classified by the Pediatric Ulcerative Colitis Activity Index (PUCAI).26,27 ShortPCDAI scores for the CD patients indicated a mean (SD) of 28.8 (19.7). A cut-off of 30 is typically used to differentiate between mild and moderate disease activity and suggested most patients had mild or moderate disease.26 Among patients with CD, there was no difference in Short PCDAI score by antibody status, luminal narrowing, or stricture. PUCAI scores were high for both IC and UC patients and did not differ significantly, with an overall average of 32.2 (17.9) indicating moderate disease activity.
Luminal Narrowing and Stricturing on Enterography are Increased in CD Patients with Elevated GM-CSF Ab
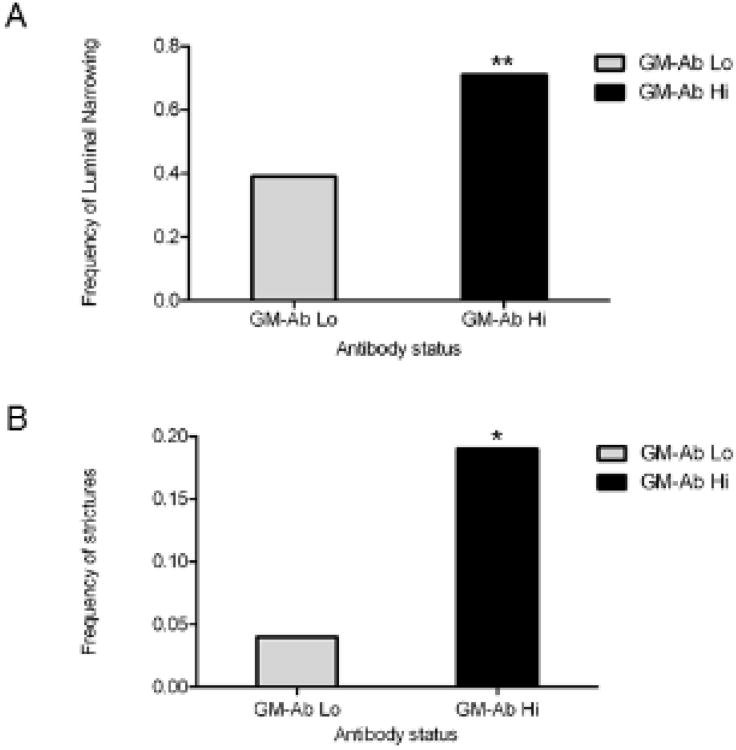
We then asked whether LN or stricturing would be more common in those with elevated GM-CSF Ab. CD patients who had previously undergone surgery for stricturing or penetrating behavior were excluded from analysis of LN, stricturing, or penetrating behavior. Luminal narrowing on CTE or MRE examination was investigated as a potential marker for future stricture sites, so patients with patients with stricturing or penetrating outcomes were not included in this analysis. Luminal narrowing was detected in 71% of patients with high GM-CSF antibody compared with 39% of patients with low GM-CSF antibody levels, p=0.004 (Figure 4A).
Figure 4.
Frequency of Primary Outcomes Among CD Patients with High vs Low Serum GM-CSF Ab Concentration. The frequency of A) LN or B) stricturing is shown with differences between groups tested using Fisher's exact test. A) Increased prevalence of luminal narrowing in patients with CD and high GM-CSF Ab (71%) compared with 39% of patients with low levels, **p=0.004. B) Increased prevalence of strictures in patients with high GM-CSF Ab (19%), compared with those with low levels (4%), *p=0.03.
While the use of standard reporting techniques is felt to provide accurate interpretation of luminal narrowing, particularly during CTE in which a limited number of images are obtained, a secondary analysis of only MRE exams was performed in an effort to remove any potential bias from CTE examinations. In this analysis, 62 MRE examinations were evaluated, and luminal narrowing was observed in 69% of patients with high GM-CSF Ab (n=35) compared with 33% of those with low antibody levels (n=27), p<0.005. Luminal narrowing was not observed in any patients with chronic abdominal pain or other diagnoses. Four patients with UC or IC had rectal luminal narrowing related to under-distension of the colon.
Multiple logistic regression analysis was conducted to observe the effect of GM-CSF Ab on luminal narrowing. Disease location, gender, duration of disease, and age at procedure were controlled for in the analysis. In the univariate analysis, only disease location and GM-CSF Ab were strongly associated with luminal narrowing, but in an effort to allow for potential confounding between variables, all variables were included in the final analysis. After controlling for these clinical and demographic variables, results confirmed that elevated GM-CSF Ab ≥ 1.6mcg/mL is associated with luminal narrowing on CTE or MRE exam (Table 2).
CTE or MRE was used to determine the presence or absence of stricturing and penetrating outcomes, and was correlated with surgical pathology as the gold standard to confirm these findings. In cases of a discordant reading between radiologists, a consensus radiologic report was obtained. Among the 100 CD patients who had not previously undergone surgery for complicated behavior, the prevalence of penetrating outcomes detected by CTE and MRE did not differ between the high and low GM-CSF antibody groups. The CD patients with elevated GM-CSF Ab were almost five times as likely to have a stricture at the time of CTE or MRE (see Figure 4B). Of the 12 patients who were identified as having stricture by CTE or MRE, 75% (n=9) ultimately underwent surgery which confirmed stricture. The remaining patients (n=3) were managed medically. Collectively, these results confirmed a higher rate of LN and stricturing on CTE and MRE exams performed in patients with elevated GM-CSF Ab.
Discussion
Previous studies and statistical models incorporating existing biomarkers such as ASCA, OmpC, and CBir1 and genetic polymorphisms in the NOD2 gene may predict complicated disease behavior.28,29 While helpful in some instances, available biomarkers have limitations, particularly in the pediatric IBD population. Biomarkers such as ASCA and pANCA may be absent in 50-70% of children who require early surgery.30 In addition, biomarker patterns may change with age and could limit their utility depending on when they are measured.31 In an effort to improve the use of biomarkers to predict disease behavior, we present the first study to combine a biomarker, GM-CSF Ab, with a sensitive radiologic technique, CTE and MRE, in order to guide future efforts to target these high-risk individuals for more aggressive screening or medications earlier in disease course.
We propose that luminal narrowing on CTE and MRE may suggest sites of early stricture formation. The results of this study confirmed a significantly higher rate of luminal narrowing in patients with high GM-CSF Ab levels. Although the approach to luminal narrowing employed by radiologists involved in this study conforms to standard of care, due to concerns that CTE may falsely identify luminal narrowing, a secondary analysis using MRE only confirmed a higher prevalence of luminal narrowing for patients with high GM-CSF Ab.32 While these patients have been followed for only a short time, both patients who progressed to stricture had elevated GM-CSF Ab and one had luminal narrowing. While this study was not powered sufficiently to determine if this progression to stricture is significant, the high correlation between GM-CSF Ab and luminal narrowing in a group at high risk for stricturing suggests that luminal narrowing and progression to stricture warrants further investigation.
Prospective evaluation of luminal narrowing as a risk for progression to future stricturing is not described in the literature, but this data serves as a first step to identify CTE or MRE features which may help improve the identification of early stricture sites in high-risk patients. The presence of both luminal narrowing and pre-stenotic dilation, or “hold-up,” on CTE and MRE examination has been used as the strict radiologic definition of stricture.21,33 Using these criteria, enterography has been shown to accurately identify strictures and is better than physician clinical assessment to determine the presence of true stricture.17,33 Patients with both luminal narrowing and “hold-up” (or pre-stenotic dilation) are less likely to respond to medical therapy than patients with luminal narrowing alone.25 Responsiveness of luminal narrowing to medical therapy supports the idea that these lesions may represent strictures at a stage when medical therapy may change progression of disease.25 These areas of luminal narrowing may progress to future stricture development in patients with high risk for stricture such as those with high GM-CSF Ab. Future studies will be necessary to determine if early treatment with medications such as infliximab may prevent progression to clinically evident stricture.
The secondary outcome of this study was to confirm that enterography would identify a higher prevalence of complicated disease behavior. While we found a significant increase in both prevalence of strictures and progression to surgery during follow-up, the prevalence of penetrating outcomes was not significantly different among patients with high versus low GM-CSF Ab. In the initial cohort published by Han et al, stricturing and penetrating outcomes were analyzed collectively.8 Further analyses of larger prospective cohorts may help define if the presence of GM-CSF Ab increases the risk for stricturing alone, or if the current findings resulted from under powering.
In addition to evaluation of stricturing and penetrating outcomes, we attempted to evaluate whether patients with elevated GM-CSF Ab showed a greater disease extent and severity on enterography. In the absence of a simple and universally accepted score to assess disease severity using enterography, we created the ISS as a cumulative score of previously reported and validated individual radiologic features associated with active CD, but for simplicity did not judge severity of individual lesions.11-13,15,16,22,24,33 Despite the excellent correlation between CTE and MRE findings and inflammation and the development of scoring tools such as the “Magnetic Resonance Index of Activity” Score (MaRIA) for adults, available tools can be cumbersome and there is no validated scoring tool for disease activity in pediatrics.22,23 We did not attempt to stratify features that have been associated with inflammation (mucosal hyper enhancement) versus fibrosis (bowel wall thickening) since these associations are not uniformly agreed upon.16,20,21,24,25 Among our cohort of patients we did not observe a statistical difference in the ISS between high and low GM-CSF Ab patients. All patients were undergoing enterography due to concerns of active disease at the time of enterography and had similar patient reported symptoms according to the Short PCDAI. While assessment of inflammation alone did not prove different between high and low antibody groups, luminal narrowing has been described in the absence of inflammation and was quite different between groups.16,19,25 The presence of similar levels of inflammation and patient reported symptoms using the short PCDAI suggests that the use of other data such as luminal narrowing may prove to be a useful tool to predict disease progression for patients at high risk of early severe disease behavior.
While cross-sectional in nature, the phenotype of CD patients enrolled in the study confirmed the expected phenotype of patients with high versus low GM-CSF Ab. Patients exhibited increased prevalence of ileal disease location (L1/L3) and early complicated disease behavior (B2, B3, or B2B3) for individuals with high GM-CSF Ab reproduced the findings in the original cohort described by Han et al in which high antibody levels were shown to be independently associated with both ileal disease and disease behavior.8 The prevalence of any complicated disease behavior was significantly different by antibody status, but the difference derived predominantly from differences in stricturing prevalence which was higher in patients with high GM-CSF Ab (see Table 1). Outcomes at enterography again suggested this trend, with a difference in stricturing but not in penetrating behavior. Our findings recapitulate the relevance of GM-CSF Ab as a risk factor for early complicated disease behavior, particularly stricturing.
Although the results of this study suggest important implications for the combination of sensitive radiologic techniques with existing biomarkers, this study did have some unique strengths and limitations. Our institution has the benefit of being both a regional referral center as well as a large quaternary medical center, so the results should be generalizable to other patients with CD. Other strengths include highly experienced radiologists with a special interest in using enterography for pediatric patients. Previous studies have found fair to good interobserver agreement for both CTE and MRE with kappa values ranging from 0.3-0.9 for most parameters, and a recently published study at our institution found interobserver agreement to be good with kappa of 0.59.12,13,16,19,34 While the use of both CTE and MRE may be a potential limitation, previous studies have shown similar sensitivities, specificities, and intermodality agreement between the two techniques, we felt that this study would provide valid results representative of clinical practice in which both techniques will be utilized.12,13,34 The combination of techniques in this study may have created the potential for selection bias, however, so we attempted to minimize this by enrolling consecutive patients undergoing either CTE or MRE. The cross-sectional study design is a slight limitation in that patients entered the study at varying stages of disease. Nonetheless, the patient characteristics described here support previously described data and suggest that the findings of this study are reproducible. While a true prospective study would determine with more certainty the prevalence of luminal narrowing and the progression to stricture in patients with high GM-CSF Ab using enterography, this data will facilitate future studies aimed at earlier identification of complications and medication response in this vulnerable population. The ISS as a tool to evaluate disease extent and activity is a limitation as well since it is not a validated instrument, but in the absence of an accepted tool, this score allowed us to gather a general idea about overall disease activity differences between patients. In this study a few healthy and disease controls had GM-CSF Ab levels higher than the 1.6mcg/mL cut-off, but mild elevations of GM-CSF Ab have been reported in healthy individuals.35,36 Despite numerically different group medians, our study was not powered to appropriately assess the difference in antibody levels by diagnostic categories that had been previously described by Han et al.8
This is the first effort to correlate enterography findings with biomarker status. Future prospective studies may help further characterize luminal narrowing as a predictor of stricturing behavior in high-risk individuals. Studies aimed at evaluation of medication response and timing of progression to surgery will provide more information about these lesions and the implication for use in clinical care. Ultimately, the incorporation of both GM-CSF Ab and enterography data into existing risk prediction models may provide additional benefit for patients with CD.
Acknowledgments
This work was supported by NIH grants R01 DK078683 (LAD) and T32 DK007727 (DD). This investigation was supported by Public Health Service research grant UL1-RR025764 and CO6-RR11234 from the National Center for Research Resources.
Thanks to James Franciosi, MD for his support of this work.
Grant Support: This work was supported by NIH grants R01 DK078683 (LAD) and T32 DK007727 (DD). This investigation was supported by Public Health Service research grant UL1-RR025764 and CO6-RR11234 from the National Center for Research Resources.
Abbreviations
- Ab
auto-antibody
- CI
confidence interval
- B1
inflammatory behavior
- B2B3
stricturing or internal penetrating behavior
- CTE
computed tomography enterography
- GM-CSF Ab
Granulocyte-Macrophage Stimulating Factor auto-antibodies
- IBD
Inflammatory Bowel Disease
- L1
ileum-only location
- L2
colon-only location
- L3
ileo-colonic location
- LN
luminal narrowing
- MRE
magnetic resonance enterography
- OR
odds ratio
- ISS
Intestinal Severity Score
Footnotes
Financial disclosures: The authors have no financial arrangement(s) with a company whose product figures prominently in the submitted manuscript or with a company making a competing product.
Writing assistance: not applicable
Competing Interest: None to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: Study concept and design: DD, LD, JH
Acquisition of data: DD, AT, EB, CC, RB, KL, DP, BT, LD
Analysis and interpretation of data: DD, AT, MK, DP, LD
Drafting of the manuscript: DD, LD
Critical revision of the manuscript for important intellectual content: DD, AT, DP, BT, LD, JH
Statistical analysis: DD, MK, LD
Obtained funding: BT, LD
Administrative, technical, or material support: EB, CC, RB, KL
Study supervision: DD, AT, BT, DP, LD
References
- 1.Malaty HM, Fan X, Opekun AR, Thibodeaux C, Ferry GD. Rising incidence of inflammatory bowel disease among children: a 12-year study. J Pediatr Gastroenterol Nutr. 2010;50:27–31. doi: 10.1097/MPG.0b013e3181b99baa. [DOI] [PubMed] [Google Scholar]
- 2.Polito JM, 2nd, Childs B, Mellits ED, Tokayer AZ, Harris ML, Bayless TM. Crohn's disease: influence of age at diagnosis on site and clinical type of disease. Gastroenterology. 1996;111:580–6. doi: 10.1053/gast.1996.v111.pm8780560. [DOI] [PubMed] [Google Scholar]
- 3.Schaefer ME, Machan JT, Kawatu D, et al. Factors that determine risk for surgery in pediatric patients with Crohn's disease. Clin Gastroenterol Hepatol. 2010;8:789–94. doi: 10.1016/j.cgh.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Van Limbergen J, Russell RK, Drummond HE, et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology. 2008;135:1114–22. doi: 10.1053/j.gastro.2008.06.081. [DOI] [PubMed] [Google Scholar]
- 5.Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011;17:1314–21. doi: 10.1002/ibd.21493. [DOI] [PubMed] [Google Scholar]
- 6.Heyman MB, Kirschner BS, Gold BD, et al. Children with early-onset inflammatory bowel disease (IBD): analysis of a pediatric IBD consortium registry. J Pediatr. 2005;146:35–40. doi: 10.1016/j.jpeds.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 7.Vernier-Massouille G, Balde M, Salleron J, et al. Natural history of pediatric Crohn's disease: a population-based cohort study. Gastroenterology. 2008;135:1106–13. doi: 10.1053/j.gastro.2008.06.079. [DOI] [PubMed] [Google Scholar]
- 8.Han X, Uchida K, Jurickova I, et al. Granulocyte-macrophage colony-stimulating factor auto antibodies in murine ileitis and progressive ileal Crohn's disease. Gastroenterology. 2009;136:1261–71. e1–3. doi: 10.1053/j.gastro.2008.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchida K, Beck DC, Yamamoto T, et al. GM-CSF auto antibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. N Engl J Med. 2007;356:567–79. doi: 10.1056/NEJMoa062505. [DOI] [PubMed] [Google Scholar]
- 10.Nylund CM, D'Mello S, Kim MO, et al. Granulocyte macrophage-colony-stimulating factor auto antibodies and increased intestinal permeability in Crohn disease. J Pediatr Gastroenterol Nutr. 2011;52:542–8. doi: 10.1097/MPG.0b013e3181fe2d93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solem CA, Loftus EV, Jr, Fletcher JG, et al. Small-bowel imaging in Crohn's disease: a prospective, blinded, 4-way comparison trial. Gastrointest Endosc. 2008;68:255–66. doi: 10.1016/j.gie.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Lee SS, Kim AY, Yang SK, et al. Crohn Disease of the Small Bowel: Comparison of CT Enterography, MR Enterography, and Small-Bowel Follow-Through as Diagnostic Techniques. Radiology. 2009;251:751–61. doi: 10.1148/radiol.2513081184. [DOI] [PubMed] [Google Scholar]
- 13.Siddiki HA, Fidler JL, Fletcher JG, et al. Prospective Comparison of State-of-the-Art MR Enterography and CT Enterography in Small-Bowel Crohn's Disease. American Journal of Roentgenology. 2009;193:113–21. doi: 10.2214/AJR.08.2027. [DOI] [PubMed] [Google Scholar]
- 14.Bruining DH, Siddiki HA, Fletcher JG, Tremaine WJ, Sandborn WJ, Loftus EV., Jr Prevalence of penetrating disease and extra intestinal manifestations of Crohn's disease detected with CT enterography. Inflamm Bowel Dis. 2008;14:1701–6. doi: 10.1002/ibd.20529. [DOI] [PubMed] [Google Scholar]
- 15.Messaris E, Chandolias N, Grand D, Pricolo V. Role of magnetic resonance enterography in the management of Crohn disease. Arch Surg. 2010;145:471–5. doi: 10.1001/archsurg.2010.68. [DOI] [PubMed] [Google Scholar]
- 16.Wallihan DB, Towbin AJ, Denson LA, Salisbury S, Podberesky DJ. Inflammatory Bowel Disease in Children and Adolescents: Assessing the Diagnostic Performance and Interreader Agreement of Magnetic Resonance Enterography Compared to Histopathology. Acad Radiol. 2012 doi: 10.1016/j.acra.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 17.Vogel J, da Luz Moreira A, Baker M, et al. CT enterography for Crohn's disease: accurate preoperative diagnostic imaging. Dis Colon Rectum. 2007;50:1761–9. doi: 10.1007/s10350-007-9005-6. [DOI] [PubMed] [Google Scholar]
- 18.Bruining DH, Siddiki HA, Fletcher JG, et al. Benefit of computed tomography enterography in Crohn's disease: effects on patient management and physician level of confidence. Inflamm Bowel Dis. 2012;18:219–25. doi: 10.1002/ibd.21683. [DOI] [PubMed] [Google Scholar]
- 19.Horsthuis K, de Ridder L, Smets AM, et al. Magnetic resonance enterography for suspected inflammatory bowel disease in a pediatric population. J Pediatr Gastroenterol Nutr. 2010;51:603–9. doi: 10.1097/MPG.0b013e3181dee5bd. [DOI] [PubMed] [Google Scholar]
- 20.Fornasa F, Benassuti C, Benazzato L. Role of Magnetic Resonance Enterography in Differentiating between Fibrotic and Active Inflammatory Small Bowel Stenosis in Patients with Crohn's Disease. J Clin Imaging Sci. 2011;1:35. doi: 10.4103/2156-7514.82339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adler J, RP D, Dillman JR, et al. Computed tomography enterography findings correlate with tissue inflammation, not fibrosis in resected small bowel Crohn's disease. Inflamm Bowel Dis. 2012;18:849–56. doi: 10.1002/ibd.21801. [DOI] [PubMed] [Google Scholar]
- 22.Horsthuis K, Bipat S, Stokkers PC, Stoker J. Magnetic resonance imaging for evaluation of disease activity in Crohn's disease: a systematic review. Eur Radiol. 2009;19:1450–60. doi: 10.1007/s00330-008-1287-0. [DOI] [PubMed] [Google Scholar]
- 23.Rimola J, Rodriguez S, Garcia-Bosch O, et al. Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn's disease. Gut. 2009;58:1113–20. doi: 10.1136/gut.2008.167957. [DOI] [PubMed] [Google Scholar]
- 24.Rimola J, Ordas I, Rodriguez S, et al. Magnetic resonance imaging for evaluation of Crohn's disease: validation of parameters of severity and quantitative index of activity. Inflamm Bowel Dis. 2011;17:1759–68. doi: 10.1002/ibd.21551. [DOI] [PubMed] [Google Scholar]
- 25.Lawrance IC, Welman CJ, Shipman P, Murray K. Correlation of MRI-determined small bowel Crohn's disease categories with medical response and surgical pathology. World J Gastroenterol. 2009;15:3367–75. doi: 10.3748/wjg.15.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kappelman MD, Crandall WV, Colletti RB, et al. Short pediatric Crohn's disease activity index for quality improvement and observational research. Inflamm Bowel Dis. 2011;17:112–7. doi: 10.1002/ibd.21452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multi center study. Gastroenterology. 2007;133:423–32. doi: 10.1053/j.gastro.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 28.Siegel CA, Siegel LS, Hyams JS, et al. Real-time tool to display the predicted disease course and treatment response for children with Crohn's disease. Inflamm Bowel Dis. 2011;17:30–8. doi: 10.1002/ibd.21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lichtenstein GR, Targan SR, Dubinsky MC, et al. Combination of genetic and quantitative serological immune markers are associated with complicated Crohn's disease behavior. Inflamm Bowel Dis. 2011;17:2488–96. doi: 10.1002/ibd.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amre DK, Lu SE, Costea F, Seidman EG. Utility of serological markers in predicting the early occurrence of complications and surgery in pediatric Crohn's disease patients. Am J Gastroenterol. 2006;101:645–52. doi: 10.1111/j.1572-0241.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- 31.Markowitz J, Kugathasan S, Dubinsky M, et al. Age of diagnosis influences serologic responses in children with Crohn's disease: a possible clue to etiology? Inflamm Bowel Dis. 2009;15:714–9. doi: 10.1002/ibd.20831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ilangovan R, Burling D, George A, Gupta A, Marshall M, Taylor SA. CT enterography: review of technique and practical tips. Br J Radiol. 2012;85:876–86. doi: 10.1259/bjr/27973476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins PD, Caoili E, Zimmermann M, et al. Computed tomographic enterography adds information to clinical management in small bowel Crohn's disease. Inflamm Bowel Dis. 2007;13:262–8. doi: 10.1002/ibd.20013. [DOI] [PubMed] [Google Scholar]
- 34.Jensen MD, Ormstrup T, Vagn-Hansen C, Ostergaard L, Rafaelsen SR. Interobserver and intermodality agreement for detection of small bowel Crohn's disease with MR enterography and CT enterography. Inflamm Bowel Dis. 2011;17:1081–8. doi: 10.1002/ibd.21534. [DOI] [PubMed] [Google Scholar]
- 35.Uchida K, Nakata K, Suzuki T, et al. Granulocyte/macrophage-colony-stimulating factor auto antibodies and myeloid cell immune functions in healthy subjects. Blood. 2009;113:2547–56. doi: 10.1182/blood-2009-05-155689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Svenson M, Hansen MB, Ross C, et al. Antibody to granulocyte-macrophage colony-stimulating factor is a dominant anti-cytokine activity in human IgG preparations. Blood. 1998;91:2054–61. [PubMed] [Google Scholar]