Abstract
Recent studies implicate the NLRP3 inflammasome pathway in elaborating sterile inflammation. CD36 serves a dual role by priming Il1b transcription and inducing assembly of the NLRP3 inflammasome complex, leading to the release of active IL-1β.
The study of innate immune responses in mammals has focused heavily on the role of various pattern recognition receptors (PRRs) in responding to microorganisms or their products. As postulated by Janeway1, the pressure that drove the evolution of modern innate immune systems was likely the need to prevent microbial colonization. PRRs such as the Toll-like receptors (TLRs), RIG-I like receptors (RLRs), NOD-like receptors (NLRs) and various C-type lectin receptors (CLRs) evolved to detect microbes in germ-free tissues of our bodies2. It is therefore logical that most studies of PRR function have focused on their role in infection. In addition to the evolutionary implications of studying PRRs in the context of infection, ample clinical evidence supports the idea that defects in TLR functions predispose humans to microbial infections that “wild-type” humans control. A new study by Sheedy et al. shows that the scavenger receptor CD36 has a critical role in activation of the NLRP3 inflammasome during sterile inflammation.
An interesting outcome of the research done on PRRs in controlling infections is the recent appreciation that PRRs also contribute to non-infectious maladies. Although not caused by microbial infections, these diseases are nevertheless associated with so-called “sterile” inflammatory responses that contribute to disease. For example, PRR-induced inflammation appears to underlie the symptoms associated with atherosclerosis, Alzheimer’s disease and type 2 diabetes. While these studies clearly underscore the clinical importance of PRRs in non-infectious disorders, we know very little about the underlying mechanisms by which PRRs operate in this context. Among the PRRs implicated in sterile inflammation, the NLRs seem to have attracted the most attention4,5. Most of the NLR family members are unusual PRRs, in that they do not function to upregulate the transcription of inflammatory cytokines, chemokines or interferons. Rather than regulating cytokine gene expression, many NLRs trigger the secretion of cytokines of the interleukin 1 (IL-1) family. Secretion of IL-1 is accomplished through the ability of NLRs to assemble cytoplasmic protein complexes called inflammasomes. The inflammasome is a protein processing machine that uses proteases to cleave pro-forms of IL-1 in the cytosol, which then somehow become secreted to induce inflammation. Because NLRs cannot activate transcription, yet they promote the secretion of inducible cytokines, NLRs usually depend on other PRRs to induce cytokine expression. As such, current models of infection-induced immune responses mediated by the IL-1 family state that two signals are necessary. Signal 1 is provided by a transcription-inducing PRR (for example, TLR signaling), which upregulates the expression of IL-1 family members and some NLRs themselves. Signal 2 is then provided that activate the NLR to assemble an inflammasome and cause the secretion of pro-inflammatory IL-1 family members6. In an interesting turn of events, Sheedy et al. now report that the scavenger receptor CD36 can provide both signal 1 and 2 to promote inflammasome activation by sterile stimuli3. This finding helps explain the importance of CD36 in atherosclerosis7, and reveals novel means by which the innate immune system can be differentially activated by microbes or endogenous triggers.
Sheedy et al.3 focused their attention of CD36 and its role in atherosclerosis because their previous work demonstrated that this scavenger receptor promotes the activation of TLR-dependent innate immune responses to oxidized LDL (oxLDL), the critical inflammatory trigger in atherosclerotic plaques8. Interestingly, their previous study revealed that oxLDL induced the assembly of an unusual dimer between TLR4 and TLR6, which was responsible for activating inflammatory cytokine expression. This data was considered in the context of two additional pieces of evidence to justify their examination of the role of CD36 in inflammasome activation. First, as stated above, TLR-dependent signals augment NLRP3 inflammasome activation by inducing Il1b and Nlrp3 expression. The fact that CD36 triggered the TLR4-TLR6 dependent expression of cytokines in response to oxLDL suggested that CD36 might facilitate inflammasome activation. Second, CD36 and other scavenger receptors bind and mediate internalization of a range of cargo of both microbial and endogenous origin9,10, several of which have been shown to activate the NLRP3 inflammasome.
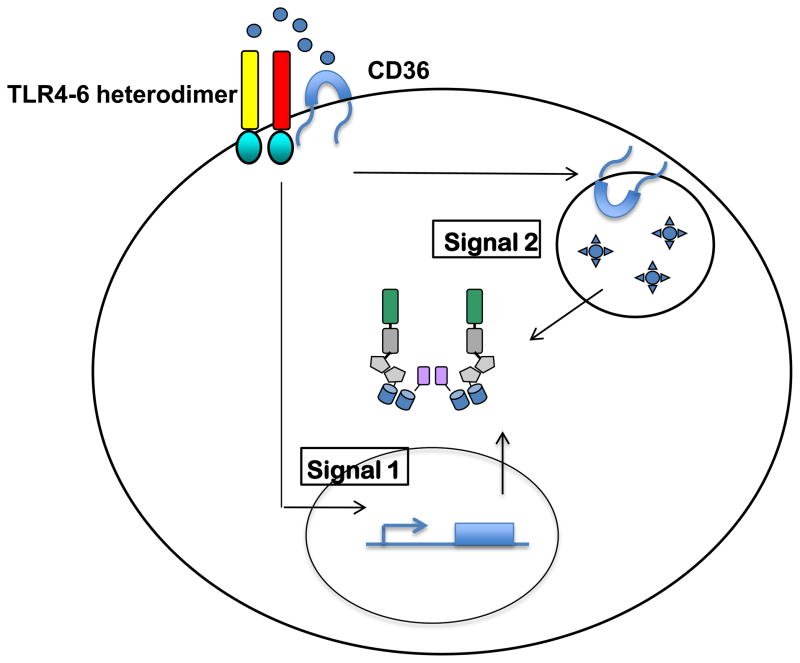
Using a classic microscopic approach, the authors made the intriguing finding that CD36 captures soluble oxLDL and delivers this molecule to lysosomes, where it is converted into a crystalline substance3. LDL crystals then somehow destabilize lysosomes, which causes them to release their contents into the cytosol. These experiments also revealed that the oxLDL capturing activity of CD36 is independent of its ability to induce TLR4-TLR6 dependent cytokine expression. In these studies, CD36 was required for intracellular LDL crystal formation, but cells lacking TLR4 or TLR6 were not. This finding is congruent with prior work demonstrating that lysosomal rupture can be a cell-intrinsic trigger of NLRP3 activation11. Consistent with this idea, the authors find that oxLDL has the capacity to induce the aggregation of the protein ASC in the cytosol of macrophages3. Since ASC aggregation is a marker for NLRP3 inflammasome activation, these data provide compelling evidence that oxLDL on its own can induce NLRP3 inflammasome formation. Taken together, it becomes clear that CD36 has two distinct activities that could be considered activators of signals 1 and 2 to mediate inflammasome-dependent cytokine secretion (Fig. 1). CD36 has the ability to recognize oxLDL and somehow activate cytokine expression mediated by TLR4 and TLR6 (signal 1). CD36 also has the ability to internalize oxLDL into lysosomes, where it becomes a crystal that ruptures the lysosomal membranes (signal 2). Convincing genetic evidence is provided to support this idea by the demonstration that CD36, TLR4 and TLR6 are each essential for NLRP3 inflammasome functions in response to oxLDL3. Thus, CD36 can use its activities as a TLR activator and an endocytosis receptor to emerge as an all-purpose NLRP3 inflammasome activator, in the absence of microbial stimuli (Fig. 1).
Figure 1. Role of CD36 in activating the NLRP3 inflammasome in the absence of infection.
CD36 and the TLR4-TLR6 heterodimer recognize oxLDL, which accumulates in atherosclerotic lesions. This interaction initiates a signaling pathway that leads to transcriptional upregulation of NLRP3 and pro-IL-1β (signal 1). CD36 also mediates internalization of oxLDL into the lysosomal compartment, where the ligands are converted into crystals that induce lysosomal rupture and NLRP3 inflammasome activation (signal 2).
The findings from Sheedy et al.3, substantiated by several complementary experiments in animal models, highlight a critical distinction between infectious and endogenous activators of the NLRP3 inflammasome. The ability of CD36 to induce both signals 1 and 2 likely explains its role in sterile inflammation resulting from atherosclerosis, and may also explain its role in other sterile inflammatory responses. While the role of CD36 in these diseases may be shared, the ligands that activate CD36-dependent responses are not. For example Sheedy et al.3 nicely demonstrate that CD36 serves a similar function in NLRP3 inflammasome activation by triggers of Alzheimer’s disease (soluble Aβ peptides) and type 2 diabetes (amyloid-containing amylin/islet amyloid polypeptides). It is also noteworthy that CD36 is able to direct soluble cargo (for example oxidized LDL and pre-fibrillar Ab) to the lysosomal compartment where subsequent crystallization leads to lysosomal destabilization, since the soluble forms are thought to make critical contributions to disease etiology. Future work will likely focus on understanding how CD36 is able to capture these seemingly diverse soluble ligands from the extracellular space, perhaps in concert with different co-receptors that can influence cargo trafficking and signaling and thus NLRP3 inflammasome activation. Another area of inquiry that is now appropriate revolves around the question of exactly how lysosomal rupture activates NLRP3. Multiple models have been proposed, including release of lysosomal cathepsins into the cytosol11,12, and most recently, Ca2+ mobilization13,14. Interestingly in the latter regard, CD36 signaling has been linked to activation of Src kinases and phospholipase C proteins that may regulate such Ca2+ mobilization. Alternatively CD36-mediated lysosome destabilization per se is sufficient for Ca2+ mobilization and associated mitochondrial reactive oxygen species production, which may promote inflammasome activation13,14. Finally, given the diverse ligand binding specificity and phagocytic ability of other scavenger receptors9,10, it would be interesting to determine if they can function similarly to CD36 in NLRP3 inflammasome activation, thus extending the model proposed here whereby the activity of an intracellular PRR is contingent upon a cell surface-resident, endocytic receptor. Conceivably, in some such cases the critical activity of the scavenger receptor is to deliver the internalized ligand to the cytosolic compartment (rather than to enable crystallization and lysosomal destabilization), especially for those molecules that do not form crystals.
Finally, there is the question of evolutionary origin. Did CD36 evolve to activate TLR4 –TLR6 dimers and induce NLRP3 activation after lysosomal rupture? Or is this simply a byproduct of scavenger receptor activity to capture ligands that happen to form crystals in lysosomes? One could imagine a scenario whereby CD36 evolved to link microbial products (for example bacterial lipoproteins) to TLRs as a means to promote inflammation to infection. CD36 may also have evolved to capture self molecules that might indicate tissue damage or dysfunction (such as oxLDL). Both of these activities would serve the body well, helping to rid infections or restore tissue homeostasis. However, in the case of sterile tissue damage, CD36 seems to be doing more harm than good in that it promotes the inflammatory symptoms associated with several human diseases. In this regard, one is left to wonder if CD36 evolved to try to do a good thing (clear dangerous molecules from circulation) but ended up causing more trouble than it is worth (promoting inflammasome activation and causing pathology). The real question then becomes: Does CD36 “know” that the ligands it internalizes will ultimately form crystals and activate NLRP3 inflammasomes? If CD36 does know that the ligands it captures will form crystals in lysosomes, then we are left with the question of how the crystal-forming potential of soluble CD36 ligands would be determined. Regardless of the answer to this question, it is clear from this study that CD36 is a major player involved in controlling NLRP3 activation in response to molecules involved in several human diseases. Sheedy et al.3 have therefore provided not only important insights into the diverse means by which inflammasomes can be activated, but revealed CD36 as an potential target for therapeutic intervention.
References
- 1.Janeway CA., Jr Cold Spring Harb Symp Quant Biol. 1989;54:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Kawai T, Akira S. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Sheedy FJ, et al. Nat Immunol. 2013;14:XXX–XXX. doi: 10.1038/ni.2711. [DOI] [PubMed] [Google Scholar]
- 4.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 5.Wen H, Ting JP, O’Neill LA. Nat Immunol. 2012;13:352–357. doi: 10.1038/ni.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rathinam VA, Vanaja SK, Fitzgerald KA. Nat Immunol. 2012;13:333–332. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Febbraio M, Hajjar DP, Silverstein RL. J Clin Invest. 2001;108:785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart CR, et al. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kzhyshkowska J, Neyen C, Gordon S. Immunobiology. 2012;217:492–502. doi: 10.1016/j.imbio.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Moore KJ, Freeman MW. Arterioscler Thromb Vasc Biol. 2006;26:1702–1711. doi: 10.1161/01.ATV.0000229218.97976.43. [DOI] [PubMed] [Google Scholar]
- 11.Hornung V, et al. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halle A, et al. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murakami T, et al. Proc Natl Acad Sci USA. 2012;109:11282–11287. doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong Z, et al. Nat Commun. 2013;4:1611. doi: 10.1038/ncomms2608. [DOI] [PMC free article] [PubMed] [Google Scholar]