Abstract
Purpose of review
Cancer patients undergoing treatment with cytotoxic chemotherapeutic agents (CCAs) often experience a cluster of treatment-related symptoms, which include fatigue, loss of appetite, disturbed sleep, depressed mood, cognitive difficulties, and changes in body composition. This symptom cluster collectively referred to herein as cancer treatment-related symptoms (CTRSs) decrease quality of life, and physical and social functioning. The preclinical and clinical studies described in this review represent important progress in understanding potential underlying mechanisms of CTRS.
Recent findings
Recent studies support a role for CCA-induced interleukin-1β (IL-1β) signaling in the cause of CTRS. CCAs may share a common ability to activate intracellular stress response pathways to trigger the synthesis, processing, and release of IL-1β from immune cells. Fatigue, sleep disturbance, and cognitive difficulties in cancer patients exposed to CCAs correlate with plasma levels of IL-6, IL-1 receptor antagonist, and soluble tumor necrosis factor receptor-I/II, surrogate markers of IL-1β-mediated central nervous system (CNS) inflammation. Additional preclinical work suggests IL-1β-mediated CNS inflammation may cause CTRS by altering hypothalamic and hippocampal functioning.
Summary
Although additional research is necessary to further establish the link between CCA exposure, IL-1β-mediated inflammatory processes and CTRS, these data provide hints for future studies and therapeutic approaches in ameliorating these symptoms in cancer patients.
Keywords: cancer, fatigue, inflammation, interleukin-1β, sickness behavior
INTRODUCTION
Cancer patients exposed to cytotoxic chemotherapeutic agents (CCAs) often experience a cluster of symptoms, which include malaise, decreased appetite, sleep disturbance, cognitive difficulties, pain, depressed mood, and changes in body composition [1,2]. These symptoms can greatly influence the subjective sensation of fatigue, which is the most common and distressing cancer treatment-related symptom (CTRS), and one that has a profoundly negative effect on physical functioning and quality of life [3]. It has long been recognized that many CTRSs are highly correlated with each other suggesting that they share a common cause. In this review, we discuss evidence that supports the view that the treatment-related symptom cluster is the same as sickness behavior, a physiological response to harmful stimuli initiated by the production of the pro-inflammatory cytokine interleukin-1β (IL-1β). The purpose of this review is to present recent clinical and preclinical evidence to support this view and to highlight areas for further research into the biological underpinnings of CTRS.
CYTOTOXIC CHEMOTHERAPEUTIC AGENTS TRIGGER THE SYNTHESIS, PROCESSING, AND RELEASE OF INTERLEUKIN-1β
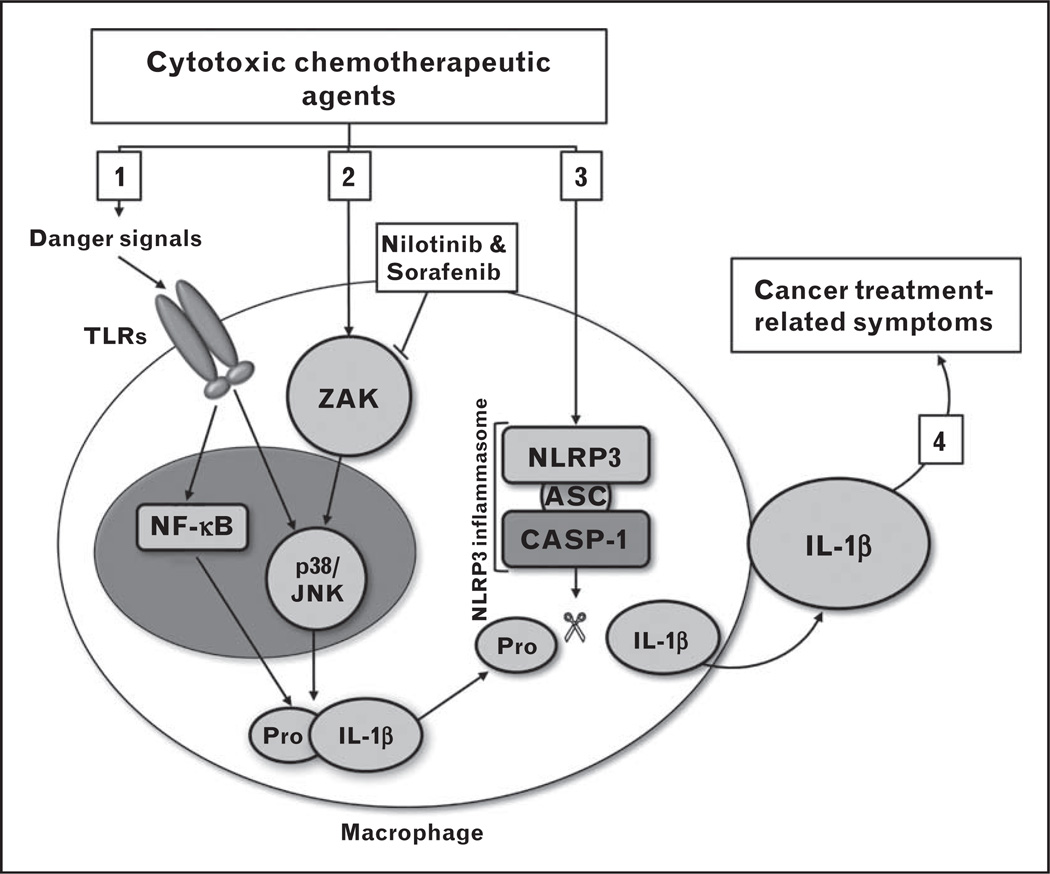
Several different types of CCAs have been shown to increase inflammatory cytokine signaling in immune cells in vitro and when administered in vivo. Recent studies suggest the ability of CCAs to induce a systemic inflammatory response is related to their capacity to trigger the synthesis, processing, and release of IL-1β, an initiator cytokine that plays a central role in the regulation of immune and inflammatory responses [4■,5■■]. IL-1β requires two distinct signals for its synthesis, processing, and secretion. The first signal is mediated by activation of the nuclear factor-κB (NF-κB) transcription factor and the stress-activated protein kinases, JNK and p38 mitogen-activated protein kinase (p38) (Fig. 1). This activation results in the production of the 35-kDa pro-IL-1β, a biologically inactive precursor of IL-1β. The second signal induces the processing of pro-IL-1β to the mature, 17-kDa biologically active IL-1β via the assembly of a multiprotein complex called the NLRP3 inflammasome (Fig. 1). The ability of CCAs to trigger inflammation is likely due in part to the cytotoxic action of these agents on tumor and healthy cells as dead and dying cells liberate a number of cell products that serve as ‘danger signals’ to prime resident tissue macrophages via activation of their surface Toll-like receptors (TLRs) [6]. Macrophage priming via TLR activation would likely occur following bacterial translocation (passage of bacteria or bacterial products across the intestinal mucosal barrier) related to drug-induced gastrointestinal damage [7,8]. These primed macrophages upregulate IL-1β gene expression via activation of the stress response proteins p38, JNK, and NF-κB. In addition to indirect priming via TLRs, chemotherapeutic agents may provide additional signals to macrophages to stimulate IL-1β synthesis, processing, and release (Fig. 1). Using bone marrow-derived macrophages as a model system, Sauter et al. [4■] showed that doxorubicin primes macrophages directly, activating p38/JNK, thereby amplifying the expression of pro-IL-1β. Wong et al. [5■■] later identified the cellular target of doxorubicin mediated p38/JNK activation as ZAK, a MAP3K upstream of p38/JNK. Doxorubicin failed to activate p38/JNK or increase intracellular pro-IL-1β levels in macrophages derived from ZAK-deficient mice [5■■]. ZAK is known to have strong binding affinities to two small molecule kinase inhibitors nilotinib and sorafenib [9]. These agents were able to block the doxorubicin-mediated activation of p38/JNK and IL-1β expression in wild-type macrophages [5■■]. Wong et al. [5■■] further demonstrated that co-administration of nilotinib with doxorubicin in mice suppressed IL-1β-mediated inflammation as evidenced by reduced levels of serum IL-6 compared to mice that were injected with doxorubicin alone. In addition to enhancing p38/JNK via ZAK, doxorubicin promotes the formation of the NLRP3 inflammasome, which is essential for the processing and release of mature IL-1β [4■]. Doxorubicin failed to stimulate the release of IL-1β from macrophages deficient in any one of the three inflammasome components, that is, ACS, NLRP3, or Caspase-1, which demonstrates a need for the NLRP3 inflammasome in the observed doxorubicin-mediated secretion of IL-1β [4■]. Although generalizing these results to all classes of CCA is premature at this time, these findings suggest that CCA-mediated induction of IL-1β synthesis, processing, and release can be targeted using drugs that block the activity of ZAK or formation of the NLRP3 inflammasome (Fig. 1). The ability of nilotinib and sorafenib to block doxorubicin-mediated upregulation of IL-1β suggests that these drugs could prove useful in reducing symptoms in cancer patients administered CCAs. Although these studies could be initiated in the clinical setting, there remain concerns about the effects of blocking IL-1β activity on the long-term efficacy of cytotoxic chemotherapy [10]. Inflammatory cytokines also play a critical role in the early stages of wound healing and blocking the activity of these cytokines in the periphery may delay regeneration of gastrointestinal and hematopoietic tissues damaged by cytoxic cancer chemotherapy [11].
FIGURE 1.
Proposed molecular mechanisms by which cytotoxic chemotherapeutic agents (CCAs) trigger the production of interleukin-1β (IL-1β) by macrophages. [1] The cytotoxic action of CCAs on tumor and healthy cells exposes tissue resident macrophages to several ‘danger signals’ including DNA, RNA, ATP, and bacterial products, and so on. These signals prime macrophages via activation of their surface Toll-like receptors (TLRs). Primed macrophages upregulate IL-1β gene expression via activation of the stress response proteins p38/JNK and nuclear factor-κB (NF-κB). The result is the accumulation of intracellular pro-IL-1β, the precursor to and biologically inactive form of IL-1β. [2] CCAs can also prime macrophages directly by activating ZAK, which prolongs and intensifies the activation of p38/JNK and synergizes with NF-κB to amplify the expression of pro-IL-1β. Nilotinib and sorafenib block ZAK and can consequently decrease CCA-mediated pro-IL-1β production. [3] CCA-induced formation of the NLRP3 inflammasome protein complex leads to cleavage of pro-IL-1β to mature IL-1β, which can then be released from the cell. [4] Release of mature IL-1β into the periphery stimulates the production of IL-1β and other inflammatory cytokines and chemokines, that is, tumor necrosis factor-α (TNF-α), IL-6, IL-1 receptor antagonist (IL-1RA), soluble tumor necrosis factor receptor-I/II (sTNFR-I/II), and so on. This IL-1β-driven systemic inflammatory response is the cause of sickness behavior, the symptoms of which are strikingly similar to cancer treatment-related symptoms.
SICKNESS BEHAVIOR: THE EFFECT OF INTERLEUKIN-1β ON SPECIFIC POPULATIONS OF HYPOTHALAMIC NEURONS
Mature IL-1β released into the periphery exerts its biological effect by binding to IL-1-specific receptors located on several different cell types including epithelial cells, hepatocytes, splenocytes, and leukocytes. In turn, these cells produce several additional inflammatory cytokines and chemokines including tumor necrosis factor (TNF)-α, IL-6, IL-1 receptor antagonist (IL-1RA), and the soluble tumor necrosis factor receptors 1 and 2 (sTNFR-1/2). This IL-1β-driven systemic inflammatory response serves to reorganize homeostatic mechanisms to favor survival [12]. The behavioral changes associated with this homeostatic reorganization include fatigue, lethargy, cognitive impairment, loss of appetite, and sleep disturbance and other symptoms, collectively known as sickness behavior. The purpose of these behavioral changes is to decrease energy demands and redirect energy stores to immune system functioning and thermogenesis [12]. Although IL-1β-induced sickness behavior promotes survival of the sick individual in the short term, prolonged production of this and other inflammatory cytokines is associated with a variety of illness states including depression [13], muscle wasting [14], and anemia [15], all of which can occur in cancer patients chronically exposed to CCAs.
Although peripheral inflammatory signaling is important for the initiation of sickness behavior, it is the activity of IL-1β and other inflammatory cytokines in the central nervous system (CNS) that mediate their behavioral effects (reviewed by [16]). The hypothalamus plays a central role in maintaining homeostasis and coordinating the physiological and behavioral changes associated with cytokine-induced sickness behavior. Indeed, specific metabolic and behavioral changes can be attributed to changes in functioning of discrete populations of hypothalamic neurons. Inflammatory cytokine induced fatigue/lethargy [17■■], fever [18], anorexia [19], and skeletal muscle catabolism [14], which can be attributed to altered functioning of specific populations of inflammatory-responsive neurons in the hypothalamus. The identification of inflammatory-responsive ‘fatigue’ neurons is of particular relevance to cancer symptom biology because fatigue is one of the most prominent and distressing symptoms associated with exposure to CCAs. These neurons located in the perifornical lateral hypothalamus secrete orexin, a neurotransmitter that promotes arousal and wakefulness by activating cholinergic, serotonergic, dopaminergic, and histaminergic signaling in the ascending arousal system [20,21]. Loss of orexin-secreting neurons causes narcolepsy, a disorder of excessive day-time sleepiness. Grossberg et al. [17■■] demonstrated that sickness behavior-associated lethargy, as evidenced by a decrease in locomotor activity (LMA), was caused by reduced orexin neuron signaling in the hypothalamus. The decline in LMA following peripheral administration of bacterial lipopolysaccharide (LPS) was associated with a reduction in orexin neuron activity and a decrease in neurotransmitter orexin-A in cerebral spinal fluid. Importantly, central (intra-cerebroventricular) orexin-A administration prevented the onset of LPS-induced lethargy and partially restored LMA in lethargic LPS-treated rats [17■■]. Although the hypothalamic neural pathways that mediate sickness behavior induced by endotoxin or LPS are becoming clear, it is not known whether the same neural pathways play a role in CTRS.
EVIDENCE OF INTERLEUKIN-1β-MEDIATED INFLAMMATORY PROCESSES IN SYMPTOMATIC CANCER PATIENTS
The striking similarity between CTRS and sickness behavior led to the idea that CTRSs were caused by inflammatory cytokines. In the last decade, a host of clinical studies have sought to establish a relationship between levels of IL-1β and other inflammatory cytokines in peripheral blood and CTRSs [22,23]. In the majority of these studies, blood levels of proinflammatory cytokines IL-1β were either undetectable or did not change in response to treatment. Rather than refute an etiological role for IL-1β-mediated inflammatory processes in initiating treatment-related symptoms, this finding highlights two challenges in examining the relationship between IL-1β, or other inflammatory mediators, and symptoms in the clinical setting. The first relates to the timing of blood sampling for cytokine measurement during treatment. In rodents and humans, the increase in blood levels of IL-1β in response to immune challenge is often transient and consequently difficult to capture [24,25]. Serial blood draws during and after chemotherapy infusion are needed to establish the kinetics of IL-1β appearance in blood and to demonstrate unequivocally that cytotoxic drugs trigger IL-1β production in people. The second challenge is that inflammatory cytokine levels in blood may not reflect their levels or activity in the CNS [26]. This is important because IL-1β-mediated inflammatory processes drive the behavioral changes characteristic of sickness. Blood levels of IL-6 and IL-1RA do increase in response to central administration of IL-1β [27,28], and blood levels of these cytokines are often considered surrogates for central IL-1β activity.
IL-6 and IL-1RA have been extensively studied as biomarkers of CTRS [29]. In a recent study, Liu et al. [30] examined sleep patterns and fatigue level in women undergoing 3-week cycles of anthracycline-based therapy. Peripheral blood and symptom data were collected during weeks 2 and 3 after the first and fourth chemotherapy infusion. These investigators observed a significant increase in fatigue, IL-6, and IL-1RA levels during treatment [30]. Mixed model results revealed a positive association between change in both sleep and fatigue and change in IL-6 and a positive association between sleep and change in IL-1RA during treatment. Taken together, these data suggest that anthracycline-based therapies can increase IL-1β signaling in the CNS of cancer patients, as evidenced by an increase in blood levels of IL-6 and IL-1RA. The association of IL-6 and IL-1RA levels with fatigue and sleep disturbance supports a role for IL-1β in the genesis of these symptoms.
Similar findings were observed in colorectal and esophageal cancer patients undergoing chemoradiation therapy [1]. Blood levels of IL-6 and sTNFR-1 were examined in patients before treatment, at the end of treatment, and 1 month thereafter. Symptom burden was assessed using the MD Anderson Symptom Inventory (MDASI), which assesses several cancer-related symptoms and their severity, at the time of blood collection (± 2 days). Although over 64% of baseline blood samples were missing, these investigators observed an increase in IL-6, sTNFR-1, and fatigue between baseline and the end of treatment and a decline in all measures 1 month after treatment. The increase in fatigue severity during treatment was associated with increased sTNFR-1 but not IL-6. However, both IL-6 and TNFR-1 were significantly associated with a composite symptom score, which included fatigue severity as well as other symptoms [1].
A recent study by Ganz et al. [31■■] also supports the idea that cognitive difficulties in cancer patients are related to CCA-induced sickness behavior. Cognitive complaints, cerebral functioning, and plasma levels of IL-1RA, sTNFR-RII, CRP, and IL-6 were examined in 93 breast cancer patients after primary cancer treatment but before the start of endocrine therapy, and at 6 and 12 months thereafter. At baseline, cognitive difficulties and fatigue were significantly higher in women who had received cytotoxic chemotherapy than those who had not. Women exposed to cytotoxic chemotherapy also had elevated plasma sTNFR-II levels, after controlling for age, BMI, and radiation exposure. Over time plasma levels of sTNFR-II decreased in chemotherapy-treated women but remained unchanged in women who were not exposed to chemotherapy. The decline in plasma sTNFR-II levels was associated with improvement in self-reported memory after controlling for age, BMI, radiation, depressive symptoms, and time since last chemotherapy. Plasma sTNFR-II was also found to be inversely associated with metabolism in the inferior frontal cortex in brain imaging studies carried out on 11 chemotherapy-exposed and five chemotherapy-naive women [31■■]. Decreased blood flow in this brain region was found to correspond to decreased cognitive performance in chemotherapy-exposed cancer patients [31■■]. Collins et al. [32] measured cognitive function in 60 women with early stage breast cancer prior to commencement of cytotoxic chemotherapy and again after each cycle of chemotherapy administration. After controlling for numerous factors, the women receiving cytotoxic chemotherapy had a significant progressive decline over time relative to a matched healthy control group in overall cognitive function, working memory, processing speed, verbal memory, and visual memory scores. A linear model best fit the trajectory of cognitive change, suggesting a dose-response relationship between cytotoxic chemotherapy and cognitive decline [32].
CYTOTOXIC CHEMOTHERAPEUTIC AGENTS IMPAIR HIPPOCAMPAL NEUROGENESIS TO CAUSE COGNITIVE DYSFUNCTION
Recent work has shown that CCA-induced cognitive impairment is associated with decreased neurogenesis in the hippocampus – a brain region involved in the consolidation of short-term memory to long-term memory, and in spatial memory and navigation. Two groups independently found that chronic administration of CCAs to rats caused a significant decrease in hippocampal neurogenesis [33■,34]. The deleterious effects of chemotherapy on hippocampal and nonhippocampal-dependent learning tasks suggest that multiple areas of the brain are affected by chronic administration of cytotoxic chemotherapeutics [33■,34]. Impaired learning and memory following chronic chemotherapy treatment was also associated with histone modifications in the hippocampus and prefrontal cortex, indicating that cytotoxic chemotherapeutics can induce epigenetic modifications that alter learning and memory [33■,34]. Combined treatment with 5-fluorouracil and oxaliplatin chemotherapy in rats impaired hippocampal-dependent tasks such as spatial reference memory in the water maze and contextual fear recall. These impairments were prevented by 4 weeks of running activity following the last dose of chemotherapy [35]. Aerobic exercise is known to increase hippocampal neurogenesis and spatial learning in rodents [36]. Although IL-1β is known to impair hippocampal neurogenesis [36], the specific role of IL-1β in mediating the effects of CCAs on hippocampal neurogenesis has not been examined.
CONCLUSION
Evidence presented here supports a role for treatment-related increases in IL-1β in the genesis of CTRS. First, CTRSs are similar to IL-1β-induced sickness behavior that occurs as a result of the action of IL-1β and other inflammatory cytokines on the activity of specific neural populations in the hypothalamus. Second, CCAs enhance the synthesis, processing, and release of IL-1β from immune cells. Finally, CCAs cause cognitive dysfunction by impairing hippocampal neurogenesis. Animal models will be essential to further understanding the molecular mechanisms underlying CTRS. This knowledge will facilitate the development of targeted therapies for the management of CTRS.
KEY POINTS.
CCAs may initiate CTRS by enhancing the synthesis, processing, and release of IL-1β, a pro-inflammatory cytokine that plays a central role in the induction of sickness behavior.
IL-1β induces sickness behavior via its influence on specific populations of hypothalamic neurons.
IL-1β may be responsible for CCA-related cognitive dysfunction caused by impairment of hippocampal neurogenesis. Additional work is needed to determine the role of IL-1β signaling in cognitive dysfunction.
Animal models will be essential to further understanding the molecular mechanisms underlying CTRS.
Therapeutic agents that decrease IL-1β-mediated inflammatory processes (i.e., nilotinib or sorafenib) or replace neuronal products (i.e., orexin) may be effective at ameliorating CTRS.
Acknowledgements
None.
This work was supported in part by R01NR012479 (to L.J.W.) from the National Institutes of Health.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 000–000).
- 1.Wang XS, Williams LA, Krishnan S, et al. Serum sTNF-R1, IL-6, and the development of fatigue in patients with gastrointestinal cancer undergoing chemoradiation therapy. Brain Behav Immun. 2012;26:699–705. doi: 10.1016/j.bbi.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oh HS, Seo WS. Systematic review and meta-analysis of the correlates of cancer-related fatigue. Worldviews Evid Based Nurs. 2011;8:191–201. doi: 10.1111/j.1741-6787.2011.00214.x. [DOI] [PubMed] [Google Scholar]
- 3.Berger AM, Gerber LH, Mayer DK. Cancer-related fatigue: implications for breast cancer survivors. Cancer. 2012;118(8 Suppl):2261–2269. doi: 10.1002/cncr.27475. [DOI] [PubMed] [Google Scholar]
- 4. Sauter KA, Wood LJ, Wong J, et al. Doxorubicin and daunorubicin induce processing and release of interleukin-1beta through activation of the NLRP3 inflammasome. Cancer Biol Ther. 2011;11:1008–1016. doi: 10.4161/cbt.11.12.15540. This article demonstrates for the first time that a CCA initiates the formation of the NLRP3 inflammasome to promote the secretion of IL-1β by immune cells. This finding supports the idea that symptoms related to exposure to cytotoxic agents may be caused by IL-1β.
- 5. Wong J, Smith LB, Magun EA, et al. Small molecule kinase inhibitors block the ZAK-dependent inflammatory effects of doxorubicin. Cancer Biol Ther. 2012;14:56–63. doi: 10.4161/cbt.22628. This article demonstrates the central role that ZAK plays in doxorubicin-induced IL-1β production by immune cells. Nilotinib and sorafenib, which target ZAK, block doxorubicin-induced IL-1β production and consequently may be useful in reducing CTRSs.
- 6.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuji E, Hiki N, Nomura S, et al. Simultaneous onset of acute inflammatory response, sepsis-like symptoms and intestinal mucosal injury after cancer chemotherapy. Int J Cancer. 2003;107:303–308. doi: 10.1002/ijc.11196. [DOI] [PubMed] [Google Scholar]
- 8.Cerci C, Ergin C, Eroglu E, et al. Effects of granulocyte-colony stimulating factor on peritoneal defense mechanisms and bacterial translocation after administration of systemic chemotherapy in rats. World J Gastroenterol. 2007;13:2596–2599. doi: 10.3748/wjg.v13.i18.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karaman MW, Herrgard S, Treiber DK, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 10.Ghiringhelli F, Apetoh L, Tesniere A, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 11.Bamias G, Corridoni D, Pizarro TT, Cominelli F. New insights into the dichotomous role of innate cytokines in gut homeostasis and inflammation. Cytokine. 2012;59:451–459. doi: 10.1016/j.cyto.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adelman JS, Martin LB. Vertebrate sickness behaviors: adaptive and integrated neuroendocrine immune responses. Integr Comp Biol. 2009;49:202–214. doi: 10.1093/icb/icp028. [DOI] [PubMed] [Google Scholar]
- 13.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 14.Braun TP, Zhu X, Szumowski M, et al. Central nervous system inflammation induces muscle atrophy via activation of the hypothalamic-pituitary-adrenal axis. J Exp Med. 2011;208:2449–2463. doi: 10.1084/jem.20111020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morceau F, Dicato M, Diederich M. Pro-inflammatory cytokine-mediated anemia: regarding molecular mechanisms of erythropoiesis. Mediat Inflamm. 2009;2009:405016. doi: 10.1155/2009/405016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grossberg AJ, Zhu X, Leinninger GM, et al. Inflammation-induced lethargy is mediated by suppression of orexin neuron activity. J Neurosci. 2011;31:11376–11386. doi: 10.1523/JNEUROSCI.2311-11.2011. This article demonstrates that suppressed orexin neuron activity is associated with the measured fatigue/lethargy outcome and that central replacement of the orexin neurotransmitter partially ameliorates lethargy following endotoxin treatment.
- 18.Harden LM, du Plessis I, Poole S, Laburn HP. Interleukin (IL)-6 and IL-1 beta act synergistically within the brain to induce sickness behavior and fever in rats. Brain Behav Immun. 2008;22:838–849. doi: 10.1016/j.bbi.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Scarlett JM, Zhu X, Enriori PJ, et al. Regulation of agouti-related protein messenger ribonucleic acid transcription and peptide secretion by acute and chronic inflammation. Endocrinology. 2008;149:4837–4845. doi: 10.1210/en.2007-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 21.Kukkonen JP. Physiology of the orexinergic/hypocretinergic system: a revisit in 2012. Am J Physiol Cell Physiol. 2013;304:C2–C32. doi: 10.1152/ajpcell.00227.2012. [DOI] [PubMed] [Google Scholar]
- 22.Saligan LN, Kim HS. A systematic review of the association between immunogenomic markers and cancer-related fatigue. Brain Behav Immun. 2012;26:830–848. doi: 10.1016/j.bbi.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schubert C, Hong S, Natarajan L, et al. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun. 2007;21:413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Kemna E, Pickkers P, Nemeth E, et al. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood. 2005;106:1864–1866. doi: 10.1182/blood-2005-03-1159. [DOI] [PubMed] [Google Scholar]
- 25.Sheng WS, Hu S, Ding JM, et al. Cytokine expression in the mouse brain in response to immune activation by Corynebacterium parvum. Clin Diagn Lab Immunol. 2001;8:446–448. doi: 10.1128/CDLI.8.2.446-448.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilbertson-White S, Aouizerat BE, Miaskowski C. Methodologic issues in the measurement of cytokines to elucidate the biological basis for cancer symptoms. Biol Res Nurs. 2011;13:15–24. doi: 10.1177/1099800410379497. [DOI] [PubMed] [Google Scholar]
- 27.Kitamura H, Okamoto S, Shimamoto Y, et al. Central IL-1 differentially regulates peripheral IL-6 and TNF synthesis. Cell Mol Life Sci. 1998;54:282–287. doi: 10.1007/s000180050151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao E, Xia L, Ferin M, Wardlaw SL. Intracerebroventricular injection of interleukin-1 stimulates the release of high levels of interleukin-6 and interleukin-1 receptor antagonist into peripheral blood in the primate. J Neuroimmunol. 1999;97:70–76. doi: 10.1016/s0165-5728(99)00050-8. [DOI] [PubMed] [Google Scholar]
- 29.Schubert C, Hong S, Natarajan L, et al. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun. 2007;21:413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Liu L, Mills PJ, Rissling M, et al. Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain Behav Immun. 2012;26:706–713. doi: 10.1016/j.bbi.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ganz PA, Bower JE, Kwan L, et al. Does tumor necrosis factor-alpha (TNF-alpha) play a role in postchemotherapy cerebral dysfunction? Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.07.015. [Epub ahead of print] This article represents one of the few clinical studies that support the review that cytotoxic chemotherapy-related cognitive dysfunction in cancer patients may be similar to sickness behavior and therefore caused by treatment-related increases in pro-inflammatory cytokines.
- 32.Collins B, Mackenzie J, Tasca GA, et al. Cognitive effects of chemotherapy in breast cancer patients: a dose-response study. Psychooncology. 2012 doi: 10.1002/pon.3163. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33. Briones TL, Woods J. Chemotherapy-induced cognitive impairment is associated with decreases in cell proliferation and histone modifications. BMC Neurosci. 2011;12:124. doi: 10.1186/1471-2202-12-124. This article demonstrates usefulness of an animal model in studying the effects of chemotherapy on brain neuron proliferation and cognitive function.
- 34.Christie LA, Acharya MM, Parihar VK, et al. Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin Cancer Res. 2012;18:1954–1965. doi: 10.1158/1078-0432.CCR-11-2000. [DOI] [PubMed] [Google Scholar]
- 35.Fardell JE, Vardy J, Shah JD, Johnston IN. Cognitive impairments caused by oxaliplatin and 5-fluorouracil chemotherapy are ameliorated by physical activity. Psychopharmacology (Berl) 2012;220:183–193. doi: 10.1007/s00213-011-2466-2. [DOI] [PubMed] [Google Scholar]
- 36.Mustroph ML, Chen S, Desai SC, et al. Aerobic exercise is the critical variable in an enriched environment that increases hippocampal neurogenesis and water maze learning in male C57BL/6J mice. Neuroscience. 2012;219:62–71. doi: 10.1016/j.neuroscience.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]