Abstract
In order to investigate the relevance of the left posterior parietal cortex (PPC) for precise sensorimotor timing we applied 1 Hz repetitive transcranial magnetic stimulation (rTMS) over left PPC, right PPC and visual cortex of healthy participants for ten minutes, respectively. The impact on sensorimotor timing of the right hand was assessed using a synchronization task that required subjects to synchronize their right index finger taps with respect to constant auditory, visual or auditory-visual pacing. Our results reveal reduced negative tap-to-pacer asynchronies following rTMS of the left PPC in all pacing conditions. This effect lasted for about 5 minutes after cessation of rTMS. Right PPC and visual cortex stimulation did not yield any significant behavioural effects. Since suppression of left PPC modified right-hand synchronization accuracy independent of the pacing signal’s modality, the present data support the significance of left PPC for anticipatory motor control over a primary role in multisensory integration. The present data suggest that 1 Hz rTMS might interrupt a matching process of anticipated and real sensorimotor feedback within PPC. Alternatively, downregulation of left PPC activity may affect M1 excitability via functional connections leading to a delay in motor output and, thus, smaller tap-to-pacer asynchronies.
Keywords: repetitive transcranial magnetic stimulation (rTMS), posterior parietal cortex (PPC), sensorimotor timing, anticipatory motor control, synchronization
1 Introduction
Precise sensorimotor timing is essential for everyday activities, especially when quick and flexible adjustment of movements with respect to external changes is required. A well-established behavioural paradigm to study sub-second sensorimotor timing is the synchronization task requiring subjects to tap with their index finger in synchrony with a regularly occurring pacing signal. From auditory pacing it is known that subjects usually tap prior to the actual pacing signal while having the impression of tapping in exact synchrony, a phenomenon known as negative asynchrony (Repp, 2005). For visual pacing, both positive and negative asynchronies have been observed. In general, visually-guided synchronization seems to be closer to the pacing signal as compared to auditory synchronization, although tap-to-tap variability is increased (Repp, 2005, Pollok et al., 2009, Krause et al., 2010a).
The brain network subserving sensorimotor timing comprises primary sensorimotor cortices (S1/M1), premotor and supplementary motor cortices (PMC/SMA) as well as posterior parietal cortex (PPC), thalamus and cerebellum (Jancke et al., 2000, Pollok et al., 2005, Schnitzler et al., 2006, Pollok et al., 2009, Krause et al., 2010b). A critical role within this cerebello-thalamo-cortical network has been ascribed to the PPC which is assumed to fulfil two main functions: (i) integration of multisensory information; as well as (ii) anticipatory motor control (Andersen and Buneo, 2002, Blakemore and Sirigu, 2003, Culham et al., 2006, Culham and Valyear, 2006, Andersen and Cui, 2009, Creem-Regehr, 2009). Anticipation of external cues as well as feedback of one’s own movements is assumed to be due to an internal model located in the cerebellum. The PPC may hold the anticipation until reafferent information from the actual movement is available and matches both information (Blakemore and Sirigu, 2003). Information may then be sent back to the cerebellum in order to update the internal model in favour of subsequent movements. In line with this hypothesis, alterations of the functional interplay between PPC and cerebellum depending on the predictability of the pacing signal were shown (Pollok et al., 2008a). Furthermore, PPC is assumed to be relevant for the integration of multisensory information (Andersen and Buneo, 2003, Creem-Regehr, 2009). Since auditory, visual and tactile-kinaesthetic information converge in parietal regions, PPC has been proposed as sensorimotor interface responsible for both the multisensory conversion and its integration with ongoing movements and movement intentions (Andersen and Buneo, 2002, Buneo and Andersen, 2006).
Application of repetitive transcranial magnetic stimulation (rTMS) with different frequencies and protocols allows the investigation of causal relationships between stimulated brain regions and behavioural outcomes. While there is definite intra- and inter-subject variability in the effects of rTMS (Maeda et al., 2000, Maeda et al., 2002), in most instances, low-frequency, continuous rTMS with 1 Hz yields a transient suppression of activity in the directly targeted brain region, while high-frequency rTMS results in a transient facilitation (Pascual-Leone et al., 1994, Chen, 2000, Valero-Cabre et al., 2005, Hallett, 2007, Valero-Cabre et al., 2008). Thus, downregulation of left PPC using rTMS with 1 Hz offers an opportunity to assess its causal role in sensorimotor timing.
Additionally, it remains elusive to what extent early information processing in primary sensory cortices is involved in sensorimotor timing. Since sensory processing is supposed to be more important for visual synchronization as opposed to auditory synchronization (Jancke et al., 2000, Pollok et al., 2009), it is likely that the visual cortex is involved in sensorimotor synchronization with respect to visual pacing. Contrasting rTMS effects on cortical regions associated with early visual and higher cognitive processing, like PPC, promises insights into the control of sensorimotor timing in modality-specific synchronization tasks.
The aim of the present study was to shed further light on the distinct role of the PPC in precise sensorimotor timing. To this end, activity in left PPC, right PPC and visual cortex was modulated using rTMS and the impact on a synchronization task was assessed. Assuming that PPC is crucial for integration of multisensory information, we hypothesized that sensorimotor timing should be affected by PPC rTMS particularly for auditory-visual pacing. On the contrary, sensorimotor timing should be affected independently of the pacing signal’s modality in case PPC is rather relevant for anticipatory motor control. In case sensorimotor timing with respect to visual pacing rather relies on processing in early sensory as compared to higher cognitive cortices, visual cortex rTMS is hypothesized to influence visual synchronization performance.
2 Materials and Methods
2.1 Subjects
We studied 13 healthy, right-handed subjects (9 male, 4 female; age 24.08 ± .87 years; mean ± standard error of mean; range 20–31 years) who did not have contraindications to receive TMS (Rossi et al., 2009). All subjects gave their written informed consent prior to the study, which had been approved by the local ethics committee and was conducted in accordance with the Declaration of Helsinki. All subjects had normal or corrected to normal sight and were classified as right-handed (1.94 ± .03) by means of a modified version of the Edinburgh Handedness Inventory (Oldfield, 1971). For right-handedness a minimum score of was required (minimum value −2 indicating left-handedness, maximum value indicating right-handedness). At the time of the study subjects reported not to be taking any medications or drugs that might have affected cortical excitability or altered cognitive function. All subjects had normal physical and neurological exams and had participated in previous TMS sessions tolerating TMS without any side-effects or complications.
2.2 TMS equipment
We employed a frameless stereotactic navigation system (Nexstim Ltd, Helsinki, Finland) equipped with a magnetic stimulator (MagPro, MagVenture A/S, Farum, Denmark) and a Nexstim 59 mm mean winding diameter figure-of-eight TMS coil type (201383P) delivering biphasic pulses. Subjects remained silent during the study to avoid speech-induced modulation of cortical excitability. Subjects were asked to keep their eyes open throughout the experiment. Prior to TMS all subjects underwent a high-resolution T1-weighted structural magnetic resonance imaging (MRI) scan (3.0 Tesla GE MRI scanner, GE Healthcare). Imaging data were fed to the navigation software (eXimia 3.1, Nexstim Ltd, Helsinki, Finland) for automatic 3-D brain reconstruction that was used to guide navigation and deliver TMS over the targeted regions (left PPC vs. right PPC vs. visual cortex). At the end of each session, the location of the stimulated sites was plotted using Nexstim stereotactic infrared registration to each subject’s structural MRI scan.
2.3 Identification of rTMS brain targets
For rTMS of left and right PPC, stimulation was applied over locations corresponding to the anatomical delineation of left and right angular gyrus. Right PPC stimulation served as control condition since subjects performed the task with the right hand only. The stimulation sites were identified on each subject's MRI scan and co-registered with scalp coordinates. Visual cortex was defined as the occipital brain region encompassing the striate cortex from which TMS induced phosphenes in central visual field (Walsh and Pascual-Leone, 2003). Mean Talairach coordinates of stimulation sites were −40 ± 1.41 (mean ± standard deviation), −50 ± 1.28, 51 ± 1.79 (left PPC); 40 ± 1.15, −51 ± 1.30, 50 ± 1.30 (right PPC) and 18 ± 1.59, −98 ± 1.24, 2 ± .90 (visual cortex).
Intensity of rTMS was adjusted to 90% of individual phosphene thresholds measured using single pulse TMS and the adaptive staircase method, i.e. stimulation intensity was decreased when subjects reported phosphenes, and was increased when absence of phosphenes was reported. Mean phosphene threshold was 59.38% ± 1.48% of stimulator output. Mean stimulation intensity was 57.88% ± 1.92% of stimulator output. 1 Hz rTMS was applied for 10 minutes in three separate sessions targeting right PPC or left PPC or visual cortex. The order of stimulation sessions was counterbalanced across subjects.
2.4 Behavioural paradigm
Sensorimotor synchronization performance was assessed using a synchronization task differing with respect to the pacing signals’ modalities (auditory (A), visual (V), or auditory-visual (AV)). Subjects performed the synchronization tasks in separate runs with their right index finger. The duration of each run was 35 sec resulting in 315 sec throughout the experimental session. The pacing signals were presented regularly with a constant inter-stimulus interval of 800 msec. In the A condition, the auditory signal consisted of a binaural click (sine-wave, duration 10 msec). In the V condition, the visual signal was a red circle appearing in the middle of the screen with a diameter of 3 cm corresponding to 3.4 deg of visual angle and a duration of 10 msec. In the AV condition, the signal comprised both the auditory click and the visual circle presented with the same onset.
2.5 Experimental set-up and data collection
Subjects were comfortably seated in the TMS chair with a distance of 0.5 m to a computer screen. They were asked to fixate a grey cross on a black background in the middle of the screen in order to minimize eye movements. Subjects performed continuous flexions and extensions of the right index finger - thereby pressing the space bar on the computer keyboard as closely synchronized with the pacing signals’ onsets as possible. The onset of finger taps was determined as soon as the space bar was pressed. Stimuli were presented and controlled with the help of a Windows laptop using PresentationP software (Neurobehavioral Systems, Inc., Albany, CA, USA).
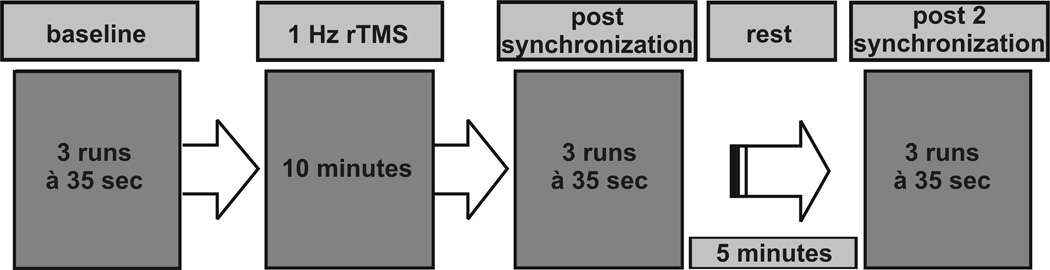
Prior to task recording, subjects were given the opportunity to get familiarized with the task for one trial of the AV condition. Subsequently, each subject participated in a baseline synchronization trial consisting of three runs (A, V, AV) before rTMS was administered. Immediately after rTMS intervention, subjects were required to perform the three synchronization runs again followed by a rest period of 5 minutes. Then the three runs were repeated (Fig. 1). The order of synchronization runs was counterbalanced across sessions and subjects, but within one session the order of runs remained constant.
Figure 1.
Experimental set-up.
2.6 Data analysis
Sensorimotor timing accuracy was determined by the so-called mean negative asynchrony - corresponding to the mean temporal distance between onset of finger taps and pacing signals - and the mean tap-to-tap variability. In each run, the first three taps were discarded from further analysis. Values below and above the limits of an individually determined confidence interval (mean ± 2 standard deviations) were also excluded from data analysis as statistical outliers. Baseline A, V and AV synchronization performance did not differ across the three sessions (p > .05) and was therefore pooled as mean baseline synchronization performance for statistical analysis. We completed a single analysis of variance (ANOVA) for factors stimulation target, modality of the pacing signal, and time using Greenhouse-Geisser correction where appropriate. Post-hoc paired t-tests were then done. All t-tests were corrected for multiple testing with the sequential Bonferroni procedure (Holm, 1979).
3 Results
3.1 Mean negative tap-to-pacer asynchrony
ANOVA with factors stimulation (left PPC vs. right PPC vs. visual cortex), modality (A = auditory vs. V = visual vs. AV = auditory-visual) and time (baseline vs. post stimulation vs. post 2 (plus 5 min)) revealed significant main effects of factors stimulation (F(2, 24) = 6.20, p = .007) and modality (F(2, 24) = 7.00, p = .004) and stimulation×time interaction (F(4, 48) = 3.00, p = .046). The ANOVA did not reveal a significant main effect of factor time (F(2, 24) = 4.18, p = .057) nor significant interactions of stimulation×modality (F(4, 48) = 2.25, p = .144), modality×time (F(4, 48) =.59, p = .603) and stimulation×modality×time (F(8, 96) = 1.59, p = .219).
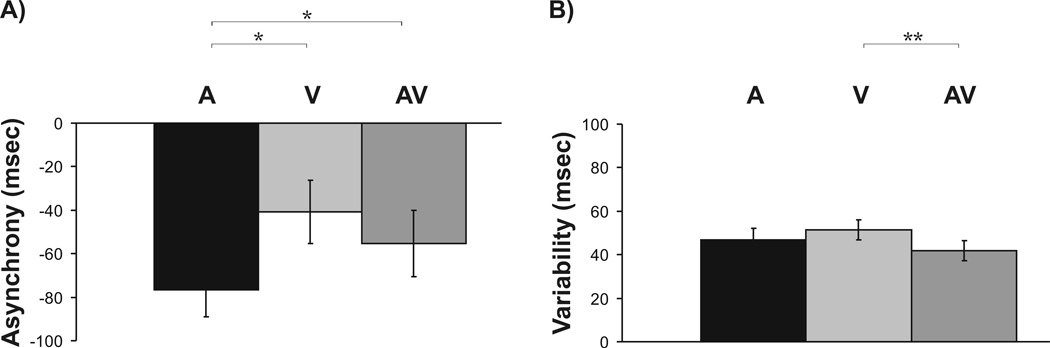
Post-hoc paired t-tests for factor modality revealed that the asynchrony during auditory pacing (−76.51 msec 7 12.66 msec) was significantly larger than during visual pacing (−40.78 msec 4 14.41 msec; t(12) = −3.52, p = .012) - replicating previous data (Repp, 2005, Pollok et al., 2009). Additionally, the asynchrony during auditory pacing was significantly larger than during auditory-visual pacing (−55.31 msec 5 15.37 msec; t(12) = −2.70, p = .038). Data are illustrated in Figure 2A.
Figure 2.
Main effect of factor modality of the pacing signal for A) mean negative asynchrony and B) mean tap-to-tap variability. Shown are mean values. Error bars indicate standard error of mean. Asterisk indicates p-values < .05. Two asterisks indicate p-values < .01.
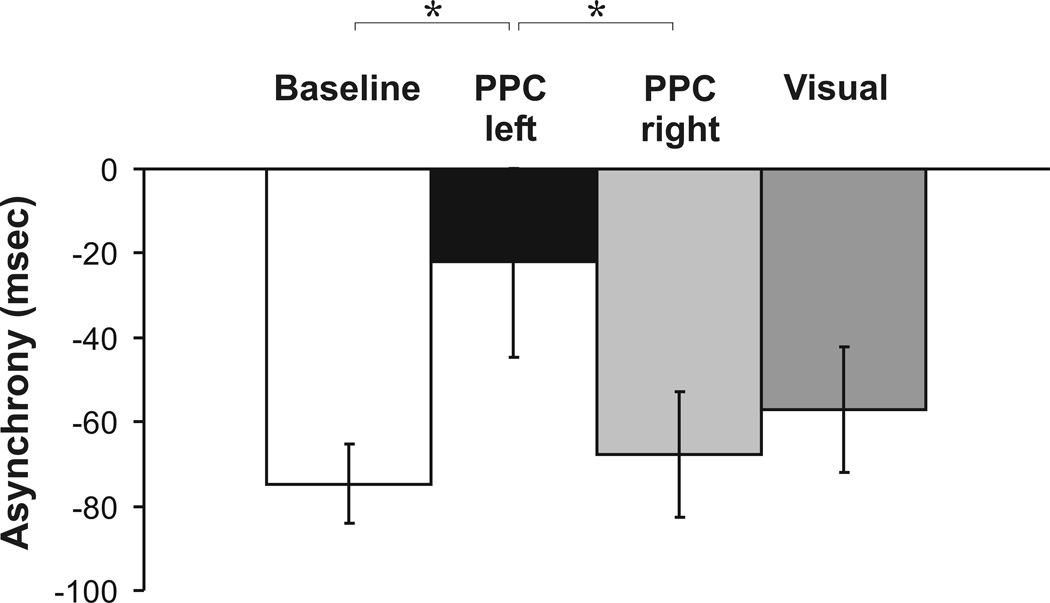
Post-hoc t-tests for factor stimulation revealed a significantly smaller asynchrony following left PPC stimulation as compared to baseline (t(12) = −2.76, p = .017; Fig. 3) and as compared to right PPC rTMS (t(12) = 3.07, p = .030; Fig. 3), whereas right PPC (t(12) = -.61, p = .553; Fig. 3) and visual cortex stimulation (t(12) = −1.75, p = .212; Fig. 3) did not reveal any significant effects as compared to baseline.
Figure 3.
Main effect of factor stimulation for mean negative asynchrony. Shown are mean values. Error bars indicate standard error of mean. Asterisk indicates p-values < .05.
An additional ANOVA contrasting left and right PPC rTMS effects with factors stimulation (left PPC vs. right PPC), modality (A vs. V vs. AV) and time (baseline vs. post vs. post 2) confirms the above result by revealing significant main effects of stimulation (F(1, 12) = 9.40, p = .010) and time (F(2, 24) = 5.32, p = .031) and a significant stimulation×time interaction (F(2, 24) = 4.24, p = .026). The asynchrony was smaller following left as compared to right PPC rTMS (left PPC post vs. right PPC post: t(12) = 2.43, p = .032: left PPC post 2 vs. right PPC post 2: t(12) = 2.94, p = .024).
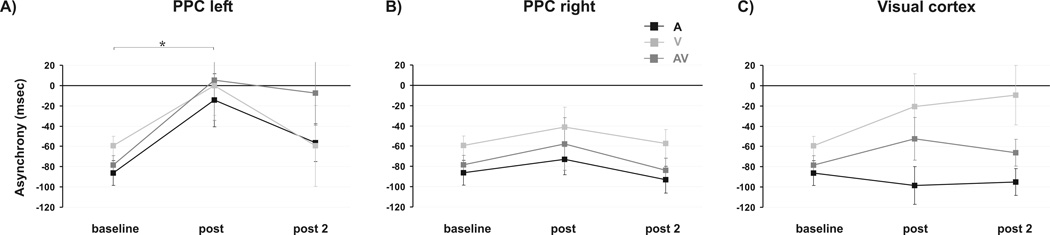
In order to elucidate specific effects of stimulation over time, separate ANOVAs for left PPC, right PPC and visual cortex were performed in a subsequent step. A single ANOVA for left PPC with factors modality (A vs. V vs. AV) and time (baseline vs. post vs. post 2) revealed a significant main effect of factor time (F(2, 24) = 6.32, p = .006). Neither a main effect of modality (F(2, 24) = .80, p = .405) nor a modality×time interaction (F(4, 48) = .98, p = .377) were found to be significant. Post-hoc tests showed a significantly reduced asynchrony across all pacing conditions immediately after left PPC stimulation as compared to baseline (t(12) = −2.79, p = .032; Fig. 4A) showing a tendency back to baseline level in the subsequent measurement (baseline vs. post 2: t(12) = −2.21, p = .047; Fig. 4A). Immediately after rTMS, subjects were able to precisely synchronize their finger taps with the pacing signals’ onsets - indicating a null-asynchrony in all three pacing conditions (t-tests: A vs. zero: t(12) = −.55, p = .592; V vs. zero: t(12) = .004, p = .997; AV vs. zero: t(12) = .14, p = .892). This effect lasted until the subsequent measurement in the AV and V condition but not the A condition (t-tests: A vs. zero: t(12) = −3.06, p = .030; V vs. zero: t(12) = −1.49, p = .324; AV vs. zero: t(12) = −.22, p = .827). The data show the most pronounced behavioural rTMS effect after stimulation termination diminishing back to baseline level five minutes later.
Figure 4.
Main effect of factor stimulation over time for mean negative asynchrony separately for (A) PPC left, (B) PPC right and (C) visual cortex. Shown are mean values. Error bars indicate standard error of mean. Asterisk indicates p-values < .05.
The ANOVA for right PPC stimulation with factors modality (A vs. V vs. AV) and time (baseline vs. post vs. post 2) showed a significant main effect of modality (F(2, 24) = 11.74, p < .001). But neither a main effect of time (F(2, 24) = 1.55, p = .238) nor the interaction modality×time (F(4, 48) = .22, p = .928) were significant. Post-hoc tests for modality showed that V asynchrony was smallest (A vs. V: t(12) = −4.09, p = .006; A vs. AV: t(12) = −1.88, p = .084; V vs. AV: t(12) = 3.31, p = .012; Fig. 4B).
The ANOVA for visual cortex stimulation with factors modality (A vs. V vs. AV) and time (baseline vs. post vs. post 2) revealed a significant main effect of modality (F(2, 24) = 8.74, p = .001). Neither a main effect of time (F(2, 24) = .78, p = .468) nor the interaction modality×time (F(4, 48) = 2.21, p = .082) were rendered significant. Post-hoc tests for modality showed that V asynchrony was significantly smaller than A asynchrony (A vs. V: t(12) = −3.90, p = .006; A vs. AV: t(12) = −2.29, p = .082; V vs. AV: t(12) = 2.13, p = .055; Fig. 4C). Noteworthy, the present data imply a descriptive trend of closer to zero synchronization only with respect to visual pacing after visual cortex rTMS. But this effect is not statistically significant.
3.2 Mean tap-to-tap variability
For tap-to-tap variability of synchronization performance, a single ANOVA with factors stimulation (left PPC vs. right PPC vs. V1), modality (A vs. V vs. AV) and time (baseline vs. post stimulation vs. post 2 (plus 5 min)) revealed a significant main effect of modality (F(2, 24) = 3.67, p = .041) but no significant main effects of stimulation (F(2, 24) = .83, p = .409) and time (F(2, 24) = 2.87, p = .106). No significant interactions of factors were found either (p > .05). Post-hoc t-tests for factor modality revealed that V synchronization was significantly more variable than AV synchronization (t(12)= 3.79, p = .009; Fig. 2B).
In sum, rTMS of left PPC, right PPC and visual cortex did not exert a significant impact on tap-to-tap variability. Since no significant main effect of stimulation or stimulation×time interaction became evident, tap-to-tap variability was not further analyzed.
4 Discussion
In the present study, rTMS was applied for 10 minutes with a frequency of 1 Hz over left PPC, visual cortex and right PPC in subsequent sessions to study the functional significance of left PPC for precise sensorimotor timing of the right hand. Following left PPC stimulation only, the asynchrony was significantly reduced independent of the pacing signals’ modality. This effect was most evident immediately after rTMS. Neither right PPC nor visual cortex stimulation yielded a significant behavioural effect.
4.1 Sensorimotor timing
In synchronization tasks, subjects tend to anticipate the pacing signal by tapping prior to the actual appearance of the cue. Interestingly, subjects do not notice this so-called negative asynchrony. Synchronization performance is assumed to rely on both anticipation of the pacing signal and processing of sensory information from different modalities (i.e. the pacing signal and tactile reafferent information). Synchronization performance has been suggested to involve different central control mechanisms depending in part on the pacing signal’s modality. This is reflected by different network components and dynamic interaction patterns within the motor control network (Jancke et al., 2000, Pollok et al., 2009). Both auditory and visual synchronization rely on the anticipation of the pacing signal. However, while auditory synchronization is characterized by large asynchronies and might rely on an internally generated pace, visual synchronization goes along with smaller asynchronies and might - at least partially - be feedback-driven depending on the appearance and processing of the visual pacing signal (Jancke et al., 2000, Pollok et al., 2009). The present results corroborate previous findings that synchronization performance differs in a modality-specific manner (Jancke et al., 2000, Repp, 2005, Pollok et al., 2009, Krause et al., 2010a). V synchronization performance was more precise (= closer to zero) than A synchronization. Interestingly, AV was shown to have a smaller asynchrony than A and a smaller tap-to-tap variability than V suggesting that redundancy of multisensory information is beneficial for sensorimotor timing.
While left PPC rTMS was shown to have an effect on mean asynchrony across all pacing signals’ modalities, mean tap-to-tap variability was not influenced. Since subjects synchronized closer to the appearance of the pacing signal independent of its modality, one may hypothesize that downregulation of left PPC yielded an interruption of the internal pace underlying anticipatory motor control. Accordingly, anticipatory motor control seems to rely on an internally generated pace rather than an external pace given by the signal (Jancke et al., 2000, Pollok et al., 2009). Along this line, the present results highlight the relevance of the left PPC for anticipatory motor control over a primary role in multisensory integration at least for right-hand sensorimotor timing. But this conclusion does not rule out the multisensory integrating function of parietal cortex in general since auditory and visual processing have been shown to be modulated with 1 Hz rTMS in reaction tasks (Bolognini et al., 2009).
Since the rTMS frequency of 1 Hz was temporally almost concordant with the interstimulus interval of 800 msec (i.e. 1.2 Hz), we, furthermore, cannot rule out an entrainment of motor control due to 1 Hz left PPC rTMS. Accordingly, one might argue that baseline motor control with 800 msec has transiently shifted towards the given rTMS frequency suggesting a general delay of movement anticipation shifting towards a closer to zero performance. On the other hand, this hypothesis is weakened because one would expect the general entrainment effect to be independent of stimulation site, and it was not evident after right PPC rTMS here and after PMC stimulation (Pollok et al., 2008b).
Visual synchronization is suggested to rather rely on visual feedback processing of the pacing signal (Jancke et al., 2000, Pollok et al., 2009). To this end, we investigated the role of the visual cortex in sensorimotor timing. We hypothesized that downregulation of visual cortex activity might lead to an impairment of visual processing at an early stage and, thus, have a specific effect on visual synchronization performance. However, the present data imply a descriptive trend towards a closer to zero synchronization performance with respect to visual pacing after 1 Hz rTMS of visual cortex. This observation was not rendered significant in statistical analyses suggesting that early sensory processing may not substantially contribute to precise visually-guided sensorimotor timing. This hypothesis is corroborated by evidence from patients with peripheral somatosensory deafferentation showing impaired but basically preserved sensorimotor timing abilities despite the alteration of sensory processing (Stenneken et al., 2002, Stenneken et al., 2006).
4.2 Timing and PPC
We found the typical negative asynchrony to be reduced following rTMS-induced downregulation of left PPC but no effect of either stimulation on tap-to-tap variability. Right PPC stimulation served as control condition ruling out the possibility that unspecific effects might have contributed to the present results. Subjects were able to tap with their right index finger in exact synchrony with the pacing signal immediately after left PPC rTMS - an effect that diminished back to baseline level after five minutes, when the effect of rTMS is expected to be reduced.
The observed behavioural rTMS effects over PPC can be explained by (i) local modulation of PPC activity and - beyond this - by (ii) modulation of functional pathways between brain regions since TMS has been shown to spread towards interconnected cortical and subcortical regions (Bestmann et al., 2004, Koch et al., 2007a, Koch et al., 2008, Koch and Rothwell, 2009, Koch et al., 2009, Romei et al., 2009).
Sensorimotor synchronization has been shown to rely on functional network interactions between primary sensorimotor cortices, parietal, premotor cortices as well as cerebellum (Pollok et al., 2009, Krause et al., 2010b). The motor control network is assumed to be lateralized within the left hemisphere (Rushworth et al., 2001a, Rushworth et al., 2001b, Schluter et al., 2001). Within this network, the PPC is assumed to play an essential role for sensorimotor timing abilities by anticipatory motor control. The PPC is functionally connected with the cerebellum (Blakemore & Sirigu, 2003). While the cerebellum is supposed to be the pacemaker for movement timing especially within the sub-second range (Inhoff et al., 1989, Ivry et al., 2002, Koch et al., 2007b), the PPC has been related to matching between anticipated and real sensorimotor consequences (Andersen and Buneo, 2002, Blakemore and Sirigu, 2003). Since in the present study, PPC rTMS affected sensorimotor timing but not variability, one might assume that the cerebellum generates the internal movement pace while within the PPC this information is matched with sensory feedback for subsequent movement implementation and execution. Interruption of this process within PPC might lead to more accurate timing possibly by interruption of this feedback matching loop.
Beyond the modulation of local activity, rTMS of left PPC may also lead to a remote reorganization of functional interactions between network components. Within the motor control network, the PPC has got a direct functional connection to M1. To this end, it seems plausible that downregulation of the left PPC alters right-hand sensorimotor timing via a direct modulation of the PPC-M1-interaction and, hence, an inhibition of left M1. M1 excitability has previously been shown to be modulated due to TMS delivered to the ipsilateral PPC (Koch et al., 2007a, Koch et al., 2008). Suppression of the left PPC may directly lead to a downregulation of activity in M1. We hypothesize that this inhibition of M1 activity may then yield a retarded motor output. Since movements are initiated in M1 with a delay, subjects tap later and, thus, closer to the pacing signal. Although this modulation is likely to be disruptive within the central motor control network, the negative asynchrony is reduced by objective measures. Supporting this interpretation, following 1 Hz rTMS of M1 subjects were also shown to tap closer to the pacing signal (Doumas et al., 2005).
Alternatively, the modulation of right-hand sensorimotor timing due to downregulation of the left PPC might be explained by a modulation of posterior parietal and premotor functional pathways. Parietal and premotor cortices are assumed to be connected bidirectionally with a feedback influence of the dorsolateral PMC on parietal cortex promoting stimulus-response-mapping (Cieslik et al., 2011). Since low-frequency rTMS and also continuous theta burst TMS of the left but not the right PMC led to increased negative asynchronies during auditory synchronization with both the right and the left hand (Pollok et al., 2008b, Bijsterbosch et al., 2011), one may assume a particular role of the left PMC and left PPC for motor control. The differential findings of PPC and PMC stimulation suggest that sensorimotor timing is influenced in specific manners by distinct brain regions within the motor control network. While PMC rTMS yields larger asynchronies, M1 and as shown here PPC stimulation are associated with smaller asynchronies. Since sensorimotor timing was unaffected by right PPC stimulation, the present data corroborate the hypothesis of a left hemisphere predominance for motor control. While the right PPC may be relevant for visuospatial and orienting attention (Rushworth et al., 2001a), the left PPC may rather be responsible for anticipatory motor control and feedback matching.
4.3 Summary
The transient, global modulation of sensorimotor timing independent of the pacing signal’s modality due to downregulation of left PPC points towards a significant role of the left PPC for anticipatory motor control over a primary role in multisensory integration. PPC might be crucial for matching between anticipated and real sensorimotor consequences within a cortico-subcortical network which is lateralized to the left hemisphere with distinct brain regions contributing differentially to motor control.
1 Hz rTMS of PPC is likely to yield a transient impairment resulting in a functional reorganization within the central motor control network. We would like to highlight two main conclusions:
On the one hand, the downregulation of PPC might yield disruption within the PPC-cerebellum interconnection where the cerebellum provides the pace and the PPC matches anticipated and real sensorimotor feedback. The temporally precise functional PPC-cerebellum-interaction is likely to be essential for flexible adjustments to environmental changes within the sub-second range and for motor learning. One may hypothesize, that interference of this interactive process yields more accurate sub-second sensorimotor timing - possibly by interruption of the feedback matching loop.
On the other hand, the downregulation of PPC may in particular interrupt the functional interaction with the left M1. The inhibition of M1 activity may simply lead to slowing of motor output. While anticipatory motor control is characterized by early movement initiation several tens of milliseconds prior to the respective event, the delayed motor output results in closer to zero and, thus, more precise sensorimotor synchronization.
However, since these conclusions are speculative at the moment, further studies on the impact of PPC rTMS on the motor control network are needed to shed light on these issues. Prospectively, it remains important to further elucidate the causal and possibly distinct roles of left and right PPC for sensorimotor timing, anticipatory motor control and multisensory integration by application of high frequency rTMS and by contrasting both right and left hand performances. An adjacent study of left-handed subjects would possibly allow complementary insights into functional brain dynamics and hemispheric predominance.
Acknowledgements
Dr. Krause is grateful for a research fellowship of the German Academic Exchange Service (Deutscher Akademischer Austauschdienst; D/09/41894) and two grants from Heinrich-Heine-University (9772440, 9772467). Dr. Pollok is grateful for financial support by grants from (i) Heinrich-Heine-University (9772440, 9772467) and (ii) German Research Foundation (Deutsche Forschungsgemeinschaft; PO 806/3-1).
Dr. Pascual-Leone serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Allied Mind, Neosync, and Novavision, and is inventor on various patents and patent applications on brain stimulation and the real-time integration of transcranial magnetic stimulation (TMS) with electroencephalography (EEG) and magnetic resonance imaging (MRI). Work on this study was supported by grants from the National Center for Research Resources: Harvard Clinical and Translational Science Center (UL1 RR025758).
Reference list
- Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Annu Rev Neurosci. 2002;25:189–220. doi: 10.1146/annurev.neuro.25.112701.142922. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Buneo CA. Sensorimotor integration in posterior parietal cortex. Adv Neurol. 2003;93:159–177. [PubMed] [Google Scholar]
- Andersen RA, Cui H. Intention, action planning, and decision making in parietal-frontal circuits. Neuron. 2009;63:568–583. doi: 10.1016/j.neuron.2009.08.028. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci. 2004;19:1950–1962. doi: 10.1111/j.1460-9568.2004.03277.x. [DOI] [PubMed] [Google Scholar]
- Bijsterbosch JD, Lee KH, Dyson-Sutton W, Barker AT, Woodruff PW. Continuous theta burst stimulation over the left pre-motor cortex affects sensorimotor timing accuracy and supraliminal error correction. Brain Res. 2011;1410:101–111. doi: 10.1016/j.brainres.2011.06.062. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Sirigu A. Action prediction in the cerebellum and in the parietal lobe. Exp Brain Res. 2003;153:239–245. doi: 10.1007/s00221-003-1597-z. [DOI] [PubMed] [Google Scholar]
- Bolognini N, Miniussi C, Savazzi S, Bricolo E, Maravita A. TMS modulation of visual and auditory processing in the posterior parietal cortex. Exp Brain Res. 2009;195:509–517. doi: 10.1007/s00221-009-1820-7. [DOI] [PubMed] [Google Scholar]
- Buneo CA, Andersen RA. The posterior parietal cortex: sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia. 2006;44:2594–2606. doi: 10.1016/j.neuropsychologia.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Chen R. Studies of human motor physiology with transcranial magnetic stimulation. Muscle Nerve Suppl. 2000;9:S26–S32. doi: 10.1002/1097-4598(2000)999:9<::aid-mus6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Grefkes C, Eickhoff SB. Dynamic interactions in the fronto-parietal network during a manual stimulus-response compatibility task. Neuroimage. 2011;58:860–869. doi: 10.1016/j.neuroimage.2011.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creem-Regehr SH. Sensory-motor and cognitive functions of the human posterior parietal cortex involved in manual actions. Neurobiol Learn Mem. 2009;91:166–171. doi: 10.1016/j.nlm.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Culham JC, Cavina-Pratesi C, Singhal A. The role of parietal cortex in visuomotor control: what have we learned from neuroimaging? Neuropsychologia. 2006;44:2668–2684. doi: 10.1016/j.neuropsychologia.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Culham JC, Valyear KF. Human parietal cortex in action. Curr Opin Neurobiol. 2006;16:205–212. doi: 10.1016/j.conb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Doumas M, Praamstra P, Wing AM. Low frequency rTMS effects on sensorimotor synchronization. Exp Brain Res. 2005;167:238–245. doi: 10.1007/s00221-005-0029-7. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- Inhoff AW, Diener HC, Rafal RD, Ivry R. The role of cerebellar structures in the execution of serial movements. Brain. 1989;112(Pt 3):565–581. doi: 10.1093/brain/112.3.565. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Spencer RM, Zelaznik HN, Diedrichsen J. The cerebellum and event timing. Ann N Y Acad Sci. 2002;978:302–317. doi: 10.1111/j.1749-6632.2002.tb07576.x. [DOI] [PubMed] [Google Scholar]
- Jancke L, Loose R, Lutz K, Specht K, Shah NJ. Cortical activations during paced finger-tapping applying visual and auditory pacing stimuli. Brain Res Cogn Brain Res. 2000;10:51–66. doi: 10.1016/s0926-6410(00)00022-7. [DOI] [PubMed] [Google Scholar]
- Koch G, Fernandez Del Olmo M, Cheeran B, Ruge D, Schippling S, Caltagirone C, Rothwell JC. Focal stimulation of the posterior parietal cortex increases the excitability of the ipsilateral motor cortex. J Neurosci. 2007a;27:6815–6822. doi: 10.1523/JNEUROSCI.0598-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Fernandez Del Olmo M, Cheeran B, Schippling S, Caltagirone C, Driver J, Rothwell JC. Functional interplay between posterior parietal and ipsilateral motor cortex revealed by twin-coil transcranial magnetic stimulation during reach planning toward contralateral space. J Neurosci. 2008;28:5944–5953. doi: 10.1523/JNEUROSCI.0957-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Torriero S, Salerno S, Lo Gerfo E, Caltagirone C. Repetitive TMS of cerebellum interferes with millisecond time processing. Exp Brain Res. 2007b;179:291–299. doi: 10.1007/s00221-006-0791-1. [DOI] [PubMed] [Google Scholar]
- Koch G, Rothwell JC. TMS investigations into the task-dependent functional interplay between human posterior parietal and motor cortex. Behav Brain Res. 2009;202:147–152. doi: 10.1016/j.bbr.2009.03.023. [DOI] [PubMed] [Google Scholar]
- Koch G, Ruge D, Cheeran B, Fernandez Del Olmo M, Pecchioli C, Marconi B, Versace V, Lo Gerfo E, Torriero S, Oliveri M, Caltagirone C, Rothwell JC. TMS activation of interhemispheric pathways between the posterior parietal cortex and the contralateral motor cortex. J Physiol. 2009;587:4281–4292. doi: 10.1113/jphysiol.2009.174086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause V, Pollok B, Schnitzler A. Perception in action: the impact of sensory information on sensorimotor synchronization in musicians and non-musicians. Acta Psychol (Amst) 2010a;133:28–37. doi: 10.1016/j.actpsy.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Krause V, Schnitzler A, Pollok B. Functional network interactions during sensorimotor synchronization in musicians and non-musicians. Neuroimage. 2010b;52:245–251. doi: 10.1016/j.neuroimage.2010.03.081. [DOI] [PubMed] [Google Scholar]
- Maeda F, Gangitano M, Thall M, Pascual-Leone A. Inter- and intra-individual variability of paired-pulse curves with transcranial magnetic stimulation (TMS) Clin Neurophysiol. 2002;113:376–382. doi: 10.1016/s1388-2457(02)00008-1. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res. 2000;133:425–430. doi: 10.1007/s002210000432. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117(Pt 4):847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Pollok B, Gross J, Kamp D, Schnitzler A. Evidence for anticipatory motor control within a cerebello-diencephalic-parietal network. J Cogn Neurosci. 2008a;20:828–840. doi: 10.1162/jocn.2008.20506. [DOI] [PubMed] [Google Scholar]
- Pollok B, Gross J, Muller K, Aschersleben G, Schnitzler A. The cerebral oscillatory network associated with auditorily paced finger movements. Neuroimage. 2005;24:646–655. doi: 10.1016/j.neuroimage.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Pollok B, Krause V, Butz M, Schnitzler A. Modality specific functional interaction in sensorimotor synchronization. Hum Brain Mapp. 2009;30:1783–1790. doi: 10.1002/hbm.20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollok B, Rothkegel H, Schnitzler A, Paulus W, Lang N. The effect of rTMS over left and right dorsolateral premotor cortex on movement timing of either hand. Eur J Neurosci. 2008b;27:757–764. doi: 10.1111/j.1460-9568.2008.06044.x. [DOI] [PubMed] [Google Scholar]
- Repp BH. Sensorimotor synchronization: a review of the tapping literature. Psychon Bull Rev. 2005;12:969–992. doi: 10.3758/bf03206433. [DOI] [PubMed] [Google Scholar]
- Romei V, Thut G, Ramos-Estebanez C, Pascual-Leone A. M1 contributes to the intrinsic but not the extrinsic components of motor-skills. Cortex. 2009;45:1058–1064. doi: 10.1016/j.cortex.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Ellison A, Walsh V. Complementary localization and lateralization of orienting and motor attention. Nat Neurosci. 2001a;4:656–661. doi: 10.1038/88492. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Krams M, Passingham RE. The attentional role of the left parietal cortex: the distinct lateralization and localization of motor attention in the human brain. J Cogn Neurosci. 2001b;13:698–710. doi: 10.1162/089892901750363244. [DOI] [PubMed] [Google Scholar]
- Schluter ND, Krams M, Rushworth MF, Passingham RE. Cerebral dominance for action in the human brain: the selection of actions. Neuropsychologia. 2001;39:105–113. doi: 10.1016/s0028-3932(00)00105-6. [DOI] [PubMed] [Google Scholar]
- Schnitzler A, Timmermann L, Gross J. Physiological and pathological oscillatory networks in the human motor system. J Physiol Paris. 2006;99:3–7. doi: 10.1016/j.jphysparis.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Stenneken P, Aschersleben G, Cole J, Prinz W. Self-induced versus reactive triggering of synchronous movements in a deafferented patient and control subjects. Psychol Res. 2002;66:40–49. doi: 10.1007/s004260100072. [DOI] [PubMed] [Google Scholar]
- Stenneken P, Prinz W, Cole J, Paillard J, Aschersleben G. The effect of sensory feedback on the timing of movements: evidence from deafferented patients. Brain Res. 2006;1084:123–131. doi: 10.1016/j.brainres.2006.02.057. [DOI] [PubMed] [Google Scholar]
- Valero-Cabre A, Pascual-Leone A, Rushmore RJ. Cumulative sessions of repetitive transcranial magnetic stimulation (rTMS) build up facilitation to subsequent TMS-mediated behavioural disruptions. Eur J Neurosci. 2008;27:765–774. doi: 10.1111/j.1460-9568.2008.06045.x. [DOI] [PubMed] [Google Scholar]
- Valero-Cabre A, Payne BR, Rushmore J, Lomber SG, Pascual-Leone A. Impact of repetitive transcranial magnetic stimulation of the parietal cortex on metabolic brain activity: a 14C-2DG tracing study in the cat. Exp Brain Res. 2005;163:1–12. doi: 10.1007/s00221-004-2140-6. [DOI] [PubMed] [Google Scholar]
- Walsh V, Pascual-Leone A. Transcranial Magnetic Stimulation: A Neurochronometrics of Mind. Boston, MA: MIT Press; 2003. [Google Scholar]