Abstract
Purpose:
Even after a mild traumatic brain injury (TBI) symptoms may be long lasting and never resolve completely. The neurophysiologic substrate for such lasting deficits remains unclear. There is a lack of objective measures of early brain abnormalities following mild TBI, which could shed light on the genesis of these lasting impairments.
Methods:
Here we report findings in a previously healthy man tested 2 and 6 weeks after a well-documented concussion. Findings were compared with 12 control subjects. All subjects underwent brain magnetic resonance imaging (MRI) and diffusion-tensor imaging (DTI). Testing included neuropsychological evaluation and physiological assessment with TMS and EEG, excitatory/inhibitory balance and brain plasticity.
Results:
While the MRI, DTI and neuropsychological evaluations showed no abnormalities, neurophysiologic tests revealed subclinical abnormalities in our patient: (1) Significantly higher intracortical facilitation than the control group at both time points; (2) Intracortical inhibition presumably mediated by GABAB receptors was absent at week 2, but returned to normal value at week 6; (3) Abnormal mechanisms of plasticity at week 2, that normalize at week 6.
Conclusions:
These findings demonstrate a transient alteration of brain cortical physiology following concussion independent of anatomical findings and neuropsychological function. This case study suggests that TMS measures may serve as sensitive biomarkers of physiologic brain abnormalities after concussion.
Keywords: Theta burst stimulation, motor cortex, excitability, EEG
1. Introduction
Following a concussion (mild traumatic brain injury), it is often unclear when recovery is complete and thus when normal activities can resume. We lack reliable biomarkers to gauge recovery prognosis, and the neurobiological substrate for the potentially long-lasting neurobiological consequences of concussions remains unresolved (Ellemberg et al., 2009). Moreover, even if symptoms abate, data is lacking on whether residual neurophysiologic abnormalities may affect the consequences from a subsequent concussion and even render the individual more prone to a subsequent concussion.
Animal and human studies suggest that traumatic brain injury (TBI) may trigger two seemingly contradictory processes: excessive glutamate accumulation that may lead to NMDA-mediated excitotoxicity, and excess in GABA-mediated inhibition that may contribute to lasting cognitive deficits (Hoskison et al., 2009; Kobori et al., 2006; Rao et al., 1998). These processes can be studied by noninvasive brain stimulation techniques such as transcranial magnetic stimulation (TMS) (De Beaumont et al., 2009).
We report the findings of neuroimaging, neuropsychological and neurophysiologic assessment at 2 and 6 weeks after a concussion in an otherwise healthy man, and compare the findings with those of 12 healthy volunteers. This case sheds light onto the neurophysiologic impact of a concussion, and illustrates the utility of serial TMS studies, even in the absence of structural damage as revealed by brain MRI and DTI.
2. Case report
This 44-year old, right-handed male sustained a concussion while playing soccer. After a head-to-head collision with another player he lost consciousness for approximately 90 seconds, followed by confusion, and about 1–2 minutes of retrograde and 3–4 minutes of anterograde amnesia. Approximately 20 minutes after the event, his physical and neurological exams were normal, and remained normal 10 days later. For 2 weeks following the accident, he complained of fatigue and poor concentration, memory problems, a mild headache and some difficulty sleeping. These symptoms had markedly improved by week 6, although he still complained of mild headaches, slight fatigue, and intermittent memory difficulties. Four weeks later, 10 weeks after the concussion, he was completely asymptomatic and had fully resumed normal daily activities. On the Immediate Postconcussion Assessment and Cognitive Test (ImPACT), he had a total symptom score of 13 at week 2 and 8 at week 6. No daily medications were administered for any of these symptoms, but he was given acetaminophen to be taken as needed for the headaches. He was not taking any drugs known to alter brain excitability, plasticity, or excitation/inhibition balance. He worked as a manager of a multimedia department, where he continued working after the accident. He had a history of four prior episodes diagnosed as concussions while an athlete in college, over 20 years prior to the present episode. In two of these episodes there was no actual loss of consciousness, but they all had been associated with varying degrees of retrograde and anterograde amnesia, mild and transient concentration and memory difficulties, headaches, and dizziness that had completely subsided within 2 months from the episode. The last episode was 21 years prior. Past medical history, review of system and family history were otherwise negative.
He was tested twice, 2 and 6 weeks after the present concussion. His findings were compared to a control group of 12 healthy adults (5 females), aged between 19 and 55 years old (30±14). We looked for control subjects spanning a wide age range to minimize the potential impact of age-related effects. The age of the patient was included within this age range. Comparison of the results in our patient with a subset of the control subjects closer to his age (mean 44 years of age) did not affect any of the reported findings. The control subjects had no psychiatric or neurological conditions, normal neurological and medical exams, no history of concussions or TBI, were not taking any medications, and had no contraindications to receive TMS (Rossi et al., 2009).
All participants tolerated the procedures, including TMS, without any side effect or complication. In particular, the patient did not have any headache either just before or at the end of each of the session. No other adverse events (pain, difficulty in concentrating, thinking, change in mood) were reported. All participants gave written informed consent for participation in the study, which had been approved by the local Institutional Review Board.
2.1. Brain imaging, neuropsychological and neurophysiologic results
Brain MRI and DTI studies were obtained using a 3T GE scanner. Anatomical images were acquired using a T1-weighted, three-dimensional, magnetization-prepared, rapid-acquisition, gradientecho (MPRAGE) volume acquisition with a voxel resolution of 1 mm3. Diffusion images were acquired using a diffusion-weighted, single-shot, spin-echo, echo-planar imaging sequence (TE = min, TR = 17,000 ms, FOV 96 mm, voxel size = 2.2 mm 3, 30 nonlinear directions with b-value of 1,000 s/mm2). For precise quantitative measurement of changes in structural MRI, voxel-based morphometry (VBM) was done using FSL-VBM toolbox implemented in the FSL. Brain was extracted and created the study-specific template. All the images were non-linearly registered to the template and GLM using permutation testing was carried out. For diffusion tensor imaging analysis, we used Tract-Based Spatial Statistics (TBSS), which is widely used for whole-brain voxelwise comparison of white matter. First, data were preprocessed for head motion, and distortions due to eddy currents. Brain was extracted and DTI data was calculated for each voxel after fitting the diffusion tensor model to each voxel. This mean image was thresholded at an FA value of 0.2 and skeletonized to generate a white matter tract skeleton representing the center of the tracts common to all subjects. Permutation testing was used to calculate the t-statistics maps (Nichols and Holmes, 2002) (uncorrected p<0.05).
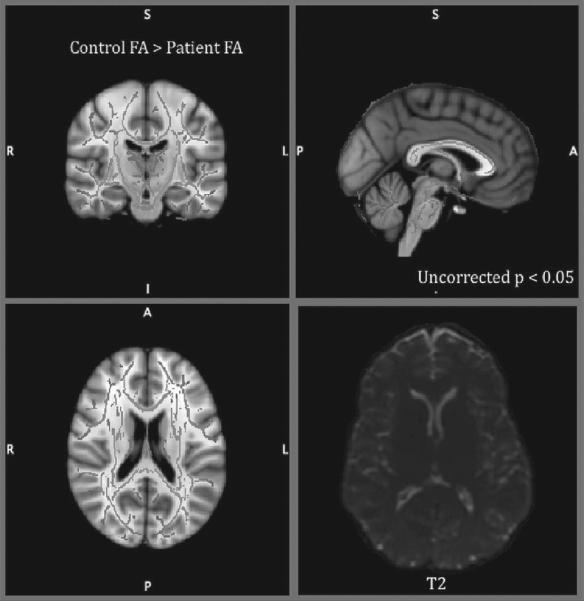
No significantly different areas were found in VBM analysis (uncorrected p<0.05). DTI analysis revealed that changes in fractional anisotropy (FA) in the TBI patient did not differ from the findings in the controls (uncorrected p<0.05) (Smith et al., 2006) (Fig. 1). Importantly, as part of the acute clinical evaluation after the concussion, the patient underwent a more complete clinical MRI study, including T2 weighted images that ruled out small hemorrhagic intraparenchymal lesions, intracranial hematomas and other signs of pathology.
Fig. 1.
Voxelwise differences in fractional anisotrophy between TBI patient and control group overlaid on mean FA skeleton (in green in the online version) didn’t show any significant differences in TBSS analysis (uncorrected p<0.05). No lesion was observed on T2-weighted MRI (right inferior).
Neuropsychological function was tested with the Immediate Postconcussion Assessment and Cognitive Test (ImPACT) and the CANTAB battery (Cambridge Cognition) and did not reveal any measurable cognitive deficits in the patient despite his subjective complaints.
Single- and paired-pulse TMS were applied using biphasic and monophasic figure-of-eight coils respectively (each wing, mean diameter 50 mm and outer diameter 70 mm) attached to a Nexstim stimulator (Nexstim Ltd, Helsinki, Finland). Continuous theta burst stimulation (cTBS) was applied using a biphasic figure-of-eight coil (each wing inner diameter 35 mm and outer diameter75 mm) attached to a MagPro stimulator (MagVenture A/S, Farum, Denmark).
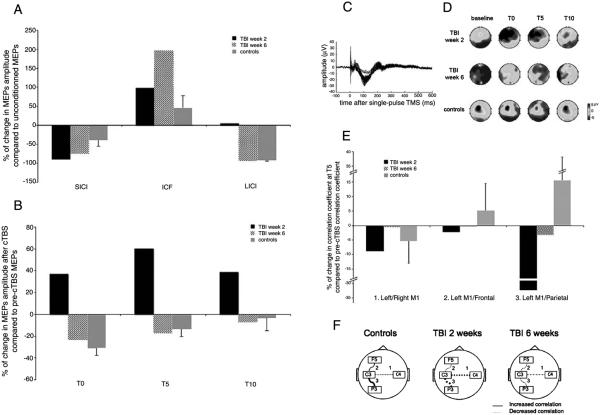
Paired-pulse TMS over the left primary motor cortex (M1) (Kujirai et al., 1993; Ziemann et al., 1996) was used to assess intracortical facilitation (ICF, tested with 12 ms between pulses) and short/long-interval intracortical inhibition (SICI and LICI, 3 and 100 ms between pulses respectively). The patient had significantly greater ICF at both time points. LICI was absent at week 2, but returned to normal at week 6. SICI in our patient was not different from the control group, which showed expected patterns of SICI, ICF and LICI (Fig. 2A).
Fig. 2.
A: Percentage of changes in MEPs amplitude after paired-pulse compared to unconditioned TMS pulse, for the TBI patient at week 2, at week 6 and for the group of control subjects (vertical bars indicate standard errors). Positive values indicate facilitation whereas negative values indicate suppression. SICI: short intracortical inhibition is expected with an inter-stimulus interval (ISI) of 3 ms. ICF: intracortical facilitation is expected with an ISI of 12 ms. LICI: long intracortical inhibition is expected with an ISI of 100 ms. The TBI patient had significantly greater ICF at both time points (p<0.01). LICI was absent in the TBI patient at week 2 (p<0.05), but returned to normal at week 6. Healthy controls showed expected patterns of SICI, ICF and LICI. B: Percentage of changes in MEPs amplitude at 0, 5 and 10 minutes after cTBS compared to pre-cTBS, for the TBI patient at week 2, at week 6 and for the group of control subjects (vertical bars indicate standard errors). Positive values indicate facilitation where as negative values indicate suppression. TBI patient had a significant (p<0.01) motor evoked potential (MEP) facilitation at week 2, but displayed predicted MEP suppression at week 6 (p<0.01). Healthy controls show expected MEP depression after cTBS. C: Example of TMS-evoked potentials at all electrodes in healthy controls. D: Topographical maps 50 ms after the TMS pulses, at baseline and at 0, 5 and 10 minutes after cTBS, for controls, and for TBI patient at week 2 and week 6. E: Changes in correlation between the temporal EEG signal recorded at the two contralateral M1 s (electrodes C3-C4), at the stimulated M1 and the ipsilateral frontal area (C3-F5) and at the stimulated M1 and the ipsilateral parietal (C3-P3), were computed at T5 compared to baseline (pre-cTBS) for the TBI patient at week 2, at week 6 and for the group of control subjects (vertical bars indicate standard errors). F: Illustration of the changes in correlation after cTBS.
Plasticity was evaluated with cTBS (Huang et al., 2005), expected to induce inhibition of the stimulated left M1 as indexed by a depression of motor-evoked potentials (MEPs) in contralateral hand muscles in response to subsequent single-pulse TMS. EEG was recorded simultaneously using the Nexstm eXimia TMS-compatible system. Contrary to the findings in controls, our patient had significant MEP facilitation at week 2, at 0, 5, 10 and 20 minutes after cTBS, but displayed normal inhibition of MEPs at week 6 (see Fig. 2B for the values up to 10 minutes after cTBS).
EEG raw data were high-pass filtered (1 Hz), epoched around each TMS pulse (−0.2 to 0.6 s, baseline: pre-TMS). For each subject, an Independent Component Analysis was run to identify and eliminate artifacts and remaining noisy channels were interpolated. Topographical EEG maps, 50 ms after single-pulse TMS, showed that at week 2, the patient had less intense and more widespread activation in response to single-pulse TMS over left M1 than the control group. This activation over left M1 was increased after cTBS, whereas it was decreased for the control group. At week 6, although the activation remained widespread, there was no more massive increase after cTBS (Fig. 2C).
In control subjects, the interhemispheric cross-correlation between temporal signals recorded over the two motor cortices (0 to 600 ms after the single-pulse TMS) tended to decrease 5 min after cTBS compared to baseline, whereas correlation between the stimulated M1 and the ipsilateral parietal and frontal areas tended to increase. The TBI patient significantly differed from the control subjects with a cTBS-induced increase of left M1-left parietal correlation at week 2, but not at week 6 (Fig. 2D and E).
3. Discussion
TMS may provide new insights into the physiologic impact of a mild TBI and the course of recovery. Our findings support the hypothesis of abnormal balance of cortical excitation and inhibition for several weeks after a concussion, even in the absence of any structural brain abnormalities as assessed by MRI or DTI, or objective neuropsychologic deficits. Two weeks after the concussion there was an increased ICF and a decreased LICI. These results are further supported by the apparent inversion of the modulatory effects of cTBS, with MEP facilitation instead of suppression. In addition, EEG correlation abnormalities suggest altered functional connectivity after mild TBI. All these measures became more similar to the measures from healthy controls at week 6, paralleling the clinical improvement. Further studies are needed to determine if this pattern of neurophysiologic abnormalities is consistent across all mild-TBI patients.
Alteration of cTBS-induced plasticity and excitatory/inhibitory balance might reflect adaptive or maladaptive plasticity mechanisms in response to the injury. One might argue that acutely after an injury increased in hibition may limit the lesion by preventing the spread of damage, later on there is a need to restore brain plasticity to reorganize networks in response to the injury (Landi and Rossini, 2010; Pascual-Leone et al., 2011; Demirates-tatlidede et al., 2011). Longitudinal studies in patients following TBI patients are thus important to understand this injury-related plasticity.
We believe that the changes in TMS measures between the two visits in our patient reflect recovery processes, rather than intra-individual test-retest variability. Single- and paired-pulse TMS measures show high test-retest reliability (Kobayashi and Pascual-Leone, 2003; Rossini et al., 1999). In regards to TBS effects, Huang et al. (2008) described high correlations in test-retest results of post-TBS modulation (up to 15 minutes) in seven normal subjects (Huang et al., 2008). Our own data (unpublished) similarly reveal high test-retest within-subject reliability of TBS-effects. Nontheless, further studies on test-retest variability within healthy subjects are needed and thus our findings should be considered preliminary.
Repetitive TMS in the study protocol did not cause any adverse events in this 44-year old patient. Although our findings are promising, we cannot conclude that this dose of rTMS is safe for all persons presenting with similar injuries. TBI is a heterogeneous injury and the safety and efficacy of this technique must be studied further in this population.
Although the physical and neurological symptoms following a concussion may resolve within a one-week period (Echemendia et al., 2001), subjective complaints may persist and brain physiology may remain abnormal. Epidemiologic studies suggest that these physiologic derangements may represent an important risk factor for the development of dementia (Guskiewicz et al., 2005). Our findings suggest that TMS measures may offer valuable markers of subclinical abnormalities that may be important for longer term prognosis and return to full activity decisions.
Acknowledgements and Grants
This work was supported in part by grants from CIMIT (Dr. Pascual-Leone), the National Center for Research Resources: Harvard Clinical and Translational Science Center (UL1 RR025758). Dr. Pascual-Leone serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Allied Mind, Neosync, and Novavision, and holds intellectual property on the real-time integration of transcranial magnetic stimulation (TMS) with electroencephalography (EEG) and magnetic resonance imaging (MRI). Dr. Vernet was supported by the Fyssen Foundation (France). Dr. Yoo was supported by a National Research Foundation of Korea grant funded by the Korean Government MEST, Basic Research Promotion Fund (NRF-013-2010-1-E00018). Mr. Mizrahi was supported by the Doris Duke Charitable Foundation. The authors thank Andrea Vatulas, Cara Burzynski and Ann Connor for their administrative support.
References
- De Beaumont L, Theoret H, Mongeon D, Messier J, Leclerc S, Tremblay S, et al. Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain. 2009;132:695–708. doi: 10.1093/brain/awn347. Pt 3. [DOI] [PubMed] [Google Scholar]
- Demirtas-Tatlidede A, Vahabzadeh-Hagh AM, Bernabeu M, Tormos JM, Pascual-Leone A. Noninvasive brain stimulation in traumatic brain injury. J Head Trauma Rehabil. 2011 doi: 10.1097/HTR.0b013e318217df55. DOI: 10.1097/HTR.0b013e318217df55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echemendia RJ, Julian LJ. Mild traumatic brain injury in sports: Neuropsychology’s contribution to a developing field. Neuropsychol Rev. 2001;11(2):69–88. doi: 10.1023/a:1016651217141. [DOI] [PubMed] [Google Scholar]
- Ellemberg D, Henry LC, Macciocchi SN, Guskiewicz KM, Broglio SP. Advances in sport concussion assessment: From behavioral to brain imaging measures. J Neurotrauma. 2009;26(12):2365–2382. doi: 10.1089/neu.2009.0906. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Cantu RC, Randolph C, et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57(4):719–726. doi: 10.1093/neurosurgery/57.4.719. discussion -26. [DOI] [PubMed] [Google Scholar]
- Hoskison MM, Moore AN, Hu B, Orsi S, Kobori N, Dash PK. Persistent working memory dysfunction following traumatic brain injury: Evidence for a time-dependent mechanism. Neuroscience. 2009;159(2):483–491. doi: 10.1016/j.neuroscience.2008.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC, Edwards MJ, Chen RS. Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cereb Cortex. 2008;18(3):563–570. doi: 10.1093/cercor/bhm087. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2(3):145–156. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- Kobori N, Dash PK. Reversal of brain injury-induced prefrontal glutamic acid decarboxylase expression and working memory deficits by D1 receptor antagonism. J Neurosci. 2006;26(16):4236–4246. doi: 10.1523/JNEUROSCI.4687-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi D, Rossini PM. Cerebral restorative plasticity from normal ageing to brain diseases: A “never ending story”. Restor Neurol Neurosci. 2010;28:349–366. doi: 10.3233/RNN-2010-0538. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Human Brain Mapping. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Freitas C, Oberman L, Horvath JC, Halko M, Eldaief M, et al. Characterizing brain cortical plasticity and network dynamics across the age-span in health and disease with TMS-EEG and TMS-fMRI. Brain Topogr. 2011;24:302–315. doi: 10.1007/s10548-011-0196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VL, Baskaya MK, Dogan A, Rothstein JD, Dempsey RJ. Traumatic brain injury down-regulates glial glutamate transporter (GLT-1 and GLAST) proteins in rat brain. J Neurochem. 1998;70(5):2020–2027. doi: 10.1046/j.1471-4159.1998.70052020.x. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Berardelli A, Deuschl G, Hallett M, Maertens de Noordhout AM, Paulus W, Pauri F. Applications of magnetic cortical stimulation. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:171–185. [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. Pt 3. [DOI] [PMC free article] [PubMed] [Google Scholar]