Abstract
Purpose
A Phase l trial of intravesical recombinant adenovirus-mediated interferon-α2b gene therapy (rAd-IFNα) formulated with the excipient SCH Syn3 was conducted in patients with non-muscle invasive bladder cancer (NMIBC) who recurred after Bacillus Calmette-Guerin (BCG). The primary objective was to determine the safety of rAd-IFNα/Syn3; secondary endpoints were to demonstrate effective rAd-IFNα gene expression and preliminary evidence of clinical activity at three months.
Patients and Methods
Seventeen patients with recurrent NMIBC after BCG were enrolled. A single treatment of rAd-IFNα (3×109 to 3×1011 particles/mL) formulated with the excipient Syn3 was administered. Patient safety was evaluated for ≥12 weeks. Efficacy of gene transfer was determined by urine IFNα protein concentrations. Preliminary drug efficacy was determined at 3 months.
Results
Intravesical rAd-IFNα/Syn3 was well tolerated as no dose limiting toxicity (DLT) was encountered. Urgency was the most common adverse event and all were grade 1 or 2. rAd-IFNα DNA was not detected in the blood, however, transient low serum IFNα and Syn3 levels were measured. High and prolonged dose-related urine IFNα levels were achieved with the initial treatment. Of the 14 patients treated at doses ≥ 1010 particles/mL with detectable urine IFNα, 6 (43%) experienced a complete response at 3 months and 2 remained disease free at 29.0 and 39.2 months respectively.
Conclusion
Intravesical rAd-IFNα/Syn3 was well tolerated with no DLT encountered. Dose dependent urinary IFNα concentrations confirmed efficient gene transfer and expression. Intravesical rAd-IFNα/Syn3 demonstrated promising clinical activity in NMIBC recurring after BCG.
Keywords: gene therapy, bladder cancer, interferon
Introduction
Adjuvant Bacillus Calmette-Guerin therapy (BCG) is the most effective treatment for reducing recurrence and preventing progression of high-risk non-muscle-invasive bladder cancer (NMIBC), resulting in complete response (CR) rates approaching 70% at 24 months (1–3). Despite optimal maintenance therapy, most patients eventually recur, some with advanced disease (4). Several agents have been tested as second-line treatment for BCG failure; however, none provided durable outcomes (5–7). Despite the possibility of interval disease progression many patients refuse extirpative therapy. Effective salvage intravesical therapy is needed to avoid cystectomy associated morbidity and improve disease specific outcomes (4, 5–7).
Recombinant interferon α2b protein (IFNα) has pleiotropic effects that contribute to antitumor activity. Intravesical IFNα has demonstrated insufficient durability as monotherapy for NMIBC after BCG failure (9), possibly related to short exposure during standard intravesical dwells of 1–2 hours. INFα was combined with BCG to enhance the cellular immune response to BCG and improve efficacy (7). Despite initial responses, many later relapsed, and ultimately required radical cystectomy. Intravesical gene therapy provides the potential for high, sustained levels of IFNα that may enhance efficacy by inducing endogenous production (4) and the ease of local, targeted delivery makes NMIBC an ideal disease for intravesical gene therapy. Due to the presence of an anti-adherence barrier that protects the bladder against infection, initial efforts to transduce the urothelium utilizing adenoviral vectors were unsuccessful (10). SCH 209702 (Syn3), a novel excipient, markedly enhances adenoviral-mediated transduction of normal urothelium and NMIBC (11, 12). Consequently, recombinant adenovirus-mediated interferon-α2b (rAd-IFNα) gene transfer and expression in both urothelium and human bladder cancer cells growing in mice is dramatically enhanced with Syn3, which results in high, durable urine IFNα concentrations and tumor regression (13, 14).
Based on preclinical studies, a Phase I trial of rAd-IFNα/Syn3 for NMIBC that recurred after BCG was initiated (13–15). Although the evaluation of rAd-IFNα/Syn3 safety was the primary objective, we also demonstrate dose-dependent adenovirus gene transfer and expression in the bladder, confirming prolonged urinary concentrations of IFNα. Additionally we evaluated the clinical activity of rAd-IFNα/Syn3.
Materials and Methods
Patients
We performed a non-randomized, open-label, dose-escalating, multi-center phase l trial of intravesical rAd-IFNα/Syn3, in patients > 18 years with histologically confirmed urothelial NMIBC (Ta, Tis or T1). Patients with T1 disease were not enrolled unless they explicitly declined cystectomy despite managing physician recommendation. Patients were required to have histologically proven disease recurrence after at least 2 cycles of BCG, with or without recombinant IFNα protein, and a minimum of 3 months since last treatment. We defined 2 cycles of BCG therapy as a minimum of one 6-week induction course followed by a 3-week maintenance course. Patients who received a second 6-week induction course were also eligible. Participants were required to have a Karnofsky score ≥ 70%. Patients with psychiatric conditions, significant cardiovascular or pulmonary disease, uncontrolled diabetes, or immune diseases were excluded. Laboratory requirements included: a hemoglobin count ≥10 gm/dL, white blood cell count ≥3000 cells/μL, absolute neutrophil count of ≥1500 cells/μL, platelet count of ≥100,000/μL, international normalized ratios and partial thromboplastin times within normal limits, alanine and aspartate aminotransferase levels ≤1.5 times upper limit of normal, normal bilirubin level, and creatinine level ≤1.5 mg/dL. No previous intravesical gene therapy was permitted.
The trial was approved by the institutional review board of the participating institutions. Two study exceptions were granted; an 85 year old male with significant co-morbidities who refused cystectomy and a 54 year old male who was considered BCG intolerant due to arthropathy after a single 6-week induction course.
Treatment and biosafety
SCH 721015 (rAd-IFNα) is a non-replicating recombinant adenoviral interferon-α2b gene transfer vector encoding human IFNα2b cDNA. Expression is driven by the cytomegalovirus immediate-early promoter and the adenovirus 2 tripartite leader enhancer sequence. The excipient SCH 209702 (Syn3) is a polyamide surfactant that enhances adenoviral gene transfer to the bladder epithelium (11–15).
At least 3 patients were assigned to one of five dosing cohorts, using a standard Phase 1 dose-escalation design (3×109 to 3×1011 particles/mL of rAd-IFN, Syn3 1mg/mL in all patients; total volume 75mL; dwell time 1hr). Patients were required to undergo a cystoscopy with baseline photographs, biopsies, urinary cytology and bladder capacity evaluation 14 to 21 days prior to treatment. Patients remained hospitalized for approximately 48 hours after treatment. Daily monitoring for adverse event (AE) occurred for 5 days post-treatment.
Trial Objectives
Safety of rAd-IFNα/Syn3 was the primary objective. To be evaluable for toxicity, patients must have undergone 4 weeks of follow-up or developed a DLT within this period. AEs were graded according to Common Terminology Criteria, and treatment-related grade 3 or 4 toxicities were considered DLTs. Patients were required to return to the study center approximately 12 weeks after therapy for cystoscopy with bladder biopsy, urine cytology, and bladder capacity measurement.
Secondary endpoints evaluated gene transduction (urinary IFNα excretion), and clinical activity in those patients with detectable urine IFNα. A CR was defined as no visual evidence of disease, negative biopsy of the prior scar site (or any visually identified lesion) and negative cytology at 3-month cystoscopy. Patients who achieved a CR 3 months after administration were eligible to receive a second administration of rAd-IFNα/Syn3 at the same dose level. Following treatment, participants were contacted at months 6, 9, 12 and yearly for 2 years to assess for AEs and disease status.
Pharmacodynamics and pharmacokinetics of adenoviral shedding and systemic absorption of rAd-IFNα
To assess systemic exposure to the adenoviral vector, whole blood samples were collected at 2, 6 and 72 hours post dose (in EDTA) for determination of rAd-IFNα DNA by quantitative polymerase chain reaction (qPCR) (Lower limit of quantitation (LLOQ); 1×104 copies/mL). To assess shedding of rAd-IFNα, urine samples were collected daily from Day 7 to Day 14 post dose (rAd-IFNα DNA measured by qPCR; LLOQ 5×104 copies/mL).
Pharmacokinetics and systemic absorption of Syn3 (SCH 209702)
To assess systemic absorption of Syn3, plasma isolated from whole blood samples (prior to dosing, at the end of dosing and 2,3,4,6 and 24 hours post installation) were evaluated using a validated liquid chromatography with tandem mass spectrometric detection (LC-MS/MS) (LLOQ of 5 ng/mL).
Anti-adenoviral and anti-inteferon antibody titers
Whole blood samples were drawn prior to dosing, at Day 7 (anti-adenoviral antibodies only), at 4 weeks (days 28 or 29), and at 12 weeks post dose. Samples were allowed to clot at room temperature for 30 minutes then centrifuged to separate serum for analysis. Both the anti-adenoviral and anti-interferon antibody assays were non-quantitative electrochemiluminescent methods.
Serum IFNα levels
Blood for determination of serum IFNα protein concentration was collected prior to dosing, 6 hours post dose and daily for 7 days. Samples were analyzed by a validated electrochemiluminescent immunoassay (LLOQ 10 – 18IU/mL).
Urine IFNα levels
Urine IFNα concentrations were determined using a validated electrochemiluminescent assay (LLOQ of 30 IU/ML). Samples were collected on ice and were stabilized by the addition of buffer containing 10% bovine serum albumin, 50 mM (4-{2-hydroxyethyl}-1-piperazineethanesulfonic acid) HEPES (1 mL buffer to 10 mL of urine). Patients had 24-hour urine collections for the first 2 days post dose. Thereafter, patients had 2-hour urine collections for IFN concentration.
Pharmacokinetic Methods
Pharmacokinetic parameters were estimated by noncompartmental methods from plasma or urine concentration-time data using WinNonlin Enterprise Version 5.2 (Pharsight Corporation a Division of the Cetara Corporation, Mountain View, California). Maximum observed concentration (Cmax) and the time to Cmax (tmax) were obtained directly from the experimental data. The area under the plasma and urine concentration-time curve (AUC) from 0 to the time of the last quantifiable concentration (AUC0-last) was calculated using the linear trapezoidal method. Actual sampling times, as recorded in the case report forms, were used for calculation of AUC.
Results
Patient characteristics
17 patients participated in the study. All were evaluated for toxicity and outcome at 3 months. One (CR at 3 months) was lost to follow-up 6 months post-therapy. Baseline characteristics are shown in Table 1. Prior intravesical therapies are designated by the number of cycles (i.e BCG × 2 = one 6-week cycle and either a 3-week maintenance cycle or repeat 6-week cycle of BCG). One exception was granted, an 85 yr old man with TaHG and CIS with prostatic urethral involvement who declined cystectomy. The patient with T1HG disease and CIS was granted an exception after suffering BCG-induced arthropathy after a 6-week induction course. 5 of the 17 subjects received a second instillation after the week 12 evaluation for response.
Table 1.
Patient characteristics
| Patient (Gender/Age) | Stage at Treatment | Previous Intravesical Treatments | Status At 3 Months | Current Status | Current Status Follow Up (months) |
|---|---|---|---|---|---|
| DOSE LEVEL 1 | |||||
| 1 (M/60) | C1S | Thiotepa, BCG, BCG/IFN | CR | TaHG at 8.8mo, Cystectomy at 9.8mo, Currently NED | 55.5 |
| 2 (M/57) | Ta HG, CIS | BCG × 3 | Ta LG | Ta LG at 3.1mo, BCG+INF for 3yrs, Currently NED | 61.6 |
| 3 (M/78) | Ta LG | BGG, BCG/IFN | Ta LG. | Ta LG at 3.1mo, Cystectomy at 3.6mo, Currently NED | 51.8 |
| DOSE LEVEL 2 | |||||
| 4 (M/72) | CIS | BCG, BCG/IFN/Proleukin × 2 | CR | CIS at 53.2mo, Cystectomy at 55.8mo, Currently NED | 58.0 |
| 5 (F/56) | Ta HG, CIS | BCG × 2 | Ta LG | Ta HG at 3.4mo, Cystectomy at 4.8mo, Currently NED | 49.1 |
| 6 (M/78) | Ta HG, CIS | BCG × 2 | CIS | T1 HG at 9.6mo, Cystectomy at 9.8mo, Currently NED | 55.6 |
| DOSE LEVEL 3 | |||||
| 7 (M/38) | Ta HG | BCG, BCG/IFN | CR | DOC* w/o any TCC | 31.3 |
| 8 (M/67) | CIS | BCG, BCG/IFN | CR | CIS at 20.9mo, Cystectomy at 23.5mo, Currently NED | 50.5 |
| 9 (M/81) | CIS | BCG, BCG/IFN | T1HG | T1HG at 3.0mo, Cystectomy at 5.1mo, Died of Metastatic disease at 24.8mo | 24.8 |
| DOSE LEVEL 4 | |||||
| 10 (M/65) | Ta HG | MVAC, BCG, BCG/IFN, Mitomycin, Thiotepa | CR | Ta LG at 11.9mo treated with TURBT and Mitomycin, currently NED | 41.9 |
| 11 (M/85) | Ta HG, CIS* | BCG × 3 | Ta HG | Ta HG at 3.3mo, refused cystectomy, currently AWD | 39.8 |
| 12 (M/77) | CIS | BCG × 3 | CR | CR | 29.0 |
| 13 (M/79) | Ta HG | BCG, BCG/INF, Gemcitabine + Mitomycin, Adriamycin + Docetaxel | CIS | CIS at 3mo, BCG/INF + Proleukin + GMCSF, Left upper tract recurrence and left nephroureterectomy at 12mo, progression at 17mo, Died of metastatic disease at 18.5mo | 18.5 |
| DOSE LEVEL 5 | |||||
| 14 (M/78) | Ta HG | BCG × 2 | CR | CR | 39.2 |
| 15 (M/63) | CIS | BCG, BCG + IFN × 2 | CIS | CIS at 3.0mo, Cystectomy at 4.5mo, Currently NED | 32.6 |
| 16 (M/79) | Ta LG | BCG × 3 | Ta LG | Ta LG at 3.2mo, Cystectomy at 15.5mo, Currently AWD (urethral recurrently Ta LG) | 30.7 |
| 17 (M/55) | T1 HG, CIS# | BCG | Ta HG | Ta HG at 3.3mo, Cystectomy 8.5, Currently NED | 28.5 |
Adverse events
There were no DLTs, and no significant treatment-related adverse events were observed. Thus, no maximum tolerated dose was defined. Common treatment-related adverse events included micturition urgency (88%) headache (59%), fatigue (47%), and nausea (35%). The micturition urgency was successfully managed by pretreatment with anticholinergics. No dose-limiting changes in laboratory parameters were reported, but transient decreases in total white blood cell counts, neutrophil counts, and lymphocyte counts were observed. Although all adverse events were grade 1 or 2, all patients did have at least one adverse event. These were universally well tolerated or controllable with anticholenergics. One participant was hospitalized for AEs not considered treatment related (viral infection with concurrent flank pain, fever, nausea and vomiting). The patient’s symptoms resolved without urologic intervention.
Serum pharmacokinetics and urine shedding of rAd-IFNα
No rAd-IFNα-specific DNA was detected in blood samples from the 17 patients. 5 of 17 patients had occasional occurrence of anti-adenovirus titers greater than 10-fold over baseline with no measurable adverse clinical manifestations.
Pharmacokinetics of Syn3
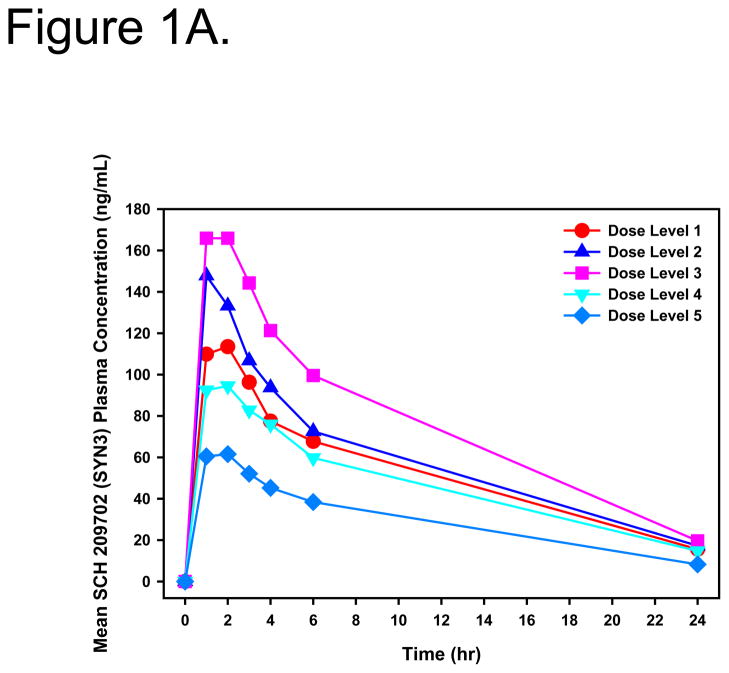
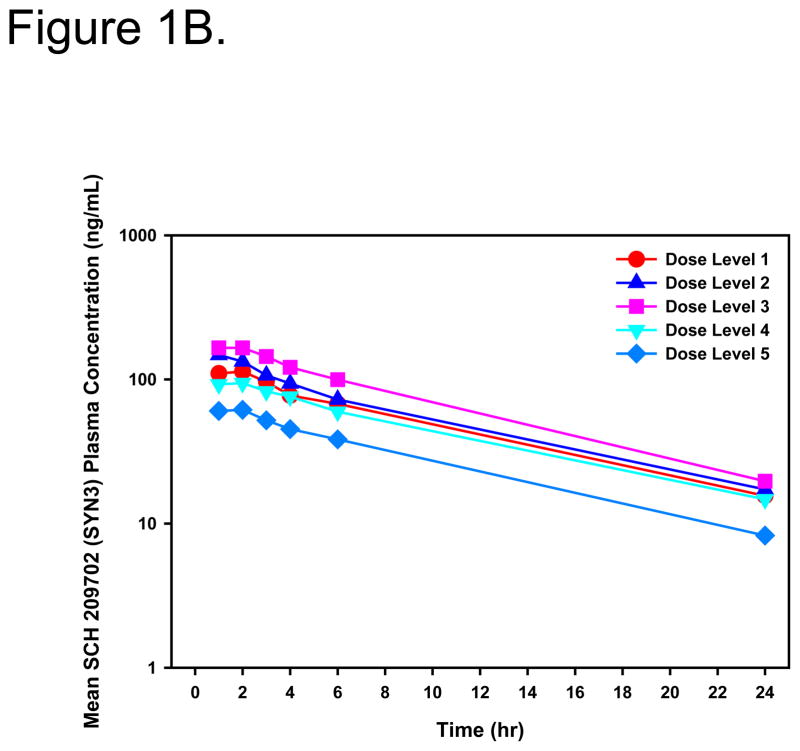
Systemic exposure to Syn3 was transient with no evidence of retention. Peak concentrations occurred 1–2 hours post dose and the mean systemic elimination, t½, was 8.4 hours (range, 6.0 to 11.4 hours) (Figure 1A and B).
Figure 1.
Linear (A) and log-linear (B) mean plasma Syn3 concentration-time profiles for all patients after intravesical administration of rAd-IFNα/Syn3. Dose Level 1 = 3×109, Dose Level 2 = 1×1010, Dose Level 3 = 3×1010, Dose Level 4 = 1×1011, Dose Level 5 = 3×1011 particles/mL in 75mL.
Serum IFNα levels
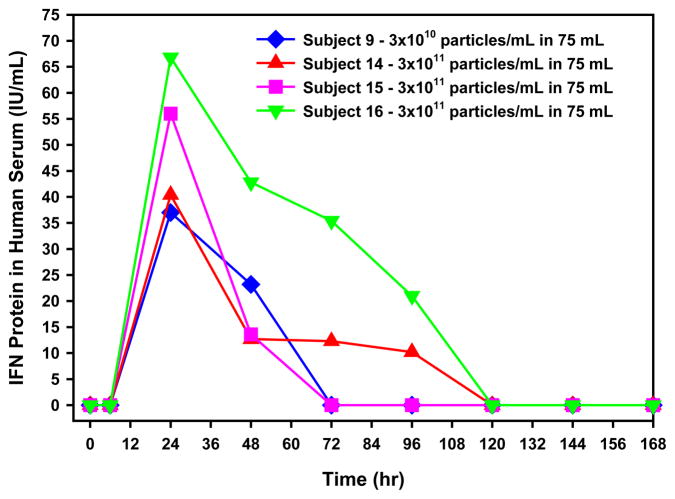
Low level, transient, dose-related serum IFNα was quantifiable in the serum of 4 of 17 patients (one at level 3, and 3 at the highest dose). Peak concentrations occurred at 24 hours post dose and remained above the LLOQ of the assay for 72–96 hours after treatment (Fig. 2).
Figure 2.
Individual serum IFNα concentration detected in the hours following intravesical administration of rAd-IFNα/Syn3. Results are reported as IFN international units (IU) per mL. One IU is approximately equal to 4 pg.
Serum Antibody Detection
Anti rAd-antibody titers were measured in nine participants (data not shown).. The majority of samples remained stable and only one remained elevated at day 90. Serum anti-INF-antibody analysis was performed in the same patients (qualitative assay; either negative or positive) with only one positive sample in a subject who had a positive pre-treatment sample.
Urine IFNα levels
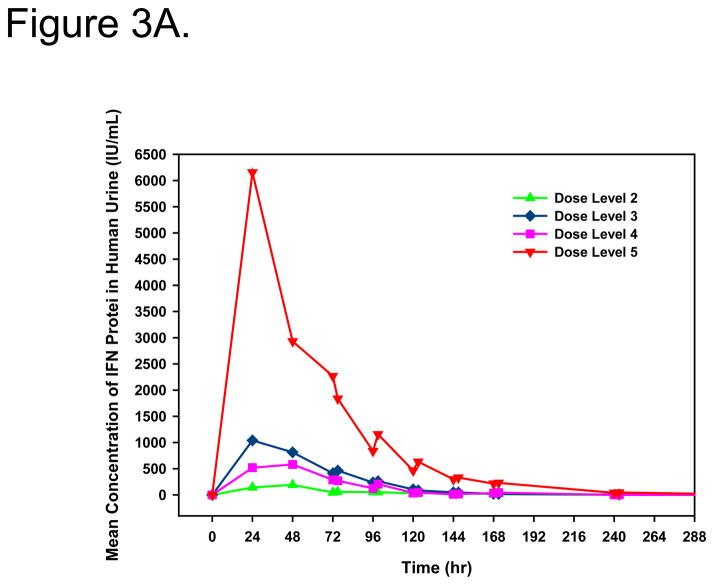
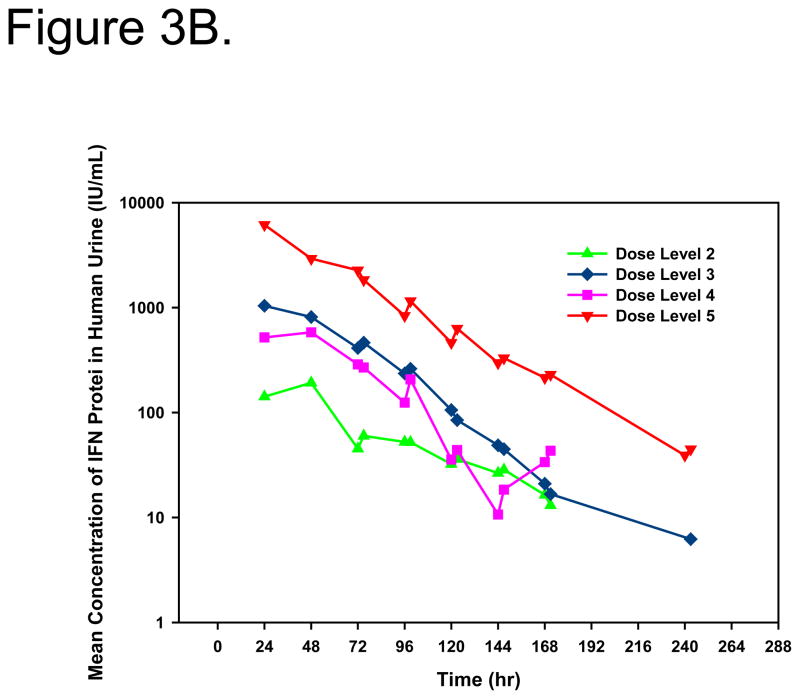
IFNα protein in the urine served as an indication of the efficiency of gene transfer and urothelial expression. With the exception of dose level 1, quantifiable urine concentrations of IFNα protein were measured in all patients. Peak IFNα concentrations and total IFNα exposure in urine were dose-dependent (Figure 3A/B). Measurable urine concentrations were observed up to 10 days after treatment. Mean IFNα in urine pharmacokinetic parameters are shown in Table 2.
Figure 3.
Linear (A) and log-linear (B) mean IFNα concentrations in urine after intravesical administration of rAd-IFNα/Syn3. Dose Level 1 = 3×109, Dose Level 2 = 1×1010, Dose Level 3 = 3×1010, Dose Level 4 = 1×1011, Dose Level 5 = 3×1011 particles/mL in 75mL.
Table 2.
Mean urine IFNα protein pharmacokinetic parameters following first intravesical administration of rAd-IFNα/Syn3.
| Dose Level (Number of subjects) | Cmax (IU/mL) | Tmaxa (hr) | Clast (IU/mL) | Tlasta (hr) | AUC0-last (IU*hr/mLc) |
|---|---|---|---|---|---|
| Dose Level 1 3 × 109 particles/mL (n=3) |
0 | NAb | NAb | NAb | 0 |
| Dose Level 2 1 × 1010 particles/mL (n=3) |
268 (97)e | 48 (48–99)d | 213 (137)e | 99 (48–171)d | 14100 (85)e |
| Dose Level 3 3 × 1010 particles/mL (n=3) |
1740 (80)e | 24 (24–48)d | 42.5 (38)e | 147 (48–243)d | 110000 (98)e |
| Dose Level 4 1 × 1011 particles/mL (n=4) |
1030 (68)e | 36 (24–99)d | 90.5 (56)e | 147 (99–171)d | 50600 (54)e |
| Dose Level 5 3 × 1011 particles/mL (n=4) |
7640 (82)e | 24 (24–48)d | 66 (73)e | 207 (120–243)d | 393000 (74)e |
Cmax = maximum observed concentration; Tmax = time to maximum observed concentration; Clast = last quantifiable concentration; Tlast = time to last quantifiable concentration; AUC0-last = area under the concentration-time curve from time 0 to the last quantifiable concentration
Median reported for Tmax and Tlast; arithmetic mean reported for Cmax, Clast, AUC0-last
NA: Not applicable
International units (IU) per milliliter multiplied by time in hours
Range
Percent coefficient of variation (%CV)
Clinical activity
7 of 17 patients experienced a CR at 3 months. All patients who achieved a CR initially presented with either CIS or TaHG disease. Six (43%) of 13 patients who were treated at doses ≥ 1×1010 particles/mL in which gene transfer resulted in detectable urine IFNα levels experienced a CR, the average duration was 31 months. Three of these 6 patients received a second intravesical dose of rAd-IFNα/Syn3. Five (36%) maintained a CR at 12 months.
Three patients remained disease free at 31.3*, 29.0, and 39.2 months respectively (*patient died of a non-cancerous cause at 31.3 months without evidence of disease). Patient 14 (disease free at 39.2 months), received the highest dose of rAd-IFNα, maintained elevated urine IFNα levels for 8 days after initial treatment, and had measurable urine IFNα after a second treatment.
Of the patients receiving dose levels 2–5, 8 of 14 patients recurred or progressed by 3 months (Table 1). Three patients who achieved a CR at 3 months recurred at 11.9, 20.9, and 53.2 months respectively. Ten patients underwent radical cystectomy and one patient underwent left nephroureterectomy for de novo upper tract disease. Among patients who underwent radical cystectomy one died of metastatic bladder cancer at 24.8 months, the remainder are disease free, one who is alive at 30.7 months following a TaLG urethral recurrence. The patient who underwent left nephro-ureterectomy developed progressive retroperitoneal disease at 17 months (5 months following nephro-ureterectomy) and ultimately died of disease at 18.5 months.
Discussion
We completed a promising Phase I study of intravesical rAd-IFNα/Syn3 in BCG refractory NMIBC. The safety profile was excellent with no DLTs identified and only one serious AE. The maximum tolerated dose was not reached and all patients tolerated treatment. No patients experienced serious local or systemic toxicities requiring intervention. Urgency, the most common adverse event, was effectively treated with pre-treatment anticholinergic administration.
In preclinical investigations, at least three distinct mechanisms mediating rAd-IFNα anti-tumor activity have been identified and normal urothelial cells were shown to be resistant to these effects (16–21). After treatment, rAd-IFNα is secreted into the bladder tissue and lumen by both normal urothelium and tumor cells, providing high and prolonged IFNα protein that is considerably higher and more prolonged than after bolus intravesical administration of IFNα protein alone (13). In IFNα sensitive cancer cells, high protracted tissue and urine IFNα levels can kill tumor cells by a tumor necrosis factor-related apoptosis-inducing ligand-mediated (TRAIL) mechanism (16). In addition IFNα resistant cancer cells can be killed by soluble bystander factor(s) produced by both rAd-IFNα treated normal urothelial and bladder cancer cells (13,17–19). Finally both IFNα sensitive and resistant tumor cells but not normal urothelial cells are susceptible to endoplasmic-reticular (ER) stress-related toxicity after the direct infection and high perinuclear IFNα production (13,17,20,21). Therefore the efficacy of rAd-IFNα treatment can involve other mechanisms and factors in addition to the anticipated antitumor activity that results from high urinary IFNα protein concentrations.
Although the primary endpoint of the study was safety, we were encouraged that more than 40% of the patients treated at doses ≥1×1010 particles/mL achieved a CR, accompanied by high urine IFNα concentrations, which often were measurable for several days. Most encouraging was that all CRs occurred in patients with either CIS or TaHG disease, demonstrating efficacy in a historically difficult to treat population. Some CR’s were durable, 36% were maintained for at least 12 months. Urine IFNα levels were observed in a dose-dependent manner, with the highest dose (3×1011 particles/mL in 75 mL) resulting in the highest and most durable urine IFNα exposure.
Given the limited number of subjects, neither the maximum concentrations nor the duration of urinary IFNα exposure directly correlated with clinical response or durability. The increased number of CRs starting at dose level 3 suggest that a minimum IFNα exposure may be needed for optimal activity though conclusions cannot be drawn. Although the study was not designed for a clinical activity endpoint we feel these results in high grade NMIBC are notable.
This trial represents the first preliminary evidence of safety and effective intravesical recombinant adenoviral gene therapy. No DLTs were reached and secondary clinical activity in patients was encouraging. The Phase I study allowed only a single intravesical instillation of rAd-IFNα/Syn3, however subjects with CR at 3 months could elect to receive a second dose. Phase II studies are being designed.
Acknowledgments
Supported by Genitourinary Bladder Cancer SPORE and Cancer Center Core Grant, (CA091846 and CA016672) Schering-Plough Research Institute (MSD) CS2005-00014622JS; and an NRSA Institutional Training Grant (T32) to M.F T32CA079449
References
- 1.Sylvester RJ, Van der Meijden AP, Lamm DL. Intravesical Bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2006;168:1964–1970. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 2.Sylvester RJ, Adrian PM, van der Meijden J, et al. BCG versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. J Urol. 2005;175:186–92. doi: 10.1097/01.ju.0000162059.64886.1c. [DOI] [PubMed] [Google Scholar]
- 3.Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1, and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group study. J Urol. 2000;163:1124–1129. [PubMed] [Google Scholar]
- 4.Cookson MS, Herr HW, Zhang ZF, et al. The treated natural history of high risk superficial bladder cancer: 15-year outcome. J Urol. 1997;158:62–67. doi: 10.1097/00005392-199707000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg G, Bahnson R, Brosman S, et al. Efficacy and safety of valrubicin for the treatment of Bacillus Calmette-Guerin refractory carcinoma in situ of the bladder. The Valrubicin Study Group. J Urol. 2000;163:761–767. [PubMed] [Google Scholar]
- 6.Dalbagni G, Russo P, Sheinfeld J, et al. Phase I trial of intravesical gemcitabine in Bacillus Calmette-Guerin-refractory transitional-cell carcinoma of the bladder. J Clin Oncol. 2002;20:3193–3198. doi: 10.1200/JCO.2002.02.066. [DOI] [PubMed] [Google Scholar]
- 7.O’Donnell MA, Lilli K, Leopold C. Interim results from a national multicenter phase II trial of combination bacillus Calmette-Guerin plus interferon alfa-2b for superficial bladder cancer. J Urol. 2004;172:888–893. doi: 10.1097/01.ju.0000136446.37840.0a. [DOI] [PubMed] [Google Scholar]
- 8.Herr HW, Sogani PC. Does early cystectomy improve the survival of patients with high-risk superficial bladder tumors? J Urol. 2001;166:1296–1299. [PubMed] [Google Scholar]
- 9.Belldegrun AS, Franklin JR, O’Donnell MA, et al. Superficial bladder cancer: the role of interferon-alpha. J Urol. 1998;159:1793–1801. doi: 10.1016/S0022-5347(01)63160-4. [DOI] [PubMed] [Google Scholar]
- 10.Pagliaro LC, Keyhani A, Williams D, et al. Repeated intravesical installations of an adenoviral vector in patients with locally advanced bladder cancer: a phase 1 study of p53 gene therapy. J Clin Oncol. 2003;15:2247–2253. doi: 10.1200/JCO.2003.09.138. [DOI] [PubMed] [Google Scholar]
- 11.Connor RJ, Engler H, Machemer T, et al. Identification of polyamides that enhance adenovirous-mediated gene expression in the urothelium. Gene Ther. 2001;8:41–48. doi: 10.1038/sj.gt.3301348. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita M, Rosser CJ, Zhou JH, et al. Syn3 provides high levels of intravesical adenoviral-mediated gene transfer for gene transfer of genetically altered urothelium and superficial bladder cancer. Cancer Gene Ther. 2002;9:687–691. doi: 10.1038/sj.cgt.7700488. [DOI] [PubMed] [Google Scholar]
- 13.Benedict WF, Tao Z, Kim CS, et al. Intravesical Ad-IFN alpha causes marked regression of human bladder cancer growing orthotopically in nude mice and overcomes resistance to IFN-alpha protein. Mol Ther. 2002;10:525–532. doi: 10.1016/j.ymthe.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 14.Tao Z, Connor RJ, Ashoori F, et al. Efficacy of a single intravesical treatment with Ad-IFN/Syn 3 is dependent on dose and urine IFN concentration obtained: implications for clinical investigation. Cancer Gene Therapy. 2006;13:125–130. doi: 10.1038/sj.cgt.7700865. [DOI] [PubMed] [Google Scholar]
- 15.Connor RJ, Anderson JM, Machemer T, et al. Sustained intravesical interferon protein exposure is achieved using an adenoviral-mediated gene delivery system: a study in rats evaluating dosing regimens. Urology. 2005;66:224–229. doi: 10.1016/j.urology.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Papageorgiou A, Lashinger L, Millikan R, et al. Autocrine TRAIL production mediates interferon-induced apoptosis in human bladder cancer cells. Cancer Res. 2005;64:8973–8979. doi: 10.1158/0008-5472.CAN-04-1909. [DOI] [PubMed] [Google Scholar]
- 17.Fisher MB, Zhang XQ, McConkey DJ, et al. Measuring soluble forms of extracellular cytokeratin 18 identifies both apoptotic and necrotic mechanisms of cell death produced by adenoviral-mediated interferon alpha: possible use as a surrogate marker. Cancer Gene Therapy. 2009;16:567–572. doi: 10.1038/cgt.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang XQ, Yang Z, Dong L, et al. Adenoviral-mediated interferon α overcomes resistance to the interferon protein in various cancer types and has marked bystander effects. Cancer Gene Therapy. 2007;14:241–250. doi: 10.1038/sj.cgt.7701011. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Dong L, Chapman E, et al. Conditioned medium from Ad-IFN-alpha-infected bladder cancer and normal urothelial cells is cytotoxic to cancer cells but not normal cells: further evidence for a strong bystander effect. Cancer Gene Therapy. 2008;12:817–822. doi: 10.1038/cgt.2008.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang XQ, Yang Z, Benedict WF. Direct gene transfer of adenoviral-mediated interferon-α into human bladder cancer cells but not the bystander factors produced induces endoplasmic reticulum stress-related cytotoxicity. Cancer Gene Therapy. 2011;18(4):260–264. doi: 10.1038/cgt.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Z, Zhang X-Q, Dinney CNP, Benedict WF. Direct cytotoxicity produced by adenoviral-mediated interferon α infection in interferon resistant cancer cells Involves ER stress and caspase 4 activation. Cancer Gene Therapy. Cancer Gene Therapy. 2011;18(9):609–616. doi: 10.1038/cgt.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]