Abstract
Previous work in our laboratory demonstrated that over-expression of human insulin-like growth factor-11 (hIGF-1) in the placenta corrects fetal weight deficits in mouse, rat, and rabbit models of intrauterine growth restriction without changes in placental weight. The underlying mechanisms of this effect have not been elucidated. To investigate the effect of intra-placental IGF-1 over-expression on placental function we examined amino acid transporter expression and localization in both a mouse model of placental Insufficiency (PI) and a model of human trophoblast, the BeWo Choriocarcinoma cell line.
For in vitro human studies, BeWo Choriocarcinoma cells were maintained in F12 complete medium + 10%FBS. Cells were incubated in serum-free control media ± Ad-IGF-1 or Ad-LacZ for 48 h. MOIs of 10:1 and 100:1 were utilized. In BeWo, transfection efficiency was 100% at an MOI of 100:1 and Ad-IGF-1 significantly increased IGF-1 secretion, proliferation and invasion but reduced apoptosis compared to controls. In vitro, amino acid uptake was increased following Ad-IGF-1 treatment and associated with significantly increased RNA expression of SNAT1, 2, LAT1 and 4F2hc. Only SNAT2 protein expression was increased but LAT1 showed relocalization from a perinuclear location to the cytoplasm and cell membrane.
For in vivo studies, timed-pregnant animals were divided into four groups on day 18; sham-operated controls, uterine artery branch ligation (UABL), UABL + Ad-hIGF-1 (108 PFU), UABL + Ad-LacZ (108 PFU). At gestational day 20, pups and placentas were harvested by C-section. Only LAT1 mRNA expression changed, showing that a reduced expression of the transporter levels in the PI model could be partially rectified with Ad-hIGF1 treatment. At the protein level, System L was reduced in PI but remained at control levels following Ad-hIGF1. The System A isoforms were differentially regulated with SNAT2 expression diminished but SNAT1 increased in PI and Ad-hIGF1 groups.
Enhanced amino acid isoform transporter expression and relocalization to the membrane may be an important mechanism contributing to Ad-hIGF-1 mediated correction of placental insufficiency.
Keywords: Trophoblast, Gene therapy, Proliferation, Amino acid, Transport
1. Introduction
Appropriate placental nutrient supply is vital for the fetus to achieve its growth potential. Placental nutrient supply is maintained by both active and passive transport systems in the maternal-facing, transporting layer of the placenta [1] and regulated by many hormonal and nutritional factors. Alterations in placental amino acid availability, especially branched chain amino acids play a significant role in the development of fetal growth restriction [2].
Amino acid transport system type A mediates the sodium-dependent uptake of amino acids with small side chains such as alanine and glutamine. Three isoforms of system A have been identified: sodium coupled neutral amino acid transporter (SNAT) 1 [3], SNAT2 [4,5], and SNAT4 [6]. Amino acid uptake by system A is highly regulated in many cell types by effectors such as pH, cell volume [7] and a variety of hormones such as insulin [8], glucagon [9] and cortisol [10]. In vitro studies have demonstrated that acute incubation of primary human placental trophoblast cells and the BeWo Choriocarcinoma cell line with exogenous insulin-like growth factor-1 (IGF-1) protein increased the uptake of small neutral amino acids via amino acid transport system A [11,12].
Amino acid transport system L transports branched amino acids and is a symporter dependent on simple non-branched amino acids; and unlike system A, system L is regulated by only a few of the factors previously mentioned. System L is comprised of two transporters, each containing the long chain molecule 4F2hc (also known as CD98) and one of the short chain molecules, LAT1 or LAT2, which are thought to target the transporter to specific membranes.
In pathological pregnancies, such as those complicated by Intra-Uterine Growth Restriction (IUGR), placental insufficiency is thought to be a major cause. Studies on human IUGR have shown reduced fetal levels of amino acids and function of placental transporters [13,14]. Furthermore, Jansson et al. [15] demonstrated that a reduction in placental System A transport and isoform expression occurs prior to the onset of IUGR in a rat model.
To date, the only treatment for IUGR is delivery of the baby and feeding, however studies in animal models of growth restriction have highlighted the possibility of treatment of IUGR prior to birth with maternal, amniotic or placental supplementation of growth factors such as IGF-1 [16,17]. Recently, placental gene therapy protocols for the treatment of IUGR with growth factor genes have been suggested [18] but the effects of adenoviral-mediated IGF-1 gene delivery on trophoblast growth or function is unknown. Chen et al. [19] demonstrated successful transfection of Ad-Timp3 into BeWo cells but the time period of infection was short and their focus was on targeted death in the BeWo cell line induced by TIMP3 expression. Adenoviral-associated virus has also successfully been used to transfect trophoblast previously [20], however we chose to use Ad-hIGF-1 as it demonstrated trophic effects in mouse placenta in a previous study [18].
It is unknown if the responses in human placenta would reflect those in the mouse model or use alternate mechanisms, therefore we chose to include Ad-hIGF-1 treatments in both a mouse model of placental insufficiency and in vitro in a widely-accepted and published model of human trophoblast, the BeWo Choriocarcinoma cell line in the current study. To investigate the effects of adenoviral-mediated gene therapy on trophoblast growth and function, we examined the effects of Ad-hIGF-1 on proliferation, invasion, apoptosis and amino acid transport mechanisms in the BeWo Choriocarcinoma cell line and amino acid transport mechanisms in a murine model of placental insufficiency treated with intra-placental injection of Ad-hIGF1.
2. Materials and methods
2.1. Cell culture
The BeWo Choriocarcinoma cell line was obtained from ATCC. Cells were maintained at 37 °C under 5% CO2 and cultured in Ham’s F12 nutrient mix supplemented with 10% fetal calf serum and 2% penicillin/streptomycin (Invitrogen, Paisley, Strathclyde, UK). The medium was changed every 2 days and cells were subcultured every 7 days. All cells used were between passages 5 and 12.
2.2. Mouse procedures
All animal procedures were performed under protocols approved by the Institutional Animal Care and Use Committee of CCHMC. Timed pregnant C57 Bl/6J mice were divided into four groups: 1) Control: Sham operated; 2) uterine artery branch ligation (UABL); 3) UABL with Ad-Lacz 1 × 108 plaque forming units (pfu); and 4) UABL with Ad-IGF-I 1 × 108 pfu. At gestational day 18, through laparotomy, UABL was performed and treatment was administered as previously described [18]. Caesarean-section was performed on day 20, pups were weighed and placentas were fixed in PFA or carefully separated by peeling apart the Labyrinthine zone from the Junctional and Decidual zones and snap frozen.
2.3. Viral vectors
Ad-hIGF-1 and Ad-LacZ constructs were obtained from Dr. M. Herlyn (Institute for Human Gene Therapy, University of Pennsylvania). Both constructs are replication defective, serotype 5 adenovirus vectors [18]. Ad-LacZ and Ad-hIGF-1 were given at an MOI of 100:1 or 10:1 for 48 h in serum-free media. Control cells were incubated in serum-free media for the same time frame.
2.4. Transfection efficiency
In vitro transfection efficiency was assessed by staining for β-galactosidase enzyme activity (X-gal stain) after exposure of BeWo cells to either control media or media containing Ad-LacZ at MOIs of 10:1 and 100:1, efficiency was calculated as the percentage of positively stained cells over total cell number.
2.5. Transgene expression
To confirm transgene expression in the Ad-hIGF-1 treated cells levels of secreted IGF-1 were measured by ELISA (ALPCO Diagnostic, Salem, NH) in the media following exposure to control media or media containing Ad-hIGF-1 at MOIs of 10:1 and 100:1 for 48 h. Serum IGF-1 levels were normalized to total protein of cell lysates.
2.6. Proliferation and invasion
BeWo proliferation was assessed by crystal violet assay [21] and invasion indices were calculated using a BD Matrigel invasion chamber kit after 48 h of exposure to either control media or Ad-hIGF-1 or Ad-LacZ at MOIs of 10:1 and 100:1.
2.7. Amino acid uptake assay
Amino acid system A activity was measured as Na+-dependent uptake of 14C-labelled 2-(methylamino)isobutyric acid (MeAIB; 10 μM), and amino acid system L transporter activity was determined as 2-aminobicyclo(2.2.1)heptane-2-carboxylic acid (BCH)-inhibitable uptake of l-[3H]leucine (0.05 μM) as previously described [22].
2.8. Reverse transcription quantitative polymerase chain reaction
mRNA expression was analysed by RTqPCR as previously published [22]. Oligonucleotide primers were aligned against the human or mouse genome by Primer-BLAST (www.NCBI.org) to ensure specificity (Table 1). Gene expression was assayed, in duplicate, using 1/40th of the cDNA template and 300 nM of forward and reverse primer in a 25ul Power SYBR Green PCR Master Mix reaction in the Applied Biosystems StepOne-Plus Real-Time PCR System. Gene expression was normalized to TATA box Binding Protein (TBP) for the BeWo and both β-actin and RPS20 for the mouse placenta gene expression. Relative expression values were calculated using the Comparative Ct method [23].
Table 1.
Oligonucleotide primers for RTqPCR.
| Oligonucleotide | Sequence (5′–3′) | Amplicon (bp) | Intron (bp) |
|---|---|---|---|
| Hu SLC3A2 1F | CCCCAATTTTGGCTCCAAGG | 77 | 841 |
| Hu SLC3A2 1R | CCAGAATGACACGGATGCTC | ||
| Hu SLC7A5 1F | CCATCATCGGCATGATCTGG | 89 | 1906 |
| Hu SLC7A5 1R | GATGAAGAACACAGGCAGGG | ||
| Hu SLC7A8 1F | GCTGCTTGCATATGTCTGCT | 87 | 12,496 |
| Hu SLC7A8 1R | CTTGGCGTAAGTGAACGTGT | ||
| Hu SLC38A1 1F | GCAGTGGGATTTTGGGACTC | 87 | 20,025 |
| Hu SLC38A1 1R | ACAGCAATGTCACTGAAGTCAA | ||
| Hu SLC38A2 1F | CCTTTGTGATCCAGGCATT | 91 | 1674 |
| Hu SLC38A2 1R | CCAATGACACCAACAGAACCA | ||
| Hu TBP F | GAACCACGGCACTGATTTTC | 77 | 2285 |
| Hu TBP R | TGCCAGTCTGGACTGTTCTTC | ||
| Mo ACTB F | CTA AGG CCA ACC GTG AAA AGA T |
83 | 454 |
| Mo ACTB R | CAC AGC CTG GAT GGC TAC GT | ||
| Mo SLC2A3 1F | CAT TGT CCT CCA GCT GTC TC | 78 | 1258 |
| Mo SLC2A3 1R | GCG TCC TTG AAG ATT CCT GT | ||
| Mo SLC38A1 1F | GGA CGG AGA TAA AGG CAC TC | 85 | 1717 |
| Mo SLC38A1 1R | CAG AGG GAT GCT GAT CAA GG | ||
| Mo SLC38A2 2F | ACC TTT GGT GAT CAA GGC AT | 78 | 1374 |
| Mo SLC38A2 2R | AGG ACC AGA TAG TCA CCG TT | ||
| Mo SLC38A4 2F | CAT CGT GCT GTT CCC GAT | 67 | 6186 |
| Mo SLC38A4 2R | CCA GCT GAA GGG TTT CCT TG | ||
| Mo SLC7A5 2F | ATT CAA GAA GCC TGA GCT GG | 73 | 1214 |
| Mo SLC7A5 2R | GCA GGC CAG GAT AAA GAA CA | ||
| Mo SLC7A8 1F | AAG AAA GAG ATC GGA TTG GT | 81 | 21,434 |
| Mo SLC7A8 1R | TTT CGG TGA GAC AAA GAT TC | ||
| Mo RPS20 F | GCT GGA GAA GGT TTG TGC G | 100 | 182 and 252 |
| Mo RPS20 R | AGT GAT TCT CAA AGT CTT GGT AGG C |
2.9. Western blotting
BeWo cells were homogenised in BSS 9pH7.4) and sonicated. Total protein was separated on a 4–20% Tris–glycine gel and transferred to nitrocellulose membrane (iBlot, Invitrogen). Membranes were incubated with primary antibodies overnight at 4°C and an HRP-anti-rabbit IgG antibody (Sigma) for 2 h at room temperature. Protein bands were visualised using Super Signal West Pico Chemiluminescent substrate (Pierce, Rockford, IL, USA). Protein expression was quantified using densitometry normalised against β-actin for loading controls.
2.10. Immunohistochemistry in mouse placenta
Fixed placental tissues were embedded in paraffin, and 5 μm sections cut. Serial sections were deparaffinised, rehydrated and incubated in Target Retrieval Solution (Dako, Carpathia, CA). After washing, sections were incubated in 3% hydrogen peroxide. Nonspecific binding was blocked with 5% normal goat serum. Sections stained for 4F2hc were blocked by M.O.M. Biotinylated Ig Blocking Reagent following the protocol of the Vector M.O.M.Immunodection Kit. Sections were incubated with anti-SNAT1, 2, LAT1, 2 and 4F2hc antibodies (Abcam). After washing slides were incubated with appropriate secondary antibodies (Vector Laboratories, Inc., Burlingame, CA). Antibody binding was detected using DAB (Dako). Transporter expression was observed by light microscopy (Nikon, Melville, NY)
2.11. Immunocytochemistry in BeWo Choriocarcinoma cells
Apoptosis was investigated using anti-Caspase 3 antibody (Cell Signaling, Danvers, MA), and System A and L transporter localisation was investigated using appropriate antibodies. Following blocking in 5% FSG, primary antibody was applied to cells in 0.2% FSG/PBS and allowed to bind overnight at 4 °C in a humidified atmosphere. Following rinses, the appropriate FITC conjugated antibody (Vector Laboratories Inc, Burlingame, CA) was applied to the cells for 1 h at room temperature in a humidified atmosphere. The cells were rinsed and mounted in Vectashield mounting medium (Vector Laboratories). Cells were observed and images captured using a Nikon microscope and Elements software (Nikon). Antibody specificity was confirmed by omittance of the primary antibody during immunocytochemistry.
2.12. Data presentation and statistics
Data are presented as means ± SEM. Statistical analysis was performed using ANOVA with post hoc Tukey’s test *p < 0.05, **p < 0.01.
3. Results
3.1. Transfection efficiency
X-gal staining of Ad-LacZ-treated cells demonstrated a transduction efficiency of 85% at an MOI of 10:1 (Fig. 1a) and 100% at an MOI of 100:1 (Fig. 1b). Furthermore, IGF-1 levels in the media of Ad-hIGF-1 infected cells increased significantly (p < 0.001, n = 4) at an MOI of 100:1 of over 90% compared to control and Ad-LacZ infected cells (Fig. 1c).
Fig. 1.
β-galactosidase enzyme activity (X-gal stain) in BeWo cells cultured with Ad-LacZ at an MOI of (a) 10:1 and (b) 100:1. C: Secreted IGF-1 levels are significantly (p < 0.001, n = 4) increased after 48 h exposure of BeWo cells to Ad-IGF-1 (MOI 100:1) compared to both control and Ad-LacZ (MOI 100:1). D: BeWo proliferation was assessed in duplicate by Crystal violet uptake assay at 570 nm, data are means ± SEM, ANOVA, p < 0.01, n ≥ 4 for each treatment. E: BeWo invasion was assessed by cell counting 5 fields per replicate following invasion into matrigel over 48 h. Data are mean ± SEM, ANOVA, p < 0.05 for MOI 10, p < 0.01 MOI 100, n = 5 replicates for each treatment. F: Apoptosis was evaluated by counting Caspase-3 positive cells after 48 h exposure to control media ± Ad-IGF or Ad-LacZ (MOI 100 and 10:1). Cell counts are mean ± SEM for 5 fields for each replicate. ANOVA p < 0.01, n = 6 replicates.
3.2. Proliferation, invasion and apoptosis
To identify any global changes that transfection may have on the BeWo cell line we assessed proliferation, invasion and apoptosis rates. Ad-LacZ was included as a viral control to identify any non-transgene effects and showed no viral effects on cell proliferation, invasion or apoptosis at either of the MOIs used. Ad-hIGF-1 significantly (ANOVA p < 0.01, n ≥ 4) increased BeWo proliferation rates more than two-fold at an MOI of 100:1 compared to both control and Ad-LacZ infected cells (Fig. 1d). Invasion into matrigel was significantly (p < 0.01, n = 5) increased by 40% following Ad-hIGF-1 (MOI of 100:1) compared to control and Ad-LacZ infected cells (Fig. 1e). In contrast, apoptosis levels in BeWo cells exposed to Ad-hIGF-1 (MOI of 100:1) were significantly (ANOVA, p < 0.01, n = 6) reduced compared to those in control media and all other infections (Fig. 1f).
3.3. Amino acid uptake
Both MeAIB (system A uptake), and Leucine (system L uptake), uptakes were significantly (p < 0.05, n > 5) increased following exposure of BeWo cells to Ad-hIGF-1 at an MOI of 100:1 compared to cells exposed to control media or Ad-LacZ for 48 h (Fig. 2).
Fig. 2.

MeAIB and Leucine uptake in BeWo cells is significantly (ANOVA<0.05, post hoc test *p < 0.05, n = 6 for each treatment) increased following 48 h infection with Ad-hIGF-1 (MOI 100:1).
3.4. Amino acid transporter mRNA expression
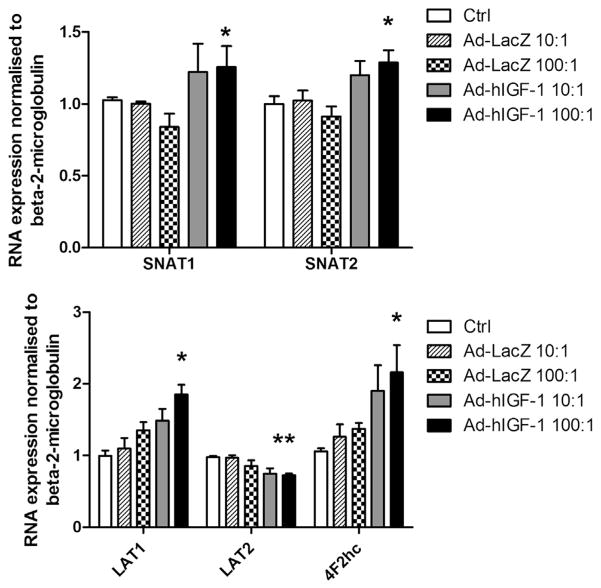
Transfection of BeWo cells with Ad-hIGF1 at an MOI of 10:1 did not alter the mRNA expression of any of the transporters compared to non-transfected or Ad-LacZ (MOI 10:1) transfected cells. However, at an MOI of 100:1, Ad-hIGF-1 transfection significantly (p < 0.05, n = 4) increased the mRNA expression of SNAT1 and 2, LAT1 and 4F2hc compared to both non-transfected and those Ad-LacZ transfected cells by 20–50% (Fig. 3). LAT2 mRNA expression was reduced by 25% following Ad-hIGF-1 compared to control cells (Fig. 3).
Fig. 3.
Exposure of BeWo cells to Ad-hIGF-1 (MOI 100:1) significantly (ANOVA p < 0.05, post hoc test *p < 0.05, n = 3 for each treatment) increased SNAT1, SNAT2, LAT1 and 4F2hc RNA expression compared to control or Ad-LacZ treated cells.
In contrast, only LAT1 mRNA expression changed in the labyrinth of the mouse placenta after intra-placental injection of Ad-hIGF-1. LAT1 was significantly reduced in UABL and Ad-hIGF1 placentas (1 ± 0.09 Vs 0.64 ± 0.09 Vs 0.6 ± 0.21 respectively, ANOVA P < 0.05, n > 3 per group).
3.5. In vitro protein expression and localisation
Following exposure of BeWo cells to Ad-hIGF-1 (MOI 100:1) for 48 h, there was a significant (p < 0.05, n = 5) increase in the protein expression of system A isoform SNAT2 but not SNAT1 or the system L isoform LAT1 (Fig. 4) as shown by Western blotting, No change was seen in LAT2 or 4F2hc levels either (data not shown).
Fig. 4.
Representative Western blot of SNAT1, SNAT2 and LAT1 in BeWo cells treated with control media (0), Ad-hIGF-1 MOI 10:1 (10), ad-hIGF-1 MOI 100:1 (100). B: Summary of SNAT2, LAT1 and SNAT1 protein expression. Exposure to Ad-IGF-1 (MOI 100) significantly increased expression of SNAT2. C: LAT1 localization is more cytoplasmic and membranous compared to control (1) following exposure to MOI 100 Ad-IGF-1 for 48 h (2). SNAT2 localization is not altered by exposure to 100 MOI Ad-IGF-1 (4) compared to control (3).
LAT1 localization was altered following exposure of BeWo cells to Ad-hIGF-1 for 48 h. In control cells the LAT1 transporter is seen primarily in the nuclear and perinuclear regions (Fig. 4C panel1), however upon treatment with Ad-hIGF-1 transporters are found in the cell cytoplasm and cell membrane (Fig. 4C panel2). In contrast, immunocytochemistry for the SNAT2 transporter showed an increase in staining following exposure to Ad-hIGF-1 MOI 100:1 as seen in the Western blot but transporters remained in the same location (Fig. 4C panel4) as in the control cells (Fig. 4C panel3).
3.6. In vivo protein expression and localization
4F2hc is localized in the trophoblast giant cells (TGC), syncytium and fetal endothelial cells (Fig. 5A) and expression is reduced in the UABL group (Fig. 5B), treatment with Ad-hIGF1 maintains expression comparable to sham (Fig. 5C). LAT1 expression was seen in the TGC nuclei and syncytium (Fig. 5D); lower expression was seen in the UABL group (Fig. 5E) compared to sham; however expression appears greater than sham levels after Ad-hIGF1 treatment (Fig. 5F). LAT2 expression was localized in the syncytium and the villus endothelial cells (Fig. 5G). Following UABL, LAT2 expression was diminished (Fig. 5H), but Ad-hIGF1 treated placentas resemble those from sham animals (Fig. 5I).
Fig. 5.
Immunohistochemistry staining at 40× magnification in SHAM, UABL, and UABL + Ad-hIGF-1 murine placentas from timed pregnant C57 B1/6J mice at gestation day 20. Expression and localization of amino acid transporters 4F2hc (A–C), LAT1 (D–F), LAT2 (G-1), SNAT1 (J–L), and SNAT2 (M–O) were assessed in the labyrinth. Reduced expression levels following UABL are seen in System L transporters LAT1 (E) and LAT2 (H) but transporter expression is restored by Ad-hIGF-1 treatment (F, I). SNAT1 levels increase in the cytoplasm following UABL (K) and remain elevated post IGF-1 treatment (L) compared to SHAM (J) whereas SNAT2 levels are reduced in the cytoplasm following UABL (N) and remain reduced post IGF-1 treatment (O).
SNAT1 was localized in the nuclear and perinuclear regions of TGCs, syncytium and villous endothelial cells in sham placentas (Fig. 5J). SNAT1 syncytial expression appears greater in the UABL and Ad-hIGF1 treated placentas (Fig. 5K and L). In contrast, SNAT2 exhibits reduced expression in the UABL (Fig. 5N) compared to sham (Fig. 5M), most notably, in the syncytial cytoplasm, but the reduction is not improved by the Ad-hIGF1 treatment (Fig. 5O).
4. Discussion
Placental Insufficiency currently has no treatment save delivery, however studies employing pre-birth growth factor supplementation have led to the development of gene therapy protocols via intra-placental injection [18]. The purpose of this study was to examine the effects of Ad-hIGF1 therapy on placental cells, both in vitro and in vivo. Here we demonstrate an increase in both proliferation and invasion, along with a reduction in apoptosis following adenoviral delivery of IGF-1 in a human trophoblast model. Furthermore, the human and murine models both show that cellular function is altered, with the regulation of amino acid transport mechanisms, following Ad-hIGF-1.
The increase in proliferation and invasion combined with the reduction in apoptosis levels seen in Ad-hIGF-1 infected BeWo cells suggests that 1) the IGF-1 secreted following transduction is biologically active and promotes viability and 2) the drop in apoptosis rates, and lack of effect of Ad-LacZ on these rates indicate the MOIs used are non-toxic. This partly reiterates previous studies by Mandl et al. [24] who demonstrated a reduction in the number of dead BeWo cells following the addition of exogenous IGF-1 protein to the cell culture media.
Since previous publications have demonstrated increased amino acid transport following exposure to stress [10] and inflammatory cytokines such as IL-6 and TNF-α [22], an Ad-LacZ viral control was included to establish the responses were due to the increased levels of IGF-1 and not to the inflammatory responses of viral infection. Ad-LacZ treatment had no effect on the transport mechanisms in either model.
Ad-hIGF-1 administration to the BeWo cell line increases system A transport and is associated with increased protein expression of the SNAT2 isoform but not the SNAT1 isoform. At an MOI of 100:1, both SNAT1 and SNAT2 showed an increase in mRNA expression. This is in contrast with the acute exposure studies in primary trophoblast of Bloxam et al. [25], Karl et al. [11] and Fang et al. [12] who showed no change in mRNA levels in response to recombinant IGF-1 and concluded the response was due to post-transcriptional regulation. The difference in IGF-1 exposure time between the previous and current studies may explain why in our study we see changes in mRNA expression of both of the transporters as well as at the protein level for SNAT2. As previously suggested [26] there may be a difference in induction mechanisms of these transporters in cases of chronic versus acute treatments.
The increase in system L uptake in BeWo cells is in contrast to that published by Roos et al. [27] who did not see an alteration in system L activity with IGF-1 protein treatment over a 24 h period. This may be due to a difference in the culture systems utilized or the treatment period length. The Ad-hIGF-1 induction of system L transport seen in the BeWo cells was not associated with an increase in LAT1 or 2 protein expression. Our localization studies of LAT1 demonstrate that following exposure to IGF-1 there is an increase in transporters localized to the BeWo cell membrane, which is consistent with the suggestions of Verrey [29] for LAT1 localization in growing cells and may underlie the increased leucine transport without an apparent increase in protein synthesis.
In our mouse model of placental insufficiency, SNAT2 is reduced in the UABL and not maintained by Ad-hIGF1 treatment; however this reduction may be compensated by the apparent increase in SNAT1, both isoforms transport the same amino acids and it is possible that SNAT1 could maintain normal levels of basic amino acids needed to supply symporters such as the System L transporters. Interestingly, LAT1 mRNA and LAT1 and 2 proteins are reduced in the UABL placentas. This disruption of transport of essential amino acids, due to a reduction in transport capacity, could be one mechanism leading to fetal growth restriction in this model. The ability of IGF-1 over-expression to maintain the expression of the transporters, via a post-transcriptional mechanism independent of mRNA levels, could contribute to appropriate fetal growth following treatment.
The differences seen in the regulation of the amino acid transport system isoforms demonstrate that the responses are not a generalised increase in global gene transcription or protein synthesis [28] in response to viral infection or over expression of IGF-1 in either of the models used.
Comparisons between the in vitro human trophoblast model and the in vivo murine placental insufficiency model are difficult, as the control BeWo cells did not undergo any kind of insult. The effects of greater than normal levels of IGF-1 on the transport systems, result in an increase in amino acid uptake most likely linked to increased transporter availability as evidenced in the BeWo. By contrast, in the mouse model, IGF-1 effects on nutrient transport mechanisms are within the context of the placental milieu and placental insufficiency. Expression of IGF-1 in an insulted placenta restores several System L amino acid transporter isoforms to levels seen in the sham-operated controls but did not alter System A. Together, these studies indicate that restoration or over-expression of IGF-1 impacts placental amino acid transport and may be one mechanism contributing to the restoration of fetal weight in the murine model of placental insufficiency treated with Ad-hIGF-1.
Despite successful transfection of undifferentiated trophoblast cells and BeWo, it is apparent that once trophoblast cells terminally differentiate into syncytiotrophoblast, transfection efficiency drops dramatically, a process likely linked to a loss of CAR receptors on the syncytium. While in the mouse model it is possible to deliver Ad-hIGF1 by direct intra-placental injection, this approach would not be ideal in a clinical setting. Therefore in order to develop a future gene therapy protocol that will be effective in late pregnancy, i.e. a time when the majority of trophoblast have undergone terminal differentiation to syncytiotrophoblast it will be necessary to develop a non-viral gene delivery system that is not dependent on the presence of viral receptors in the cell membrane.
Acknowledgments
We would like to thank Dr M. Herlyn, University of Pennsylvania, Philadelphia, PA for supplying the Adenoviral vectors and Dr S. Balaji for critical assessment of the manuscript. HNJ is funded by a Eunice Kennedy Shriver NICHD award K99HD068504.
References
- 1.Jones HN, Powell TL, Jansson T. Regulation of placental nutrient transport–a review. Placenta. 2007;28(8–9):763–74. doi: 10.1016/j.placenta.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Ogata ES, Bussey ME, Finley S. Altered gas exchange, limited glucose and branched chain amino acids, and hypoinsulinism retard fetal growth in the rat. Metabolism. 1986;35(10):970–7. doi: 10.1016/0026-0495(86)90064-8. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Huang W, Sugawara M, Devoe LD, Leibach FH, Prasad PD, et al. Cloning and functional expression of SNAT1, a subtype of amino acid transporter A, from human placenta. Biochem Biophys Res Commun. 2000;273:1175–9. doi: 10.1006/bbrc.2000.3061. [DOI] [PubMed] [Google Scholar]
- 4.Hatanaka T, Huang W, Wang H, Sugawara M, Prasad PD, Leibach FH, et al. Primary structure, functional characteristics and tissue expression pattern of human SNAT2, a subtype of amino acid transport system A. Biochim Biophys Acta. 2000;1467:1–6. doi: 10.1016/s0005-2736(00)00252-2. [DOI] [PubMed] [Google Scholar]
- 5.Sugawara M, Nakanish T, Fei YJ, Huang W, Ganapathy ME, Leibach FH, et al. Cloning of an amino acid transporter with functional characteristics and tissue expression pattern identical to that of system A. J Biol Chem. 2000a;275:16473–7. doi: 10.1074/jbc.C000205200. [DOI] [PubMed] [Google Scholar]
- 6.Desforges M, Lacey HA, Glazier JD, Greenwood SL, Mynett KJ, Speake PF, et al. The SNAT4 isoform of the system A amino acid transporter is expressed in human placenta. Am J Physiol. 2005;290:C305–12. doi: 10.1152/ajpcell.00258.2005. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz-Montasell B, Gomez-Angelats M, Casado FJ, Felipe A, McGivan JD, Pastor-Anglada M. Evidence for a regulatory protein involved in the increased activity of system A for neutral amino acid transport in osmotically stressed mammalian cells. PNAS. 1994;91:9569–73. doi: 10.1073/pnas.91.20.9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDowell HE, Eyers PA, Hundal HS. Regulation of system A amino acid transport in L6 rat skeletal muscle cells by insulin, chemical and hyperthermic stress. FEBS Lett. 1998;441:15–9. doi: 10.1016/s0014-5793(98)01508-7. [DOI] [PubMed] [Google Scholar]
- 9.Yao D, Mackenzie B, Ming H, Varoqui H, Zhu H, Hediger MA, et al. A novel system A isoform mediating Na+/neutral amino acid cotransport. J Biol Chem. 2000;275:22790–7. doi: 10.1074/jbc.M002965200. [DOI] [PubMed] [Google Scholar]
- 10.Jones HN, Ashworth CJ, Page KR, McArdle HJ. Cortisol stimulates system A amino acid transport and SNAT2 expression in a human placental cell line (BeWo) Am J Physiol. 2006;291:E596–603. doi: 10.1152/ajpendo.00359.2005. [DOI] [PubMed] [Google Scholar]
- 11.Karl PI. Insulin-like growth factor-1 stimulates amino acid uptake by the cultured human placental trophoblast. J Cell Physiol. 1995;165:83–8. doi: 10.1002/jcp.1041650111. [DOI] [PubMed] [Google Scholar]
- 12.Fang J, Mao D, Smith CH, Fant ME. IGF regulation of neutral amino acid transport in the BeWo choriocarcinoma cell line (b30 clone): evidence for MAP kinase-dependent and MAP kinase-independent mechanisms. Growth Horm IGF Res. 2006;16:318–25. doi: 10.1016/j.ghir.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Cetin I, Ronzoni S, Marconi AM, Perugino G, Corbetta C, Battaglia FC, et al. MatemRNAl concentrations and fetal-matemRNAl concentration differences of plasma amino acids in normal and intrauterine growth-restricted pregnancies. Am J Obstet Gynecol. 1996;174:1575–83. doi: 10.1016/s0002-9378(96)70609-9. [DOI] [PubMed] [Google Scholar]
- 14.Jansson T, Ylvén K, Wennergren M, Powell TL. Glucose transport and system A activity in syncytiotrophoblast microvillous and basal plasma membranes in intrauterine growth restriction. Placenta. 2002;23(5):392–9. doi: 10.1053/plac.2002.0826. [DOI] [PubMed] [Google Scholar]
- 15.Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, et al. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol. 2006;576:935–46. doi: 10.1113/jphysiol.2006.116509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sferruzzi-Perri AN, Owens JA, Standen P, Taylor RL, Heineman GK, Robinson JS, et al. Early treatment of the pregnant guinea pig with IGFs promotes placental transport and nutrition partitioning near term. Am J Physiol. 2007;292(3):E668–76. doi: 10.1152/ajpendo.00320.2006. [DOI] [PubMed] [Google Scholar]
- 17.Eremia SC, de Boo HA, Bloomfield FH, Oliver MH, Harding JE. Fetal and amniotic insulin-like growth factor-I supplements improve growth rate in intrauterine growth restriction fetal sheep. Endocrinology. 2007;148(6):2963–72. doi: 10.1210/en.2006-1701. [DOI] [PubMed] [Google Scholar]
- 18.Katz AB, Keswani SG, Habli M, Lim FY, Zoltick PW, Midrio P, et al. Placental gene transfer: transgene screening in mice for trophic effects on the placenta. Am J Obstet Gynecol. 2009;201:499, e1–8. doi: 10.1016/j.ajog.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Qian H, Zhang Y, Ma Y, Lin C, Xiang Y. Effect of Ad-TIMP3 on biologic behavior of choriocarcinoma cells in vitro. J Reprod Med. 2008;53(8):608–14. [PubMed] [Google Scholar]
- 20.Parry S, Holder J, Strauss JF., III Mechanisms of trophoblast-virus interaction. J Reprod Immunol. 1997;37:25534. doi: 10.1016/s0165-0378(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 21.Vega-Avila E, Pugsley MK. An overview of colorimetric assay methods used to assess survival or proliferation of mammalian cells. Proc West Pharmacol Soc. 2011;54:10–4. [PubMed] [Google Scholar]
- 22.Jones HN, Jansson T, Powell TL. IL-6 stimulates system A amino acid transporter activity in trophoblast cells through STAT3 and increased expression of SNAT2. Am J Physiol Cell Physiol. 2009;297(5):C1228–35. doi: 10.1152/ajpcell.00195.2009. [DOI] [PubMed] [Google Scholar]
- 23.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001 May 1;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandl M, Haas J, Bischof P, Nöhammer G, Desoye G. Serum-dependent effects of IGF-I and insulin on proliferation and invasion of human first trimester trophoblast cell models. Histochem Cell Biol. 2002;117:391–9. doi: 10.1007/s00418-002-0403-5. [DOI] [PubMed] [Google Scholar]
- 25.Bloxam DL, Bax BE, Bax CM. Epidermal growth factor and insulin-like growth factor I differently influence the directional accumulation and transfer of 2-aminoisobutyrate (AIB) by human placental trophoblast in two-sided culture. Biochem Biophys Res Commun. 1994 Mar 15;199(2):922–9. doi: 10.1006/bbrc.1994.1317. [DOI] [PubMed] [Google Scholar]
- 26.Jones HN, Ashworth CJ, Page KR, McArdle HJ. Expression and adaptive regulation of amino acid transport system A in a placental cell line under amino acid restriction. Reproduction. 2006;131:951–60. doi: 10.1530/rep.1.00808. [DOI] [PubMed] [Google Scholar]
- 27.Roos S, Lagerlöf O, Wennergren M, Powell TL, Jansson T. Regulation of amino acid transporters by glucose and growth factors in cultured primary human trophoblast cells is mediated by mTOR signaling. Am J Physiol Cell Physiol. 2009;297(3):C723–31. doi: 10.1152/ajpcell.00191.2009. [DOI] [PubMed] [Google Scholar]
- 28.Williams SF, Fik E, Zamudio S, Illsley NP. Global protein synthesis in human trophoblast is resistant to inhibition by hypoxia. Placenta. 2012;33(1):31–8. doi: 10.1016/j.placenta.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verrey F. System L: heterogenic exchangers of large, neutral amino acids involved in directional transport. Pflugers Arch. 2003;445(5):529–33. doi: 10.1007/s00424-002-0973-z. [DOI] [PubMed] [Google Scholar]