Preface
γδ T cells are a unique and conserved population of lymphocytes that have been the subject of a recent explosion of interest owing to their essential contributions to many types of immune response and immunopathology. But what does the integration of recent and long-established studies really tell us about these cells and their place in immunology? The time is ripe to consider where strong evidence exists for their unique and critical functions. We conclude that whereas αβ T cells and B cells are commonly viewed as contributing primarily to antigen-specific effector and memory phases of immunity, γδ T cells are distinct in that they combine conventional adaptive potentials, inherent in their T cell receptors (TCRs) and pleiotropic effector functions, with rapid, innate-like responses that place them in the initiation phase of immune reactions. This underpins a revised perspective on lymphocyte biology and the regulation of immunogenicity.
Introduction
As their name indicates, γδ T lymphocytes develop largely in the thymus, generating their defining receptor via RAG-mediated V(D)J recombination. The resulting potential for diversity in the γδ T cell receptor (TCR) and the consequent capacity for shaping the T cell repertoire via clonal expansion appropriately assign γδ T cells to the adaptive immune compartment1. Furthermore, there are striking connections between γδ T cells and αβ T cells. For example, the TCRδ locus in mice and in humans is embedded within the TCRα locus, and some TCR-V gene segments can be utilised interchangeably by TCRα or TCRδ. Moreover, a common thymic progenitor may give rise to either αβ or γδ T cells2, although this does not exclude the possibility that distinct subsets of γδ and αβ T cells arise from qualitatively discrete progenitors, as indicated in Figure 1. Indeed, new findings relevant to this issue will be reviewed later in this article.
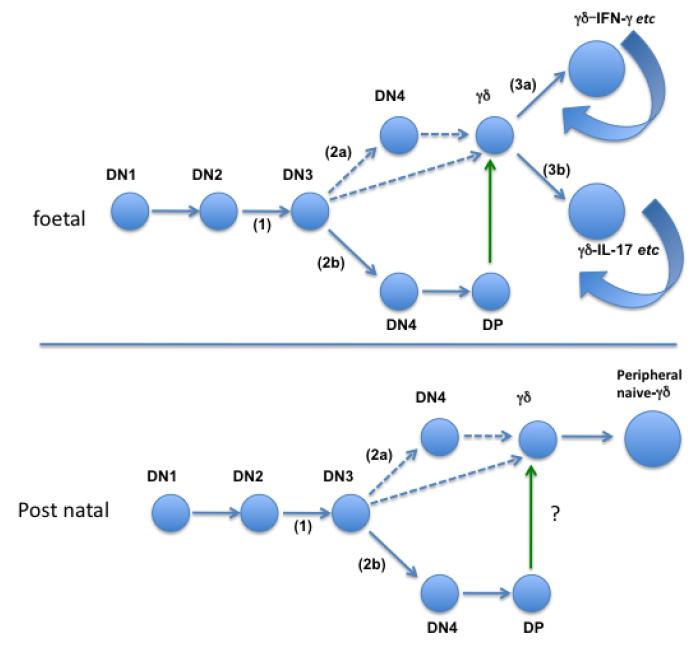
Figure 1. Overview of pre- and post-natal γδ T cell development.
Top: Mouse γδ T cell development from foetal liver progenitors. Cells undergo development through several steps of differentiation, starting at the double negative 1 (DN1) stage characterized by a CD44+ CD25− phenotype, followed by the CD44+CD25+ DN2 stage. At this point, the β, γ and δ chains of the TCR are rearranged. A functional γδ TCR expression will drive cells into the γδ lineage, supported by the expression of Sox13. Cells failing to produce a functional γδ TCR will undergo β selection supported by Notch 1, with a further rearrangement of the TCR α chain, eventually entering the Double Positive (DP) stage. These cells can support γδ T cell development via trans-conditioning (green arrow). Unlike αβ T cells, γδ T cells are functionally pre-programmed, depending on TCR and/or related signalling. It is proposed that a strong, agonist-dependent signal will result in the loss of Sox13 and expression of NFAT, NFκB, Egr3 and Tbet, and a capability to produce IFN-γ among other effector molecules while weaker TCR signalling permits the cells to maintain Sox13, increase Rorγc expression, and a adopt an IL-17 “default position”. The dashed line indicates the potential origin of γδ cells from progenitors with either a DN3 or DN4 phenotype. The curved arrows indicate the potential for lifelong self-renewal that exists in at least two pre-natally derived γδ cell compartments.
Bottom: Mouse post-natal development from bone marrow-derived progenitors. There is no evidence that innate-like CD27− IL-17-producing γδ T cells can be generated from the bone marrow, implying that a different thymic progenitor gives rise to post-natal γδ T cells, by comparison to foetus-derived γδ cells. With no evidence for IL-17-competent progenitors, the evidence for pre-programming is less, and naïve unprimed cells may emerge in the periphery, possibly with a “default potential” for IFN-γ production.
Within the adaptive compartment it seems facile to accept the complementary value of B cells, that can secrete their antigen receptors as antibodies, and αβ T cells, that use cell-bound TCRs to induce cytolytic responses and helper functions. However, it is less easy to envision the selective pressure(s) that have over 420 million years sustained the co-existence of two lineages of T cells (αβ and γδ) with surface-bound TCRs. The nihilistic view is that no such selective pressure currently exists, and that γδ T cells are en route to extinction, having been superseded by an extraordinarily potent αβ T cell compartment. Conversely, the recent increase in the study of γδ T cells has added to the established literature in providing conspicuous cases of non-redundant γδ T cell activities. Furthermore, the lamprey, an extant but primitive jawless vertebrate, uses RAG-independent mechanisms to generate an adaptive immune compartment that is also characterised by three distinct receptors with diverse potential, of which one is secreted and two are cell-surface bound3. Hence, this type of tripartite organization may be optimal for adaptive immune function.
In this light, we shall consider six properties that may collectively distinguish γδ T cells from αβ T cells, and thereby define their unique contributions to lymphocyte biology: one, that γδ TCRs recognise qualitatively distinct antigens; two, that γδ T cells contribute to immune responses with distinct kinetics; three, that γδ T cells have unique functional potentials; four, that γδ T cells are particularly suited to the protection of defined anatomical sites; five, that γδ T cells are of primary value in young animals; and six, that γδ T cells, although not invariably important, mediate critical responses to specific pathogens, in a manner similar to natural killer (NK) cells. Because γδ T cells comprise heterogeneous subsets, these six properties will not apply equally to all γδ T cells. Accepting this point, we consider here the evidence for each property, and its potential to explain the conservation of γδ T cells.
γδ TCRs recognise distinct antigens
Anatomical distribution of γδ T cells
The anatomical localization of lymphocytes has profound implications for their antigen specificity. Thus, the clonal selection and expansion of αβ T cells with very rare specificities relies on the fact that following egress from the thymus, naïve αβ T cells home to the lymph nodes (LNs) and to the T cell zones of the spleen where they regularly encounter vast numbers of dendritic cells (DCs) presenting diverse antigens. While some γδ T cells home to the LNs, many migrate directly to tissues such as the epidermis (in murine species), the dermis, the intestine, the lung and the uterus. Moreover, by contrast to αβ T cells, splenic γδ T cells are not confined to the lymphoid areas (the white pulp) but are also found throughout the red pulp4. Sequestration of γδ T cells within tissues is incompatible with their sampling diverse antigens and the consequent clonal expansion of very rare cells. Consistent with this is the limited TCR diversity of many tissue-resident γδ T cells, which in the murine epidermis or uterus are essentially monoclonal5. This implies that these cells recognise either pathogen-encoded antigens that are predictably encountered in specific tissues, or for self-encoded molecules reflecting a dysregulated state of that tissue.
The expression of a monoclonal, RAG-generated receptor by the majority of γδ T cells in a specific compartment and its use to engage only one or few antigens was unprecedented prior to studies of murine skin γδ T cells known as DETC (Dendritic Epidermal T cells)6. By permitting large numbers of T cells to be rapidly activated and their function mobilised without a requirement for prior clonal expansion, the mono- or oligo-clonal use of a TCR represents a profound cross-over of adaptive into innate immunity: hence the term “innate-like”. Since the seminal work on DETC, evidence has accrued for numerous other “innate-like” γδ T cells including many within the predominant human peripheral blood Vγ9Vδ2+ compartment. In sum, anatomical considerations suggest that γδ T cells are divisible into lymphoid-homing γδ T cells that may be primed in the circulation and clonally expand in a conventional adaptive fashion, and innate-like cells that respond rapidly and at relatively high frequency in many different sites.
Adaptive TCR specificities
γδ TCRs are not restricted to the recognition of peptides complexed to MHC, thus distinguishing them from the great majority of αβ T cells. Furthermore, the diversity in length of the CDR3, which is conferred particularly by the architecture of the TCRD locus, suggests that the γδ TCR is not structurally constrained by the recognition of cargo presented by some specific presenting element.
Instead, an antibody-like breadth in antigen recognition by the γδ TCR is suggested by the recent demonstration that some human, murine and bovine γδ TCRs can bind to phycoerythrin (PE), an algal molecule readily recognised by B cells7. PE binding induces γδ T cells to upregulate CD44, to downregulate CD62L expression, and to express cytokines, as happens when naïve αβ T cells are primed. Hence, this response to nominal exogenous antigen seemingly illustrates an adaptive potential of γδ T cells. However, compared to the priming of αβ T cells, PE-reactive γδ T cells showed conspicuously less clonal expansion, which is a defining parameter of delayed antigen-specific adaptive responses. Thus, even in this case, the functional γδ T cell response was rapidly mobilised with innate-like kinetics. Moreover, the cells quickly acquired an innate-like capacity to respond to inflammatory cytokines in the absence of further antigen.
The conversion of antigen-specific naïve cells into more rapidly-responsive memory cells is a key criterion of adaptive immunity. In this regard, BCG vaccination induced mycobacteria-specific γδ T cells with memory characteristics in macaques and in cattle 8-11. Additionally, immunoprotective murine γδ cells reactive to a Herpes Simplex virus glycoprotein were obtained from infected mice12. Nonetheless, most attempts to evoke antigen-specific γδ T cells following deliberate immunization or infection of mice have conspicuously failed, even when polyclonal γδ T cell responses were induced. Hence, much of γδ T lymphocyte biology is not captured by the conventional concept of adaptive immunity.
Self-reactivity
Few γδ TCR specificities have been deduced; even fewer are supported by biochemical binding data; and of these the general representativeness is not always clear (Table 1). Nonetheless, as hypothesized almost 25 years ago13, several γδ TCRs are reactive either to self-MHC molecules independently of their cargo or to MHC-related proteins, including murine T10/T22, a non-peptide binding MHC class 1b molecule14, 15; the murine MHC class II molecule I-Ek, but with the epitope lying outside the peptide-binding groove16; and human HLA (P. Fisch, personal communication). It has also been reported that some human γδ TCRs engage members of the MHC class I-like MICA and ULBP families (see below)17, 18. Recently, the TCR from a γδ T cell clone derived from a CMV-infected transplant patient was shown to directly bind endothelial protein C receptor (EPCR), a lipid carrier with similar structure to CD1, although again γδ TCR engagement was cargo independent19. This evoked an earlier report, albeit lacking biochemical data, that CD1c was a ligand for several human Vδ1+ T cell clones20.
Table 1.
Activating ligands for γδ T cells.
| HUMAN | ||
|---|---|---|
| Subset | Antigen | Reference |
| Vδ1 (IEL) | MICA/B | 17 |
| Vδ2 | ULBP4 | 18 |
| Vδ1 (clones) | CD1c | 20 |
| Vδ1 (Blood) | CD1d + Sulfatides | 21 |
| Vγ4Vδ5 (clone) | EPCR | 19 |
| Vδ1 (clones) | Lipohexapeptides | 118 |
| Various | Phycoerythrin | 7 |
| Vγ9Vδ2 | Phosphoantigens | 119 |
| Vγ9Vδ2 | F1-ATPase | 27 |
| Vγ1.3Vδ2 | Aminoacyl tRNA synthetases | 30 |
| MOUSE | ||
| Various | T10/T22 | 14, 15 |
| Vγ2/Vδ5 (clone) | I-Ek | 16 |
| Vγ2/Vδ8 (clone) | HSV-gI | 120 |
| Vγ1 (clones) | Cardiolipin, Apolipoprotein H | 38 |
| Various | Phycoerythrin | 7 |
| Vγ1 (clones) | Insulin peptide | 22 |
Other γδ T cells are specific for antigen presented by MHC or MHC-related molecules. For example, in healthy individuals, Vδ1+ cells (which are more prevalent in tissues than in the peripheral blood) compose the majority of T cells reactive to CD1d tetramers loaded with sulphatide, a myelin glycosphingolipid21. Also, several γδ T cell clones derived from diabetes-prone NOD mice respond to the insulin-derived peptide B:9–23, which is also presented to CD4+ αβ T cells by disease-associated MHC class II molecule I-Ag722. However, the nature of peptide recognition by γδ T cells was quite distinct from that of αβ T cells, in that MHC was not required. Thus, γδ TCRs and αβ TCRs have qualitatively distinct modes of antigen recognition, that in some cases may provide complementary means to detect a single target. γδ T cells therefore increase the scope of lymphocyte recognition.
Cross-reactivity
Some antigens appear to be unique targets of γδ cells. Thus, low molecular mass alkyl diphosphates termed phosphoantigens or phosphoagonists are the prototypical, naturally occurring moieties recognized by Vγ9Vδ2+ cells, the predominant subset of γδ T cells in peripheral blood. The most potent is hydroxymethyl-but-2-enyl-pyrophosphate (HMBPP), an intermediate in the alternative deoxyxylulose (non-mevalonate) pathway of cholesterol synthesis that is used by numerous bacterial species and by some highly significant eukaryotic pathogens, notably Plasmodium spp., but not by vertebrate cells23. However, Vγ9Vδ2+ T cells are also activated by isopentenyl pyrophosphate (IPP)24, which is an intermediate in a part of the mevalonate pathway that is conserved in prokaryotes and eukaryotes. Hence IPP is a self-antigen.
Given this cross-reactivity of human Vγ9Vδ2+ T cells to foreign and self-phosphoantigens, there is understandable interest in elucidating how TCR-signalling can be induced by such small molecules. Phosphoantigens can directly activate Vγ9Vδ2+ T cells, but such activation is greatly enhanced by monocytes25. Thus, either phosphoantigens are presented as cargo to γδ TCRs, or their cellular processing somehow sensitises cells to recognition by Vγ9Vδ2 TCRs, for example by stabilising surface expression of a TCR-binding ligand. A candidate molecule involved in intracellular phosphoantigen processing is the F1-ATPase, which was reported to directly bind a Vγ9Vδ2 TCR, and which may also interact with ApppI, an adenosine derivative of IPP26-28. Furthermore, IPP–ApppI interconversion may be catalysed by an aminoacyl tRNA synthetase29, which is interesting given that a conformational epitope on histidyl-tRNA synthetase is recognised by an autoreactive γδ TCR from a patient with a rare form of γδ T cell-mediated myositis30. Of note, histidyl-tRNA synthetase was previously implicated in autoimmunity as a self-antigen, Jo-1, targeted by autoreactive B cells. Thus, human γδ T cells may collectively monitor multiple components of pathways regulating cholesterol biosynthesis and nucleotide metabolism that are likely to be altered by infection or other forms of stress.
In this regard, it was recently found that phosphoantigen-mediated, Vγ9Vδ2+ T cell activation can be mimicked and inhibited, respectively, by different antibodies against the widely-expressed immunoglobulin superfamily member, butyrophilin 3A1 (CD277)31, 32. Although the involvement of CD277 in phosphoantigen recognition is not yet unresolved, it is interesting that mouse γδ T cells neither display phosphoantigen reactivity nor express a CD277 homolog. However, CD277 displays high structural similarity to Skint1, a murine immunoglobulin superfamily gene expressed by medullary thymic epithelial cells (mTECs) and keratinocytes and which is critical to the development of Vγ5Vδ1+ DETC (see below)33, 34. Thus conserved molecular mechanisms may underlie the activation of disparate γδ T cell subsets in mice and in humans.
The collective TCR specificities of γδ T cells permit them to respond both to infection and to dysregulated self. HMBPP clearly qualifies as a pathogen-associated molecular pattern (PAMP), and HMBPP-specific Vγ9Vδ2+ T cells in the presence of monocytes respond strongly to neutrophils that have taken up HMBPP+ but not HMBPP− clinically relevant bacterial strains35. However, upregulation of endogenous IPP in human cells in response to infection or non-infectious dysregulation36, 37 also provokes Vγ9Vδ2+ T cell reactivity, albeit at a lower sensitivity.
Similarly, murine hepatic γδ T cells respond to CD1d presenting cardiolipin, a major bacterial cell wall phospholipid that is also an enodogenous component of mitochondria38. Given the potential for diversity in adaptive immune receptors, cross-reactivity is inevitable and can be documented in B cells and ab T cells. However, the cross-reactivity reviewed here for γδ T cells is an overt functional cross-reactivity that clouds whether cells are responsive primarily to foreign or to self-antigens. This is similar to the status of B-1 cells, which have been proposed to mount rapid responses to common molecular signatures of infection or dysregulation39. Thus, even though few γδTCR specificities have so far been defined, it seems clear that peripheral γδ T cells are distinct from their TCRαβ+ counterparts both in their constellation of antigens recognised and in the strong representation of self-antigens within that constellation.
γδ T cell response kinetics
Lymphoid Stress-Surveillance
The capacity to recognise antigens rapidly displayed following infection or other forms of stress, and to respond in large numbers without requiring extensive clonal expansion permits γδ T cells to participate in the early stages of an immune response, known as the “afferent phase”. This means that they act in synchrony with innate immune cells as sensors of dysregulation, thereby setting in motion downstream efferent immune responses mediated by conventional adaptive lymphocytes. By contrast, CD4+ αβ T cells would not be able to participate in tissue immune surveillance, because their activation requires processed antigens to be presented by highly specialised antigen presenting cells. In this regard, afferent sensing is ordinarily attributed to myeloid cells, particularly DCs that are typically viewed as the primary orchestrators of adaptive immunity. Therefore, to highlight the capacity of γδ T cells to play an equivalent role, we have applied the term lymphoid stress-surveillance40.
We previously considered that epithelial cells express a set of gene-products that regulate immune cells. We termed this the Epimmunome, which encompasses cell-surface molecules upregulated in response to numerous forms of cell dysregulation41. These include murine Rae-1 and H60, and human MICA, MICB and ULBPs, which are all members of a large family of MHC class I-related molecules that engage the activating receptor, NKG2D, which is expressed by many γδ T cell subsets, NK cells, CD8+ T cells and some CD4+ T cells.
The importance of the NKG2D pathway is attested to by the plethora of strategies employed by viruses and tumours to evade it42, 43. Given the evidence that γδ T cells make TCR-dependent responses to heat-shocked but not normal cells44, it has long been hypothesized that some γδ TCRs recognize moieties upregulated on the surface of stressed cells. Indeed, tetramers composed of the Vγ5Vδ1 DETC TCR bound to a keratinocyte determinant transiently expressed adjacent to wounded skin45. Moreover, we have mentioned that MICA is reportedly a ligand for some human Vδ1+ TCRs17. However, because only few stress-regulated surface molecules have so far been identified, the generality of γδ TCRs engaging “stress antigens” is hard to assess.
A different perspective is that the γδ TCR constitutively engages self-ligand, thereby predisposing γδ T cells to respond to stress via co-stimulatory receptors such as NKG2D and/or receptors for cytokines such as IL-1 and IL-15 which are upregulated by tissue dysregulation. Consistent with this, DETCs can be activated in vivo simply by acute, keratinocyte-specific upregulation of Rae-146. Furthermore, recently published images show the Vγ5Vδ1 DETC TCR at steady state constitutively signalling at specific sites of interaction with keratinocytes 47. The steady-state ligand recognised by the Vγ5Vδ1 TCR was not identified, but Skint1 (see above) is a candidate for contributing to this interaction since it is expressed by keratinocytes at steady-state. This study also confirmed the observation that following activation DETCs make overt contacts with Langerhans cells (LC)46, supporting the possibility that LC also express a DETC TCR ligand. For DETC to respond rapidly to changes in epithelial cells and to then communicate with LC reinforces the assignment of γδ T cell function to the afferent sensing phase of an immune response, consistent with lymphoid stress-surveillance, and distinct from the biology of most αβ T cells. However, this does not imply that γδ T cells do not also contribute to downstream effector and regulatory phases of immunity.
Co-stimulatory and inhibitory receptors
Conventional αβ T cells are regulated by the coincident provision of Signals 1, 2 and 3, and if any one of these is lacking, the cells may anergise, thereby ensuring that they are activated only in cases of genuine infection and dysregulation. As was just considered, γδ T cells may respond to these signals in sequence rather than coincidentally: for example, the DETC response to NKG2D-ligands by cells with pre-engaged TCRs. Similarly, PE-primed γδ T cells acquired the capacity to respond to inflammatory cytokines, IL-1 and IL-23, alone7.
Such acquired responsiveness to Signals 2 or 3 alone raises the issue of what ordinarily limits the activation of γδ T cells so as to prevent chronic inflammation. While this issue is not resolved, it is notable that γδ T cells can express many receptors regulating their responsiveness to their environment. Among these are inhibitory Ly49 receptors, whose expression on αβ TCR CD8αα+ intraepithelial lymphocytes (IELs) is associated with hyporesponsiveness48. Innate-like γδ T cell activation may additionally require input from unconventional co-stimulators. Thus, DETCs express junctional adhesion molecule-like (JAML), which upon engagement of the coxsackie adenovirus receptor (CAR) expressed by keratinocytes activates phosphoinositide 3-kinase (PI3K) which is likewise mediates co-stimulation via CD28 or NKG2D49. DETCs also express semaphorin 4D (CD100), which has been implicated in cell migration and morphology. When plexin B2, expressed by keratinocytes, engages CD100 on DETC, it induces Erk activation and promotes DETC activation-dependent rounding and cytokine production50, possibly explaining why CD100-deficient mice show defects in wound healing. The plexin–semaphorin axis operates in many systems, and engagement of CD100 on conventional T cells by plexin B2 expressed by plasmacytoid dendritic cells affects IL-12 production and T cell priming51.
Aryl Hyrocarbon Receptor (AhR)
The AhR is highly expressed by DETCs and γδ IELs as well as by Th17 cells, where it most conspicuously regulates IL-22 production52, 53. DETCs and γδ IELs make neither IL-17 nor IL-22 (see below), but they are strongly influenced by AhR in that AhR-deficient mice fail to sustain either DETC or γδ IEL numbers over time54. AhR is broadly expressed, including by epithelial cells and Langerhans cells. However, its effects on DETCs and γδ IELs are dependent on its expression by RAG-dependent lymphoid cells, and seem to reflect the cells’ intrinsic responses to aryl hydrocarbons found in food and other components of the environment. However, the effects of AhR are not specific to either γδ T cells or IELs, with earlier studies of AhR-deficient mice reporting defects in systemic T cell compartments55. Nonetheless, AhR regulation of γδ T cells within tissues emphasises the diversity of receptor-mediated interactions that transmit to γδ T cells the status of their environment and that regulate them both positively and negatively. For example, IFN-γ production by human Vγ9Vδ2+ T cells is strongly attenuated by co-engagement of the TCR and CD46, a complement receptor for which ligands would certainly be available during infection or tissue damage56. The need now is to learn under which environmental conditions specific molecular sensors operate; which sensors are integrated with others and which function independently; and how the activities of specific sensors relate to γδ cell biology.
γδ T cell functions
Activated murine systemic γδ T cells and human peripheral blood γδ T cells can express high levels of IFN-γ, TNF, and granzymes; IL-17 is produced by distinct subsets of systemic γδ T cells and by those in the dermis and intestinal lamina propria; and CD4+ IL-4-producing γδ T cell clones have been described57. Collectively these effector functions permit γδ T cells to participate in the later, “efferent phase” of immune responses.
However, γδ T cells can also display a pleiotropy that contrasts with the functional limitations of conventional Th1, Th2, and Th17 αβ T cell subsets. For example, while DETCs can produce IFN-γ and express high levels of granzymes, they also express IL-1358 that can regulate B cells; growth factors such as IGF1 that may regulate neighbouring stromal cells59; and numerous chemokines that will recruit other leukocytes. This combination of CTL-, Th1-, and Th2-type phenotypes evokes the finding that the transcription factor NFIL3 promotes IL-13 production by chronically stimulated Th1 and NK cells60. It may enable rapidly-responsive γδ cells to contribute to the afferent phase of immune responses via pivotal interactions with other cells (Figure 2), as will now be considered.
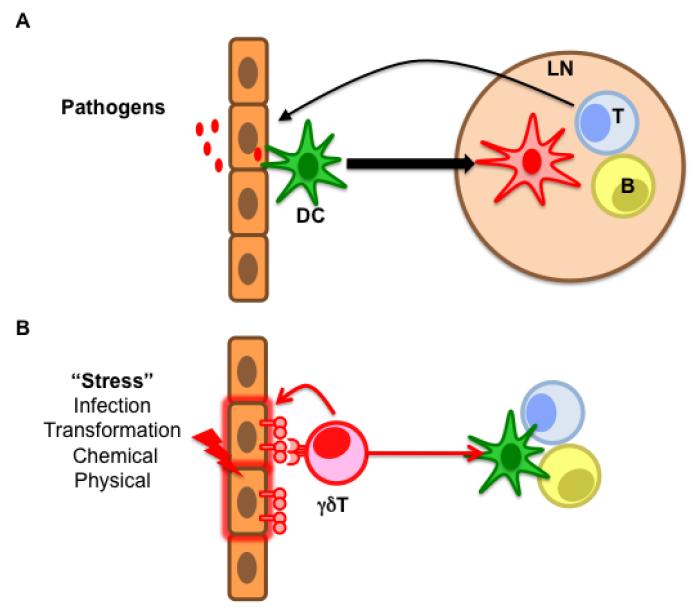
Figure 2. An alternative way to achieve broad systemic immune responses.
(A) The text-book view of the activation of an adaptive immune response. Dendritic cells (DC) capture pathogens and mature while migrating to the lymph nodes, where they prime αβ T and B cells which will migrate back to the infected tissue and mount effector responses or produce antibodies, respectively. This very specific albeit slow response is complemented by γδ T cells which, in response to various sources of stress, not only mount immediate, local effector responses but also trigger the other arms of the adaptive immune system (B).
Interactions with B cells
In interacting with other cells, a key function of γδ T cells may be their expression of chemokines (Table 2). Thus, activated Vγ9Vδ2+ T cells can produce large amounts of CXCL1361 that regulates the organization of B cells within follicles of lymphoid tissues62. Indeed, there is clear evidence for an impact of γδ T cells on B cells from both mice and humans. Thus, germinal centres (albeit small) and the production of high levels of T cell-dependent immunoglobulins, notably IgE, can be detected in αβ T cell-deficient mice, particularly following infection63. The immunoglobulins in these mice are mostly not specific for challenging antigens, reiterating that immunization seldom evokes pathogen-specific γδ T cells in a conventional sense, but rather promotes rapid γδ T cell responses to a dysregulated state, the details of which differ according to the challenge. Consistent with this, when mild physical perturbation of the skin coincides with epicutaneous exposure to antigen, high levels of IgE result that are at least partially dependent on a normal DETC compartment and upon NKG2D, to which DETC respond by making IL-13, a “Th2-cytokine” that upregulates IgG1 and IgE58.
Table 2.
Production of chemokines by γδ T cells.
| HUMAN | |||
|---|---|---|---|
| Subset | Chemokine (alternative name) | Receptor expressed by | Reference |
| Vδ2 (IPP) | CCL3 (MIP1α) | Macrophages | 61 |
| CCL4 (MIP1β) | Macrophages, NK cells, other cell types | ||
| CXCL10 | Macrophages, T cells, NK cells, other cell types | ||
| CXCL13 | B cells | ||
| Vδ1 (NKp30) | CCL3 (MIP1α) | Macrophages | 121 |
| CCL4 (MIP1β) | Macrophages, NK cells, other cell types | ||
| CCL5 (RANTES) | T cells, eosinophils, basophils | ||
| Skin Vδ2 | CCL1 | Monocytes, NK cells, immature B cells | 122 |
| MOUSE | |||
| Lung-resident | CXCL1 | Neutrophils | 123 |
| CXCL10 | Macrophages, T cells, NK cells, other cell types | ||
| Peritoneal Vδ1 | CCL3 (MIP1α) | Macrophages | 124 |
| CCL5 (RANTES) | T cells, eosinophils, basophils | ||
| DETC | CCL3 (MIP1α) | Macrophages | 125, 126 |
| CCL4 (MIP1β) | Macrophages, NK cells, other cell types | ||
| CCL5 (RANTES) | T cells, eosinophils, basophils | ||
| XCL1 | CD8+ cross-presenting DCs | ||
In humans, a hypomorphic RAG1 mutation was recently reported that results in a predominance of γδ T cells64. Despite their strong αβ T cell deficiency, patients with this mutation had normal immunoglobulin levels and responses to infectious agents and vaccination. One patient displayed a hyper-IgE syndrome concomitant with elevated numbers of circulating eosinophils, while another with very high absolute numbers of circulating γδ T cells also presented high titres of circulating IgM, IgG and IgA. Likewise, of two patients with a novel leaky CD3δ mutation that mostly affects the αβ T cell compartment65, the patient with the most responsive γδ T cells also presented with hyper-IgE syndrome and eosinophilia, further demonstrating a role for γδ T cells in antibody production. Indeed, healthy individuals harbour a subset of Vγ9Vδ2+ T cells that expresses CXCR5, has a TFH-like phenotype and can provide B cell help66, particularly in the presence of IL-21, the prototypic TFH cell-inducing cytokine67.
Interactions with Dendritic Cells
Given that tissue-associated DC can respond to numerous molecular sentinels of infection and stress; can migrate to the LNs, and can there prime efferent adaptive responses, one might reasonably question the importance of an afferent role for γδ T cells. One explanation is that γδ T cells collaborate with DC, refining the quality of information that DC receive about the status of a tissue, and thereby improving the criteria for whether or not an immunogenic or tolerogenic response ensues. For example, DC lack NKG2D and hence may only be able to sense epithelial cell stress if this status is communicated to them by responding γδ T cells. Indeed, the visualisation of DETC–Langerhans cell contacts in the skin46, 47 encourages the view that activated γδ T cells directly regulate DC function, in contrast to the conventional view that DCs always function upstream of T cells.
Aminobisphosphonates (NBPs) include clinically approved entities such as zoledronate that inhibit a downstream enzyme in the mevalonate pathway thereby causing the accumulation of IPP and sensitising cells to Vγ9Vδ2+ T cells. Thus, NBP-treated human DCs mimic DC infected with specific types of bacteria, protozoa, or viruses (see above). Vγ9Vδ2+ T cells potentiate the maturation of NBP-treated DC via their production of IFN-γ and TNF in a cell-cell contact68. Additionally, immature DCs are reciprocally efficient at promoting Vγ9Vδ2+ T cell activation 69. The significance of such interactions is suggested by the finding that activated Vγ9Vδ2+ T cells can relieve a block to DC maturation imposed by M. tuberculosis infection70.
Interactions with αβ T cells
A second explanation for the importance of γδ T cells in the afferent phase is that they may mimic the functions of DC, thereby amplifying or even substituting for them. Perhaps the signatory function of DC is their presentation to T cells of antigenic peptide-MHC complexes. B cells can also present antigen71, and whereas the case for conventional human T cells is less compelling, it is clear that human Vγ9Vδ2+ T cells can also present antigen to CD4+ T cells and cross-present antigen to CD8+ T cells72. Activated Vγ9Vδ2+ T cells efficiently take up soluble antigens and can opsonize and phagocytose target cells 73, 74. Moreover, whereas steady-state Vγ9Vδ2+ T cells localise mostly to the peripheral blood or to tissues, their activation rapidly induces CCR7, driving their migration to LNs, and upregulates MHC class I and class II and the co-stimulators CD80 and CD86 to levels equivalent to those on mature DCs72. This combination of properties is well-suited to antigen presentation to LN T cells, underlining the innate-like potential of γδ T cells to initiate antigen-specific adaptive responses against virus-infected cells and tumours.
γδ T cells may also regulate the αβ T cell repertoire. Thus, the interaction of fetal Vγ5Vδ1+ DETC progenitors with Skint1-expressing mTECs (see above) induces those epithelial cells to express AIRE which regulates the promiscuous gene expression that in turn purges the αβ T cell repertoire of strongly autoreactive cells75. Conversely, there is little evidence for either a specialised peripheral regulatory γδ T subset or one that is Foxp3, although intestinal γδ IELs produce high levels of TGFβ, that may downregulate local T cell responses76. Indeed, hyperactivity of systemic αβ T cells is a common phenotype of γδ T cell-deficient mice77, 78 (see below). In sum, γδ T cells do not have unique functional capabilities, but may be unique in their capacity to orchestrate a diversity of functions appropriate to their participation in each of the afferent, efferent, and regulatory phases of immunity.
Tissue-Associated γδ T cells
Selective homing and retention
What processes lead to the formation of tissue-localised γδ cell compartments well-suited to the afferent phase of immune responses? In mice, the answer relates in part to the schedule of γδ T cell development, with the first wave of cells homing to the epidermis; the second wave to the genital tract; and subsequent waves to the lung and to the gut, etc. Cells in successive waves developmentally acquire specific chemokine receptors: thus, upon interaction with Skint1+ mTEC in the foetal thymus, Vγ5Vδ1+ DETC progenitor thymocytes acquire CC-chemokine receptor 10 (CCR10) which in part directs them to the epidermis79. Additionally, γδ cells seemingly engage in steady-state interactions that stabilise their retention within particular tissues47, 80, although the molecules responsible for this important process are largely unelucidated.
Micro-anatomical specialisation and Pre-programming
Within tissues, γδ T cells display overt microanatomical localisation. Thus, the murine skin juxtaposes IFN-γ-producing intraepithelial T cells (DETC) with IL-17-producing dermal γδ T cells, while the intestine juxtaposes IFN-γ-producing IEL and IL-17-producing γδ T cells in the lamina propria. It is not known why these specific effector functions are best suited to the outer and inner layers of the tissue, respectively. Nonetheless, hard-wired commitment of γδ T cell compartments to particular functional programmes is important because it permits the rapid responsiveness that is essential for the afferent response. As will now be outlined, this is achieved by developmental pre-programming.
By monitoring T10/T22-specific γδ T cells (see above) developing in mice that do or do not express T10/T22, it was possible to show that agonist encounter during thymic development committed cells to a differentiation pathway characterized by IFN-γ production, in place of IL-1781. Similarly, Vγ5Vδ1+ DETC progenitors that engage Skint1+ mTECs express IFN-γ, whereas Vγ5Vδ1+ thymocytes in FVB mice from Taconic farms, which lack the Skint1 gene, continue to express IL-17. The selective impact of Skint1 is at least in part achieved by the upregulation of Nfat, Nfkb, and Egr3, which collectively promote T-bet expression and suppress transcription of Rorgc and the “γδ-marker” gene, Sox1382.
This induced gene regulatory network is not limited to Skint1-selected DETC progenitors, being detected in adult γδ thymocytes identified by their phenotypic similarity to Skint1-selected Vγ5Vδ1+ foetal thymocytes. However, the network in these cells is induced by molecules other than Skint1 that remain to be identified. This is relevant to the observations that murine peripheral γδ T cells can be sub-divided into CD27+ γδ T cells that produce IFN-γ, and CD27− γδ T cells that produce IL-17, and that many such cells are also developmentally pre-programmed83. Provocatively, the Skint1-induced gene regulatory network can be induced in adult thymocytes by direct TCR stimulation82, consistent with the prospect that TCR agonists are primary agents of functional pre-programming. This is in striking contrast to the developmental impact of agonists on αβ T cell progenitors, which is usually to promote apoptosis or the upregulation of FoxP3 that suppresses effector function.
According to these studies, IL-17-producing γδ cell progenitors may not have encountered TCR-binding agonists. However, they may still have received critical differentiation signals, possibly via ligand-independent TCR signalling. Thus, these cells are CD44hiCD62LlowCD127hiTCRhi, a phenotype consistent with pre-activation. Moreover, peripheral IL-17-producing CD27− cells can be activated simply by exposure to IL-1 and/or IL-237, that is also consistent with prior TCR signalling. However, the possibility that innate-like IL-17-producing γδ T cells develop without direct TCR-ligand engagement contradicts the common view that innate-like T cells emerge primarily as a result of positive agonist selection.
There is also a murine γδ T cell subset that is pre-programmed toward IL-4 production following interactions between signalling lymphocytic activation molecule (SLAM) on γδ T cell progenitors and SLAM-associated protein (SAP) on immature CD4+CD8+ (“DP”) αβ T cell progenitors84. Interestingly, DP cells also regulate the differentiation of IFNγ– and IL-17-producing γδ thymocytes in a process termed trans-conditioning that is in part mediated by lymphotoxin85, 86. This and other mechanisms regulating γδ T cell development have been extensively discussed elsewhere87.
Two origins of γδ T cells
As stated, the biological significance of pre-programming is not fully understood: what, for example, is the benefit of extinguishing IL-17 potential in agonist-selected γδ T cells? Moreover, many naïve peripheral γδ T cells do not display the molecular hallmarks of pre-programming, and retain the potential for IL-17 production in vivo even if they emerge from among CD27+ cells, more typically associated with IFN-γ production. Collectively, such non-pre-programmed cells may compose the adaptive γδ T cell compartment, with specificity for diverse antigens, including PE (see above). They may develop in the thymus with no selective educational input from the TCR.
The contrast of the “IL-17-default position” of distinct subsets of TCRγδ+ thymocytes that fail to engage agonists, with the “non-committed” state of many other post-natal TCRγδ+ thymocytes strongly implies distinct developmental origins of different γδ T cell subsets. Possibly the “IL-17-default position” marks γδ T cell progenitors that will form the bulk of innate-like cells, whereas other murine γδ T cells will form the predominant reservoir of lymphoid-homing, adaptive γδ T cells (Figure 1). Indeed, evidence was recently presented for the divergence of innate and adaptive T cell precursors prior to commitment to the αβ and γδ T cell lineages88. More specifically, those γδ T cells that are rapidly-responsive via innate co-stimulatory or cytokine receptors (see above) readily accommodate co-expression of TCRβ, consistent with the view that signalling via their own γδ TCR is the dominant force in their development. Conversely, TCRβ expression is selected against in those γδ T cells with more adaptive properties, such as PE-reactive γδ T cells, since in the absence of pre-commitment, such cells might readily be diverted toward an αβ T cell fate by TCRβ chain expression and the consequent formation of a preTCR. The presence of discrete progenitors for innate and adaptive γδ cells would readily explain why different gene regulatory networks are found in discrete subsets of γδTCR+ thmocytes89. Moreover, most innate-like γδ T cells may derive from the foetus and may not be reconstituted from the bone marrow. This is certainly true for DETCs and for IL-17-producing CD27− γδ cells, and may extend to a substantial numbers of human peripheral blood γδ T cells. This has profound clinical implications in terms of bone marrow transplantation, and it leads to the importance of ontogeny in γδ cell biology.
γδ T cells and ontogeny
γδ T cells are the first T cells to develop in every vertebrate in which T cell ontogeny has been examined. In cattle, from which some of the best data derive for adaptive γδcell responses, the T cell compartment throughout the first year of life can be dominated by γδ cells. As was just considered, murine DETCs and many IL-17-producing γδ T cells are exclusively generated from foetal progenitors. Indeed, the development of mouse and human IL-17-producing γδ T cells is selectively promoted by IL-7, whose expression is highest in neonates90. This may explain why human IL-17-producing γδ T cells are readily evoked from cord blood but are very difficult to evoke from the peripheral blood of healthy adults91. Moreover, although DETCs and IL-17-producing γδ T cells are functionally distinct, they clearly share a capacity for life-long self-renewal. The same may be true of many human peripheral blood, IFN-γ-producing Vγ9Vδ2+ cells that also derive from foetal progenitors and that undergo substantial expansion in early life92. Understanding such self renewal of differentiated cells may inform the biology of Langerhans cells and microglia that were also recently found to derive exclusively from foetal progenitors93.
This striking ontogeny has suggested that the primary contribution of γδ T cells is to neonatal protection, when conventional αβ T cell responses are severely functionally impaired and DCs are immature. This reasoning complies with the growing belief that the neonatal immune compartment is not simply an immature version of that which arises in adults, but is qualitatively distinct. In support of this hypothesis, human γδ T cells are functionally precocious relative to αβ T cells94, 95. Moreover, in independent cases of cytomegalovirus (CMV) transmission in utero, the dramatic expansion of human Vδ1+ T cells with highly related TCRs has been reported, suggesting a common response to a single epitope96.
In two instances of parasite infection in mice, γδ T cells were required for the protection of young mice but not adults97, 98. However, even as adults, the combined deficiency of αβ and γδ T cells increased susceptibility to parasite infection relative to TCRβ-deficiency alone, demonstrating that responsive γδ T cells persist in adults99. Additionally, the frequent association of γδ T cells with IgE induction may reflect their role in early life, since B cells that directly switch to IgE production rather than via an IgG1+ intermediate are most abundant in very young mice100. In sum, the greatest dependence of cell-mediated immunity on γδ cells may exist in newborns and may have been largely overlooked because of the scarcity of immunological studies in young animals. However, given their capacity to self-renew, γδ cells of fetal origin may variably persist in adults, and combine with those derived from post-natal progenitors to make additional, key contributions to immunoprotection and, conversely, to immunopathology.
γδ T cell responses to specific challenges
γδ T cells are present in adult animals at considerably lower numbers than αβ T cells, and are seemingly irrelevant to immunity against certain well-studied infections such as lymphocytic choriomeningitis virus (LCMV). Thus, it is easy to understand why little attention may have been paid to γδ T cells. However, it now clear that these cells are essential to myriad host processes.
For example, mice infected intraperitoneally with vaccinia virus show increased numbers of IFNγ-secreting splenic γδ T cells by 2 days post infection101. Such cells show increased reactivity toward vaccinia virus-infected cells, but there is no evidence of virus specificity. Nonetheless, γδ T cell-deficient mice show substantial increases in virus titres immediately post-infection as well as increased mortality compared with control mice. However, in surviving vaccinia virus-infected Tcrd−/− mice, immunity develops normally101, consistent with γδ T cells making innate-like contributions to the primary response, but failing to mediate adaptive memory.
By contrast, γδ T cell deficiency in mice infected with West Nile virus (an emerging mosquito-borne pathogen) impairs responses to both primary and secondary infection, not because γδ T cells form memory cells in these animals, but because they critically contribute to the quality of recall CD8+ cells that are generated during primary infection102. An analogous influence of γδ T cells exists over CD4+ T cell memory generated during intra-vaginal infection by herpes simplex virus 2103. In humans, γδ T cell population expansion is strongly associated with CMV infection104, 105, and γδ T cell clones derived from CMV-infected individuals secrete cytokines in response to both infected cells and tumour cells19.
Likewise, γδ T cells are a critical source of rapid IL-17 production in response to diverse bacterial infections, and Tcrd−/− mice show substantially increased susceptibility to infections by Noccardia, Klebsiella, Listeria, E. coli, Salmonella, Mycobacterium, and Pseudomonas, for example106. Additionally, Tcrd−/− mice show impaired responses to infection by certain parasites, including Plasmodium, where IFNγ-production by γδ T cells may be more important than IFN-γ production by NK cells or αβ T cells107, 108. Beyond infection, γδ T cells confer resistance to particular regimens of chemical carcinogenesis and to certain spontaneously arising tumours in transgenic mice, by mechanisms that have not been clarified in depth109.
As mentioned above, the primary manifestation of γδ T cell deficiency in mice is often an inflammatory pathology reflecting exaggerated αβ T cell responses. Understandably, this has been taken as evidence that γδ T cells exert a regulatory effect upon conventional T cells, possibly consistent with their production of TGFβ in the gut. However, it can also be explained by the rapid response to and limitation of infection by γδ T cells that thereby limits αβ T cell activation. In general terms, this may not be a unique means of regulation since some human αβ TCR+ Treg cells exert their effects by killing APCs in a phenomenon of linked suppression110. Importantly, this would be consistent with the sustained contribution of γδ T cells to protective immunosurveillance in adults. Naturally, aggregate functional pleiotropy of γδ T cells has clinical implications, in the cells’ potential to cause and to regulate particular immunoapathologies, and in their utility as targets in mediating immunotherapy of cancer and of chronic infection. These are active areas of investigation, as summarised in Box 1.
Box 1. γδ cells in disease and therapy.
Inevitably, functionally pleiotropic γδ T cells can contribute to and ameliorate disease and are attractive targets for clinical manipulation. The capacity of some γδ T cell activities to limit αβ T cell functions is manifest in the increased incidence of αβ T cell-dependent glomerular nephritis and lupus in MRL–lpr mice crossed with Tcrd−/− mice111. Conversely, the rapid responsiveness of IL-17- and IL-22-producing CD27− γδ T cells in response to infection is mirrored by a rapid response of dermal IL-17-producing cells to the epicutaneous application of imiquimod, thereby promoting a psoriasiform pathology112, 113. IL-17-producing γδ T cells have also been identified in psoriatic lesions but not unaffected human skin114. There are at least two interesting implications of these findings. First, disease may reflect violation of the anatomical functional micro-segregation that ordinarily excludes IL-17-producing γδ T cells from epithelia (see above). Second, immunopathology in adults may be provoked by cells that arose in the foetus. Hence, disease might be predisposed by dysregulated persistence of foetal-derived cells.
IL-17-producing γδ T cells are also implicated in experimental autoimmune encephalomyelitis (EAE), the murine model for multiple sclerosis115, 116, and in the prototypical adaptive immunopathology, type 1 diabetes (unpublished). Moreover, by promoting inflammation, IL-17-producing γδ T cells may also exacerbate tumour progression in cases where transformed cells were not eradicated by prior lymphoid stress surveillance117.
Such considerations invoke two distinct clinical approaches. On the one hand, the clear capacity of human γδ T cells to detect transformed cells via NKG2D and/or other pathways, to promote cytolysis, to present antigen, and to mobilise other components of the immune systems provides an anti-tumour potential that can be readily invoked by clinically approved aminobisphosphonates that upregulate γδ T cell-activating phosphoantigens. This approach has been pursued at multiple centres, is largely safe and has implied efficacy. However, γδ T cells become irreversibly exhausted after chronic aminobisphosphonate treatment, which will impinge upon the application of adoptive γδ T cell immunotherapy. The second approach would be to limit the activities of γδ T cells in autoimmune and autoinflammatory diseases. Whereas it is common-place yet challenging to treat such diseases with agents that suppress effector function, targeting γδ T cells offers the opportunity to target the afferent response to whatever is the chronic stimulus. Moreover, the attractiveness of these cells as a target would be considerable if, in some scenarios, the cells persist from the foetus and are not absolutely required for adult immune function.
Conclusions: six good reasons for γδ T cells
This review has considered data, much of it recently published, in the context of six explanations for the unique contributions of γδ T cells in the immune system. While much remains to be learned, there is a sufficient basis to draw some conclusions. First, γδ TCRs engage a distinct constellation of antigens, thereby widening the scope of immune responsiveness. This may reflect a particularly significant contribution of adaptive γδ T cells. At the same time, the overt inclusion of auto-antigens in their specificities illustrates a “beneficial auto-immunogenicity” that may be based in part on a novel perspective; namely constitutive TCR engagement facilitating rapid responses to stress or infection. Such afferent actions distinguish γδ T cells functionally and kinetically from most conventional T cells, and relies in large part on the capacity of the γδTCR to see self-surface moieties expressed on cells within tissues. Moreover, the utilisation of adaptive TCRs in the afferent phase potentially offers greater breadth and selectivity over the type of stimuli to respond to, by comparison to the use of DCs or NK cells. Thus, γδ T cells may be better than TLR- or NLR-dependent sensing at distinguishing between pathogenic and benign challenges and therefore at determining whether an immunogenic or tolerogenic response ensues. Consistent with this, several adaptive responses are severely impaired in the absence of γδ T cells. Perhaps γδ T cells are well placed to eventually substitute for DC in the role of afferent sensing.
Strictly speaking γδ T cells do not express unique functions, but they can offer distinct combinations of functional potentials, such as cytolysis, IgE induction, antigen presentation and the production of growth factors (Figure 3). The disposition of distinct functions to particular subsets, particularly within tissues, permits different complexions of the γδ T cell response. Thus, TCRγδ+ IEL may promote the exclusion of infectious agents or toxins by eradicating targeted cells, employing IgE-mediated expulsion mechanisms, recruiting other cells, and promoting tissue re-growth. Conversely, sub-epithelial responses may promote an intergrative microbicidal innate and adaptive response to counter agents that have penetrated the basement membrane. Such capacity to form functional compartments in particular anatomical niches may reflect a further critical contribution of γδ T cells to immunity. However, it is possible that such roles may be increasingly subsumed by unconventional αβ T cells at human body surfaces. By contrast, αβ T cells may not be able to substitute for the functional potency of γδ T cells in newborns or even in the foetus where intrinsic as opposed to maternal mechanisms of immunoprotection are increasingly considered important.
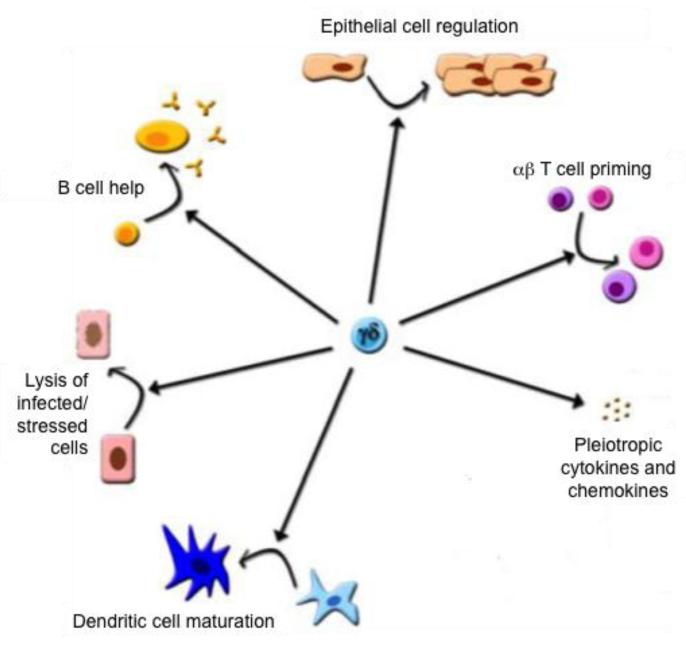
Figure 3. Six of the best γδ T cell functions.
An increasing body of literature now demonstrates that γδ T cells can play an important central role in defending the organism against a broad range of infectious and sterile stresses by directly eliminating infected or stressed cells; by producing a diversified set of cytokines and chemokines to regulate other immune and non-immune cells; by directly promoting immune cell maturation and activation by triggering B cell help, DC maturation and αβ T cell priming via antigen presentation; and finally by regulating stromal cell function.
Even in the neonate, however, the growing data-sets suggest that γδ T cells are disproportionately responsive to particular challenges, notably CMV, tuberculosis and malaria. Moreover, although we have emphasised that the expansion of rare, antigen-specific clones is not the hallmark of γδ T cell biology, both tuberculosis and CMV seemingly induce the selective expansion of clones that share reactivity for relevant PAMPs or molecular markers of infected cells. Thus, events early in life drive the expansion of specific γδ T cell populations that, albeit polyclonal, represent an alteration to the starting repertoire. This is by definition a form of adaptive immunity and it reminds us of the difficulty inherent in applying strict teleological terms to immunological processes. Perhaps the simplest reconciliation is to consider that all functional γδ T cells must at some point have received a signal through the TCR. This would clearly distinguish them from emerging cohorts of innate lymphoid cells. Those γδ T cells that develop without pre-programming and that do not receive the requisite TCR signal until peripheral exposure are most obviously adaptive; those that receive the signal in the thymus are most obviously innate-like; and those that receive it in the periphery but during very early life may be the products of adaptive processes which rapidly convert them into innate-like cells. Given this, a particular emphasis should be placed on clarifying γδ T cell biology in CMV, tuberculosis and malaria, since these agents are of great clinical significance and seem so effective at driving early γδ expansion and conversion into innate-like cells. These studies may test the utility of the mouse as a model for detailed aspects of γδ T cell biology. Indeed, like NK cells, γδ T cells show species-specific variation that is in part reflected in highly variable gene structures1.
It is evident that some immunology college courses still pay little attention to γδ T cells. This is completely unjustified given the six signatory roles of γδ T cells reviewed here. We look forward to greater consideration, more extensive investigation, and improved clinical manipulation of γδ T cells, which so clearly combine effector functions with a powerful afferent potential, and which tell us so much about lymphocyte biology as well as about the cells themselves.
Acknowledgements
We thank many colleagues, within our lab and beyond it, including Bruno Silva-Santos, Jan Kisielow, Paul Fisch, Leo Lefrancois, and Willi Born, for thoughtful input and clarification of data, and thank the Wellcome Trust and CRUK for funding. We apologise to those whose findings we may have inadvertently overlooked or that were a victim of space constraints.
Glossary terms
- AIRE
The autoimmune regulator gene whose product expressed in medullary thymic epithelial cells (mTECs) promotes the promiscuous expression of genes that are otherwise specific to specific peripheral tissues, e.g. endocrine glands or the nervous system. Peptides derived from these tissue-specific antigens are presented by mTECs to developing ab T cells, such that any with high affinity reactive TCRs may be clonally deleted as means of central tolerance
- Anergise
The conversion of a T cell to a state in which it is almost completely non-responsive to TCR engagement. This may occur when a peripheral T cell is exposed to antigen in the absence of co-stimulation and is interpreted as a means to suppress potentially auto-reactive T cell responses in the absence of infection
- Dendritic epidermal γδ T cells
(DETCs). gd T cell receptor (TCR)+ cells localized purely in the epidermis that have been described in rodents and cattle but not humans. In mice, essentially all DETCs express precisely the same TCR, forming a prototype lymphocyte repertoire of limited diversity.
- Intraepithelial lymphocytes
(IELs). Intraepithelial lymphocytes are T cells that reside in the basolateral side of the intestinal epithelium, above the basement membrane. They express either an ab TCR (T cell receptor) or a gd TCR, and in mice they frequently express the CD8aa homodimer.
- NOD mice
An inbred strain of mice that spontaneously develop T cell-mediated autoimmune diabetes, dependent on their expression of a particular MHC class 2, molecule, I-Ag7.
- B-1 cells
IgMhiIgDlowMAC1+B220lowCD23− cells that are dominant in the peritoneal and pleural cavities. Their precursors develop in the fetal liver and omentum, and in adult mice, the size of the B-1 cell population is kept constant owing to the self-renewing capacity of these cells. B-1 cells recognize self components, as well as common bacterial antigens, and they secrete antibodies that tend to have low affinity and broad specificity.
- Lymphoid stress-surveillance
The capacity of lymphocytes, as opposed to myelomonocytic cells, to sense infection or tissue dysregulation and to respond rapidly, in synchrony with innate responses.
- NKG2D
(Natural-killer group 2, member D). A lectin-type activating receptor encoded by the NK complex and expressed at the surface of most NK cells and NKT cells; many gd T cells; and antigen-experienced cytolytic CD8+ ab T cells. The ligands for NKG2D are MHC class I polypeptide-related sequence A (MICA) and MICB, and at least four related ULBP proteins, and multiple members of the structurally related retinoic acid early transcript 1 (RAE1) and H60 families, and Mult-1 and in mice. Such ligands are generally expressed at the surface of infected, stressed or transformed cells.
- Signals 1, 2 and 3
Cell signalling pathways activated by engagement of the antigen receptor (Signal 1); co-stimulatory receptors, e.g. CD28 (Signal 2); and cytokine receptors, e.g. IL-2 (Signal 3).
- CD8aa+ IELs
A type of T cell found mostly in the murine intestinal epithelium. The CD8 molecule that they express is a homodimer of CD8α, rather than the CD8αβ heterodimer that is expressed by conventional CD8+ T cells in the lymph nodes and by another distinct subset of IEL. CD8aa is a ligand of the non-classical MHC class I molecule thymus leukemia antigen (TL), which is expressed by the intestinal epithelium. It has been proposed that CD8aa+ IEL are self-reactive, TCR-agonist-selected cells that have regulatory properties.
- Germinal centres
Highly specialized and dynamic microenvironments that give rise to secondary B cell follicles during an immune response. They are the main site of B cell maturation, leading to the generation of memory B cells and plasma cells that produce high-affinity antibody.
- MRL–lpr mouse
A mouse strain that spontaneously develops glomerulonephritis and other symptoms of systemic lupus erythematosus (SLE). The lpr mutation causes a defect in CD95 (also known as FAS), preventing apoptosis of activated lymphocytes. The MRL strain contributes disease-associated mutations that have yet to be identified.
- Stress Antigens
Molecules, such as MICA or Rae-1 whose expression is upregulated by cellular dysregulation and that are functionally recognised by lymphocytes as part of a process of immune surveillance
- Imiquimod
An imidazoquinolene-based compound that is sensed by TLR7, is currently used for the treatment of basal cell carcinoma, but that has also been implicated in iatrogenic induction of psoriasis-like symptoms.
- Autoinflammatory disease
A disease characterized by seemingly unprovoked pathological activation of the innate immune system in the absence of overt autoantibodies or autoreactive T cells.
- V(D)J recombination
Somatic rearrangement of variable (V), diversity (D) and joining (J) regions of the genes that encode antigen receptors, leading to repertoire diversity of both B cell and T cell receptors.
Footnotes
This manuscript has been accepted for publication in Nature Reviews Immunology. This version has not undergone final editing. Please refer to the complete version at http://www.nature.com/nri/journal/v13/n2/abs/nri3384.html (doi:10.1038/nri3384)
References
- 1.Kazen AR, Adams EJ. Evolution of the V, D, and J gene segments used in the primate gammadelta T-cell receptor reveals a dichotomy of conservation and diversity. Proc Natl Acad Sci U S A. 2011;108:E332–40. doi: 10.1073/pnas.1105105108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley EC, Girardi M, Owen MJ, Hayday AC. Alpha beta and gamma delta T cells can share a late common precursor. Curr Biol. 1995;5:659–69. doi: 10.1016/s0960-9822(95)00131-x. [DOI] [PubMed] [Google Scholar]
- 3.Boehm T, et al. VLR-based adaptive immunity. Annu Rev Immunol. 2012;30:203–20. doi: 10.1146/annurev-immunol-020711-075038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bucy RP, Chen CL, Cihak J, Losch U, Cooper MD. Avian T cells expressing gamma delta receptors localize in the splenic sinusoids and the intestinal epithelium. J Immunol. 1988;141:2200–5. [PubMed] [Google Scholar]
- 5.Lafaille JJ, DeCloux A, Bonneville M, Takagaki Y, Tonegawa S. Junctional sequences of T cell receptor gamma delta genes: implications for gamma delta T cell lineages and for a novel intermediate of V-(D)-J joining. Cell. 1989;59:859–70. doi: 10.1016/0092-8674(89)90609-0. [DOI] [PubMed] [Google Scholar]
- 6.Asarnow DM, et al. Limited diversity of gamma delta antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell. 1988;55:837–47. doi: 10.1016/0092-8674(88)90139-0. [DOI] [PubMed] [Google Scholar]
- 7.Zeng X, et al. gammadelta T cells recognize a microbial encoded B cell antigen to initiate a rapid antigen-specific interleukin-17 response. Immunity. 2012;37:524–34. doi: 10.1016/j.immuni.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hogg AE, Worth A, Beverley P, Howard CJ, Villarreal-Ramos B. The antigen-specific memory CD8+ T-cell response induced by BCG in cattle resides in the CD8+gamma/deltaTCR-CD45RO+ T-cell population. Vaccine. 2009;27:270–9. doi: 10.1016/j.vaccine.2008.10.053. [DOI] [PubMed] [Google Scholar]
- 9.Neves PC, et al. CD8+ gamma-delta TCR+ and CD4+ T cells produce IFN-gamma at 5-7 days after yellow fever vaccination in Indian rhesus macaques, before the induction of classical antigen-specific T cell responses. Vaccine. 2010;28:8183–8. doi: 10.1016/j.vaccine.2010.09.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plattner BL, Huffman EL, Hostetter JM. Gamma-Delta T-Cell Responses During Subcutaneous Mycobacterium avium Subspecies paratuberculosis Challenge in Sensitized or Naive Calves Using Matrix Biopolymers. Vet Pathol. 2012 doi: 10.1177/0300985812463404. [DOI] [PubMed] [Google Scholar]
- 11.Shen Y, et al. Adaptive immune response of Vgamma2Vdelta2+ T cells during mycobacterial infections. Science. 2002;295:2255–8. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sciammas R, et al. Unique antigen recognition by a herpesvirus-specific TCR-gamma delta cell. J Immunol. 1994;152:5392–7. [PubMed] [Google Scholar]
- 13.Janeway CA, Jr., Jones B, Hayday A. Specificity and function of T cells bearing gamma delta receptors. Immunol Today. 1988;9:73–6. doi: 10.1016/0167-5699(88)91267-4. [DOI] [PubMed] [Google Scholar]
- 14.Crowley MP, Reich Z, Mavaddat N, Altman JD, Chien Y. The recognition of the nonclassical major histocompatibility complex (MHC) class I molecule, T10, by the gammadelta T cell, G8. J Exp Med. 1997;185:122330. doi: 10.1084/jem.185.7.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin S, et al. Antigen recognition determinants of gammadelta T cell receptors. Science. 2005;308:252–5. doi: 10.1126/science.1106480. [DOI] [PubMed] [Google Scholar]
- 16.Matis LA, et al. Structure and specificity of a class II MHC alloreactive gamma delta T cell receptor heterodimer. Science. 1989;245:746–9. doi: 10.1126/science.2528206. [DOI] [PubMed] [Google Scholar]
- 17.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science. 1998;279:1737–40. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 18.Kong Y, et al. The NKG2D ligand ULBP4 binds to TCRgamma9/delta2 and induces cytotoxicity to tumor cells through both TCRgammadelta and NKG2D. Blood. 2009;114:310–7. doi: 10.1182/blood-2008-12-196287. [DOI] [PubMed] [Google Scholar]
- 19.Willcox CR, et al. Cytomegalovirus and tumor stress surveillance by binding of a human gammadelta T cell antigen receptor to endothelial protein C receptor. Nat Immunol. 2012;13:872–9. doi: 10.1038/ni.2394. [DOI] [PubMed] [Google Scholar]
- 20.Porcelli S, et al. Recognition of cluster of differentiation 1 antigens by human CD4-CD8-cytolytic T lymphocytes. Nature. 1989;341:447–50. doi: 10.1038/341447a0. [DOI] [PubMed] [Google Scholar]
- 21.Bai L, et al. The majority of CD1d-sulfatide-specific T cells in human blood use a semiinvariant Vdelta1 TCR. Eur J Immunol. 2012;42:2505–10. doi: 10.1002/eji.201242531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, et al. Gamma delta T cell receptors confer autonomous responsiveness to the insulin-peptide B:9-23. J Autoimmun. 2010;34:478–84. doi: 10.1016/j.jaut.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 24.Morita CT, et al. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human gamma delta T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 25.Eberl M, Moser B. Monocytes and gammadelta T cells: close encounters in microbial infection. Trends Immunol. 2009;30:562–8. doi: 10.1016/j.it.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Mookerjee-Basu J, et al. F1-adenosine triphosphatase displays properties characteristic of an antigen presentation molecule for Vgamma9Vdelta2 T cells. J Immunol. 2010;184:6920–8. doi: 10.4049/jimmunol.0904024. [DOI] [PubMed] [Google Scholar]
- 27.Scotet E, et al. Tumor recognition following Vgamma9Vdelta2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity. 2005;22:71–80. doi: 10.1016/j.immuni.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Vantourout P, et al. Specific requirements for Vgamma9Vdelta2 T cell stimulation by a natural adenylated phosphoantigen. J Immunol. 2009;183:3848–57. doi: 10.4049/jimmunol.0901085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monkkonen H, et al. A new endogenous ATP analog (ApppI) inhibits the mitochondrial adenine nucleotide translocase (ANT) and is responsible for the apoptosis induced by nitrogen-containing bisphosphonates. Br J Pharmacol. 2006;147:437–45. doi: 10.1038/sj.bjp.0706628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruder J, et al. Target specificity of an autoreactive pathogenic human gammadelta-T cell receptor in myositis. J Biol Chem. 2012;287:20986–95. doi: 10.1074/jbc.M112.356709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harly C, et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human gammadelta T-cell subset. Blood. 2012;120:2269–79. doi: 10.1182/blood-2012-05-430470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palakodeti A, et al. The molecular basis for modulation of human Vgamma9Vdelta2 T cell responses by CD277/butyrophilin-3 (BTN3A)-specific antibodies. J Biol Chem. 2012;287:32780–90. doi: 10.1074/jbc.M112.384354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyden LM, et al. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal gammadelta T cells. Nat Genet. 2008;40:656–62. doi: 10.1038/ng.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis JM, et al. Selection of the cutaneous intraepithelial gammadelta+ T cell repertoire by a thymic stromal determinant. Nat Immunol. 2006;7:843–50. doi: 10.1038/ni1363. [DOI] [PubMed] [Google Scholar]
- 35.Davey MS, et al. Human neutrophil clearance of bacterial pathogens triggers anti-microbial gammadelta T cell responses in early infection. PLoS Pathog. 2011;7:e1002040. doi: 10.1371/journal.ppat.1002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gober HJ, et al. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–8. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kistowska M, et al. Dysregulation of the host mevalonate pathway during early bacterial infection activates human TCR gamma delta cells. Eur J Immunol. 2008;38:2200–9. doi: 10.1002/eji.200838366. [DOI] [PubMed] [Google Scholar]
- 38.Born WK, et al. Hybridomas expressing gammadelta T-cell receptors respond to cardiolipin and beta2-glycoprotein 1 (apolipoprotein H) Scand J Immunol. 2003;58:374–81. doi: 10.1046/j.1365-3083.2003.01315.x. [DOI] [PubMed] [Google Scholar]
- 39.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 40.Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–96. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Swamy M, Jamora C, Havran W, Hayday A. Epithelial decision makers: in search of the ‘epimmunome’. Nat Immunol. 2010;11:656–65. doi: 10.1038/ni.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–8. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 43.Lodoen MB, Lanier LL. Viral modulation of NK cell immunity. Nat Rev Microbiol. 2005;3:59–69. doi: 10.1038/nrmicro1066. [DOI] [PubMed] [Google Scholar]
- 44.O’Brien RL, Born W. Heat shock proteins as antigens for gamma delta T cells. Semin Immunol. 1991;3:81–7. [PubMed] [Google Scholar]
- 45.Komori HK, et al. Cutting edge: dendritic epidermal gammadelta T cell ligands are rapidly and locally expressed by keratinocytes following cutaneous wounding. J Immunol. 2012;188:2972–6. doi: 10.4049/jimmunol.1100887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strid J, et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol. 2008;9:146–54. doi: 10.1038/ni1556. [DOI] [PubMed] [Google Scholar]
- 47.Chodaczek G, Papanna V, Zal MA, Zal T. Body-barrier surveillance by epidermal gammadelta TCRs. Nat Immunol. 2012;13:272–82. doi: 10.1038/ni.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taveirne S, et al. Inhibitory receptors specific for MHC class I educate murine NK cells but not CD8alphaalpha intestinal intraepithelial T lymphocytes. Blood. 2011;118:339–47. doi: 10.1182/blood-2011-01-331124. [DOI] [PubMed] [Google Scholar]
- 49.Witherden DA, et al. The junctional adhesion molecule JAML is a costimulatory receptor for epithelial gammadelta T cell activation. Science. 2010;329:1205–10. doi: 10.1126/science.1192698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Witherden DA, et al. The CD100 receptor interacts with its plexin B2 ligand to regulate epidermal gammadelta T cell function. Immunity. 2012;37:314–25. doi: 10.1016/j.immuni.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holl EK, et al. Plexin-B2 and Plexin-D1 in dendritic cells: expression and IL-12/IL-23p40 production. PLoS One. 2012;7:e43333. doi: 10.1371/journal.pone.0043333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147:629–40. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 53.Veldhoen M, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–9. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 54.Kadow S, et al. Aryl hydrocarbon receptor is critical for homeostasis of invariant gammadelta T cells in the murine epidermis. J Immunol. 2011;187:3104–10. doi: 10.4049/jimmunol.1100912. [DOI] [PubMed] [Google Scholar]
- 55.Fernandez-Salguero P, et al. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–6. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 56.Cardone J, et al. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat Immunol. 2010;11:862–71. doi: 10.1038/ni.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wen L, et al. Primary gamma delta cell clones can be defined phenotypically and functionally as Th1/Th2 cells and illustrate the association of CD4 with Th2 differentiation. J Immunol. 1998;160:1965–74. [PubMed] [Google Scholar]
- 58.Strid J, Sobolev O, Zafirova B, Polic B, Hayday A. The intraepithelial T cell response to NKG2D-ligands links lymphoid stress surveillance to atopy. Science. 2011;334:1293–7. doi: 10.1126/science.1211250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharp LL, Jameson JM, Cauvi G, Havran WL. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat Immunol. 2005;6:73–9. doi: 10.1038/ni1152. [DOI] [PubMed] [Google Scholar]
- 60.Motomura Y, et al. The transcription factor E4BP4 regulates the production of IL-10 and IL-13 in CD4+ T cells. Nat Immunol. 2011;12:450–9. doi: 10.1038/ni.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vermijlen D, et al. Distinct cytokine-driven responses of activated blood gammadelta T cells: insights into unconventional T cell pleiotropy. J Immunol. 2007;178:4304–14. doi: 10.4049/jimmunol.178.7.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ansel KM, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–14. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 63.Wen L, et al. Germinal center formation, immunoglobulin class switching, and autoantibody production driven by “non alpha/beta” T cells. J Exp Med. 1996;183:2271–82. [Google Scholar]
- 64.Ehl S, et al. A variant of SCID with specific immune responses and predominance of gamma delta T cells. J Clin Invest. 2005;115:3140–8. doi: 10.1172/JCI25221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gil J, et al. A leaky mutation in CD3D differentially affects alphabeta and gammadelta T cells and leads to a Talphabeta-Tgammadelta+B+NK+ human SCID. J Clin Invest. 2011;121:3872–6. doi: 10.1172/JCI44254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caccamo N, et al. CXCR5 identifies a subset of Vgamma9Vdelta2 T cells which secrete IL-4 and IL-10 and help B cells for antibody production. J Immunol. 2006;177:5290–5. doi: 10.4049/jimmunol.177.8.5290. [DOI] [PubMed] [Google Scholar]
- 67.Caccamo N, et al. IL-21 regulates the differentiation of a human gammadelta T cell subset equipped with B cell helper activity. PLoS One. 2012;7:e41940. doi: 10.1371/journal.pone.0041940. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Conti L, et al. Reciprocal activating interaction between dendritic cells and pamidronate-stimulated gammadelta T cells: role of CD86 and inflammatory cytokines. J Immunol. 2005;174:252–60. doi: 10.4049/jimmunol.174.1.252. [DOI] [PubMed] [Google Scholar]
- 69.Devilder MC, et al. Potentiation of antigen-stimulated V gamma 9V delta 2 T cell cytokine production by immature dendritic cells (DC) and reciprocal effect on DC maturation. J Immunol. 2006;176:1386–93. doi: 10.4049/jimmunol.176.3.1386. [DOI] [PubMed] [Google Scholar]
- 70.Caccamo N, et al. gammadelta T cells condition dendritic cells in vivo for priming pulmonary CD8 T cell responses against Mycobacterium tuberculosis. Eur J Immunol. 2006;36:2681–90. doi: 10.1002/eji.200636220. [DOI] [PubMed] [Google Scholar]
- 71.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–9. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 72.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science. 2005;309:264–8. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 73.Brandes M, et al. Cross-presenting human gammadelta T cells induce robust CD8+ alphabeta T cell responses. Proc Natl Acad Sci U S A. 2009;106:2307–12. doi: 10.1073/pnas.0810059106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Himoudi N, et al. Human gammadelta T lymphocytes are licensed for professional antigen presentation by interaction with opsonized target cells. J Immunol. 2012;188:1708–16. doi: 10.4049/jimmunol.1102654. [DOI] [PubMed] [Google Scholar]
- 75.Roberts NA, et al. Rank signaling links the development of invariant gammadelta T cell progenitors and Aire(+) medullary epithelium. Immunity. 2012;36:427–37. doi: 10.1016/j.immuni.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bhagat G, et al. Small intestinal CD8+TCRgammadelta+NKG2A+ intraepithelial lymphocytes have attributes of regulatory cells in patients with celiac disease. J Clin Invest. 2008;118:281–93. doi: 10.1172/JCI30989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hayday A, Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nat Rev Immunol. 2003;3:233–42. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- 78.Lahn M, et al. gammadelta T cells as regulators of airway hyperresponsiveness. Int Arch Allergy Immunol. 2001;125:203–10. doi: 10.1159/000053817. [DOI] [PubMed] [Google Scholar]
- 79.Jin Y, et al. Cutting edge: Intrinsic programming of thymic gammadeltaT cells for specific peripheral tissue localization. J Immunol. 2010;185:7156–60. doi: 10.4049/jimmunol.1002781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kyes S, Pao W, Hayday A. Influence of site of expression on the fetal gamma delta T-cell receptor repertoire. Proc Natl Acad Sci U S A. 1991;88:7830–3. doi: 10.1073/pnas.88.17.7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jensen KD, et al. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Turchinovich G, Hayday AC. Skint-1 identifies a common molecular mechanism for the development of interferon-gamma-secreting versus interleukin-17-secreting gammadelta T cells. Immunity. 2011;35:59–68. doi: 10.1016/j.immuni.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 83.Ribot JC, et al. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol. 2009;10:427–36. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Verykokakis M, et al. Inhibitor of DNA binding 3 limits development of murine slam-associated adaptor protein-dependent “innate” gammadelta T cells. PLoS One. 2010;5:e9303. doi: 10.1371/journal.pone.0009303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Powolny-Budnicka I, et al. RelA and RelB transcription factors in distinct thymocyte populations control lymphotoxin-dependent interleukin-17 production in gammadelta T cells. Immunity. 2011;34:364–74. doi: 10.1016/j.immuni.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 86.Silva-Santos B, Pennington DJ, Hayday AC. Lymphotoxin-mediated regulation of gammadelta cell differentiation by alphabeta T cell progenitors. Science. 2005;307:925–8. doi: 10.1126/science.1103978. [DOI] [PubMed] [Google Scholar]
- 87.Turchinovich G, Pennington DJ. T cell receptor signalling in gammadelta cell development: strength isn’t everything. Trends Immunol. 2011;32:567–73. doi: 10.1016/j.it.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 88.Kisielow J, Tortola L, Weber J, Karjalainen K, Kopf M. Evidence for the divergence of innate and adaptive T-cell precursors before commitment to the alphabeta and gammadelta lineages. Blood. 2011;118:6591–600. doi: 10.1182/blood-2011-05-352732. [DOI] [PubMed] [Google Scholar]
- 89.Narayan K, et al. Intrathymic programming of effector fates in three molecularly distinct gammadelta T cell subtypes. Nat Immunol. 2012;13:511–8. doi: 10.1038/ni.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Michel ML, et al. Interleukin 7 (IL-7) selectively promotes mouse and human IL-17-producing gammadelta cells. Proc Natl Acad Sci U S A. 2012;109:17549–54. doi: 10.1073/pnas.1204327109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ness-Schwickerath KJ, Jin C, Morita CT. Cytokine requirements for the differentiation and expansion of IL-17A- and IL-22-producing human Vgamma2Vdelta2 T cells. J Immunol. 2010;184:7268–80. doi: 10.4049/jimmunol.1000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Parker CM, et al. Evidence for extrathymic changes in the T cell receptor gamma/delta repertoire. J Exp Med. 1990;171:1597–612. doi: 10.1084/jem.171.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schulz C, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 94.De Rosa SC, et al. Ontogeny of gamma delta T cells in humans. J Immunol. 2004;172:1637–45. doi: 10.4049/jimmunol.172.3.1637. [DOI] [PubMed] [Google Scholar]
- 95.Gibbons DL, et al. Neonates harbour highly active gammadelta T cells with selective impairments in preterm infants. Eur J Immunol. 2009;39:1794–806. doi: 10.1002/eji.200939222. [DOI] [PubMed] [Google Scholar]
- 96.Vermijlen D, et al. Human cytomegalovirus elicits fetal gammadelta T cell responses in utero. J Exp Med. 2010;207:807–21. doi: 10.1084/jem.20090348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ramsburg E, Tigelaar R, Craft J, Hayday A. Age-dependent requirement for gammadelta T cells in the primary but not secondary protective immune response against an intestinal parasite. J Exp Med. 2003;198:1403–14. doi: 10.1084/jem.20030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Waters WR, et al. Cryptosporidium parvum-induced inflammatory bowel disease of TCR-beta- x TCR-delta-deficient mice. J Parasitol. 1999;85:1100–5. [PubMed] [Google Scholar]
- 99.Smith AL, Hayday AC. An alphabeta T-cell-independent immunoprotective response towards gut coccidia is supported by gammadelta cells. Immunology. 2000;101:325–32. doi: 10.1046/j.1365-2567.2000.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wesemann DR, et al. Immature B cells preferentially switch to IgE with increased direct Smu to Sepsilon recombination. J Exp Med. 2011;208:2733–46. doi: 10.1084/jem.20111155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Selin LK, Santolucito PA, Pinto AK, Szomolanyi-Tsuda E, Welsh RM. Innate immunity to viruses: control of vaccinia virus infection by gamma delta T cells. J Immunol. 2001;166:6784–94. doi: 10.4049/jimmunol.166.11.6784. [DOI] [PubMed] [Google Scholar]
- 102.Wang T, et al. Gamma delta T cells facilitate adaptive immunity against West Nile virus infection in mice. J Immunol. 2006;177:1825–32. doi: 10.4049/jimmunol.177.3.1825. [DOI] [PubMed] [Google Scholar]
- 103.Nishimura H, et al. Intraepithelial gammadelta T cells may bridge a gap between innate immunity and acquired immunity to herpes simplex virus type 2. J Virol. 2004;78:4927–30. doi: 10.1128/JVI.78.9.4927-4930.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lafarge X, et al. Cytomegalovirus infection in transplant recipients resolves when circulating gammadelta T lymphocytes expand, suggesting a protective antiviral role. J Infect Dis. 2001;184:533–41. doi: 10.1086/322843. [DOI] [PubMed] [Google Scholar]
- 105.Pitard V, et al. Long-term expansion of effector/memory Vdelta2-gammadelta T cells is a specific blood signature of CMV infection. Blood. 2008;112:1317–24. doi: 10.1182/blood-2008-01-136713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sutton CE, Mielke LA, Mills KH. IL-17-producing gammadelta T cells and innate lymphoid cells. Eur J Immunol. 2012;42:2221–31. doi: 10.1002/eji.201242569. [DOI] [PubMed] [Google Scholar]
- 107.Tsuji M, et al. Development of antimalaria immunity in mice lacking IFN-gamma receptor. J Immunol. 1995;154:5338–44. [PubMed] [Google Scholar]
- 108.Tsuji M, et al. Gamma delta T cells contribute to immunity against the liver stages of malaria in alpha beta T-cell-deficient mice. Proc Natl Acad Sci USA. 1994;91:345–9. doi: 10.1073/pnas.91.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Girardi M, et al. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–9. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 110.Tree TI, et al. Naturally arising human CD4 T-cells that recognize islet autoantigens and secrete interleukin-10 regulate proinflammatory T-cell responses via linked suppression. Diabetes. 2010;59:1451–60. doi: 10.2337/db09-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Peng SL, Madaio MP, Hayday AC, Craft J. Propagation and regulation of systemic autoimmunity by gammadelta T cells. J Immunol. 1996;157:5689–98. [PubMed] [Google Scholar]
- 112.Cai Y, et al. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity. 2011;35:596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Van Belle AB, et al. IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice. J Immunol. 2012;188:462–9. doi: 10.4049/jimmunol.1102224. [DOI] [PubMed] [Google Scholar]
- 114.Laggner U, et al. Identification of a novel proinflammatory human skin-homing Vgamma9Vdelta2 T cell subset with a potential role in psoriasis. J Immunol. 2011;187:2783–93. doi: 10.4049/jimmunol.1100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Petermann F, et al. gammadelta T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity. 2010;33:351–63. doi: 10.1016/j.immuni.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sutton CE, et al. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–41. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 117.Wakita D, et al. Tumor-infiltrating IL-17-producing gammadelta T cells support the progression of tumor by promoting angiogenesis. Eur J Immunol. 2010;40:1927–37. doi: 10.1002/eji.200940157. [DOI] [PubMed] [Google Scholar]
- 118.Vincent MS, et al. Lyme arthritis synovial gamma delta T cells respond to Borrelia burgdorferi lipoproteins and lipidated hexapeptides. J Immunol. 1998;161:5762–71. [PubMed] [Google Scholar]
- 119.Constant P, et al. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–70. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 120.Johnson RM, et al. A murine CD4-, CD8- T cell receptor-gamma delta T lymphocyte clone specific for herpes simplex virus glycoprotein I. J Immunol. 1992;148:983–8. [PubMed] [Google Scholar]
- 121.Hudspeth K, et al. Engagement of NKp30 on Vdelta1 T cells induces the production of CCL3, CCL4, and CCL5 and suppresses HIV-1 replication. Blood. 2012;119:4013–6. doi: 10.1182/blood-2011-11-390153. [DOI] [PubMed] [Google Scholar]
- 122.Ebert LM, Meuter S, Moser B. Homing and function of human skin gammadelta T cells and NK cells: relevance for tumor surveillance. J Immunol. 2006;176:4331–6. doi: 10.4049/jimmunol.176.7.4331. [DOI] [PubMed] [Google Scholar]
- 123.Pociask DA, et al. gammadelta T cells attenuate bleomycin-induced fibrosis through the production of CXCL10. Am J Pathol. 2011;178:1167–76. doi: 10.1016/j.ajpath.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tagawa T, et al. Vdelta1+ gammadelta T cells producing CC chemokines may bridge a gap between neutrophils and macrophages in innate immunity during Escherichia coli infection in mice. J Immunol. 2004;173:5156–64. doi: 10.4049/jimmunol.173.8.5156. [DOI] [PubMed] [Google Scholar]
- 125.Boismenu R, Feng L, Xia YY, Chang JC, Havran WL. Chemokine expression by intraepithelial gamma delta T cells. Implications for the recruitment of inflammatory cells to damaged epithelia. J Immunol. 1996;157:985–92. [PubMed] [Google Scholar]
- 126.Matsue H, Bergstresser PR, Takashima A. Reciprocal cytokine-mediated cellular interactions in mouse epidermis: promotion of gamma delta T-cell growth by IL-7 and TNF alpha and inhibition of keratinocyte growth by gamma IFN. J Invest Dermatol. 1993;101:543–8. doi: 10.1111/1523-1747.ep12365938. [DOI] [PubMed] [Google Scholar]