Abstract
The impact of the rate of carbohydrate absorption, as measured by the carbohydrate’s glycemic index (GI) on cognitive performance, is not clear. The aim of this review was to systematically assess the relevant research studies. A systematic review of English-language articles using Medline, Cochrane Central Register of Controlled Trials, EMBASE, PsycINFO, and PsycARTICLES (up to July 2012) using the search terms “glyc(a)emic index” or “glycaemic load” combined with “cognitive function” or “cognition” or “memory” was carried out. Inclusion and exclusion criteria were prespecified. Eligibility of the identified studies was assessed independently by the 2 reviewers. Independent extraction of data was carried out by the 2 authors using predefined data fields. The primary outcome measure was the effect on cognitive function (CF) after the consumption of meals varying in GI. Eleven eligible studies were identified. The age range of the participants varied from 6 to 82 y old. Overall, the findings were inconsistent, with some studies showing benefits toward either the high-GI or the low-GI meal, others not finding any differences between the 2 meals, and other studies showing a positive or negative effect on performance on only some cognitive domain or domains after consumption of 1 of the 2 meals. A number of methodologic and confounding factors were identified that could explain these inconsistencies. These include the study design, the selected sample (size, age, blood glucose regulation), the timing of testing, the cognitive domain being examined, the number and type of cognitive tests used, the meals provided (composition, size), the timing of blood samples collected, as well as the possibility of bias because participants and investigators were not blinded to randomization. A low-GI meal may favor CF in adults, but the findings at present are inconclusive. On the basis of this review, it is suggested that future studies address the identified methodologic issues and some recommendations are proposed to this effect.
Introduction
Glucose is the main energy source for the brain and thus essential for its function (1). Human studies have shown that the performance of difficult tasks requiring intensive cognitive resources results in a measurable decline in peripheral blood glucose (BG)4 concentration, which is suggested to be due to increased neural energy expenditure (2–4). In animals, it has been shown that at a high cognitive load, hippocampal glucose demand exceeds supply, whereas exogenous glucose supply enhances performance (5). This is also supported by a number of human studies that have shown that glucose consumption, compared with placebo or breakfast omission, enhances cognitive performance both in healthy participants and in participants with memory deficits and those with poor glucose regulation (6, 7). The optimal glucose dose for enhancing verbal episodic memory, relative to placebo in elderly participants, was found to be 25 g, or a BG concentration of ~8–10 mmol/L (8), whereas in healthy young women the optimal glucose dosage was found to be 300 mg/kg body weight (9).
It is worth noting that the glucose-enhancing effect on memory is more consistent in healthy elderly participants (10) and in patients with Alzheimer disease (11) who present with memory decline (12) than in healthy young participants. In the latter group, glucose reliably facilitates memory when the cognitive demand of the task is high or under conditions of divided attention (13). In addition, it is now well established that poor glucose regulation is a risk factor for impaired cognitive functioning (CF), as shown in patients with diabetes mellitus and in persons with poor glucose regulation. A systematic review on this issue concluded that poor glucose tolerance affects cognitive performance, with some cognitive domains being more sensitive than others (14). The evidence is stronger, although not entirely consistent, for CFs such as verbal memory, working memory, vigilance, and attention, which appear to be the most vulnerable functions in the case of brain impairment and the most susceptible to hyperglycemia and insulin resistance (14). On the contrary, although repeated episodes of hypoglycemia observed in insulin-treated diabetic patients were thought to cause cognitive dysfunction in patients with diabetes (15), this was not supported by the Diabetes Control and Complications Trial (16) or its 18-y follow-up (17). Thus, as concluded by Lamport et al. (14), there still remains to be determined which variables of glucose tolerance are most strongly associated with cognition.
Glucose is rarely consumed as part of the normal diet; instead, it is obtained from carbohydrate-containing foods, which are then broken down to glucose, supplying the brain and other organs with the necessary energy. A number of studies investigated the hypothesis that the rate of glucose release would affect CF by using the established carbohydrate classification of the glycemic index (GI). The GI is a way of classifying carbohydrate-containing foods on the basis of the rate of glucose release by measuring the 2-h postprandial BG concentration after consumption of a portion of food containing 50 g of carbohydrate and comparing it with the 2-h BG concentration after consumption of 50 g of glucose (18). A slowly absorbed carbohydrate-containing food or low-GI (LGI) food (e.g., rye bread) would lead to a slower release of glucose than a quickly absorbed or high-GI (HGI) food (e.g., white or brown bread). It should be noted that, by definition, the GI only provides a measure of carbohydrate quality (19); if both the quality and quantity of carbohydrate need to be considered, the glycemic load (GL) is used, which is defined as GL = (GI × amount of carbohydrate /100) (20).
A previously published review critically evaluated the effect of the diet’s GL on cognitive performance and concluded that the data at the time were insufficient to support an effect of GL on short-term cognitive performance due to the inconsistent findings of the available studies (21). The review, however, included a study in which the meals varied in macronutrient content (22) and a study in which the GL was reduced by manipulating the carbohydrate content of the meal and not the GI (i.e., the carbohydrate quantity but not the quality) (23). The conclusions were thus confounded by these studies. To avoid this, the present review only includes studies that assess the effect of the quality of carbohydrates consumed (i.e., the dietary GI) and their effect, if any, on CF. Establishing if the quality of the carbohydrate influences CF may assist in the provision of nutritional recommendations both for children and adults aiming to enhance their CF. This can have a potential influence on studying, working, and performing everyday activities more efficiently and effectively.
Thus, the aim of the present systematic research review was to ascertain whether the rate of glucose release after consumption of carbohydrate-containing foods, as assessed by the dietary GI, affects CF. We did this by reviewing studies in adults and children that assessed the effect of dietary GI on CF using an objective measure (e.g., a CF test) without setting any limitations on study duration or study design and with the primary outcome being the effect (if any) of changing the dietary GI on CF.
Methods
The methods and the inclusion and exclusion criteria were specified in advance and documented in a protocol as explained below. A literature search was carried out independently by the 2 reviewers (E.P. is a clinical dietitian and M.C. is a clinical neuropsychologist) for short- or long-term studies assessing the effect of food(s) with differing GI on CF using the following search terms: “glyc(a)emic index” or “glycaemic load” combined with “cognitive function” or “cognition” or “memory.” The following search engines were used: Medline (PubMed) (restriction: English language), the Cochrane Central Register of Controlled Trials (CENTRAL), and EMBASE for all years until July 2012 (with the exception of EMBASE, which was searched for the years 1980–July 2012). In addition, PsycINFO and PsycARTICLES (up to July 2012, with the same search terms) were used to search for publications in cognitive psychology and neuropsychology. Relevant studies cited either in review articles or in the articles identified through the search were also considered. The inclusion criteria were as follows: 1) comparisons between LGI and HGI foods/diets, 2) studies in which the food or dietary GI was either estimated or measured, 3) studies conducted in humans of all ages, and 4) studies published in English. The exclusion criteria were as follows: 1) review articles, 2) studies in which the effect of GI on CF was not the main outcome measure, 3) studies in which only GL and not GI was assessed, 4) studies in which water was used as a placebo and there was no comparison between HGI and LGI foods/diets, and 5) studies in which the same carbohydrate food/drink was consumed at different rates to simulate a LGI or HGI response. The primary outcome measure was the effect on CF (as assessed by CF tests) after the consumption of meals varying in GI (i.e., LGI or HGI meal). Eligibility assessment of the identified studies was performed independently by the 2 reviewers in an unblended manner, and any disagreements were resolved by consensus.
Data extraction was carried out by 1 of the reviewers (E.P.), with the exception of “cognitive function tests used” and “cognitive function or domain assessed,” which were extracted from the studies by a second reviewer (M.C.). This procedure was thought to be preferable due to the different specializations of the 2 reviewers (see above). Both reviewers checked all data extracted. Any disagreements were resolved through discussion between the 2 reviewers. One study author was contacted to provide information on GI and GL values and responded promptly. The following data were extracted from each included trial: 1) characteristics of trial participants (number of participants, gender, and age), 2) study design, 3) carbohydrate intervention used, 4) GI and GL of the intervention(s) used (based on a glucose standard), 5) timing of BG sampling, 6) CF tests used, 7) CF or domain assessed, 8) timing of the administered tests, and 9) findings or outcomes and authors’ comments. To ascertain the validity of the eligible trials, the 2 reviewers independently assessed the adequacy of randomization, blinding of participants, and blinding of investigators in all trials.
Results
Study selection
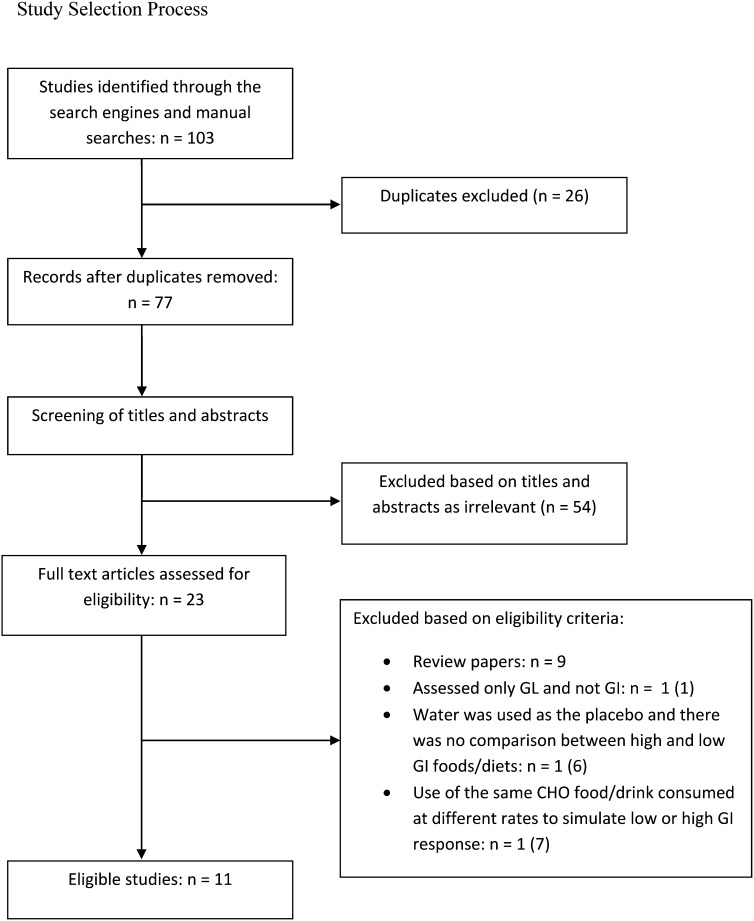
On the basis of the exclusion and inclusion criteria, 11 studies were identified as eligible. In summary, the independent search of the 2 reviewers identified 103 trials, and after adjusting for duplicates, 77 trials remained. Of these, 54 were discarded because after reviewing the titles and abstracts, it appeared that they did not meet the criteria. The full text of the remaining 23 articles was examined more carefully, and it appeared that 12 studies did not meet the inclusion criteria for the reasons given in Fig. 1. The total number of eligible studies was 11. The flow diagram in Fig. 1 shows the study selection process.
FIGURE 1.
Study selection process. CHO, carbohydrate; GI, glycemic index; GL, glycemic load.
Study characteristics
Table 1 shows the data extracted from each study. In summary, the age range of the participants in the included studies varied, with 6 studies including either children (24, 25) or adolescents (26–29), 2 studies including young adults (30, 31), 1 study middle-aged to elderly participants aged 49–71 y (32), and 2 studies only elderly participants (33, 34). All participants were healthy, with the exception of 1 study that included type 2 diabetic participants (34). In all studies, CF was examined in the morning after an overnight fast and the provision by the investigators of a breakfast meal/drink. In the Lamport et al. (31) study, the evening meals rather than the breakfast were manipulated to examine the second meal effect—i.e., whether the GI of the previous meal (in this case, the evening meal) affected CF the following morning both before and after a standardized HGI breakfast. Most studies used a within-subject design with participants acting as their own control (24, 25, 27, 31–34). Three studies used a between-subjects design (26, 28, 30), and in 1 study, participants were categorized into 4 groups on the basis of the GI and GL of their own breakfast consumed on that day (29). Randomization was not concealed in any study. In addition, no study attempted to blind the participants or the investigators with regard to the GI of the meal provided with the exception of the study by Micha et al. (28) in which both participants and investigators were blinded.
TABLE 1.
Eligible studies on the effect of dietary GI on cognitive function1
| Source | Sample | Study design | CHO intervention | GI and/or GL of intervention (based on glucose) | Timing of BG sampling | Cognitive function tests used | Assessed cognitive function/domain | Timing of cognitive function tests | Outcomes/findings and comments | |
| Kaplan et al. (33) | 20 elderly; M = 10; age 60–82 y | Repeated-measures crossover; blinded to placebo and glucose | 50 g CHO provided as glucose drink or instant mashed potatoes (HGI) or barley (LGI) or placebo (sweetened water) | Glucose: 100; potatoes: 83; barley: 25; placebo: 0 | 0, 15, 60, 105 min after breakfast | 1) Self-developed test as word list recall; | 1) Verbal Recall and Learning; | 15, 60, 105 min | No differences in performance were observed. | |
| 2) Wechsler Memory Scale–3rd Edition; | 2) Prose Memory; | Worse performance by individuals with poorer glucose regulation. | ||||||||
| 3) Trail Making Test A & B; | 3) Attention, Mental Flexibility, Graphomotor and Visual Spatial Skills; | Poor β-cell-function individuals were most sensitive to the cognitive-enhancing effects of CHOs. | ||||||||
| 4) self-developed attention test | 4) Sustained Attention | |||||||||
| Micha et al. (29) | 60 children; M = 24; age 11–14 y | Participants were categorized into 4 groups based on GI and GL of their breakfast | Breakfast and snacks eaten on study day categorized as LGI+HGL, HGI+HGL, LGI+LGL, HGI+LGL2 | GI: < or >61; GL: < or >27 | 105, 149 min after breakfast | 1) Word Generation Task; | 1) Verbal Fluency | 90 min | HGI: better short-term memory | |
| 2) Immediate Word Recall; | 2) Immediate Verbal Recall; | HGL: better inductive reasoning | ||||||||
| 3) Strool Test; | 3) Alternating Attention, Selective Attention, & Impulsivity; | LGI and HGL: better vigilance, sustained attention, and inductive reasoning | ||||||||
| 4) Matrices; | 4) Visual Reasoning & Nonverbal Intelligence; | LGI and HGL: associated with the 2 CF tasks that were reported to be most difficult (i.e., mentally demanding) for the participants. | ||||||||
| 5) Number Search; | 5) Visual Attention; | A LGI+HGL breakfast could selectively facilitate mentally demanding CF tasks. GI may be differentially associated with different domains. | ||||||||
| 6) Serial Sevens; | 6) Sustained Attention; | |||||||||
| 7) Delayed Word Recall; | 7) Delayed Verbal Recall; | |||||||||
| 8) Profile of Mood States bipolar form (POMS-BI) | 8) State Psychological Assessment | |||||||||
| Papanikolaou et al. (34) | 21 Type 2 diabetic patients; M = 10; age 65 ± 7.29 y | Within-individual | 50 g CHO: pasta (LGI) or white bread (HGI) or water | LGI: GI, 43; GL, 22 | −5, 15, 62, 100, 138 min after breakfast | 1) Hopkins Verbal Learning Test-Revised; | 1) Verbal Recall; | 15, 62, 100 min (Digit span, Trail Making and Test of Everyday Attention: between 62 and 100 min) | Worse working memory, executive function, and auditory selective attention after HGI than after LGI breakfast. | |
| HGI: GI, 73; GL, 373 | 2) Wechsler Memory Scales (Revised and 3rd Edition); | 2) Prose Memory; | No differences for sustained attention. | |||||||
| 3) Verbal Paired Associates; | 3) Learning and Retrieving Verbal Information | A LGI meal relative to an HGI meal generally resulted in better cognitive performance in the postprandial period in participants, particularly in those with the greatest food-induced elevations in BG. | ||||||||
| 4) Digit Span; | 4) Immediate Recall, Immediate Attention, & Working Memory; | |||||||||
| 5) Test of Everyday Attention | 5) Sustained and Selective Attention | |||||||||
| Ingwersen et al. (24) | 64 children; M = 26; age 6–11 y | Crossover | 35 g of HGI or LGI cereal with milk4 | LGI: 42; HGI: 77; GL: not provided (calculated as LGI: GL, 7; HGI: GL, 23) | Not done | Cognitive Drug Research (CDR) Computerized Assessment Battery | Speed of Attention (reaction times in attending to stimuli); Memory (reaction times in recalling and recognition and immediate and delayed recall); Accuracy of Attention (accuracy of responses); Working Memory | −30, 10, 70, 130 min | A significant decline in accuracy of attention (reflecting ability to sustain attention) 2 h after the HGI cereal compared with the LGI cereal. Better secondary memory performance (reflecting ability to store, hold, and retrieve information) after the LGI than after the HGI meal. No effect of GI on speed of attention, speed of memory, and working memory. | |
| Benton et al. (30) | 106 students; all female; mean age: 21 y, 1 mo | Random allocation to 1 of 2 breakfasts (BS) | Two breakfasts differing in type of CHO (SAG vs. RAG) | Diet 1: GI, 42.3 | 0, 20, 50, 80, 140, 200, and 230 min | Self-developed memory test | Word recall in different time intervals | 30, 90, 150, and 210 min | Recall of more concrete words at 210 min after the LGI meal, but not earlier. | |
| Rat study: not included | Diet 2: GI, 65.9 | Better memory throughout the morning for abstract words after the LGI breakfast. | ||||||||
| Lamport et al. (31) | 14 participants; all male; age 19–28 y | WS; LGI and HGI evening meals and HGI standard breakfast | Evening meals: HGI: white bread, banana, and Lucozade;5 LGI: pasta, pear, and apple juice | Evening meals: HGI: GI, 72; GL, 96; LGI: GI, 47; GL, 63 | Evening meal: 30, 45, 60, 75, 90 min | 1) Visual Verbal Learning Test (VVLT); | 1) Recall of visually presented words; | −15, 30 min | The HGI evening meal was associated with a nonsignificant trend for better verbal recall the following morning and a trend for better word recognition after but not before the breakfast. No differences in attention. Note that the above were non–statistically significant. | |
| HGI breakfast: white bread and Lucozade | Breakfast: GI, 75; GL,106 | 2) Attention Switching Test; | 2) Alternating Attention; | |||||||
| Breakfast: −15, 0, 15, 30, 45, 60, 75 min | 3) Word Recognition Test | 3) Visual Recognition Memory | ||||||||
| Smith et al. (26) | 38 adolescents; M = 18; age 15.6 ± 0.9 y | BS | LGI breakfast: 30 g Kellog's All-Bran with milk | LGI: 30; HGI: 77 | −10, 10, 50, and 90 min | 1) California Verbal Learning; | 1) Verbal Recall; | CVLT: 20, 60, 100 mins | No differences in CVLT, BG concentration or alertness, calmness, contentedness, and satiety. | |
| HGI breakfast: 30 g cornflakes with milk | 2) Bond-Lader Questionnaire | 2) Psychological State | Bond-Lader Scale: −10, 10, 50, 90 min | HGI group remembered significantly more items after the long delay relative to the short delay and remembered significantly more items than the LGI group after the long delay. | ||||||
| Breakfasts differed in composition | The HGI breakfast was associated with reduced forgetting of previously encoded verbal episodic memory materials under conditions of divided attention. May be related to a more rapid supply of glucose to the bloodstream. There were no differences in BG concentrations between the 2 meals. | |||||||||
| Mahoney et al. (25)6 | Expt. 1: 30 students; M = 15; age 9–11 y | WS | Breakfast: oatmeal (LGI) or ready-to-eat (HGI) cereal with milk or no breakfast7 | NA | Not done | 1) Self-developed spatial map test; | 1) Spatial Recall and Learning; | 75 min after start of breakfast; Visual Attention: 75, 95, 125 min | Expt. 1: Better performance on short-term memory task after consuming LGI breakfast compared with HGI breakfast or no breakfast. | |
| Expt. 2: 30 students; M = 15; age 6–8 y | 2) Digit Span Test; | 2) Immediate Recall, Immediate Attention, & Working Memory; | Expt. 2: Better performance on a short-term memory task and an auditory attention task after consuming LGI breakfast than HGI breakfast. | |||||||
| 3) Rey Complex Figure Test (RCFT); | 3) Visual Spatial Perception; | Younger children may be affected more by breakfast composition. | ||||||||
| 4) Continuous Performance Test (CPT); | 4) Visual Attention and Vigilance and Auditory Attention; | Greater benefits in girls than boys from LGI meal, but reason is unclear. | ||||||||
| 5) self-developed prose memory test; | 5) Prose Memory; | |||||||||
| 6) Mini Questionnaire (Likert Scale) | 6) Psychological State | |||||||||
| Nilsson et al. (32) | 40 adults; M = 12; age 49–71 y | WS | HGI: WWB | WWB (ref): GI, 100 | 0, 15, 30, 45, 60, 90, 120, 150 min after a 50-g glucose drink to assess glucoregulation | 1) Reading Sentences (self-developed test); | 1) Working Memory; | Working Memory: 90, 135, 180, 225 min | Selective Attention test: Significantly better performance at 120 min and between 75 and 235 min (late postprandial period) after the LGI than after the HGI meal. | |
| LGI: G-WWB8 | G-WWB: GI, 45 | 2) Picture Test (computerized self-developed test) | 2) Selective Attention and Reaction Time | Selective Attention: 75, 120, 165, 210 min | No significant differences between breakfast in Reaction Time or in the Working Memory tests. | |||||
| Glucose (ref) | Superior performance in participants with better glucoregulation. Superior performance in the Selective Attention test in participants with better than worse glucoregulation after the WWB test. | |||||||||
| WWB; GI, 70 | Comment: Significant improvements after LGI meal predominantly occurred in the late postprandial period. | |||||||||
| G-WWB GI: 32 (own calculations) | ||||||||||
| Cooper et al. (27) | 41 participants; M = 18; age 12–14 y | WS | Provided 1.5 g/kg body mass available CHO; matched for energy, protein, and fat | HGI: GI, 72; GL, 54 | 0, 15, 30, 60, 90, 120 min | 1) Stroop Test; | 1) Alternating Attention, Selective Attention, & mpulsivity; | 30, 120 min | The LGI breakfast enhanced cognitive function in adolescents when compared with both the HGI breakfast and breakfast omission. For all 3 cognitive function tests, the LGI breakfast enhanced both response times and accuracy later in the morning when compared with the HGI breakfast or breakfast omission, particularly on the more cognitively demanding levels of the tests. | |
| HGI: cornflakes, white bread, margarine, milk | LGI; GI, 48; GL, 36 | 2) Sternberg Paradigm; | 2) Working Memory and Information Processing Speed; | Higher BG concentrations enhance response times, but this is to the detriment of accuracy (speed-accuracy trade-off). | ||||||
| LGI: muesli, milk, apple | 3) Flanker Task | 3) Attention and Response Times | ||||||||
| Micha et al. (28) | 74 children; M = 37; age 11–14 y | Matched, random allocation to HGL or LGL group; each group given an HGI and LGI breakfast | Varied amounts of muesli, cornflakes, milk, apple juice and sugar: 1) LGI+HGL, | 1) GI, 48; GL, 41; | 0, 90 min (salivary cortisol also assessed at same times) | 1) Word Generation Task; | 1) Verbal Fluency; | 0, 90 min | LGI meals: Better performance on the Word Generation task | |
| 2) HGI+HGL, | 2) GI, 61; GL, 55; | 2) Immediate Word Recall; | 2) Immediate Verbal Recall; | HGI meals increased cortisol concentration both before and after the CF tests. | ||||||
| 3) LGI+LGL, | 3) GI, 48; GL, 21; | 3) Stroop Test; | 3) Alternating Attention, Selective Attention, & Impulsivity; | HGI meals: Better performance on the Stroop Test (in the high GL meals only), speed of information processing, and Serial Sevens task. | ||||||
| 4) HGI+LGL | 4) GI, 61; GL, 28 | 4) Matrices; | 4) Visual Reasoning & Nonverbal Intelligence; | A LGI+HGL breakfast meal improved learning, possibly through its effects on glucose and cortisol concentrations. | ||||||
| 5) Number Search; | 5) Visual Attention; | HGL and LGI meals decreased fatigue and increase alertness. | ||||||||
| 6) Serial Sevens; | 6) Sustained Attention; | |||||||||
| 7) Delayed Word Recall; | 7) Delayed Verbal Recall; | |||||||||
| 8) Profile of Mood States bipolar form (POMS-BI) | 8) State Psychological Assessment |
BG, blood glucose; BS, between-subject; CF, cognitive functioning; CHO, carbohydrate; CVLT, Modified California Verbal Learning Test; G-WWB, guar gum–enriched white wheat bread; GI, glycemic index; GL, glycemic load; HGI, high glycemic index; HGL, high glycemic load; LGI, low glycemic index; LGL, low glycemic load; NA, not available; RAG, rapidly available glucose; SAG, slowly available glucose; ST, short-term; WS, within-subject; WWB, white wheat bread.
LGI+HGL was 32% higher in energy than the HGI+HGL breakfast.
GI and GL values were obtained through personal communication with Yianni Papanikolaou, Kunin-Lunenfeld Applied Research Unit, Baycrest, Toronto, Canada.
Differed in energy, protein, CHO, fat, and fiber.
Lucozade (Suntory Beverage and Food) is an energy drink containing glucose.
GI was not the primary aim of the study.
Differed in nutrient composition (CHO, fiber, and protein).
The aim of G-WWB was low, sustained BG for 240 min postconsumption.
CF tests
In all studies, the assessment of CF was carried out by using a battery of tests, ranging from 2 to 8 tests, with the exception of Benton et al. (30), in which only 1 test, namely word recall, was used. The utilized CF tests were in some studies published and well-known instruments, such as the Wechsler Memory Scale-IV (35), Trail Making Test A & B (36), and Hopkins Verbal Learning Test (37). In a number of studies, however, instruments developed by the investigators were used that assessed many neuropsychological/cognitive functions or domains such as memory (verbal recall, prose memory, working memory, visual recognition memory), attention (sustained and selective attention, speed of attention, visual attention), learning (learning and retrieving verbal information, information processing skill), reasoning and intelligence (mental flexibility, verbal fluency, visual reasoning, graphomotor and visual spatial skills, nonverbal intelligence), and psychopathology. There was no consistency among studies in the timing of the administration of tests, namely the timing between food intake and cognitive assessment, although in most studies CF was assessed at least 3 times starting from before breakfast consumption to ≤210 min postprandially. The exceptions were Micha et al. (29), in which CF was tested only once (at 90 min), and 3 other studies (27, 28, 31), in which tests were administered only twice.
Meal interventions
In all studies, except in Micha et al. (29) and Lamport et al. (31), a standardized LGI or HGI breakfast meal or drink were provided. In the Micha et al. study (29), participants consumed their usual breakfast and were categorized on the basis of its GI and GL into 4 groups, whereas in the study by Lamport et al. (31), instead of manipulating the breakfast’s GI, there was a manipulation of the GI of the evening meal, and all participants were provided with an HGI breakfast. In the studies by Mahoney et al. (25) and Papanikolaou et al. (34), in addition to examining the influence of dietary GI, cognition was also assessed in the fasted state.
The nutritional composition of the meals was not reported in 3 studies (29, 31, 32), whereas in most of the remaining studies, the composition of the meals provided to the 2 groups was not matched, mainly because of differences in the amounts of fiber and protein between the meals. The exception was the study by Cooper et al. (27), in which the HGI and LGI meals both contained 1.5 g/kg body mass available carbohydrate and were matched for energy, protein, and fat content.
In most studies, GI values were calculated by using the international GI tables (38) with additional values based on U.K. products (39) used by some U.K. investigators (28, 31). Papanikolaou et al. (34) based their GI and GL values on the average given by the University of Sydney website (40) (Yianni Papanikolaou, Kunin-Lunenfeld Applied Research Unit, Baycrest, Toronto, Canada; personal communication). Benton et al. (30) calculated the dietary GI on the basis of the amount of rapidly and slowly available glucose as suggested by Englyst et al. (41). In the study by Nilsson et al. (32), the bread products used were developed as part of the research protocol and their GI was assessed and compared with that of white bread in healthy young participants. The GI values of the LGI interventions ranged from 25 to <61 and those of the HGI interventions from >61 to 83. The main findings and outcomes of the studies are summarized in Table 1.
Studies in children
The findings of the reviewed studies were not consistent across all studies with regard to the effects of meals on CF. In Micha et al. (29), the LGI + high GL (HGL) breakfast was associated with better performance in the 2 most mentally demanding assessments of CF, namely speed of information processing and the Serial Sevens task, the HGI breakfast was associated with better short-term memory and the HGL breakfast with better inductive reasoning, leading the authors to conclude that GI “may be differentially associated with different domains.” In a study by the same investigators on similar-aged children (28), LGI meals resulted in a better performance on the Word Generation task, but in contrast to their previous findings the authors found that HGI meals led to better performance on the speed of information processing and the Serial Sevens task, whereas the LGI, HGL meal improved learning (28).
In the Ingwersen et al. study (24), the results were also mixed because there was a significant decline in attention 2 h after consumption of HGI cereal and better verbal recall after the LGI than after the HGI meal. However, the breakfast GI had no effect on cognitive processing speed in measures of attention, memory, and working memory. Similarly, in the Mahoney et al. (25) study, there was improved performance in a short-term memory task and a verbal auditory attention task after the LGI compared with the HGI breakfast, whereas for unclear reasons girls benefited more from the LGI meal than did boys. The findings of Cooper et al. (27) also favored the LGI meal because it appeared to enhance CF via the improvement of both response times and accuracy in cognitive tasks, in comparison to the HGI meal and breakfast omission. In contrast, Smith et al. (26) found that the HGI group recalled significantly more after a long delay compared with the LGI group and the HGI breakfast was associated with reduced memory decay of previously learned materials under conditions of divided attention.
Studies in adults
Kaplan et al. (33) found no differences between meals of different GI in performance in elderly adults. Lamport et al. (31), who studied whether the GI of the evening meal had any effects on CF the following morning, also found no statistically significant differences between the HGI and LGI evening meals, although they observed a statistical trend favoring the HGI evening meal. On the other hand, the results by Benton et al. (30), who studied young women, favored the LGI meals because verbal recall of abstract words appeared to improve throughout the morning after the LGI breakfast, whereas more concrete words could be recalled in the late postprandial phase (210 min) after consumption of the LGI meal. Nilsson et al. (42) also showed that performance was better in the late postprandial period after consumption of a LGI than an HGI meal but only in selective attention. In this study, no significant differences were observed in cognitive reaction time or working memory. Last, in the study by Papanikolaou et al. (34), which was the only one performed in adults diagnosed with type 2 diabetes (as opposed to healthy participants in the other studies), the results also favored the LGI breakfast because general CF was better in the postprandial period after the LGI than after the HGI meal.
Discussion
Overall, the findings of the studies both in children and in adults were inconsistent, with some showing benefits toward either the HGI or the LGI meal, others not finding any differences between the 2 meals, and yet others showing a positive or negative effect on performance on only 1 or some cognitive domain(s) after the consumption of 1 of the 2 meals. A number of possible reasons might explain the inconsistencies between the studies’ findings and the differences observed between adults and children, and these are discussed below. First, it should be noted that, on average, there were ~37 participants per study, which signifies a low power in most, if not all, studies. Also, there was a wide demographic variation between the populations studied (e.g., in age, gender, health status, etc.). In addition, there were other variations with regard to the meals provided, the timing of testing, and the blinding of participants and investigators, which are reported below.
With regard to age, it is known that children are more susceptible to glucose provision than adults because, per gram of weight, a child’s brain tissue uses more glucose than that of an adult and, compared with adults, children’s brains are relatively bigger and more active per unit weight, which may make them more responsive to glucose provision (43). This may partly explain why the findings of the studies in children and adults differed because if, for children, the amount rather than the type of glucose is more important, then the cognitive domains that require a quick release of glucose would be more likely to benefit from HGI (and/or HGL) meals, whereas if testing was carried out at a late postprandial period, then the LGI meal (i.e., slowly released meal) and the HGL meal (i.e., the meal containing the most glucose) would most likely be more beneficial because these meals would provide the brain with the most available glucose. This points to the necessity of a better understanding of the impact of carbohydrate manipulation on different cognitive domains because it is clear that they are not all affected in the same way. One way to achieve this would be to concentrate more on testing 1 specific cognitive domain by using different cognitive instruments rather than testing many domains in the same study. Such an approach could decipher the most sensitive cognitive assessment instruments for assessing the impact of the quality of the consumed carbohydrate.
The inconsistencies in the findings might also be due to the differences in meal composition. For example, in Micha et al. (29), the energy content of the meals was significantly different, with the LGI + HGL breakfast having the highest energy content of all meals—on average, double that of the HGI + low GL (LGL) breakfast (LGI + HGL vs. HGI + LGL: 502 vs. 240 kcal). It is not known, however, whether the difference in energy content of the LGI and HGI meals with an HGL was significant. In the other study conducted by the same investigators in similar-aged children, the HGL meals again had a higher energy content than the rest of the meals (mainly because of differences in carbohydrate, protein, and fat content) and the energy content of meals also differed substantially (LGI + HGL vs. HGI + LGI: 470 vs. 276 kcal) (28). Thus, as the authors themselves pointed out, the differences in GL cannot be differentiated from the differences in energy and macronutrient content. However, at least for these 2 studies, within the same GL group the energy and macronutrient composition of HGI and LGI meals was similar, and thus any GI effects could be differentiated from energy and macronutrient content differences. In any case, there needs to be an account in all studies of not only the GI but also the GL of the meal as well as the meal’s energy and macronutrient content (29).
Another possible factor that might have affected the results may be the individual differences between the participants’ usual breakfast GI (and GL). At present, it is not clear if the consumption of meals under experimental conditions can have a different impact on individuals when the experimental meal is of a similar GI to their usual one as opposed to a meal with a higher or a lower GI than the one they regularly consume. The effect of breakfast consumption per se, irrespective of GI, on the CF of individuals who usually skip this meal is also not known. These variables are important and should be taken into consideration in future studies, given that meal composition may have an impact on factors such as fullness, bloating, satiety, meal rating, or even mood, which subsequently affects motivation and arousal and thus test performance (29). A different experimental approach could have been to test (in the same participants) meals with a similar GI, GL, and total energy content as a way to determine the extent by which the above confounders (e.g., fullness) contribute to the inconsistency in the findings. In addition to the previous factors, the timing of testing is equally crucial and could also explain some of the inconsistencies between studies because timing in the reported studies varied.
With regard to the adult studies, there are additional reasons that might have contributed to the observed inconsistencies. The first is the variation in the participants’ age; the second is the participants’ BG regulation and the interaction with age. Cognition (44) and perhaps glucose tolerance (14) are affected by age. As shown in a review by Lamport et al. (14), individuals with poor glucose tolerance benefit more from glucose consumption than those with better glucoregulation. Moreover, in studies in older adults, glucose is more beneficial irrespective of their glucose tolerance, but older, better glucoregulators are more likely to benefit from glucose consumption than younger ones (14, 45, 46). On the other hand, in individuals with good cognition and good glucose tolerance, glucose consumption could hamper performance (33). Irrespective of age, however, the cognitive benefits of glucose consumption increase with worsening glucose tolerance (14). These factors are important because in the studies included in the present review, the age range was very wide (19–82 y old); and although in all but 1 study (34) the participants were healthy, glucose tolerance was not necessarily assessed before enrollment. For example, in the study that included elderly participants aged 60–82 y, evidence of diabetes (fasting plasma glucose ≥7.0 mmol/L) was used as an exclusion criterion, but there was no reference that participants with glucose intolerance or insulin resistance were excluded (33). Similarly, Lamport et al. (31) excluded diabetes, but there is no mention of assessing glucose tolerance. Even in the study in type 2 diabetic participants, there seems to be a wide variation in glucose tolerance because some of the participants were taking oral hypoglycamic agents (2 different categories) and some were controlled by diet alone (34). Last, Benton et al. (30) did not refer to glucose assessment at all as part of the inclusion/exclusion criteria, whereas the only study that refers to only including participants with normal fasting glucose was that of Nilsson et al. (32). Although the question being asked in this review is how the rate of glucose release rather than glucose provision itself affects CF, there were clearly many differences between participants in terms of their glucose tolerance that might in turn have affected their CF.
In addition to these limitations, this review is limited in that there was much interstudy methodologic variability in the included studies, which also complicates the extraction of conclusions (e.g., use of between- or within-subjects design, the timing of testing, the cognitive domain being examined, the number and type of cognitive tests used, the meals provided, as well as the timing of blood samples collected). In addition, studies, with the exception of Lamport et al. (31), did not control for the previous evening’s meal, known to affect the glycemic response the next morning (47, 48). Moreover, none of the studies referred to restricting physical activity the previous day, which is important because exercise increases glucose muscle uptake (49) and again could influence the glycemic response to the meals. Another limitation of all of the studies, except for Micha et al. (28), is that there was no blinding of the participants and the investigators to the meal GI. Furthermore, randomization was not concealed in any of the included studies. Although due to the nature of the intervention in nutrition studies (i.e., meals provided), concealment and blinding may not always be possible, and this is a source of bias. Thus, it is recommended that in future studies involving dietary GI, both the participants and the investigators are blinded, if the type of meal allows, and standard conditions are adhered to particularly on the day before the test day, ensuring that the type and timing of the evening meal consumed is the same and exercise and alcohol consumption are avoided (50).
Possible mechanisms explaining the effect of dietary GI on cognitive function
Even though the findings of the studies were inconsistent, there are many possible mechanisms that might explain why altering the dietary GI may affect cognition, although these are thought to be complex and have not been fully elucidated. A review of the relation of cognitive performance with postprandial metabolic changes after ingestion of different macronutrients referred to 3 targets with direct or indirect influences on CF, as follows: energy supply to nerve cells, neurotransmitter and hormone modulations, and activation or deactivation of the nervous system (51). As is known, the brain is very sensitive to changes in nutrient supply; nevertheless, it has been suggested that not the amount of glucose but the BG concentration after glucose delivery is the most relevant factor in determining the glucose memory-enhancement effect (8, 13). Thus, a potential mechanism in favor of the LGI as opposed to the HGI meal could be the more constant postprandial BG concentration associated with the ingestion of the first type of meal. On the other hand, consumption of an HGI meal leads to a rapid increase in plasma glucose concentration followed by a concomitant high insulin response, resulting in a rapid BG disposal, which may cause the BG concentration to decrease to below the fasting concentration in the later postprandial period (52). In support of this are the findings of studies showing that the changes in BG concentration rather than the absolute concentrations are critical for a modulation of CF (53). Moreover, studies examining the effect of GI on CF have shown that significant improvements occur in the late (rather than early) postprandial phase (30, 32), presumably because of the more stable glucose (and insulin) profile resulting from consumption of the LGI meal.
With regard to hormone modulation, a hormone that might affect CF both through short- but also longer-term mechanisms is insulin. It has already been proven that the more stable postprandial glucose profile characteristic of a LGI meal is beneficial to whole-body insulin sensitivity (54). It is also known that the brain contains insulin receptors with important roles in CF that are affected by insulin resistance (55). As mentioned above, individuals with type 2 diabetes but also those with glucose regulation abnormalities have a higher risk of Alzheimer disease and cognitive dysfunction (14). It is possible that a LGI diet results in better CF in the long term through improvements in insulin sensitivity. In support of this hypothesis is the finding that a LGI diet has been shown to improve glucose tolerance and reduce glycated hemoglobin (HbA1c) and fructosamine concentrations in diabetic patients (56). These are important findings with regard to cognition because, among others, both hypo- and hyperglycemia as well as insulin resistance and poor glucose regulation are implicated as possible mechanisms in cognitive dysfunction (15). Moreover, in patients with type 1 diabetes, better glycemic control was related to improved cognitive performance as shown in the 18-y follow-up of the Diabetes Control and Complications Trial in which the performance on CF tests of those patients with a time-weighted mean HbA1c of <7.4% was better than in those with an HbA1c of 8.8% (17). Thus, the diet’s GI could potentially influence CF both in the short term through the variation in the rate of glucose release and in the long term through its effects on the mechanisms linking glucose regulation and cognition.
Another hormone that has been explored as a potential mechanism linking GI and cognition is cortisol, and its role has been investigated in a recent study (28). In theory, a difference between GI and GL should not have affected cortisol concentration, but it was found that an HGI meal resulted in higher cortisol concentrations both before and after the CF tests, suggesting that LGI meals may be associated with a reduced response to stressful stimuli (28). It was argued that the lower BG concentration after consumption of a LGI meal could result in lower activation of the hypothalamic-pituitary-adrenal axis and thus lower cortisol concentrations. The outcome was that the participants felt less stressed or nervous before carrying out the CF tests, which ultimately improved their performance on memory tests. On the other hand, the HGI meal caused an increase in cortisol concentration, and the resulting nervousness is thought to have led to a better performance on vigilance tasks (i.e., how quickly the participants could process information). It would be interesting to further explore the role of this hormone: for example, in a recent study, greater cortisol responses during a test were related to enhanced memory in children 2 wk later (57). The contradictory findings might be due to the CF being assessed and the type of tests used to assess it. For example, in the study by Cooper et al. (27), the higher BG concentrations after the ingestion of an HGI meal enhanced response times, but resulted in a detrimental effect on accuracy, thus as explained by the authors, possibly causing a speed-accuracy trade-off. If stress hormones were indeed involved in this study, the findings might be explained by the large body of evidence showing that stress enhances memory for information that is directly related to the cause of the stress at the expense of memory for unrelated, peripheral details (58). It is clear that further studies are needed to shed more light on stress hormones as a possible mechanism with regard to dietary GI and cognition.
Conclusions
The results of the reviewed studies assessing the effect of dietary GI on CF were inconsistent. The inconsistencies could be due to the small number of reviewed eligible studies and the many methodologic differences between studies (e.g., type of design, meal composition, types and timing of CF tests) as well as the many confounding factors (e.g., age, glucose tolerance), which did not allow for a larger consensus in the findings. Future studies should aim to use more consistent methodologies and try to eliminate all potential aforementioned confounders, with the most important being meal composition, type and timing of CF tests, and participants studied. There should also be an attempt to identify specific mechanisms that might link dietary GI and cognition. In this way, it would be possible to both compare the findings of different studies but also understand how CF is (if at all significantly) affected by the manipulation of dietary carbohydrates. Ultimately, this will allow for the setting of recommendations on this issue and potentially the development of food products aiming to enhance CF. In addition, the authors believe that it is imperative that future studies use some of the following recommendations when designing a study of the effect of dietary GI on CF:
1. Use cognitive/neuropsychological instruments that are standardized and normed in the studied population so that the conclusions drawn are more confident and reliable as well as easily comparable to the greater population.
2. Use cognitive/neuropsychological instruments with high specificity and sensitivity that are known to detect even small, but significant for everyday functioning, variations in cognition. For example, the Trail Making Test, particularly the second part of the test (B) is very sensitive to even small declines in cognition. Another example is a short battery of cognitive tests (which includes a variation of the Trail Making Test) called MoCA (Montreal Cognitive Assessment) (59), which is used for detecting small cognitive declines in adults.
3. Concentrate on testing 1 specific domain by using different appropriate (see above) instruments rather than assessing many domains with the aim of assessing the effect of dietary GI on the particular domain.
4. Ensure that the composition of the HGI and LGI meals used for testing is as closely matched as possible for energy, macronutrients, and fiber to reduce the effect of confounding.
5. Ensure that standardized conditions are adhered to on the day before the test day. In particular, the participants should not engage in heavy exercise, should not consume alcohol, and should consume the same evening meal before each of the test days if the study has a within-subject design.
6. Ensure that other potentially confounding factors such as glucose tolerance and medication that affects glucose regulation are taken into account as part of the inclusion/exclusion criteria and that these are sufficiently assessed. Aim for, as far as possible, a homogeneous population.
7. Take into account the participants’ usual breakfast composition and size because factors such as fullness, bloating, satiety, and meal rating, as well as whether the participants usually consume breakfast, might be confounding to the study’s findings.
Last, the authors suggest that the involved disciplines introduce some common guidelines, perhaps at an international conference, to assess and develop a consistent methodology for researching this vastly important area.
Acknowledgments
We would like to thank Dr. Yianni Papanikolaou, Kunin-Lunenfeld Applied Research Unit, Baycrest, Toronto, Canada for providing us with the GI and GL values of the meals used in the study they performed following personal communication. Both authors read and approved the final version of the manuscript.
Footnotes
Abbreviations used: BG, blood glucose; CF, cognitive function(ing); GI, glycemic index; GL, glycemic load; HbA1c, glycated hemoglobin; HGI, high glycemic index; HGL, high glycemic load; LGI, low glycemic index; LGL, low glycemic load.
Literature Cited
- 1.Amiel SA. Nutrition of the brain: macronutrient supply. Proc Nutr Soc. 1994;53:401–5 [DOI] [PubMed] [Google Scholar]
- 2.Donohoe RT, Benton D. Cognitive functioning is susceptible to the level of blood glucose. Psychopharmacology (Berl). 1999;145:378–85 [DOI] [PubMed] [Google Scholar]
- 3.Reivich M, Alavi A. Positron emission tomographic studies of local cerebral glucose metabolism in humans in physiological and pathophysiological conditions. Adv Metab Disord. 1983;10:135–76 [DOI] [PubMed] [Google Scholar]
- 4.Scholey AB, Laing S, Kennedy DO. Blood glucose changes and memory: effects of manipulating emotionality and mental effort. Biol Psychol. 2006;71:12–9 [DOI] [PubMed] [Google Scholar]
- 5.McNay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proc Natl Acad Sci USA. 2000;97:2881–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korol DL, Gold PE. Glucose, memory, and aging. Am J Clin Nutr. 1998;67 Suppl:764S–71S [DOI] [PubMed] [Google Scholar]
- 7.Smith A, Kendrick A, Maben A, Salmon J. Effects of breakfast and caffeine on cognitive performance, mood and cardiovascular functioning. Appetite. 1994;22:39–55 [DOI] [PubMed] [Google Scholar]
- 8.Parsons MW, Gold PE. Glucose enhancement of memory in elderly humans: an inverted-U dose-response curve. Neurobiol Aging. 1992;13:401–4 [DOI] [PubMed] [Google Scholar]
- 9.Messier C, Pierre J, Desrochers A, Gravel M. Dose-dependent action of glucose on memory processes in women: effect on serial position and recall priority. Brain Res Cogn Brain Res. 1998;7:221–33 [DOI] [PubMed] [Google Scholar]
- 10.Manning CA, Parsons MW, Cotter EM, Gold PE. Glucose effects on declarative and non-declarative memory in healthy elderly and young adults. Psychobiology. 1997;25:103–8 [Google Scholar]
- 11.Manning CA, Ragozzino ME, Gold PE. Glucose enhancement of memory in patients with probable senile dementia of the Alzheimer's type. Neurobiol Aging. 1993;14:523–8 [DOI] [PubMed] [Google Scholar]
- 12.Meneilly GS, Dawson K, Tessier D. Alterations in glucose metabolism in the elderly patient with diabetes. Diabetes Care. 1993;16:1241–8 [DOI] [PubMed] [Google Scholar]
- 13.Smith MA, Riby LM, van Eekelen AM, Foster JK. Glucose enhancement of human memory: a comprehensive research review of the glucose memory facilitation effect. Neurosci Biobehav Rev. 2011;35:770–83 [DOI] [PubMed] [Google Scholar]
- 14.Lamport DJ, Lawton CL, Mansfield MW, Dye L. Impairments in glucose tolerance can have a negative impact on cognitive function: a systematic research review. Neurosci Biobehav Rev. 2009;33:394–413 [DOI] [PubMed] [Google Scholar]
- 15.Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev. 2008;29:494–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin EJ, Deary IJ. Effects of repeated hypoglycemia on cognitive function: a psychometrically validated reanalysis of the Diabetes Control and Complications Trial data. Diabetes Care. 1999;22:1273–7 [DOI] [PubMed] [Google Scholar]
- 17.Jacobson AM, Musen G, Ryan CM, Silvers N, Cleary P, Waberski B, Burwood A, Weinger K, Bayless M, Dahms W, Harth J. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med. 2007;356:1842–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362–6 [DOI] [PubMed] [Google Scholar]
- 19.Wolever TM, Vorster HH, Bjorck I, Brand-Miller J, Brighenti F, Mann JI, Ramdath DD, Granfeldt Y, Holt S, Perry TL, Venter C, Xiaomei Wu. Determination of the glycaemic index of foods: interlaboratory study. Eur J Clin Nutr. 2003;57:475–82 [DOI] [PubMed] [Google Scholar]
- 20.Salmerón J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA. 1997;277:472–7 [DOI] [PubMed] [Google Scholar]
- 21.Gilsenan MB, de Bruin EA, Dye L. The influence of carbohydrate on cognitive performance: a critical evaluation from the perspective of glycaemic load. Br J Nutr. 2009;101:941–9 [DOI] [PubMed] [Google Scholar]
- 22.Nabb S, Benton D. The influence on cognition of the interaction between the macro-nutrient content of breakfast and glucose tolerance. Physiol Behav. 2006;87:16–23 [DOI] [PubMed] [Google Scholar]
- 23.Benton D, Maconie A, Williams C. The influence of the glycaemic load of breakfast on the behaviour of children in school. Physiol Behav. 2007;92:717–24 [DOI] [PubMed] [Google Scholar]
- 24.Ingwersen J, Defeyter MA, Kennedy DO, Wesnes KA, Scholey AB. A low glycaemic index breakfast cereal preferentially prevents children's cognitive performance from declining throughout the morning. Appetite. 2007;49:240–4 [DOI] [PubMed] [Google Scholar]
- 25.Mahoney CR, Taylor HA, Kanarek RB, Samuel P. Effect of breakfast composition on cognitive processes in elementary school children. Physiol Behav. 2005;85:635–45 [DOI] [PubMed] [Google Scholar]
- 26.Smith MA, Foster JK. The impact of a high versus a low glycaemic index breakfast cereal meal on verbal episodic memory in healthy adolescents. Nutr Neurosci. 2008;11:219–27 [DOI] [PubMed] [Google Scholar]
- 27.Cooper SB, Bandelow S, Nute ML, Morris JG, Nevill ME. Breakfast glycaemic index and cognitive function in adolescent school children. Br J Nutr. 2012;107:1823–32 [DOI] [PubMed] [Google Scholar]
- 28.Micha R, Rogers PJ, Nelson M. Glycaemic index and glycaemic load of breakfast predict cognitive function and mood in school children: a randomised controlled trial. Br J Nutr. 2011;106:1552–61 [DOI] [PubMed] [Google Scholar]
- 29.Micha R, Rogers PJ, Nelson M. The glycaemic potency of breakfast and cognitive function in school children. Eur J Clin Nutr. 2010;64:948–57 [DOI] [PubMed] [Google Scholar]
- 30.Benton D, Ruffin M-P, Lassel TNS, Lassel T, Nabb S, Messaoudi M, Vinoy S, Desor D, Lang V. The delivery rate of dietary carbohydrates affects cognitive performance in both rats and humans. Psychopharmacology (Berl). 2003;166:86–90 [DOI] [PubMed] [Google Scholar]
- 31.Lamport DJ, Hoyle E, Lawton CL, Mansfield MW, Dye L. Evidence for a second meal cognitive effect: glycaemic responses to high and low glycaemic index evening meals are associated with cognition the following morning. Nutr Neurosci. 2011;14:66–71 [DOI] [PubMed] [Google Scholar]
- 32.Nilsson A, Radeborg K, Bjorck I. Effects on cognitive performance of modulating the postprandial blood glucose profile at breakfast. Eur J Clin Nutr. 2012;66:1039–43 [DOI] [PubMed] [Google Scholar]
- 33.Kaplan RJ, Greenwood CE, Winocur G, Wolever TM. Cognitive performance is associated with glucose regulation in healthy elderly persons and can be enhanced with glucose and dietary carbohydrates. Am J Clin Nutr. 2000;72:825–36 [DOI] [PubMed] [Google Scholar]
- 34.Papanikolaou Y, Palmer H, Binns MA, Jenkins DJ, Greenwood CE. Better cognitive performance following a low-glycaemic-index compared with a high-glycaemic-index carbohydrate meal in adults with type 2 diabetes. Diabetologia. 2006;49:855–62 [DOI] [PubMed] [Google Scholar]
- 35.Wechsler D. Wechsler Memory Scale. 4th ed. San Antonio (TX): Pearson; 2009. [Google Scholar]
- 36.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19:203–14 [DOI] [PubMed] [Google Scholar]
- 37.Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test-Revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12:43–55 [Google Scholar]
- 38.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002;76:5–56 [DOI] [PubMed] [Google Scholar]
- 39.Henry CJ, Lightowler HJ, Strik CM, Renton H, Hails S. Glycaemic index and glycaemic load values of commercially available products in the UK. Br J Nutr. 2005;94:922–30 [DOI] [PubMed] [Google Scholar]
- 40.The University of Sydney. Glycemic index website. 2013. Available from: www.glycemicindex.com. Accessed 15 March 2011.
- 41.Englyst KN, Vinoy S, Englyst HN, Lang V. Glycaemic index of cereal products explained by their content of rapidly and slowly available glucose. Br J Nutr. 2003;89:329–40 [DOI] [PubMed] [Google Scholar]
- 42.Nilsson A, Radeborg K, Bjorck I. Effects of differences in postprandial glycaemia on cognitive functions in healthy middle-aged subjects. Eur J Clin Nutr. 2009;63:113–20 [DOI] [PubMed] [Google Scholar]
- 43.Chugani HT. A critical period of brain development: studies of cerebral glucose utilization with PET. Prev Med. 1998;27:184–8 [DOI] [PubMed] [Google Scholar]
- 44.Brayne C, Calloway P. Normal ageing, impaired cognitive function, and senile dementia of the Alzheimer's type: a continuum. Lancet. 1988;1:1265–7 [DOI] [PubMed] [Google Scholar]
- 45.Messier C, Tsiakas M, Gagnon M, Desrochers A, Awad N. Effect of age and glucoregulation on cognitive performance. Neurobiol Aging. 2003;24:985–1003 [DOI] [PubMed] [Google Scholar]
- 46.Awad N, Gagnon M, Desrochers A, Tsiakas M, Messier C. Impact of peripheral glucoregulation on memory. Behav Neurosci. 2002;116:691–702 [DOI] [PubMed] [Google Scholar]
- 47.Wolever TM, Jenkins DJ, Ocana AM, Rao VA, Collier GR. Second-meal effect: low-glycemic-index foods eaten at dinner improve subsequent breakfast glycemic response. Am J Clin Nutr. 1988;48:1041–7 [DOI] [PubMed] [Google Scholar]
- 48.Granfeldt Y, Wu X, Bjorck I. Determination of glycaemic index; some methodological aspects related to the analysis of carbohydrate load and characteristics of the previous evening meal. Eur J Clin Nutr. 2006. 60:104–12 [DOI] [PubMed] [Google Scholar]
- 49.Malkova D, Evans RD, Frayn KN, Humphreys SM, Jones PR, Hardman AE. Prior exercise and postprandial substrate extraction across the human leg. Am J Physiol Endocrinol Metab. 2000;279:E1020–8 [DOI] [PubMed] [Google Scholar]
- 50.Brouns F, Bjorck I, Frayn KN, Gibbs AL, Lang V, Slama G, Wolever TM. Glycaemic index methodology. Nutr Res Rev. 2005;18:145–71 [DOI] [PubMed] [Google Scholar]
- 51.Fischer K, Colombani PC, Langhans W, Wenk C. Cognitive performance and its relationship with postprandial metabolic changes after ingestion of different macronutrients in the morning. Br J Nutr. 2001;85:393–405 [DOI] [PubMed] [Google Scholar]
- 52.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287:2414–23 [DOI] [PubMed] [Google Scholar]
- 53.Owens DS, Benton D. The impact of raising blood glucose on reaction times. Neuropsychobiology. 1994;30:106–13 [DOI] [PubMed] [Google Scholar]
- 54.Rizkalla SW, Taghrid L, Laromiguiere M, Huet D, Boillot J, Rigoir A, Elgrably F, Slama G. Improved plasma glucose control, whole-body glucose utilization, and lipid profile on a low-glycemic index diet in type 2 diabetic men: a randomized controlled trial. Diabetes Care. 2004;27:1866–72 [DOI] [PubMed] [Google Scholar]
- 55.Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol Ther. 2012;136:82–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2003;26:2261–7 [DOI] [PubMed] [Google Scholar]
- 57.Quas JA, Yim IS, Edelstein RS, Cahill L, Rush EB. The role of cortisol reactivity in children's and adults’ memory of a prior stressful experience. Dev Psychobiol. 2011;53:166–74 [DOI] [PubMed] [Google Scholar]
- 58.Deffenbacher KA, Bornstein BH, Penrod SD, McGorty EK. A meta-analytic review of the effects of high stress on eyewitness memory. Law Hum Behav. 2004;28:687–706 [DOI] [PubMed] [Google Scholar]
- 59.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9 [DOI] [PubMed] [Google Scholar]