Abstract
More than one-half of young adults aged 18–24 y have at least 1 coronary heart disease (CHD) risk factor and nearly one-quarter have advanced atherosclerotic lesions. The extent of atherosclerosis is directly correlated with the number of risk factors. Unhealthy dietary choices made by this age group contribute to weight gain and dyslipidemia. Risk factor profiles in young adulthood strongly predict long-term CHD risk. Early detection is critical to identify individuals at risk and to promote lifestyle changes before disease progression occurs. Despite the presence of risk factors and pathological changes, risk assessment and disease prevention efforts are lacking in this age group. Most young adults are not screened and are unaware of their risk. This review provides pathological evidence along with current risk factor prevalence data to demonstrate the need for early detection. Eighty percent of heart disease is preventable through diet and lifestyle, and young adults are ideal targets for prevention efforts because they are in the process of establishing lifestyle habits, which track forward into adulthood. This review aims to establish the need for increased screening, risk assessment, education, and management in young adults. These essential screening efforts should include the assessment of all CHD risk factors and lifestyle habits (diet, exercise, and smoking), blood pressure, glucose, and body mass index in addition to the traditional lipid panel for effective long-term risk reduction.
Introduction
Coronary heart disease (CHD)5 risk in young adults aged 18–24 y is underestimated despite the high prevalence of CHD risk factors (1–4) and early signs of atherosclerosis in this age group (5, 6). Obesity has more than doubled in children and more than tripled in adolescents over the past 30 y (7). This weight gain tracks forward and worsens in young adulthood (8). Heart disease risk increases by 2–4% for each year a young adult is obese (9). As many as 33% of young adults are overweight (1), and this excess weight leads to dyslipidemia (10) and increases in metabolic syndrome (11), diabetes (12), and CHD (3) risk. CHD accounts for 50% of cardiovascular disease (CVD) deaths and is 1 of the leading causes of death in young adults (13). CHD costs the United States $108.9 billion each year in health care services, medications, and lost productivity (14), which is more than for any other disease. A death occurs from CVD every 40 s in the United States, which would wipe out a college campus of 25,000 in <12 d (15).
More than half of young adults have at least 1 CHD risk factor, and this greatly increases lifetime heart disease risk (16). Because many CHD risk factors surface in adolescence (13, 17–19) and track forward to adulthood (20), the AHA’s 2020 Strategic Impact Goals along with the National Heart, Lung, and Blood Institute’s (NHLBI’s) 2012 Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents (21) emphasize primordial prevention beginning in childhood and adolescence (16). This concept of primordial prevention was introduced by Strasser (22) in 1978 and focuses on preventing the development of risk factors themselves (16). Dietary modifications are central to this approach (16).
Despite screening recommendations for all adults aged >20 y (23, 24), <50% of women and <40% of men of this age are screened for CHD risk (25). In addition, the majority of young adults are unaware of their risk (26). Until primordial prevention strategies are implemented to avoid risk factor development in the first place, there is a need for improved screening, risk assessment, management, and education in this age group. Early detection and intervention are critical because 80% of CVD events are preventable through diet and lifestyle (27). Diets low in saturated fat and high in fruits and vegetables reduce the risk of new cardiac events by 73% (28). Despite this evidence, young adults have high intakes of solid fats, added sugars (29), and sodium (1, 30), along with inadequate intakes of fruits and vegetables (31), whole grains (32, 33), and fiber (30). The AHA recently issued a scientific statement recommending reductions in added sugar intake in response to research linking sugar to excess energy intake, obesity, dyslipidemia, and CHD risk (34). Sugar consumption has increased by nearly 20% from 1970 to 2005, supplying almost 500 kcal/d (35). Adolescents consume more sugar than any other age group (549 kcal) (34), and this continues into young adulthood (29). Collectively, these poor dietary choices contribute to the high prevalence of CHD risk factors in this age group (36–39).
In 2011, Magnussen et al. (40) reviewed findings from 2 population-based studies in Finland that support the ability to avoid or delay premature atherosclerosis by prevention efforts early in life. In 2012, Rubin and Borden (41) reviewed atherosclerotic versus nonatherosclerotic causes of CHD in young adults. Although these 2 recent reviews examined the causes of CHD in young adults (40, 41), there is a need for a review of pathological evidence along with recent risk factor and screening data to highlight the need for increased screening, risk assessment, education, and management in this age group.
The purpose of this review is to demonstrate the need for improved screening and risk awareness of CHD in young adults by revealing pathological changes that start in childhood and manifest themselves in young adult CHD risk factors. In addition, successful population-based prevention/treatment strategies used in other populations will be discussed with a focus on how these strategies can be applied to this age group.
Current Status of Knowledge
Progression of atherosclerosis
Childhood risk factors correlated with extent of lesions.
Research indicates that atherosclerosis has roots in childhood. In the 1950s and 1960s, Holman et al. (42) and Strong and McGill (43, 44) were the first to show that fatty streaks were present in the aortas of children as young as 3 y of age without a congenital heart condition, and progressed to fibrous plaques by the second decade of life. This evidence of atherosclerosis early in life led to large observational studies in the 1970s and 1980s (45–48) that examined childhood CVD risk factors, lifestyle patterns, and the development of CVD later in life.
The Muscatine, Bogalusa Heart, Cardiovascular Risk in Young Finns, and Childhood Determinants of Adult Health (CDAH) studies are the largest cohorts that tracked childhood risk factors into adulthood, with an average follow-up time of 30 y (49) (Table 1). The Muscatine Study (1970) indicated that risk factors predictive of CHD in adulthood, such as total cholesterol (TC), TGs, blood pressure (BP) and obesity, are prevalent in school-aged children (48). The Bogalusa Heart Study (1973) linked these childhood risk factors with atherosclerosis in young adults. This autopsy study showed that the extent of atherosclerotic lesions was directly correlated to antemortem concentrations of TC, TGs, LDL cholesterol, and HDL cholesterol; BP; BMI; and cigarette smoking in young adults (47, 50). The Cardiovascular Risk in Young Finns Study (1980) provided longitudinal data to show that CHD risk factors such as TC, HDL cholesterol, LDL cholesterol, TGs, BMI, and systolic blood pressure (SBP) track forward to adulthood (8, 45). Associations between childhood risk factors and those measured 27 y later were strongest for TC and LDL cholesterol. In addition, dietary intake and patterns showed significant tracking over time because individuals in the highest quintiles of either a traditional Finnish dietary pattern or a health-conscious dietary pattern remained in the same quintile 21 y later (51). The CDAH study (1985) supported the findings from the previous cohort studies and further demonstrated that healthy lifestyle behaviors such as consuming a diet low in saturated fat and sodium and being physically active were associated with a better cardiovascular risk profile even in young adults (52). Each of these studies contributed to the understanding that early life factors influence the development of adult CVD (40).
TABLE 1.
Cohort studies1
| Authors (ref) and study name (country) | Baseline year | No. and age of participants | Years of follow-up | Key outcomes |
| Lauer et al. (48) | ||||
| Muscatine Study (United States) | 1970 | 11,337; 5–18 y | 1970–1981 | TC: 37% >200 mg/dL; TGs: 15% >140 mg/dL; BP: 21% ≥140/90 mm Hg; weight: 33% ≥110% relative weight; elevated TC, TGs, BP, and relative weight in youth predict CHD in adults |
| 1982–1991 | ||||
| 1992–ongoing | ||||
| Berenson et al. (47) | ||||
| Bogalusa Heart Study (United States) | 1973 | 12,164; 4–17 y | 1977–1996 | Pathological changes occur by 5–8 y of age; extent of lesions significantly related to concentrations of TC, LDL-C, TGs, BMI, and HDL-C and BP |
| 2001–2002 | ||||
| 2003–2005 | ||||
| 2007–ongoing | ||||
| Raitakari et al. (45) | ||||
| Cardiovascular Risk in Young Finns Study (Finland) | 1980 | 3596; 3–18 y | 1983–ongoing | CVD risk factors (elevated TC, LDL-C, BP, smoking) early in life lead to structural and functional vascular changes related to atherosclerosis; increased LDL-C, BP, obesity and cigarette smoke in adolescence predict increased cIMT and decreased elasticity in adulthood |
| Gall et al. (46) | ||||
| Childhood Determinants of Adult Health Study (United States) | 1985 | 8498; 7–15 y | 2004–2006 | Childhood physical activity, obesity, and TC are important determinants of adult CVD risk factors (obesity, IR, dyslipidemia, cIMT) |
| 2013–ongoing |
BP, blood pressure; CHD, coronary heart disease; cIMT, carotid intima media thickness; CVD, cardiovascular disease; HDL-C, HDL cholesterol; IR, insulin resistance; LDL-C, LDL cholesterol; ref, reference; TC, total cholesterol.
Further evidence was provided by the Pathobiological Determinants of Atherosclerosis (PDAY) Study (1987), which examined the onset and progression of atherosclerosis in >3000 individuals aged 15–34 y in the United States (53). Although earlier autopsy studies (1970s and 1980s) indicated that risk factors for CHD were associated with atherosclerosis in adults, the PDAY and Bogalusa Heart studies provided evidence for this in children and young adults (47, 53). PDAY found intimal lesions in all aortas and in more than half of the right coronary arteries of adolescents aged 15–19 y (5). These lesions progress to more advanced, clinically significant lesions by young adulthood (53).
As many as 10–20% of young adults have advanced atherosclerotic lesions (54). This progression correlates with the number of CHD risk factors: young adults with ≥3 childhood risk factors had a 9-fold increase in atherosclerotic plaque area compared with those with no risk factors (6). As shown in Table 1, risk factors in childhood were shown to be strong predictors of preclinical atherosclerosis, even after adjustment for adult risk factors (55, 56). These findings are critical from a prevention standpoint because those at risk of developing atherosclerosis can be identified and treated decades before clinical manifestation of disease.
Childhood risk factors associated with preclinical disease markers.
Hyperlipidemia early in life is directly related to pathological changes and functional abnormalities and strongly predicts CHD in adulthood (57). The development of noninvasive techniques in the 1990s to measure preclinical markers such as carotid artery intima media thickness (cIMT), arterial endothelial function, and coronary artery calcification allowed for the assessment of structural and functional changes indicative of preclinical atherosclerosis (58, 59). The Muscatine, Bogalusa Heart, Cardiovascular Disease Risk in Young Finns, and CDAH studies provided evidence that these preclinical markers are associated with risk factors in childhood. Preclinical markers are strongly associated with the risk of CVD events (58), but longer follow-up times are needed to directly link childhood risk factors with clinical events (40). In the absence of these data, these surrogate disease markers serve as intermediate endpoints to assess the effects of risk factors and risk factor interventions before the clinical manifestation of disease and provide a better understanding of the evolution of CVD across the life span (40, 49).
In an attempt to address the difficulties in obtaining sufficient follow-up CVD events data, the International Childhood Cardiovascular Risk Consortium (i3C) was developed in 2011 to pool data previously collected from childhood to adulthood in large multicountry cohort studies for a meta-analysis to increase the power to link longitudinal risk data with CVD events. Data from the 4 largest cohort studies (Muscatine, Bogalusa Heart, Cardiovascular Disease Risk in Young Finns, and CDAH) and from similar smaller studies (Minneapolis Childhood Cohort Studies, Princeton Lipid Research Clinics Study; Princeton; National Heart, Lung, and Blood Institute Growth and Health Study) were combined for a total number of 12,000 participants with major CVD risk factors measured at least once in childhood and adulthood. In an effort to determine the effects of child and adult elevated BP on cIMT, data were pooled from the Bogalusa Heart, Muscatine, Young Finns, and CDAH studies with a mean follow-up of 23 y. Participants were 6–18 y old at baseline and 27–45 y old at follow-up. Results indicated that elevated BP that persisted from childhood into adulthood increased cIMT (60). In a similar analysis using the same 4 cohort studies (n = 4380 participants aged 3–18 y at baseline; mean follow-up = 22 y), the influence of age on the associations between childhood risk factors and cIMT in adulthood was examined (61). Risk factors (TC, TGs, BMI, and SBP) measured in the oldest children (15–18 y olds) at baseline were the strongest predictors of increased cIMT >20 y later. These findings demonstrate that late adolescence is the optimal age for screening and these screenings can effectively identify those at risk of atherosclerosis in adulthood (61).
Another recent meta-analysis (2013) in young adults from the i3C (Bogalusa Heart, Young Finns, and CDAH studies) and from the Minneapolis Childhood Cohort Studies and the Princeton Follow-Up Study assessed the association of ideal cardiovascular health with cIMT in 5785 participants aged 20–38 y (62). Ideal cardiovascular health is emphasized in the AHA’s 2020 Strategic Impact Goals and is defined as BP <120/80 mm Hg, glucose <100 mg/dL, TC <200 mg/dL, BMI <25 kg/m2, physical activity >150 min/wk of moderate/vigorous activity or >75 min/wk vigorous activity, nonsmoking, and meeting 4–5 components of a healthy diet score (16). Ideal cardiovascular health was achieved by only 1% of young adults. The least commonly met goal was diet-related; only 7% met the criteria for ideal diet. Compliance was particularly poor for sodium and saturated fat intakes. The number of ideal cardiovascular health criteria was inversely associated with cIMT, demonstrating that these 7 health metrics are related to vascular health in young adults. The goal of future analyses from i3C data is to determine the independent effects of childhood and early adult number of CVD risk factors on subsequent CVD occurrence (49). This will involve collecting CVD morbidity and mortality follow-up data, examining gene variants that increase disease risk, and harmonizing noninvasive vascular measures to obtain a better understanding of causal pathways to CVD events (49).
Although diet was not the main outcome in any of the studies in the i3C, it was measured in all studies. Future research should involve a pooled analysis to better understand the role that dietary intake in childhood and adolescence has on present and future CVD risk. Because diet is considered the first line of defense, this research would guide the development of both population-based and individual prevention efforts.
Poor dietary choices negatively affect CHD risk factors
Adolescents.
Unhealthy diet choices are a major determinant of CHD risk (34, 63, 64). Recent NHANES data in 4673 adolescents ages 12–19 y show an alarmingly high prevalence of adolescents in poor and intermediate CHD risk factor categories (65). Adherence to the 5 components of the healthy diet score was assessed: >4.5 cups (0.001 m3) of fruits and vegetables/d, >2 servings [3.5 oz (99.2 g)] of fish/wk, >3 servings [1 oz (28.4 g)] of fiber-rich whole grains (>1.1 g of fiber per 10 g of carbohydrate)/d, <1500 mg of sodium/d, and <450 kcal (1884.1 kJ) from sugar-sweetened beverages/wk. Healthy diet score was the least prevalent component of ideal cardiovascular health (65). Less than 1% met the criteria for an ideal healthy diet score and 90% had diets classified as poor. Adolescents consume as much as 34% of energy from solid fats and added sugars (66), exceeding recommendations by >200%. The consumption of excess calories from solid fats and added sugars is a major contributor to weight gain, which increases CHD risk in a dose-response manner (67). Although not the focus of this article, these data highlight the most prevalent dietary quality issues in this age group.
Dietary patterns established early in life carry into adulthood and are strongly associated with CHD risk (51). The transition from adolescence to young adulthood is considered a high-risk period because of declines in diet quality and increases in body weight (68–70). This transition period is often marked by students entering college, living away from home for the first time, and experiencing increased independence and responsibility for food choices (68, 71). If adolescents enter this transition period with poor diet quality, their chances of making positive dietary changes without intervention/education are slim.
College students.
College students consume excessive calories from high-fat snack foods (cookies, cake, chips, and ice cream), frequently skip meals, avoid certain nutrient-dense foods (fruits, vegetables, and low-fat dairy), and practice unhealthy weight-loss techniques (72–74). These unhealthy dietary choices and eating behaviors contribute to the declines in diet quality observed during this period. College students’ diets exceed recommendations of total fat (46% vs. 35% of energy) and saturated fat (13% vs. 10% of energy) (30). Intakes of total (24% of energy) and added (17% of energy) sugar also surpass guidelines (<10% of energy) (29, 75). College students also fail to meet whole-grain recommendations (32, 33), consuming just over 10% (10.5 g) of the recommended 3 ounces (85.1 g) (33). Similarly, fiber intake is inadequate, with only 43% of females and 51% of males meeting recommendations (30). More than 90% of college students exceed sodium recommendations (1). Dietary patterns high in solid fats, added sugars, and sodium and low in whole grains and fiber are known to exacerbate CHD risk factors (37, 63).
Changes in the college dining environment may play an important role in the worsening of eating behaviors and dietary intake during the transition from adolescence to young adulthood (76). Most dining halls are “all you can eat” styles and allow unlimited meal frequency. The campus food environment is no longer restricted to dining halls; students now have access to a variety of on-campus restaurants, cafes, snack bars, convenience stores, and vending machines (77, 78). Although there are a greater variety of options both on and off campus, there are few healthful options (77, 79).
In 2012, Horacek et al. (78) assessed the on-campus and off-campus dining environment at 15 universities. Unhealthy dining environments were widespread. Fast-food restaurants had significantly greater portion sizes and were more likely to have “combo meal” pricing compared with snack bars/cafes, dining halls and other sit down, fast casual and student union dining venues. Signs to encourage unhealthy or overeating were most common at fast-food restaurants and at snack bars/cafes. Dining halls had significantly more healthy entrees, nonfried vegetables, no-sugar-added fruits, vegetarian options, whole-wheat bread, and low-fat milk compared with all other dining settings. Dining halls, however, had 1 of the biggest barriers to healthy eating: “all you can eat” pricing. This all-you-can-eat environment and the wide variety of foods available in dining halls leads to larger portion sizes, increased energy intake, and weight gain (80). In the first semester, college students gain weight up to 11 times faster compared with young adults not in college (72) and maintain this weight throughout college (81) and into adulthood. This additional weight, most of which is excess body fat, can lead to dyslipidemia and increased heart disease risk (10).
Prevalence of CHD risk factors in college students
CHD risk factors in young adulthood can be the result of pathological changes from childhood. Only 20% of CHD in young adults is related to nonatherosclerotic factors (41). Results from the few cross-sectional studies that have assessed CHD risk in college students aged 18–24 y show an alarmingly high prevalence of young adults with abnormal risk factor profiles (Table 2). Huang et al. (82) reported that the most prevalent risk factors in a sample of 163 college students were elevated TC (12%) and low HDL cholesterol (14%). Impaired glucose metabolism was also a concern because just over 6% had prediabetes. Overweight students had worse risk factor profiles (waist circumference, BP, TC, LDL cholesterol, VLDL cholesterol, TGs, leptin, and insulin) compared with normal-weight students and were nearly 3 times as likely to have at least 1 metabolic syndrome component.
TABLE 2.
CHD risk factor prevalence in college students1
| Authors (ref) | TC (≥200 mg/dL) | LDL-C (≥100 mg/dL) | HDL-C (<40 M, <50 F mg/dL) | TGs (≥150 mg/dL) | Glu (≥100 mg/dL) | BP (≥130/85 mm Hg) | WC (>102 M, >88 F cm) |
| Fernandes and Lofgren (2), % | |||||||
| M | — | — | 3.2 | 3.7 | 2.1 | 2.1 | 1.1 |
| F | — | — | 16.9 | 13.8 | 5.3 | 0.0 | 6.3 |
| Huang et al. (4), % | |||||||
| M | — | — | 22.5 | 15.7 | 14.7 | 13.7 | 2.9 |
| F | — | — | 25.3 | 5.6 | 7.6 | 0.5 | 2.5 |
| Burke et al. (1), % | |||||||
| M | 27.0 | 63.0 | 29.0 | — | 8.0 | — | 4.0 |
| F | 27.0 | 47.0 | 23.0 | — | 5.0 | — | 4.0 |
| Morrell et al. (3), % | |||||||
| M | 24.7 | 61.9 | 30.6 | 12.2 | 13.7 | 62.1 | 5.2 |
| F | 25.8 | 45.5 | 23.7 | 18.3 | 6.4 | 21.2 | 4.2 |
BP, blood pressure; CHD, coronary heart disease; F, females; Glu, glucose; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; M, males; TC, total cholesterol; WC, waist circumference; –, data not reported or not reported in this format.
Fernandes et al. (2) assessed the prevalence of metabolic syndrome criteria in 189 first-year college students and found that 18% had elevated TGs and 20% had low HDL cholesterol for gender. Metabolic syndrome risk was also high: 28% met at least 1 of the criteria for metabolic syndrome and 4% had metabolic syndrome. Obese students were more likely to meet ≥3 metabolic syndrome criteria and had a higher prevalence of abnormal HDL cholesterol, WC, and BP compared with participants with a BMI <30 kg/m2. Gender differences were also noted, with males having a higher prevalence of risk factors (Table 2).
In a similar study by Huang et al. (4) that examined the prevalence of metabolic risk and gender differences in a sample of 300 students, 24% had low HDL cholesterol, 9% had elevated fasting glucose, and 9% had elevated TGs. The overall prevalence of metabolic syndrome was low (1%), but one-third of the sample had at least 1 component. As shown in Table 2, males had a worse metabolic profile than did females.
In a larger study conducted in 1701 college students, Burke et al. (1) reported that more than half had at least 1 CHD risk factor. The sample had high rates of overweight/obesity (33%) and elevated LDL cholesterol (53%), TC (27%), and BP (47%). Males also had a worse risk factor profile (BMI, glucose, TC, HDL cholesterol, LDL cholesterol, SBP, and diastolic blood pressure) than did females in this study. In a subsequent analysis of the same data but with a larger sample size (n = 2103), nearly one-third had low HDL cholesterol, nearly two-thirds had high BP, and approximately one-fourth had elevated TC or LDL cholesterol (3). Metabolic syndrome was observed in up to 10% of the sample, and those with a higher BMI had a substantially greater number of individual metabolic syndrome risk factors. In addition, males had a higher risk prevalence (BMI, HDL cholesterol, LDL cholesterol, TGs, and BP).
The differences in prevalence rates between studies can be partially attributed to demographic differences between universities. Risk factor profiles can be expected to vary because of different ethnic breakdowns and lifestyle factors across geographically dispersed university samples (2). There were also gender differences: a higher prevalence of CHD risk factors was found in men. Risk factor profiles were worse in overweight and obese individuals, regardless of gender. Collectively, these studies demonstrate that dyslipidemia and metabolic dysfunction are a common and major concern in young adults. As previously discussed, poor dietary choices made by this age group contribute to the high prevalence of risk factors. These data underscore the need to identify those at risk, especially male and overweight/obese young adults, so that steps can be taken to prevent future CHD risk and manage existing risk factors. Data collected to date demonstrate that college students are at risk of heart disease, but additional research needs to be conducted in young adults who are not in college to get a more comprehensive profile of this age group.
CHD risk factor screening in young adults
Historically conflicting guidelines.
Data from the cross-sectional studies mentioned above demonstrate that CHD risk factor prevalence is high in this age bracket, yet universal risk assessment for primordial and primary prevention is lacking. Although the importance and need for screening for early detection and management of dyslipidemia are recognized from public health organizations, including the NHLBI, AHA, American Academy of Pediatrics (AAP), and U.S. Preventive Services Task Force (USPSTF), the majority of young adults are not screened (25). The absence of apparent disease in young adults contributes to the underestimation of risk in this age group by both young adults themselves and by health professionals (26, 83, 84). This underestimation of risk and historically differing risk assessment guidelines contribute to this problem (85).
A variety of approaches and attitudes toward screening in young adults has existed among health professionals over the past 2 decades (85, 86). This can be traced back to the 1990s, with the release of the National Cholesterol Education Program (NCEP) Adult Treatment Panel III guidelines in 1993, which recommended universal lipid screening, regardless of risk level, every 5 y for all adults older than age 20 y. The rationale for these recommendations was to detect individuals at risk early on so that early intervention could reduce long-term CHD risk. Although these guidelines have been endorsed by representatives from >40 different medical and health organizations, the American College of Physicians argued against the need for screening in young adults due to the low short-term risk of CHD is this age group (87). Despite the presence of detractors early on, however, the strength of these screening recommendations was evidenced by their inclusion in the 2004 NCEP Adult Treatment Panel III guidelines (17) and in more recent 2012 NHLBI Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents (21) and 2013 American College of Cardiology (ACC)/AHA Guidelines on Assessment of CVD Risk (23).
Different recommendations over the past 20 y from other organizations have also led to inconsistent screening practices (85). The 2008 guidelines from the USPSTF recommend screening in all men older than age 35 and in men 20–35 y of age and in women older than age 45 who are at increased risk (88). The USPSTF makes no recommendation, however, for or against routine screening in men and women >20 y of age who are not at increased risk of CHD and states that the optimal screening interval is uncertain. Young adults in the 18–24-y age bracket span both children/adolescent and adult recommendations, which further complicates the issue. Screening guidelines for children and adolescents have also been conflicting since 1992 due to different recommendations by the NCEP (89), AHA (90), USPSTF (91), AAP (92), and the National Lipid Association (93). This conflicting guidance over the past 20 y has made it difficult for a uniform screening protocol to be followed by doctors and other health professionals (85).
Much needed progress was made, however, with the release of the 2012 NHLBI Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents (21) and the ACC/AHA Guidelines on Assessment of CVD Risk in 2013 (23). The NHLBI’s comprehensive, evidence-based guidelines represent a change in approach from targeted screening to universal screening, with an emphasis on primordial and primary prevention. This change was supported by the inability of previous high-risk, targeted screening approaches to detect up to 60% of children and adolescents with hypercholesterolemia (94). The 2012 evidence-based recommendations for lipid assessment recommend universal lipid screening by using a nonfasting non–HDL-cholesterol concentration between ages 9–11 and 17–21 y. Targeted screening is recommended between 2–8 and 12–16 y of age if risk factors are present. These new lipid screening guidelines are endorsed by the AAP, but the new expanded screening guidelines have not been without their detractors (85, 95–97). There are concerns that the new guidelines may result in overdiagnosis, false-positives, and overuse of statins in children (95–97). Although some experts disagree with the conservative nature of the guidelines, they are a pivotal step in the shift toward primordial, population-based prevention strategies that are needed to reduce future risk (16, 23, 65, 98, 99).
More recent 2013 ACC/AHA CVD assessment guidelines also support the need for risk assessment early in life to motivate lifestyle changes in younger individuals who may be at low short-term risk but who could benefit from long-term risk assessment. Long-term risk assessment of traditional CVD risk factors is recommended every 4–6 y beginning at age 20 for those who are free from atherosclerotic CVD (23).
Inadequate screening in young adults.
NHANES data from 1999–2006 on 2587 young adults aged 20–45 y indicated that two-thirds have at least 1 CVD risk factor. This is alarming because <50% of women and <40% of men reported being screened before the assessment visit. The screening rate for young adults in the 18–24-y age bracket can be expected to be even lower because screening rates increase with age (100). Younger males, in particular, are >50% less likely than their female counterparts to obtain preventive services (101). Data from NHANES show that women are more likely to have health insurance and see a health care provider (25). These low screening rates are especially concerning among young adults with multiple risk factors because the extent of atherosclerosis is directly correlated with the number of risk factors.
The AHA supports population-based strategies such as screenings at universities to identify at-risk individuals (16, 98, 102). Policy changes are needed to promote increased screening in primary care settings, clinics, schools, worksites, and community sites. These screenings are particularly important in the young-adult age group who may go otherwise undetected by the health care system (103), partly due to the underestimation of risk (26, 83, 84). As discussed in the AHA’s 2013 Science Advisory, screenings should include the assessment of all CHD risk factors including lifestyle habits (diet, exercise, and smoking), BP, glucose, and BMI in addition to the traditional lipid panel (98). Screening, however, must be accompanied by reliable interpretation of results, provision of appropriate educational material, and referral to a physician for those who need it for follow-up to be most effective. Young adults should be informed of the meaning of their results, the importance of dietary changes, and the appropriate follow-up steps that need to be taken depending on their other risk factors (103) (Fig. 1). As outlined in the 2013 AHA/ACC Guidelines on Lifestyle Management to Reduce Cardiovascular Risk and in the 2013 ACC/AHA Guidelines on Assessment of Cardiovascular Risk, heart healthy nutrition and physical activity behaviors are recommended for all adults older than age 18 for both prevention and treatment (23, 104). These preventive efforts are essential for reducing CHD events later in life and for reducing the burden of CHD on a population level (98). Future research is needed to better understand and eliminate barriers to screening. This needs to be done at the policy, provider, and patient level to improve suboptimal screening in young adults (105).
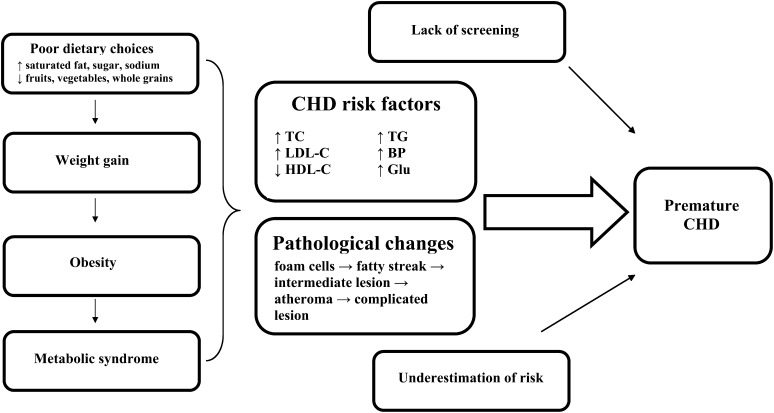
FIGURE 1.
Progression of atherosclerosis and prevention targets. BP, blood pressure; CHD, coronary heart disease; Glu, glucose; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; TC, total cholesterol.
Population-based nutrition interventions in college students
Until primordial prevention strategies are successful in avoiding risk factor development all together, risk factor screening needs to work in tandem with education and management for effective disease prevention. Strategies that focus on high-risk individuals are effective in reducing CHD events, but population-level strategies are needed to produce wide-scale risk reductions (16, 98). Population-based interventions on college campuses are cost-effective strategies to manage existing risk factors by promoting lifestyle changes, which are the foundation for risk reduction efforts (104). The college setting is an ideal forum to reach large numbers of the young adult population because 12.5 million (nearly 50%) of those aged 18–24 y were enrolled in U.S. colleges and universities in 2010 (106). Interventions aimed at the college population represent an opportunity to promote healthy eating while lifestyle habits are still being formed and to target CHD risk factors before disease progression occurs.
Previous population-based strategies have proven to be successful in reducing CHD risk in other populations (16). In the late 1980s, a population-based approach was used to lower CHD risk in the island nation of Mauritius. The FA composition of imported cooking oil was changed to contain higher amounts of polyunsaturated fat instead of saturated fat. The mean TC concentration decreased from 225 mg/dL in 1987 to 182 mg/dL in 1992, decreasing the prevalence of hypercholesterolemia from 25% to 6% in men and from 22% to 5% in women (107, 108). This intervention was a classic example of a population-based strategy that effectively shifted the entire distribution of risk. Estimates from the World Heart Federation show that a universal reduction in sodium intake by 1 g/d would lead to a 50% reduction in the number of individuals needing treatment for hypertension, a 22% decrease in deaths from stroke, and a 16% decrease in deaths from CHD (28).
Similar population-based strategies can be applied to the college setting. Although cafeterias can contribute to an obesogenic environment on college campuses, they also represent an opportunity to influence students’ diets for the better by providing nutrition information to guide healthy choices (109). To motivate students to choose healthier options, colleges need to identify healthy choices, provide nutrition information, and use point-of-selection signage (78). This nutrition information may provide the stimulus for students to reevaluate and change their eating habits (110). Pyramids that displayed energy and nutrient content of menu offerings at a university cafeteria led 71% of patrons to change their lunch selections by choosing meals lower in energy and fat (111).
Peterson et al. (112) reported increased awareness of healthy foods as the primary reason for selecting healthier food choices in a dining hall intervention consisting of signs, table tents, flyers, and benefit-based messages. Similar studies have also found that point-of-selection nutrition labels in dining halls resulted in better food choices and decreased energy intake at meals (113, 114). In another study, students with the highest nutrition knowledge were 12 times more likely to meet dietary recommendations compared with those with the lowest knowledge (115). Drawing attention to nutrition and health in a campus dining hall setting has a positive affect on food choices (112). Relatively small changes in the physical environment can produce behavioral changes (116). For example, placing healthy foods in more prominent places and removing trays from dining halls are other inexpensive ways to prompt healthier dietary choices.
Recently, technology has been used to promote behavior change. Technology-based interventions are particularly appealing to the young-adult population and are quick, cost-effective, and convenient ways to transmit information to a large audience (117). For example, messages displayed on computer screens at “point of decision” spots in a college dining hall influenced students to increase their fruit intake (118). Poddar et al. (119) demonstrated that 8 wk of e-mail messages as part of a dairy intake intervention were effective in increasing dairy intake in college students relative to the comparison group. Greene et al. (31) found that a 10-lesson Web-based nutrition and physical activity intervention resulted in higher fruit and vegetable intake and greater physical activity in 1689 college students from 8 universities.
Other studies have also reported success with mobile technology–based interventions (120–124). Text messaging, in particular, has been used in a variety of behavioral intervention studies to provide reminders, cues, and positive reinforcement and enhance self-monitoring (125–128). All of these features are recognized as keys to successful maintenance of dietary changes (124). Text messaging is an especially appealing intervention mode for college students because 99.8% of college students own a cell phone and 97% of college students rely on text messaging as their main form of communication (129).
In conclusion, this review highlights the need for improved risk assessment and increased awareness in young adults. Cross-sectional studies provide evidence of the high prevalence of CHD risk factors in this age group. It is well established that these risk factors are associated with pathological changes and substantially increase lifetime CHD risk. Until successful primordial prevention strategies are part of the public health care infrastructure and prevent risk factors, the focus must be on improving education about and screening, assessment, and treatment of CHD risk factors. Targeting young adults at a time in their lives when lifelong habits are being developed is critical to prevent disease progression.
The low screening rates in this age group are concerning in light of the high prevalence of risk factors. Increased screening is the first step because young adults at risk must first be identified before treatment approaches can be initiated. College campuses provide an opportunity for population-based screening approaches. College students and health professionals on campus must first be made aware of the need for risk assessment and then risk reduction through lifestyle changes.
Future research needs to be done to identify the most effective and efficient ways of screening large numbers of young adults. Screenings embedded into course curricula in health courses, as part of university wellness programs or as a part of freshmen orientation, are potential avenues to increase screening rates in this age group. Increased screening needs to work in conjunction with education to effectively identify and manage CHD risk.
Acknowledgments
All authors read and approved the final manuscript. This paper is a contribution of the Rhode Island Agricultural Experiment Station (no. 5356).
Footnotes
Abbreviations used: AAP, American Academy of Pediatrics; ACC, American College of Cardiology; BP, blood pressure; CDAH, Childhood Determinants of Adult Health; CHD, coronary heart disease; cIMT, carotid artery intima media thickness; CVD, cardiovascular disease; i3C, International Childhood Cardiovascular Risk Consortium; NCEP, National Cholesterol Education Program; NHLBI, National Heart, Lung, and Blood Institute; PDAY, Pathobiological Determinants of Atherosclerosis; SBP, systolic blood pressure; TC, total cholesterol; USPSTF, U.S. Preventive Services Task Force.
Literature Cited
- 1.Burke JD, Reilly RA, Morrell JS, Lofgren IE. The University of New Hampshire's Young Adult Health Risk Screening Initiative. J Am Diet Assoc. 2009;109:1751–8 [DOI] [PubMed] [Google Scholar]
- 2.Fernandes J, Lofgren IE. Prevalence of metabolic syndrome and individual criteria in college students. J Am Coll Health. 2011;59:313–21 [DOI] [PubMed] [Google Scholar]
- 3.Morrell JS, Lofgren IE, Burke JD, Reilly RA. Metabolic syndrome, obesity, and related risk factors among college men and women. J Am Coll Health. 2012;60:82–9 [DOI] [PubMed] [Google Scholar]
- 4.Huang TT, Shimel A, Lee RE, Delancey W, Strother ML. Metabolic risks among college students: prevalence and gender differences. Metab Syndr Relat Disord. 2007;5:365–72 [DOI] [PubMed] [Google Scholar]
- 5.Strong JP, Malcom GT, McMahan CA, Tracy RE, Newman WP, 3rd, Herderick EE, Cornhill JF. Prevalence and extent of atherosclerosis in adolescents and young adults: implications for prevention from the Pathobiological Determinants of Atherosclerosis in Youth Study. JAMA. 1999;281:727–35 [DOI] [PubMed] [Google Scholar]
- 6.Berenson GS, Srinivasan SR, Bao W, Newman WP, 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults: the Bogalusa Heart Study. N Engl J Med. 1998;338:1650–6 [DOI] [PubMed] [Google Scholar]
- 7.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juhola J, Magnussen CG, Viikari JS, Kahonen M, Hutri-Kahonen N, Jula A, Lehtimaki T, Akerblom HK, Pietikainen M, Laitinen T, et al. Tracking of serum lipid levels, blood pressure, and body mass index from childhood to adulthood: the Cardiovascular Risk in Young Finns Study. J Pediatr. 2011;159:584–90 [DOI] [PubMed] [Google Scholar]
- 9.Reis JP, Loria CM, Lewis CE, Powell-Wiley TM, Wei GS, Carr JJ, Terry JG, Liu K. Association between duration of overall and abdominal obesity beginning in young adulthood and coronary artery calcification in middle age. JAMA. 2013;310:280–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boreham C, Twisk J, Murray L, Savage M, Strain JJ, Cran G. Fitness, fatness, and coronary heart disease risk in adolescents: the Northern Ireland Young Hearts Project. Med Sci Sports Exerc. 2001;33:270–4 [DOI] [PubMed] [Google Scholar]
- 11.Sacheck JM, Kuder JF, Economos CD. Physical fitness, adiposity, and metabolic risk factors in young college students. Med Sci Sports Exerc. 2010;42:1039–44 [DOI] [PubMed] [Google Scholar]
- 12.Narayan KM, Boyle JP, Thompson TJ, Gregg EW, Williamson DF. Effect of BMI on lifetime risk for diabetes in the U.S. Diabetes Care. 2007;30(6):1562–6 [DOI] [PubMed] [Google Scholar]
- 13. U.S. Department of Health and Human Services. Health, United States, 2010. [Cited 2011 Jun 22]. Available from: http://www.cdc.gov/nchs/data/hus/hus10.pdf. [DOI] [PubMed]
- 14.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–44 [DOI] [PubMed] [Google Scholar]
- 15.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's Strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613 [DOI] [PubMed] [Google Scholar]
- 17.National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): final report. Circulation. 2002;106:3143–421 [PubMed] [Google Scholar]
- 18. National Heart, Lung, and Blood Institute. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, MD: NIH; 2004. [PubMed]
- 19. National Center for Health Statistics. Health, United States, 2008, with special feature on the health of young adults. Hyattsville, MD: USDHHS; 2009. [Cited 2013 Jun 10]. Available from http://www.cdc.gov/nchs/data/hus/hus08.pdf. 2008. [PubMed]
- 20.Camhi SM, Katzmarzyk PT. Tracking of cardiometabolic risk factor clustering from childhood to adulthood. Int J Pediatr Obes. 2010;5:122–9 [DOI] [PubMed] [Google Scholar]
- 21. National Heart, Lung, and Blood Institute. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents. Bethesda; MD: NIH; 2012. [Cited 2013 Jun 12]. Available from http://www.nhlbi.nih.gov/guidelines/cvd_ped/peds_guidelines_full.pdf. 2012.
- 22.Strasser T. Reflections on cardiovascular-diseases. Interdiscip Sci Rev. 1978;3:225–30 [Google Scholar]
- 23.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Sr, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, et al. ACC/AHA Guideline on the Assessment of Cardiovascular Risk: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2013; doi: 10.1161:2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97 [DOI] [PubMed] [Google Scholar]
- 25.Kuklina EV, Yoon PW, Keenan NL. Prevalence of coronary heart disease risk factors and screening for high cholesterol levels among young adults, United States, 1999–2006. Ann Fam Med. 2010;8:327–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muñoz LR, Etnyre A, Adams M, Herbers S, Witte A, Horlen C, Baynton S, Estrada R, Jones ME. Awareness of heart disease among female college students. J Womens Health (Larchmt). 2010;19:2253–9 [DOI] [PubMed] [Google Scholar]
- 27.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343:16–22 [DOI] [PubMed] [Google Scholar]
- 28. Diet and cardiovascular disease. World Heart Federation. Geneva, Switzerland. [Cited 2013 Sept 23]. Available from: http://www.world-heart-federation.org. 2013.
- 29.Hirshberg S, Fernandes J, Melanson K, Dwiggins J, Lofgren I. Dietary sugars predict chronic disease risk factors in college students. Topics Clin Nutr. 2011;26:324–34 [Google Scholar]
- 30.Irazusta A, Hoyos I, Irazusta J, Ruiz F, Diaz E, Gil J. Increased cardiovascular risk associated with poor nutritional habits in first-year university students. Nutr Res. 2007;27:387–94 [Google Scholar]
- 31.Greene GW, White AA, Hoerr SL, Lohse B, Schembre SM, Riebe D, Patterson J, Kattelmann KK, Shoff S, Horacek T, et al. Impact of an online healthful eating and physical activity program for college students. Am J Health Promot. 2012;27:e47–58 [DOI] [PubMed] [Google Scholar]
- 32.Rose N, Hosig K, Davy B, Serrano E, Davis L. Whole-grain intake is associated with body mass index in college students. J Nutr Educ Behav. 2007;39:90–4 [DOI] [PubMed] [Google Scholar]
- 33.Ha EJ, Caine-Bish N. Interactive introductory nutrition course focusing on disease prevention increased whole-grain consumption by college students. J Nutr Educ Behav. 2011;43:263–7 [DOI] [PubMed] [Google Scholar]
- 34.Johnson RK, Appel LJ, Brands M, Howard BV, Lefevre M, Lustig RH, Sacks F, Steffen LM, Wylie-Rosett J. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2009;120:1011–20 [DOI] [PubMed] [Google Scholar]
- 35.Wells HF, Buzby JC. Dietary assessment of major trends in US food consumption, 1970–2005. Economic Research Service; USDA; Washington D.C. 2008.
- 36.Anderson JW, Hanna TJ, Peng X, Kryscio RJ. Whole grain foods and heart disease risk. J Am Coll Nutr. 2000;19(3 Suppl):291S–9S [DOI] [PubMed] [Google Scholar]
- 37.Grundy SM, Denke MA. Dietary influences on serum lipids and lipoproteins. J Lipid Res. 1990;31:1149–72 [PubMed] [Google Scholar]
- 38.Anderson JW, Hanna TJ. Whole grains and protection against coronary heart disease: what are the active components and mechanisms? Am J Clin Nutr. 1999;70:307–8 [DOI] [PubMed] [Google Scholar]
- 39.Fernandez ML, West KL. Mechanisms by which dietary fatty acids modulate plasma lipids. J Nutr. 2005;135:2075–8 [DOI] [PubMed] [Google Scholar]
- 40.Magnussen CG, Niinikoski H, Juonala M, Kivimaki M, Ronnemaa T, Viikari JS, Simell O, Raitakari OT. When and how to start prevention of atherosclerosis? Lessons from the Cardiovascular Risk in the Young Finns Study and the Special Turku Coronary Risk Factor Intervention Project. Pediatr Nephrol. 2011;27:1441–52 [DOI] [PubMed] [Google Scholar]
- 41.Rubin JB, Borden WB. Coronary heart disease in young adults. Curr Atheroscler Rep. 2012;14:140–9 [DOI] [PubMed] [Google Scholar]
- 42.Holman RL, Mc Gill HC, Jr, Strong JP, Geer JC. The natural history of atherosclerosis: the early aortic lesions as seen in New Orleans in the middle of the 20th century. Am J Pathol. 1958;34:209–35 [PMC free article] [PubMed] [Google Scholar]
- 43.Strong JP, McGill H C., Jr The natural history of coronary atherosclerosis. Am J Pathol. 1962;40:37–49 [PMC free article] [PubMed] [Google Scholar]
- 44.Strong JP, McGill HC., Jr The pediatric aspects of atherosclerosis. J Atheroscler Res. 1969;9:251–65 [DOI] [PubMed] [Google Scholar]
- 45.Raitakari OT, Juonala M, Ronnemaa T, Keltikangas-Jarvinen L, Rasanen L, Pietikainen M, Hutri-Kahonen N, Taittonen L, Jokinen E, Marniemi J, et al. Cohort profile: the Cardiovascular Risk in Young Finns Study. Int J Epidemiol. 2008;37:1220–6 [DOI] [PubMed] [Google Scholar]
- 46.Gall SLJK, Smith K, Dwyer T, Venn A. The Childhood Determinants of Adult Health Study: a profile of a cohort study to examine the childhood influences on adult cardiovascular health. Austral Epidemiol. 2009;16:35–9 [Google Scholar]
- 47.Lauer RM, Connor WE, Leaverton PE, Reiter MA, Clarke WR. Coronary heart disease risk factors in school children: the Muscatine study. J Pediatr. 1975;86:697–706 [DOI] [PubMed] [Google Scholar]
- 48.Dwyer T, Sun C, Magnussen CG, Raitakari OT, Schork NJ, Venn A, Burns TL, Juonala M, Steinberger J, Sinaiko AR, et al. Cohort profile: the International Childhood Cardiovascular Cohort (i3C) Consortium. Int J Epidemiol. 2013;42:86–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frerichs RR, Srinivasan SR, Webber LS, Berenson GR. Serum cholesterol and triglyceride levels in 3,446 children from a biracial community: the Bogalusa Heart Study. Circulation. 1976;54:302–9 [DOI] [PubMed] [Google Scholar]
- 50.Mikkilä V, Rasanen L, Raitakari OT, Pietinen P, Viikari J. Consistent dietary patterns identified from childhood to adulthood: the Cardiovascular Risk in Young Finns Study. Br J Nutr. 2005;93:923–31 [DOI] [PubMed] [Google Scholar]
- 51.Gall SL, Jamrozik K, Blizzard L, Dwyer T, Venn A. Healthy lifestyles and cardiovascular risk profiles in young Australian adults: the Childhood Determinants of Adult Health Study. Eur J Cardiovasc Prev Rehabil. 2009;16:684–9 [DOI] [PubMed] [Google Scholar]
- 52.Zieske AW, Malcom GT, Strong JP. Natural history and risk factors of atherosclerosis in children and youth: the PDAY study. Pediatr Pathol Mol Med. 2002;21:213–37 [DOI] [PubMed] [Google Scholar]
- 53.Stary HC. Evolution and progression of atherosclerotic lesions in coronary arteries of children and young adults. Arteriosclerosis. 1989;9(1 Suppl):I19–32 [PubMed] [Google Scholar]
- 54.Juonala M, Jarvisalo MJ, Maki-Torkko N, Kahonen M, Viikari JS, Raitakari OT. Risk factors identified in childhood and decreased carotid artery elasticity in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 2005;112:1486–93 [DOI] [PubMed] [Google Scholar]
- 55.Raitakari OT, Juonala M, Kahonen M, Taittonen L, Laitinen T, Maki-Torkko N, Jarvisalo MJ, Uhari M, Jokinen E, Ronnemaa T, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290:2277–83 [DOI] [PubMed] [Google Scholar]
- 56.McCrindle BW. Hyperlipidemia in children. Thromb Res. 2006;118:49–58 [DOI] [PubMed] [Google Scholar]
- 57.Cohn JN, Quyyumi AA, Hollenberg NK, Jamerson KA. Surrogate markers for cardiovascular disease: functional markers. Circulation. 2004;109(25 Suppl 1):IV31–46 [DOI] [PubMed] [Google Scholar]
- 58.Mancini GB, Dahlof B, Diez J. Surrogate markers for cardiovascular disease: structural markers. Circulation. 2004;109(25 Suppl 1):IV22–30 [DOI] [PubMed] [Google Scholar]
- 59.Juhola J, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, Srinivasan SR, Daniels SR, Davis PH, Chen W, et al. Combined effects of child and adult elevated blood pressure on subclinical atherosclerosis: the International Childhood Cardiovascular Cohort Consortium. Circulation. 2013;128:217–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Juonala M, Magnussen CG, Venn A, Dwyer T, Burns TL, Davis PH, Chen W, Srinivasan SR, Daniels SR, Kahonen M, et al. Influence of age on associations between childhood risk factors and carotid intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study, the Childhood Determinants of Adult Health Study, the Bogalusa Heart Study, and the Muscatine Study for the International Childhood Cardiovascular Cohort (i3C) Consortium. Circulation. 2010;122:2514–20 [DOI] [PubMed] [Google Scholar]
- 61.Oikonen M, Laitinen TT, Magnussen CG, Steinberger J, Sinaiko AR, Dwyer T, Venn A, Smith KJ, Hutri-Kahonen N, Pahkala K, et al. Ideal cardiovascular health in young adult populations from the United States, Finland, and Australia and its association with cIMT: the International Childhood Cardiovascular Cohort Consortium. J Am Heart Assoc. 2013;2:e000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Welsh JA, Sharma A, Cunningham SA, Vos MB. Consumption of added sugars and indicators of cardiovascular disease risk among US adolescents. Circulation. 2011;123:249–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu FB, Manson JE, Willett WC. Types of dietary fat and risk of coronary heart disease: a critical review. J Am Coll Nutr. 2001;20:5–19 [DOI] [PubMed] [Google Scholar]
- 64.Shay CM, Ning H, Daniels SR, Rooks CR, Gidding SS, Lloyd-Jones DM. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005–2010. Circulation. 2013;127:1369–76 [DOI] [PubMed] [Google Scholar]
- 65.Slining MM, Popkin BM. Trends in intakes and sources of solid fats and added sugars among U.S. children and adolescents: 1994–2010. Pediatr Obes. 2013;8:307–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.May AL, Kuklina EV, Yoon PW. Prevalence of cardiovascular disease risk factors among US adolescents, 1999–2008. Pediatrics. 2012;129:1035–41 [DOI] [PubMed] [Google Scholar]
- 67.Nelson MC, Story M, Larson NI, Neumark-Sztainer D, Lytle LA. Emerging adulthood and college-aged youth: an overlooked age for weight-related behavior change. Obesity (Silver Spring). 2008;16:2205–11 [DOI] [PubMed] [Google Scholar]
- 68.Gordon-Larsen P, Adair LS, Nelson MC, Popkin BM. Five-year obesity incidence in the transition period between adolescence and adulthood: the National Longitudinal Study of Adolescent Health. Am J Clin Nutr. 2004;80:569–75 [DOI] [PubMed] [Google Scholar]
- 69.Gordon-Larsen P, The NS, Adair LS. Longitudinal trends in obesity in the United States from adolescence to the third decade of life. Obesity (Silver Spring). 2010;18:1801–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McDade TW, Chyu L, Duncan GJ, Hoyt LT, Doane LD, Adam EK. Adolescents’ expectations for the future predict health behaviors in early adulthood. Soc Sci Med. 2011;73:391–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Levitsky DA, Halbmaier CA, Mrdjenovic G. The freshman weight gain: a model for the study of the epidemic of obesity. Int J Obes Relat Metab Disord. 2004;28:1435–42 [DOI] [PubMed] [Google Scholar]
- 72.Hendricks KM, Herbold NH. Diet, activity, and other health-related behaviors in college-age women. Nutr Rev. 1998;56:65–75 [DOI] [PubMed] [Google Scholar]
- 73.Ha EJ, Caine-Bish N, Holloman C, Lowry-Gordon K. Evaluation of effectiveness of class-based nutrition intervention on changes in soft drink and milk consumption among young adults. Nutr J. 2009;8:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nishida C, Uauy R, Kumanyika S, Shetty P. The joint WHO/FAO Expert Consultation on Diet, Nutrition and the Prevention of Chronic Diseases: process, product and policy implications. Public Health Nutr. 2004;7(1A):245–50 [DOI] [PubMed] [Google Scholar]
- 75.Kobayashi T, Umemura U, Iso H, Ishimori M, Tamura Y, Iida M, Shimamoto T. Differences in dietary habits, serum fatty acid compositions and other coronary risk characteristics between freshmen and fourth-year male university students. Environ Health Prev Med. 2001;6:143–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Horacek TM, Erdman MB, Reznar MM, Olfert M, Brown-Esters ON, Kattelmann KK, Kidd T, Koenings M, Phillips B, Quick V, et al. Evaluation of the food store environment on and near the campus of 15 postsecondary institutions. Am J Health Promot. 2013;27:e81–90 [DOI] [PubMed] [Google Scholar]
- 77.Horacek TM, Erdman MB, Byrd-Bredbenner C, Carey G, Colby SM, Greene GW, Guo W, Kattelmann KK, Olfert M, Walsh J, et al. Assessment of the dining environment on and near the campuses of fifteen post-secondary institutions. Public Health Nutr. 2013;16:1186–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Byrd-Bredbenner C, Johnson M, Quick VM, Walsh J, Greene GW, Hoerr S, Colby SM, Kattelmann KK, Phillips BW, Kidd T, et al. Sweet and salty: an assessment of the snacks and beverages sold in vending machines on US post-secondary institution campuses. Appetite. 2012;58:1143–51 [DOI] [PubMed] [Google Scholar]
- 79.Levitsky DA, Youn T. The more food young adults are served, the more they overeat. J Nutr. 2004;134:2546–9 [DOI] [PubMed] [Google Scholar]
- 80.Holm-Denoma JM, Joiner TE, Vohs KD, Heatherton TF. The "freshman fifteen" (the "freshman five" actually): predictors and possible explanations. Health Psychol. 2008;27(1 Suppl):S3–9 [DOI] [PubMed] [Google Scholar]
- 81.Huang TT, Kempf AM, Strother ML, Li C, Lee RE, Harris KJ, Kaur H. Overweight and components of the metabolic syndrome in college students. Diabetes Care. 2004;27:3000–1 [DOI] [PubMed] [Google Scholar]
- 82.Collins KM, Dantico M, Shearer NBC, Mossman KL. Heart disease awareness among college students. J Community Health. 2004;29:405–20 [DOI] [PubMed] [Google Scholar]
- 83.Cleeman JI, Grundy SM. National Cholesterol Education Program recommendations for cholesterol testing in young adults: a science-based approach. Circulation. 1997;95:1646–50 [DOI] [PubMed] [Google Scholar]
- 84.Saenger AK. Universal lipid screening in children and adolescents: a baby step toward primordial prevention? Clin Chem. 2012;58:1179–81 [DOI] [PubMed] [Google Scholar]
- 85.Peterson AL, McBride PE. A review of guidelines for dyslipidemia in children and adolescents. WMJ. 2012;111:274–81; quiz 282 [PubMed] [Google Scholar]
- 86.Garber AM, Browner WS. Cholesterol screening guidelines: consensus, evidence, and common sense. Circulation. 1997;95:1642–5 [DOI] [PubMed] [Google Scholar]
- 87.U.S. Preventive Services Task Force Screening for lipid disorders in adults: recommendation statement. Am Fam Physician. 2009;80:1273–4 [Google Scholar]
- 88.National Cholesterol Education Program. Highlights of the report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics. 1992;89:495–501 [PubMed] [Google Scholar]
- 89.Kavey RE, Allada V, Daniels SR, Hayman LL, McCrindle BW, Newburger JW, Parekh RS, Steinberger J. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2006;114:2710–38 [DOI] [PubMed] [Google Scholar]
- 90.U.S. Preventive Services Task Force. Screening for lipid disorders in children: recommendation statement. 2007. [Cited 2013 July 12]. Available from: http://www.uspreventiveservicestaskforce.org/uspstf07/chlipid/chlipidrs.htm.
- 91.Daniels SR, Greer FR. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122:198–208 [DOI] [PubMed] [Google Scholar]
- 92. Goldberg AC, Hopkins PN, Toth PP, Ballantyne CM, Rader DJ, Robinson JG, Daniels SR, Gidding SS, de Ferranti SD, Ito MK, et al. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5(35):S1–58. [DOI] [PubMed]
- 93.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: summary report. Pediatrics. 2011;128 Suppl 5:S213–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gillman MW, Daniels SR. Is universal pediatric lipid screening justified? JAMA. 2012;307:259–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Psaty BM, Rivara FP. Universal screening and drug treatment of dyslipidemia in children and adolescents. JAMA. 2012;307:257–8 [DOI] [PubMed] [Google Scholar]
- 96.Newman TB, Pletcher MJ, Hulley SB. Overly aggressive new guidelines for lipid screening in children: evidence of a broken process. Pediatrics. 2012;130:349–52 [DOI] [PubMed] [Google Scholar]
- 97.Spring B, Ockene JK, Gidding SS, Mozaffarian D, Moore S, Rosal MC, Brown MD, Vafiadis DK, Cohen DL, Burke LE, et al. Better population health through behavior change in adults: a call to action. Circulation. 2013;128:2169–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Laitinen TT, Pahkala K, Magnussen CG, Viikari JS, Oikonen M, Taittonen L, Mikkila V, Jokinen E, Hutri-Kahonen N, Laitinen T, et al. Ideal cardiovascular health in childhood and cardiometabolic outcomes in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 2012;125:1971–8 [DOI] [PubMed] [Google Scholar]
- 99.Disparities in screening for and awareness of high blood cholesterol—United States, 1999–2002. MMWR Morb Mortal Wkly Rep. Centers for Disease Control and Prevention. 2005;54:117–9 [PubMed] [Google Scholar]
- 100.U.S. Department of Health and Human Services. Utilization of ambulatory medical care by women: United States, 1997–98. 2001. [Cited 2013 Oct 10]. Available from: http://www.cdc.gov/nchs/data/series/sr_13/sr13_149.pdf.
- 101. American Heart Association. American Heart Association Scientific Position: public cholesterol screening (adults and children). 2012 [Cited 2013 Sept 27]. Available from: http://www.heart.org/HEARTORG/Conditions/Cholesterol/SymptomsDiagnosisMonitoringofHighCholesterol/Public-Cholesterol-Screening-Adults-and-Children_UCM_305617_Article.jsp.
- 102.Carleton RA, Dwyer J, Finberg L, Flora J, Goodman DS, Grundy SM, Havas S, Hunter GT, Kritchevsky D, Lauer RM. Report of the Expert Panel on Population Strategies for Blood Cholesterol Reduction: a statement from the National Cholesterol Education Program, National Heart, Lung, and Blood Institute, National Institutes of Health. Circulation. 1991;83:2154–232 [DOI] [PubMed] [Google Scholar]
- 103.Eckel RH, Jakicic JM, Ard JD, Hubbard VS, de Jesus JM, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Miller NH, et al. AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013; doi: 10.1161/:2013 [Google Scholar]
- 104.Robbins CL, Dietz PM, Bombard JM, Gibbs F, Ko JY, Valderrama AL. Blood pressure and cholesterol screening prevalence among U.S. women of reproductive age: opportunities to improve screening. Am J Prev Med. 2011;41:588–95 [DOI] [PubMed] [Google Scholar]
- 105. U.S. Department of Education. Digest of education statistics, 2011. National Center for Education Statistics. Institute of Education Sciences. Washington D.C. 2012.
- 106.Dowse GK, Gareeboo H, Alberti KG, Zimmet P, Tuomilehto J, Purran A, Fareed D, Chitson P, Collins VR. Changes in population cholesterol concentrations and other cardiovascular risk factor levels after five years of the non-communicable disease intervention programme in Mauritius. Mauritius Non-communicable Disease Study Group. BMJ. 1995;311:1255–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Uusitalo U, Feskens EJ, Tuomilehto J, Dowse G, Haw U, Fareed D, Hemraj F, Gareeboo H, Alberti KG, Zimmet P. Fall in total cholesterol concentration over five years in association with changes in fatty acid composition of cooking oil in Mauritius: cross sectional survey. BMJ. 1996;313:1044–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gorman N, Lackney JA, Rollings K, Huang TT. Designer schools: the role of school space and architecture in obesity prevention. Obesity (Silver Spring). 2007;15:2521–30 [DOI] [PubMed] [Google Scholar]
- 109.Conklin MT, Cranage DA, Lambert CU. College students’ use of point of selection nutrition information. Topics Clin Nutr. 2005;20:97–108 [Google Scholar]
- 110.Turconi G, Bazzano R, Roggi C, Cena H. Helping consumers make a more conscious nutritional choice: acceptability of nutrition information at a cafeteria. Public Health Nutr. 2012;15:792–801 [DOI] [PubMed] [Google Scholar]
- 111.Peterson S, Duncan DP, Null DB, Roth SL, Gill L. Positive changes in perceptions and selections of healthful foods by college students after a short-term point-of-selection intervention at a dining hall. J Am Coll Health. 2010;58:425–31 [DOI] [PubMed] [Google Scholar]
- 112.Freedman MR, Connors R. Point-of-purchase nutrition information influences food-purchasing behaviors of college students: a pilot study (reprinted from American Dietetic Association, vol 110, pg 1222–1226, 2010). J Am Diet Assoc. 2011;111:S42–6 [DOI] [PubMed] [Google Scholar]
- 113.Driskell JA, Schake MC, Detter HA. Using nutrition labeling as a potential tool for changing eating habits of university dining hall patrons. J Am Diet Assoc. 2008;108:2071–6 [DOI] [PubMed] [Google Scholar]
- 114.Kresić G, Kendel Jovanovic G, Pavicic Zezel S, Cvijanovic O, Ivezic G. The effect of nutrition knowledge on dietary intake among Croatian university students. Coll Antropol. 2009;33:1047–56 [PubMed] [Google Scholar]
- 115.Kremers SPJ, Eves FF, Andersen RE. Environmental changes to promote physical activity and healthy dietary behavior. J Environ Public Health. 2012:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sirriyeh R, Lawton R, Ward J. Physical activity and adolescents: an exploratory randomized controlled trial investigating the influence of affective and instrumental text messages. Br J Health Psychol. 2010;15:825–40 [DOI] [PubMed] [Google Scholar]
- 117.Reed JA, Powers A, Greenwood M, Smith W, Underwood R. Using "point of decision" messages to intervene on college students’ eating behaviors. Am J Health Promot. 2011;25:298–300 [DOI] [PubMed] [Google Scholar]
- 118.Poddar KH, Hosig KW, Anderson-Bill ES, Nickols-Richardson SM, Duncan SE. Dairy intake and related self-regulation improved in college students using online nutrition education. J Acad Nutr Diet. 2012;112:1976–86 [DOI] [PubMed] [Google Scholar]
- 119.Spring B, Duncan JM, Janke EA, Kozak AT, McFadden HG, Demott A, Pictor A, Epstein LH, Siddique J, Pellegrini CA, et al. Integrating technology into standard weight loss treatment: a randomized controlled trial. Arch Intern Med. 2012;173:105–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Spring B, Schneider K, McFadden HG, Vaughn J, Kozak AT, Smith M, Moller AC, Epstein LH, Demott A, Hedeker D, et al. Multiple behavior changes in diet and activity: a randomized controlled trial using mobile technology. Arch Intern Med. 2012;172:789–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schembre SM, Yuen J. Project TwEATs: a feasibility study testing the use of automated text messaging to monitor appetite ratings in a free-living population. Appetite. 2011;56:465–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kerr DA, Pollard CM, Howat P, Delp EJ, Pickering M, Kerr KR, Dhaliwal SS, Pratt IS, Wright J, Boushey CJ. Connecting Health and Technology (CHAT): protocol of a randomized controlled trial to improve nutrition behaviours using mobile devices and tailored text messaging in young adults. BMC Public Health. 2012;12:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Patrick K, Raab F, Adams MA, Dillon L, Zabinski M, Rock CL, Griswold WG, Norman GJ. A text message-based intervention for weight loss: randomized controlled trial. J Med Internet Res. 2009;11:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Armstrong AW, Watson AJ, Makredes M, Frangos JE, Kimball AB, Kvedar JC. Text-message reminders to improve sunscreen use: a randomized, controlled trial using electronic monitoring. Arch Dermatol. 2009;145:1230–6 [DOI] [PubMed] [Google Scholar]
- 125.Hanauer DA, Wentzell K, Laffel N, Laffel LM. Computerized Automated Reminder Diabetes System (CARDS): e-mail and SMS cell phone text messaging reminders to support diabetes management. Diabetes Technol Ther. 2009;11:99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hou MY, Hurwitz S, Kavanagh E, Fortin J, Goldberg AB. Using daily text-message reminders to improve adherence with oral contraceptives: a randomized controlled trial. Obstet Gynecol. 2010;116:633–40 [DOI] [PubMed] [Google Scholar]
- 127.Prabhakaran L, Chee WY, Chua KC, Abisheganaden J, Wong WM. The use of text messaging to improve asthma control: a pilot study using the mobile phone short messaging service (SMS). J Telemed Telecare. 2010;16:286–90 [DOI] [PubMed] [Google Scholar]
- 128.Hanley M. Student smartphone use doubles; instant messaging loses favor. 2009 [Cited 2013 Feb]. Available from: http://chronicle.com/blogs/wiredcampus/student-smartphone-use-doubles-instant-messaging-loses-favor/24876.