Abstract
AIM: To investigate the influence of tumor grade on sentinel lymph node (SLN) status in patients with gastric cancer (GC).
METHODS: We retrospectively studied 71 patients with GC who underwent SLN mapping during gastric surgery to evaluate the relationship between SLN status and tumor grade.
RESULTS: Poorly differentiated tumors were detected in 50/71 patients, while the other 21 patients had moderately differentiated tumors. SLNs were identified in 58/71 patients (82%). In 41 of the 58 patients that were found to have stained nodes (70.7%), the tumor was of the poorly differentiated type (group I), while in the remaining patients with stained nodes 17/58 (29.3%), the tumor was of the moderately differentiated type (group II). Positive SLNs were found in 22/41 patients in group I (53.7%) and in 7/17 patients in group II (41.2%) (P = 0.325). The rate of positivity for the SLNs in the two groups (53.7% vs 41.2%) was not statistically significant (P = 0.514).
CONCLUSION: Most of our patients were found to have poorly differentiated adenocarcinoma of the stomach and there was no correlation between tumor grade and SLN involvement.
Keywords: Gastric cancer, Sentinel lymph nodes, Tumor differentiation, Sentinel lymph node mapping, Prognosis
Core tip: The application of sentinel lymph node (SLN) sampling in gastric cancer is limited to the early stages of the disease. The results of the sampling, which is usually not one node but rather a group of nodes, might influence the extent of lymphadenectomy to be performed. In a previous study, we clearly showed that the accuracy of SLN testing is inversely proportionate to the T stage of the tumor. In this retrospective study, we evaluated the level of tumor differentiation as related to the SLN status. Our study showed that there was no correlation between tumor differentiation and SLN status.
INTRODUCTION
Although first described for patients with penile cancer[1] and now used routinely in patients with malignant melanoma and breast cancer, the evaluation of sentinel lymph nodes (SLNs) has also gradually entered the field of gastrointestinal cancer[2]. SLN mapping of the gastrointestinal tract has been studied extensively, especially in patients with gastric cancer (GC) and today, SLN status plays an important role in the decision-making process regarding the extent of lymphadenectomy in selected groups of patients with early stage GC[3,4].
In a previous study, we investigated the accuracy of SLN mapping according to the T stage of the tumor and showed that in T1-2 tumors SLN mapping may be of assistance, but that in patients with T3 it will be misleading in a third of the patients and should not be attempted[5].
We used this particular group of patients to retrospectively study whether or not tumor grade also has an influence on SLN status.
MATERIALS AND METHODS
This study was performed under the authorization of the Institutional Review Board of our medical center (Assaf Harofeh Medical Center; Approval No. 82/12).
Data was retrieved from a computerized data base. Out of 80 patients, nine patients with well differentiated tumors were omitted so that only 71 patients with poorly and moderately differentiated GC entered the study. Pre-op evaluation included gastroscopy, intravenous contrast computed tomography (CT) and endoscopic ultrasound in a selected group of patients with a gastroesophageal junction location.
Surgery started with exploration of the abdominal cavity, disease staging and resectability assessment. Before any dissection was performed, patent blue (Guerbet Patent Blue V Sodium 2.5%; Guerbet, Roissy, France) diluted with 2 mL of normal saline was injected subserosally in four different opposing points adjacent to the tumor site. Ten minutes following dye injection, dye spread was evaluated and blue nodes were marked by a stitch. The type of D2 resection was based on tumor location and the extent of the disease.
A detailed focused pathological assessment was performed with special attention to all areas marked by patent blue. All blue-stained lymph nodes were sectioned into 0.2 cm thick slices. Two 3 μm thick sections were serially cut at 0.25 mm levels from these lymph node slices: the first was stained with hematoxylin and eosin and the second was placed on a Superfrost Plus Slide (Menzel GmbH and Co KG, Braunschweig, Germany). If the hematoxylin and eosin slides were negative for metastatic involvement, the unstained consecutive slides were stained with a pan cytokeratin antibody (CKMNF116; Dako, Carpinteria, CA, United States) to highlight micrometastases. All relevant sections were examined. The total sampling of the SLNs with systematic serial sectioning and cytokeratin immunohistochemistry enabled a relatively optimal estimation of the metastatic status of the SLNs.
The non-stained (not sentinel) lymph nodes were routinely submitted either in toto when less than 0.2 cm in diameter or sectioned into 0.2cm thick slices. Two levels of 3 μm thickness were performed on each of these tissue fragments, which were then stained with hematoxylin and eosin only.
All pathological slides were re-evaluated by the senior pathologist with respect to tumor grade. Tumor grade was matched to SLN status and statistically evaluated.
Statistical analysis
Statistical analysis was performed at the Department of Statistics of the Tel Aviv University using the χ2 Test, Fisher’s Exact Test and the Mann-Whitney Test.
RESULTS
Our cohort included 71 patients (30 women and 41 men) with GC with no evidence of spread (by computer tomography scan). The age range varied from 26 to 88 years (mean, 67.4 years).
The tumor was located in the lower third of the stomach in 32 patients, the middle third in 15 patients and the upper third or the gastroesophageal junction in 18 patients. Four patients had linitis plastica and two patients had gastric stump carcinoma that developed many years after a subtotal gastrectomy for benign disease.
Forty-four patients underwent distal subtotal gastrectomy, 14 patients underwent proximal gastrectomy, 11 patients underwent total gastrectomy and two patients underwent gastric stump resection, one of them with en-bloc transverse colon resection.
Poorly differentiated tumors were detected in 50/71 patients, while the other 21 patients had moderately differentiated tumors (Figure 1).
Figure 1.
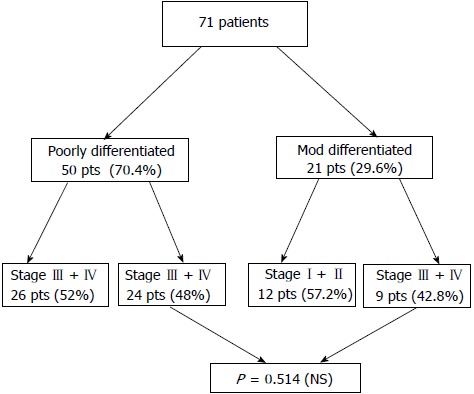
Distribution of the 71 patients according to tumor differentiation and state of disease. NS: No significant.
A total of 1114 regional lymph nodes were harvested in the group of patients with poorly differentiated tumors (22.7 nodes per person) with a positivity ratio of 20.3% (226/1114). In the group of patients with moderately differentiated tumors, the overall number of harvested nodes was 401 (19.1 nodes per person) with a positivity ratio of 16.5% (66/401).
SLNs were identified in 58/71 patients (82%), of which 41 (70.7%) were of the poorly differentiated type (group I) and 17 (29.3%) were of the moderately differentiated type (group II).
Positive SLNs were found in 22/41 patients in group I (53.7%) and in 7/17 patients in group II (41.2%), P = 0.325 NS (Figure 2).
Figure 2.
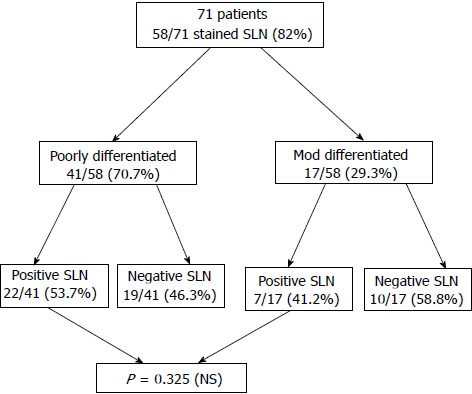
Distribution of sentinel lymph nodes according to tumor differentiation. SLN: Sentinel lymph node; NS: No significant.
The patients with poorly differentiated tumors were more likely to have a higher T stage (3-4) than those with moderately differentiated tumors, 68% vs 47.6%.
DISCUSSION
The precise evaluation of SLN status is one of the most important factors in determining the clinical outcome when treating gastrointestinal cancer. Nodal involvement in gastric cancer is defined by two main systems: the American Joint Committee on Cancer staging system, which is based on the number of positive nodes, and the Japanese system, which is based on the location of positive nodes[6-8].
In recent years, the SLN concept has been widely investigated in different types of malignant disease as an alternative to routine lymph node dissection[9-11]. The SLN concept postulates that if the first draining node is negative for metastasis, the remaining lymph nodes in this particular nodal basin are negative for metastases. Thus one can predict the status of the nodal basin with a high degree of accuracy[12].
Numerous authors have described the successful use of SLN status in colon, rectal, gastric, esophageal and anal canal malignancies, with a high degree of accuracy when using a detailed pathological analysis of the SLNs[13].
The reported number of harvested SLNs varies according to the primary organ. For example, in patients with breast cancer, the quoted numbers vary from 1.87 to 2.14 nodes[14,15]. This number increases significantly in patients with GC and figures as high as 16 harvested SLNs have been quoted[5].
The logic of using SLN evaluation in patients with GC is related to the decision regarding the extent of the lymphadenectomy that should be performed: formal D2 lymphadenectomy in cases of positive SLNs vs limited lymphadenectomy for negative SLNs. The extent of gastric resection will depend on tumor location and SLN status[1,5,16].
Unfortunately, most of the patients with GC in the Western hemisphere present with advanced disease and the idea of using the SLN technique to tailor the extent of lymphadenectomy and resection to a minimum has not proven worthwhile due to the multidirectional nature of gastric lymphatic drainage[17-21].
In our previous study, we showed clearly that the SLN status depends on the T stage of the tumor. Stained nodes were detected in around 90% in T1 and T2 tumors, but in only 68.8% in T3 tumors. Based on these findings, we decided to retrospectively study the effect of tumor grade on SLN status in the same group of patients.
To the best of our knowledge (including a thorough literature search), there are no other studies dealing with this subject and we were therefore unable to compare our results with those of other reports.
In 82% of the patients (58/71), SLNs were stained (70.7% in the group of patients with poorly differentiated group compared to only 29.3% in the moderately differentiated group). There was no statistical difference in the staging based on tumor grade (P = 0.514). There was no statistical significance difference between positive SLNs in both groups 53.7% vs 41.2% (P = 0.325).
In conclusion, based on our previous study, we expected to see a higher rate of SLN involvement in the group of patients with poorly differentiated tumors but, despite the fact that there was a difference, this was with no statistical significance. The clinical significance of the connection between tumor grading and the SLN status was supposed to guide us how to limit the SLN procedure to a specific group of patients with gastric cancer. The results of this study failed to provide that information.
COMMENTS
Background
Sentinel lymph node (SLN) status plays an important role in the decision-making process regarding the extent of lymphadenectomy in selected groups of patients with early stage gastric cancer.
Research frontiers
This is a retrospective study on whether or not tumor grade has an influence on SLN status.
Innovations and breakthroughs
In a previous study, the authors investigated the accuracy of SLN mapping according to the T stage of the tumor and showed that in T1-2 tumors, SLN mapping may be of assistance, but in patients with T3 it will be misleading in a third of the patients and should not be attempted.
Applications
The evaluation of tumor grade may aid in predicting the outcome in patients with gastric cancer and the need for sentinel node evaluation.
Peer review
The manuscript titled “Tumor differentiation as related to sentinel lymph node status in patients with gastric cancer” by Lavy et al, was performed to investigate the influence of tumor grade on sentinel lymph node status in patients with gastric cancer. The paper is well written.
Footnotes
P- Reviewers: El-Tawil AM, Nowicki MJ, Xia HHX S- Editor: Cui XM L- Editor: A E- Editor: Wu HL
References
- 1.Cabanas RM. An approach for the treatment of penile carcinoma. Cancer. 1977;39:456–466. doi: 10.1002/1097-0142(197702)39:2<456::aid-cncr2820390214>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 2.Fujii H, Kitagawa Y, Kitajima M, Kubo A. Sentinel nodes of malignancies originating in the alimentary tract. Ann Nucl Med. 2004;18:1–12. doi: 10.1007/BF02985608. [DOI] [PubMed] [Google Scholar]
- 3.Kitagawa Y, Fujii H, Mukai M, Kubo A, Kitajima M. Sentinel lymph node mapping in esophageal and gastric cancer. Cancer Treat Res. 2005;127:123–139. doi: 10.1007/0-387-23604-x_6. [DOI] [PubMed] [Google Scholar]
- 4.Kitagawa Y, Fujii H, Kumai K, Kubota T, Otani Y, Saikawa Y, Yoshida M, Kubo A, Kitajima M. Recent advances in sentinel node navigation for gastric cancer: a paradigm shift of surgical management. J Surg Oncol. 2005;90:147–151; discussion 151-152. doi: 10.1002/jso.20220. [DOI] [PubMed] [Google Scholar]
- 5.Rabin I, Chikman B, Lavy R, Poluksht N, Halpern Z, Wassermann I, Gold-Deutch R, Sandbank J, Halevy A. The accuracy of sentinel node mapping according to T stage in patients with gastric cancer. Gastric Cancer. 2010;13:30–35. doi: 10.1007/s10120-009-0532-9. [DOI] [PubMed] [Google Scholar]
- 6.Aikou T, Kitagawa Y, Kitajima M, Uenosono Y, Bilchik AJ, Martinez SR, Saha S. Sentinel lymph node mapping with GI cancer. Cancer Metastasis Rev. 2006;25:269–277. doi: 10.1007/s10555-006-8507-3. [DOI] [PubMed] [Google Scholar]
- 7.Kim DH, Oh CA, Oh SJ, Choi MG, Noh JH, Sohn TS, Bae JM, Kim S. Validation of seventh edition AJCC gastric cancer staging modifications. J Surg Oncol. 2012;105:26–30. doi: 10.1002/jso.22026. [DOI] [PubMed] [Google Scholar]
- 8.Zhang M, Zhu G, Ma Y, Xue Y. Comparison of four staging systems of lymph node metastasis in gastric cancer. World J Surg. 2009;33:2383–2388. doi: 10.1007/s00268-009-0214-0. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi H, Kitagawa Y. Preoperative diagnosis of lymph node metastases and sentinel node navigation surgery in patients with upper gastrointestinal cancer. Nihon Geka Gakkai Zasshi. 2008;109:90–94. [PubMed] [Google Scholar]
- 10.Snider H, Dowlatshahi K, Fan M, Bridger WM, Rayudu G, Oleske D. Sentinel node biopsy in the staging of breast cancer. Am J Surg. 1998;176:305–310. doi: 10.1016/s0002-9610(98)00207-4. [DOI] [PubMed] [Google Scholar]
- 11.Barnwell JM, Arredondo MA, Kollmorgen D, Gibbs JF, Lamonica D, Carson W, Zhang P, Winston J, Edge SB. Sentinel node biopsy in breast cancer. Ann Surg Oncol. 1998;5:126–130. doi: 10.1007/BF02303845. [DOI] [PubMed] [Google Scholar]
- 12.Kitagawa Y, Burian M, Kitajima M. Methods of sentinel lymph node mapping. Chirurg. 2004;75:751–755. doi: 10.1007/s00104-004-0908-7. [DOI] [PubMed] [Google Scholar]
- 13.Saha S, Dan AG, Bilchik AJ, Kitagawa Y, Schochet E, Choudhri S, Saha LT, Wiese D, Morton D, Kitajima M. Historical review of lymphatic mapping in gastrointestinal malignancies. Ann Surg Oncol. 2004;11:245S–249S. doi: 10.1007/BF02523638. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen KR, Oturai PS, Friis E, Hesse U, Callesen T, Nielsen MB, Chakera AH, Hesse B. Axillary sentinel node identification in breast cancer patients: degree of radioactivity present at biopsy is critical. Clin Physiol Funct Imaging. 2011;31:288–293. doi: 10.1111/j.1475-097X.2011.01015.x. [DOI] [PubMed] [Google Scholar]
- 15.Hundley JC, Shen P, Shiver SA, Geisinger KR, Levine EA. Lymphatic mapping for gastric adenocarcinoma. Am Surg. 2002;68:931–935. [PubMed] [Google Scholar]
- 16.Cozzaglio L, Bottura R, Di Rocco M, Gennari L, Doci R. Sentinel lymph node biopsy in gastric cancer: possible applications and limits. Eur J Surg Oncol. 2011;37:55–59. doi: 10.1016/j.ejso.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Kitagawa Y, Kitajima M. Diagnostic validity of radio-guided sentinel node mapping for gastric cancer: a review of current status and future direction. Surg Technol Int. 2006;15:32–36. [PubMed] [Google Scholar]
- 18.Wong J, Jackson P. Gastric cancer surgery: an American perspective on the current options and standards. Curr Treat Options Oncol. 2011;12:72–84. doi: 10.1007/s11864-010-0136-y. [DOI] [PubMed] [Google Scholar]
- 19.Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis. Cancer. 2000;88:921–932. [PubMed] [Google Scholar]
- 20.Tsubono Y, Hisamichi S. Screening for gastric cancer in Japan. Gastric Cancer. 2000;3:9–18. doi: 10.1007/pl00011692. [DOI] [PubMed] [Google Scholar]
- 21.Lambert R, Guilloux A, Oshima A, Pompe-Kirn V, Bray F, Parkin M, Ajiki W, Tsukuma H. Incidence and mortality from stomach cancer in Japan, Slovenia and the USA. Int J Cancer. 2002;97:811–818. doi: 10.1002/ijc.10150. [DOI] [PubMed] [Google Scholar]


