An estimated 1 million times per day, someone in the United States uses ultraviolet (UV) radiation for skin tanning. According to the indoor tanning industry, tanning beds are used by 30 million Americans, or about 10% of the U.S. population, each year (www.theita.com/indoor/). These users include minors, who often have ready access to tanning beds. In response to considerable grassroots and political opposition to indoor tanning, in late March the Food and Drug Administration (FDA) convened an advisory panel to review the safety of the procedure. The FDA is expected to announce a decision soon on whether and how to reclassify tanning lamps and possibly to address minors’ access to them.
The concern arises from the increases in the incidence of melanoma and its related mortality. In the United States, the incidence of melanoma is increasing more rapidly than that of any other cancer. From 1992 through 2004, there was a particularly alarming trend in new melanoma diagnoses among girls and women between the ages of 15 and 39. Data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results registry show an estimated annual increase of 2.7% in this group.1 Researchers suspect that the increase results at least partially from the expanded use of tanning beds. The possibility that changes in diagnostic criteria may have contributed to the increased incidence is lessened by the fact that the trend is specific to a certain age range and sex. The incidence of thicker cutaneous melanomas (>1 mm) also increased, and the incidence of regional and distant tumors has increased at an estimated annual rate of 9.2% — a change that could portend a surge in advanced melanomas in young women. Although substantial advances have been made in melanoma therapies, the risk of death from advanced disease remains high.
Abundant epidemiologic data have been examined to assess potential connections between indoor tanning and both melanoma and non-melanoma cutaneous cancers. According to a 2006 meta-analysis by the International Agency for Research on Cancer (IARC), among people who first used indoor tanning before 35 years of age, the relative risk of melanoma was 1.75 – a finding that prompted the World Health Organization to classify tanning beds as a group I carcinogen. Similarly, a recent case–control study in Minnesota showed an adjusted odds ratio of 1.74; the risk of melanoma increased as the number of years of tanning and hours of tanning sessions increased.2
An even more dramatic association has been found between UV-radiation and non-melanoma skin cancers. In the IARC study, history of any indoor tanning was associated with a relative risk of 2.25 for squamous-cell carcinoma. Although most of these lesions are successfully treated at an early stage, metastisis persistently occurs in a small minority of such lesions, at which point cure is rare. Although the overall rate of death from sqaumous-cell carcinoma is low, the high incidence of this form of cancer means that accounts for 25 to 35% of skin-cancer-related deaths.
In response to such data, numerous countries have tightened their restrictions on indoor tanning; France, Germany, Austria, Finland, and Britain, for instance, ban indoor tanning for people under the age of 18. Some U.S. states have also enacted restrictions on access for minors, but many have not. The FDA classifies tanning beds as medical devices and designates them as class I, the same class as tongue depressors and adhesive bandages — in contrast to tampons, for example, which are considered class II devices. Although no formal vote was reported at the March meeting of the FDA advisory committee, its members appeared to be unanimous in suggesting a change of classification.
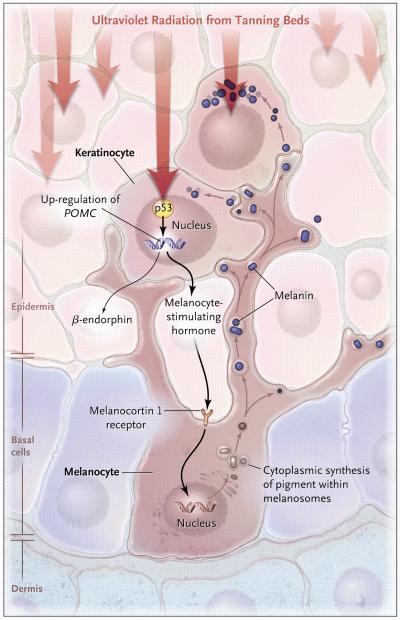
Advances in the molecular understanding of signaling pathways in skin have yielded insights into the relationship between tanning and cancer, showing that the common molecular intermediate for both is DNA damage, which activates melanin synthesis, even when triggered by the singular, precise activity of restriction endonucleases.3 Studies have also revealed the involvement of p53 and proopiomelanocortin in the processing and secretion of melanocyte stimulating hormone (MSH), which activates pigment production in epidermal melanocytes4 (see diagram). The tanning response is thus an offshoot of the p53 tumor-suppressor pathway. A striking example of the carcinogenic activity of UV radiation is also seen in xeroderma pigmentosum, a condition involving a DNA-repair deficiency.
The tanning industry argues that indoor tanners avoid sunburn better than outdoor tanners. This difference could arise from increased use of UVA, rather than UVB, wavelengths, but UVA radiation also damages DNA and induces discrete mutations. Moreover, UV radiation may be carcinogenic without causing sunburn. The precise roles of specific UV wavelengths in both tanning and carcinogenesis remain to be fully elucidated, but since DNA damage appears to be the key intermediate for both, tanning induced by UV radiation that is devoid of carcinogenic risk may be scientifically impossible.
Some evidence suggests that repeated UV irradiation, and the use of indoor tanning beds specifically, may have important systemic and behavioural consequences, including mood changes, pain, and physical dependency. In a series of studies, Feldman and colleagues identified the ability of frequent tanners to perceive the difference between UV-radiating and sham devices, suggesting the presence of a reinforcing stimulus, and found that the administration of an opiate-receptor blocker induced withdrawal-like symptoms among frequent tanners, suggesting the presence of opiate-like addiction. More recent data have shown addictive features of indoor tanning in a large cohort of college-age tanning-bed users.5 A mechanistic explanation may lie in the fact that MSH production in the UV-tanning response is accompanied by the release of β-endorphin, which shares the same precursor peptide (proopiomelanocortin)4 (see diagram).
One plausible model for the evolution of such behavioral sun-seeking effects involves the participation of UV radiation in the cutaneous production of vitamin D. In settings such as high latitudes, where exposure to such radiation is limited, a behavioral inclination toward sun exposure might have historically provided a survival advantage by averting lethal vitamin D deficiency at pre-reproductive ages.
Is cutaneous vitamin D synthesis a justifiable defense of indoor tanning in 2010? In addition to the tight overlap between UV radiation’s action spectra for DNA damage and vitamin D synthesis, a key reason why such radiation is a poor choice for vitamin D replacement is the the many (sometimes uncontrollable) variables involved in its use, including the quantity of skin exposed, the darkness or pigmentation of that skin, the wavelength or energy of the source (which varies with the time of year and latitude), and the degree of one’s vitamin D deficiency. It is difficult to consume sufficient vitamin D from typical diets, but oral supplements and intermittent testing of blood levels would appear to be significantly more effective than tanning, without carcinogenic risk.
An estimated six of every seven melanomas are now being cured, thanks to early detection, but the U.S. Preventive Services Task Force does not recommend skin-cancer screening, since the evidence for its benefit has not been validated in large, prospective, randomized trials. Meanwhile, a number of promising new drugs for metastatic melanoma are progressing slowly through clinical trials to satisfy the FDA’s stringent safety and efficacy criteria – requirements that, remarkably, have not been applied to indoor tanning devices. Relatively few human cancers are tightly linked to a known environmental carcinogen. Given the mechanistic and epidemiologic, we believe that regulation of this industry may offer one of the most profound cancer-prevention opportunities of our time.
Fig 1.
Molecular Mechanism of Skin Pigmentation Induced by UV Radiation
Ultraviolet light triggers DNA damage in the nucleus of keratinocytes, resulting in the activation of p53, which transcriptionally up-regulates expression of the gene encoding proopiomelanocortin (POMC). POMC is post-translationally processed to produce melanocyte stimulating hormone (MSH) and β-endorphin. After secretion, MSH acts on its receptor, the melanocortin 1 receptor, located on melanocytes at the basal layer of the epidermis, thereby inducing the production of pigment, which is subsequently transported out of melanocytes to overlying keratinocytes, where the pigment vesicles coalesce over the sun-exposed side of the nucleus, resulting in tanning.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Purdue MP, Beane, Freeman1 LE, Anderson WF, Tucker MA. Recent Trends in Incidence of Cutaneous Melanoma among US Caucasian Young Adults. J Invest Dermatology. 2008;128:2905–2908. doi: 10.1038/jid.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazovich D, Vogel RI, Berwick M, Weinstock MA, Anderson K, Warshaw E. Indoor tanning and risk of melanoma: a case-control study in a highly exposed population. Cancer Epidemiol Biomarkers Prev. 2010;19:1557–68. doi: 10.1158/1055-9965.EPI-09-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eller MS, Ostrom K, Gilchrest BA. DNA damage enhances melanogenesis. Proc Natl Acad Sci U S A. 1996;93:1087–92. doi: 10.1073/pnas.93.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, D’Orazio J, Fung CY, Schanbacher CF, Granter SR, Fisher DE. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128(5):853–864. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 5.Mosher CE, Danoff-Burg S. Addiction to indoor tanning: relation to anxiety, depression, and substance use. Arch Dermatol. 2010;146:412–7. doi: 10.1001/archdermatol.2009.385. [DOI] [PMC free article] [PubMed] [Google Scholar]