Abstract
In this study, we propose that diprophylline exerts bidirectional modulation (BM) on the isolated rat jejunal segment depending on its contractile state. The results supported the hypothesis. Diprophylline (20 µM) exerted stimulatory effects on the contractility of jejunal segment in six low contractile states while inhibitory effects in six high contractile states, showing the characteristics of BM. Diprophylline-induced stimulatory effect was significantly blocked by atropine, indicating the correlation with cholinergic activation. Diprophylline-induced inhibitory effect was partially blocked by phentolamine, propranolol, and L-N-Nitro-Arginine respectively, indicating their correlation with sympathetic activation and nitric oxide-mediated relaxing mechanisms. Diprophylline-induced BM was abolished by tetrodotoxin or in a Ca2+ free condition or pretreated with tyrosine kinase inhibitor imatinib, suggesting that diprophylline-induced BM is Ca2+ dependent, and that it requires the presence of enteric nervous system as well as pacemaker activity of interstitial cells of Cajal. Diprophylline significantly increased the reduced MLCK expression and myosin extent in constipation-prominent rats and significantly decreased the increased MLCK expression and myosin extent in diarrhea-prominent rats, suggesting that the change of MLCK expression may also be involved in diprophylline-induced BM on rat jejunal contractility. In summary, diprophylline-exerted BM depends on the contractile states of the jejunal segments, requires the presence of Ca2+, enteric nervous system, pacemaker activity of interstitial cells of Cajal, and MLCK-correlated myosin phosphorylation. The results suggest the potential implication of diprophylline in relieving alternative hypo/hyper intestinal motility.
Keywords: Bidirectional modulation, Contractile state, Diprophylline, Jejunal segment, Phosphorylation
INTRODUCTION
Diprophylline is the N7-substituted derivative of theophylline naturally found in tea and cocoa beans [1,2]. Diprophylline and theophylline are both methylxanthines usually employed for symptomatic relief or prevention of bronchi asthma and chronic obstructive pulmonary disease clinically [3]. Compared with theophylline, diprophylline causes fewer adverse drug reactions [4] and has a wider therapeutic window and a more effective relaxant effect [5,6].
Diprophylline decreases the pulmonary vascular resistance by reducing the mean pulmonary arterial pressure without a significant change in cardiac output and causes a significant decrease in arterial carbon dioxide tension [7]. Diprophylline, which is not converted to free theophylline in vivo, has its own pharmacokinetic and pharmacodynamic properties [8].
The effects of diprophylline on gastrointestinal tract belong to commonly encountered adverse drug reactions, including nausea and vomiting [9]. However, the characteristics of diprophylline-induced effects on gastrointestinal motility and whether the effects could have potential therapeutic value for certain gastrointestinal disorders need to be investigated.
Based upon the evidence and observations from previous and our studies respectively, we propose that diprophylline can induce bidirectional modulation (BM) on jejunal motility depending on its contractile state. To verify the hypothesis, the present study was designed to characterize diprophylline-induced effects, correlated mechanisms, and evaluate the potential implication for the treatment of abnormal hypo-/hyper gut motility. Isolated rat jejunal segments were used in the assay, as the modulation on intestinal motility by enteric nervous system (ENS) is characterized by its ability to fulfill pivotal functions even when isolated from the body [10]. Jejunal segments in different high and low contractile states in the assay were prepared by changing ionic concentration or by using exogenous stimulators and inhibitors respectively, by using stimulatory and inhibitory neurotransmitters respectively, or by using jejunal segment from rat models of diarrhea-prominent (DP) and constipation-prominent (CP) bowel dysfunction respectively. The role of Ca2+, enteric nerve system, pacemaker activity of interstitial cells of Cajal (ICCs), stimulation and inhibition-related receptors, extent of phosphorylation of 20-kDa myosin light chain (P-MLC20), and myosin light chain kinase (MLCK) were investigated to reveal diprophylline-exerted BM. The results supported our hypothesis. The present study found two types of BM exerted by diprophylline: one was dose dependant and the other contractile state dependant. The later was characterized, and the potential correlated mechanisms were investigated in the study.
METHODS
Animals
Sixty Sprague-Dawley rats, 180~220 g, half males and half females, were provided by Experimental Animal Center, Dalian Medical University (Certificate of Conformity: No. SCXK (Liao) 2008-0002). Proper handling of the animals was carried out according to the principles issued by Dalian Medical Animal Care and Ethics Committee. All animals used were maintained in accordance with National Institutes of Health Guide for Care and Use of Laboratory Animals. Animals were housed 1 per cage in a temperature-controlled room with a 12-h light-dark cycle. Food and water were available ad libitum.
Drugs
Diprophylline was obtained from Tianjin Jinyao Amino Acid Co., Ltd (Tianjin, China). The concentration of pre-diprophylline solution was 10 mM, and pH was adjusted to 7.4 before use. The proper volume of diprophylline solution was chosen to get the final required concentration. Tetrodotoxin (TTX) was from Aladdin Chemistry Co.Ltd (Shanghai, China). TTX was dissolved in acetic acid-acetate buffer. Unless otherwise indicated, chemicals were obtained from Sigma (USA).
Experimental models of diarrhea and constipation
Sprague-Dawley rats were randomly divided into normal control, DP, CP, diprophylline-treated DP, and diprophylline-treated CP groups. DP rats were built up by intracolonic acetic acid (4%) instillation and restraint stress, and normal rats were received intracolonic saline instillation [11]. CP rats were built up by daily gavage with cool water (0~4℃) for 14 days, and normal rats were established by daily gavage with water at room temperature [12]. The body mass was recorded every 2 days, the moisture content and granule number of the feces from the normal, CP, and DP rats were measured daily. The granules and the moisture content of the feces from normal, DP, and CP rats were measured to determine whether experimental rat models were successfully made. After successful establishment of CP and DP rats, diprophylline (20 mg/kg) was gavaged to DP and CP rats once daily. After 7 days of diprophylline administration, rats were sacrificed.
Tissue preparation and contractility determination
Jejunal segments isolated from normal, CP, and DP rats were cut into approximately 2 cm in length (tubes) and assayed in a 20 ml bath, filled with pre-aerated Krebs solution at 37℃ [13]. Jejunal segment was allowed to equilibrate for 1 hour until a stable baseline was achieved; the bath solution was replaced every 10 min. Jejunal contractility measured from the baseline to the peak was recorded using a BL-420F physiological recording system and expressed as a percentage of normal contractile amplitude. Same time-interval of each assay with identical start and stop time was selected to compare the amplitude before and after drug treatment in different assay conditions. The mean amplitude was calculated from results of 6 independent assays.
Jejunal contractility measured in different contractile states
Jejunal contractility determined in normal Krebs buffer was chosen as the normal contractile state. Jejunal contractility measured in a modified low Ca2+ (1.25 mM) and high Ca2+ (5.0 mM) Krebs solution was chosen as the representative low contractile state (RLCS) and representative high contractile state (RHCS) respectively, as spontaneous intestinal contractility is paralleled to intracellular Ca2+ concentration [14]. One pair of low-high contractile states was built up by using the jejunal segments isolated from CP and DP rats respectively. The other 5 pairs of low-high contractile states were prepared by incubating jejunal segments in modified low K+ (2.5 mM)-high K+ (10.0 mM) Krebs buffer, low Na+ (100 mM)-high Na+ (150 mM) Krebs buffer, adrenaline (5.0 µM)-acetylcholine (ACh) (5.0 µM) Krebs buffer and nitric oxide donor sodium nitroprusside (SNP) (5 µM)-erythromycin (10 µM) Krebs buffer [15]. After a stable contractile state of jejunal contraction was established, diprophylline was added to make a final concentration of 20 µM, unless otherwise indicated.
Western blot analysis
The extent of P-MLC20 and protein content of MLCK in jejunal segment were determined using Western blot analysis as described previously [16,17]. Rat jejunal segments were immediately isolated and stored in liquid nitrogen. Ground product was incubated for 30 min in ice-cold homogenization buffer. The blots on nitrocellulose filter membrane were probed with phosphor-myosin light chain 2 (Ser 19) antibody (1:1,000) (No. 3671, Cell Signaling Technology, Inc (CST) USA), myosin light chain 2 (total myosin light chain) antibody (1:1,000) (#3672, CST, USA) and MLCK antibody (1:1,000) (No. ab76092, abcam (Hong Kong) Ltd. UK) respectively, at 4℃ over night. Anti-rabbit IgG secondary antibodies were used at 1:1,000 for 90 min at room temperature. MultiSpectral imaging system (UVP, USA) was used to detect and quantify the bands.
PT-PCR analysis of myosin light chain kinase mRNA expression
Total RNA was extracted from rat jejunal segment using TRIzol® reagent (Life Technologies, Rockville, MD). For cDNA synthesis, Oligo (dT) primers were used to prime the reverse transcription reactions. Based on the published sequences, the primers (Takara Biotechnology (Dalian) Co., Ltd) were designed to determine MLCK transcripts. The house-keeping gene GAPDH served as an internal control. Real-time PCR primer sequences used for amplifications were as follows: MLCK: 5'-AATGGTGTTGCTGGAGATCGAGGT-3' (forward) and 5'-GCTGGATCAAATTGCGGTGGTTCA-3' (reverse). GAPDH: 5'-GGCAAGTTCAATGGCACAGT-3' (forward) and 5'-TGGTGAAGACGCCAGTAGACTC-3' (reverse). Real-time PCR was performed by using SYBR green method for fluorescence detection during amplification on an ABI 7500 Fast Real-Time PCR System (Applied Biosystems). PCR conditions were as follows: initial denaturation at 95℃ for 30 sec, 40 thermal cycles (95℃ for 5 sec, 60℃ for 34 sec and 72℃ for 30 sec). The amplification of MLCK was normalized to GAPDH mRNA amplification in the same sample (ΔCt, the difference between CtMLCK and CtGAPDH). The ΔCt was then transformed to absolute values (2-ΔCt) and expressed as percentage of expression in normal control samples [18].
Statistical analysis
The results were expressed as the mean±SEM. Paired Student t test was used to compare the means in two groups. The One-Way ANOVA was used where three or more groups were compared. All experiments were repeated at least three times. p<0.05 was considered to denote statistical significance.
RESULTS
Concentration-effects relationship of diprophylline
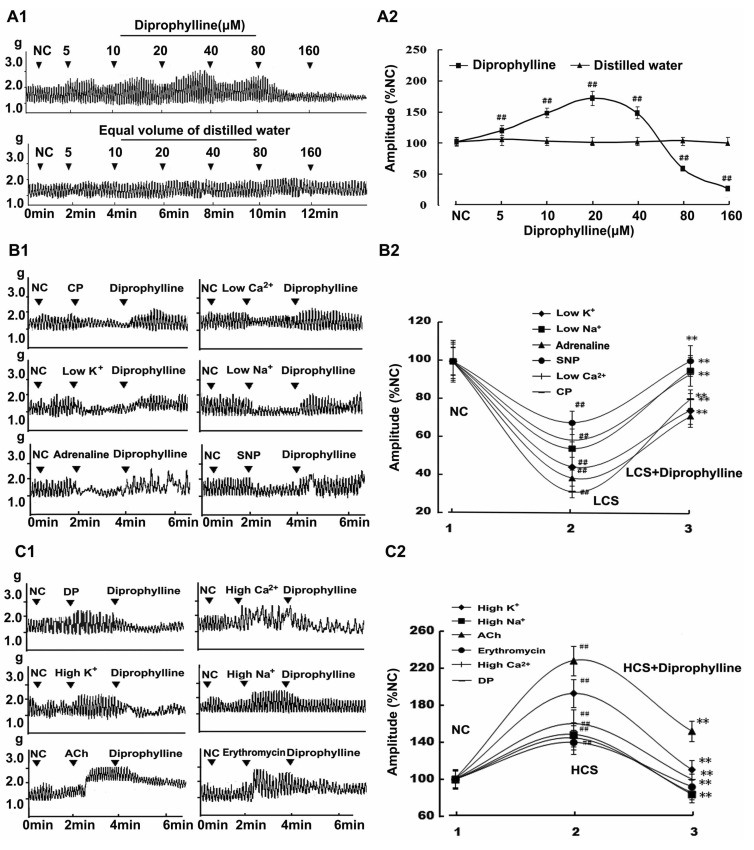
Diprophylline-induced effects on jejunal contractility in normal contractile state were shown by its concentration-response relationship. Diprophylline increased the contractile amplitude of jejunal segment in a concentration range of 5~40 µM with an ED50 of 10 µM and decreased the contractile amplitude of jejunal segment in a concentration range of 80~160 µM with an ED50 of 80 µM (Fig. 1A).
Fig. 1.
Diprophylline-induced bidirectional modulation (BM) on jejunal contractility. (A1) Representative traces and (A2) statistical analysis of concentration-response relationship for diprophylline-indued effects on jejunal contractility. (B1) Representative traces and (B2) statistical analysis of diprophylline-exerted stimulatory effects on the contractility of jejunal segments in 6 low contractile states (LCS). (C1) Representative traces and (C2) statistical analysis of diprophylline-exerted inhibitory effects on the contractility of jejunal segments in 6 high contractile states (HCS). Contractile amplitude of jejunal segment in normal contractile state is set to 100% (NC). Data are expressed as the mean±SEM (% NC, n=6). ##p<0.01 compared with NC. **p<0.01 compared with the contractile amplitudes in LCS and the contractile amplitudes in HCS before diprophylline treatment respectively. CP, constipation-prominent; DP, diarrhea-prominent; SNP, sodium nitropr usside; ACh, acetylcholine.
Relationship between jejunal contractile state and diprophylline-induced effects
To characterize diprophylline-induced effects, 6 high and 6 low contractile states of rat jejunal segments were established. The jejunal contractility in both low and high contractile states was statistically different from that in normal contractile state. Diprophylline (20 µM) was used in the contractility determination, based on the observation that diprophylline could induce BM on jejunal contractility in a concentration range of 10~40 µM.
Diprophylline exerted stimulatory effects on the jejunal contractility in all 6 low contractile states (Fig. 1B), and conversely, exerted inhibitory effects on the jejunal contractility in all 6 high contractile states (Fig. 1C).
Underlying mechanisms involved in diprophylline-induced bidirectional modulation
The following assays were designed to reveal the underlying mechanisms involved in diprophylline-induced BM, including the role of enteric nervous system, pacemaker activity of ICCs, Ca2+, contraction/relaxation-related receptors, activity of nitric oxide synthases, and myosin light chain phosphorylation.
1. Links between enteric nervous system and diprophylline-induced bidirectional modulation
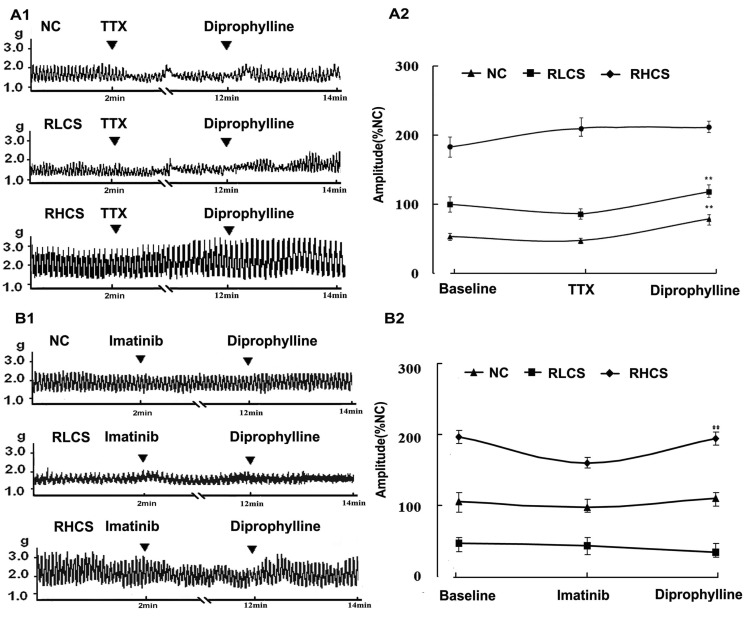
To characterize the effects of diprophylline on the jejunal contractility in the absence of neural control, TTX, a potent neurotoxin, was used to block action potentials by inhibiting the movement of Na+ across neural membranes. Diprophylline exerted significant stimulatory effects on jejunal contractility in RLCS (Fig. 1B) and significant inhibitory effects on jejunal contractility in RHCS (Fig. 1C). However, in the presence of TTX, diprophylline only exerted stimulatory effects on jejunal contractility in normal contractile state, RLCS, and RHCS (Fig. 2A).
Fig. 2.
Effects of diprophylline on jejunal contractility pretreated with tetrodotoxin (TTX) and imatinib respectively. (A1) Representative traces and (A2) statistical analysis of diprophylline (20 µM)-induced effects on jejunal segment pretreated with tetrodotoxin (0.1µM). (B1) Representative traces and (B2) statistical analysis of diprophylline on the contractility of jejunal segment pretreated with c-Kit tyrosine kinase blocker imatinib. Contractile amplitude of jejunal segment in normal contractile state is set to100% (normal control, NC). Data are expressed as the mean±SEM (% NC, n=6). **p<0.01 compared with contractile amplitude of jejunal segment after treatment with TTX or imatinib. RLCS, representative low contractile state; RHCS, representative high contractile state.
2. Links between pacemaker activity of interstitial cells of Cajal and diprophylline-induced bidirectional modulation
Tyrosine kinase inhibitor imatinib (5 µM, 5 min) did not affect jejunal contractility in either RLCS or RHCS. However, in the presence of imatinib (5 µM, 5 min), both diprophylline-exerted stimulatory effects on jejunal segment in RLCS and inhibitory effects on jejunal segment in RHCS were abolished (Fig. 2B).
3. Links between Ca2+ and diprophylline-induced bidirectional modulation
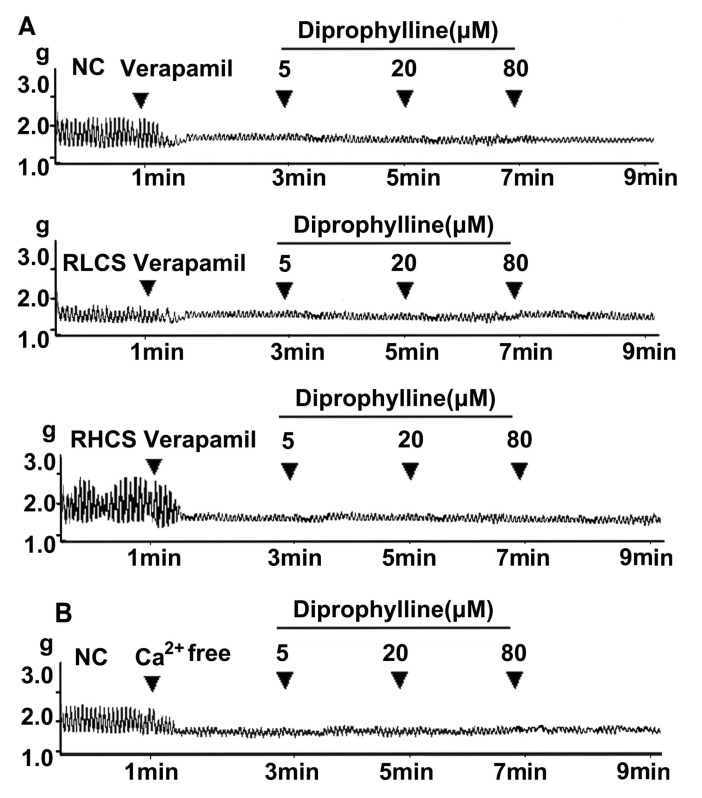
Diprophylline, at final concentrations of 5, 20, and 80 µM, did not affect jejunal contractility in a Ca2+-free assay condition (Fig. 3B), and diprophylline (20 µM) did not affect jejunal contractility pre-incubated with the Ca2+channel blocker verapamil in normal contractile state, RLCS, and RHCS (Fig. 3A).
Fig. 3.
Links between Ca2+ and diprophylline-induced effects. (A) Representative traces of diprophylline (5~80 µM)-induced effects on the contractility of jejunal segment pre-treated with verapamil (0.1 µM) in normal contractile state (NC), representative low contractile state (RLCS) and representative high contractile state (RHCS). (B) Representative traces of the effects of diprophylline (5~80 µM) on the contractility of jejunal segment assayed in Ca2+-free Krebs buffer.
4. Receptors and enzymes potentially involved in diprophylline-induced bidirectional modulation
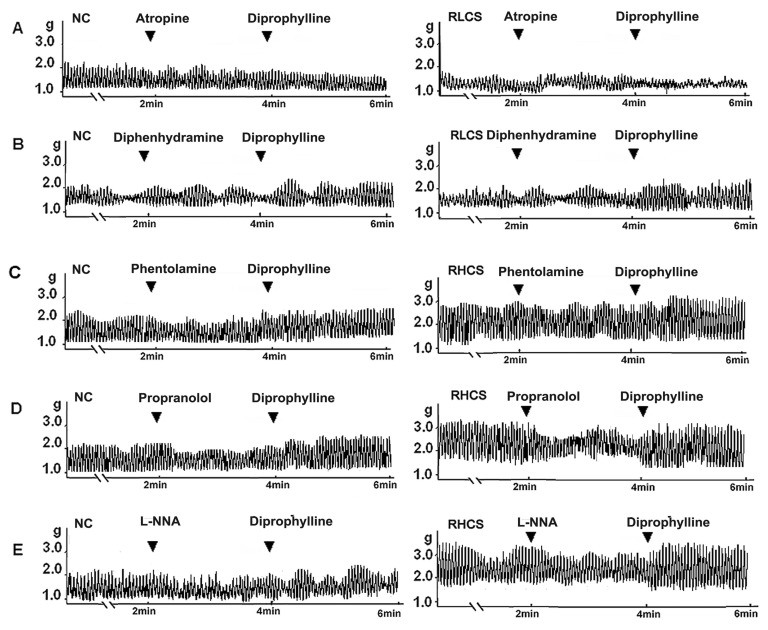
Muscarinic receptor antagonist atropine blocked the stimulatory effects of diprophylline on jejunal contractility in RLCS. Histamine H1-receptor antagonist diphenhydramine did not affect diprophylline-induced stimulatory effects on jejunal contractility in RLCS. Alpha-adrenergic receptor antagonist phentolamine, beta-adrenergic receptor antagonist propranolol, and nitric oxide synthase inhibitor L-N-Nitro-Arginine (L-NNA) blocked diprophylline-induced inhibitory effects on jejunal contractility in RHCS, respectively (Fig. 4).
Fig. 4.
Receptors and enzymes potentially involved in diprophylline-induced bidirectional modulation. Effects of diprophylline on the jejunal contractility pretreated with (A) 10 µM atropine and (B) 10 µM diphenhydramine respectively in normal contractile state (NC) and in representative low contractile state (RLCS). Effects of diprophylline on jejunal contractility, pretreated with (C) 10 µM phentolamine, (D) 5 µM propranolol, and (E) 10 µM L-NG-nitroargenine (L-NNA) respectively in NC and representative high contractile state (RHCS).
5. Diprophylline-induced bidirectional modulation on myosin-phosphorylation
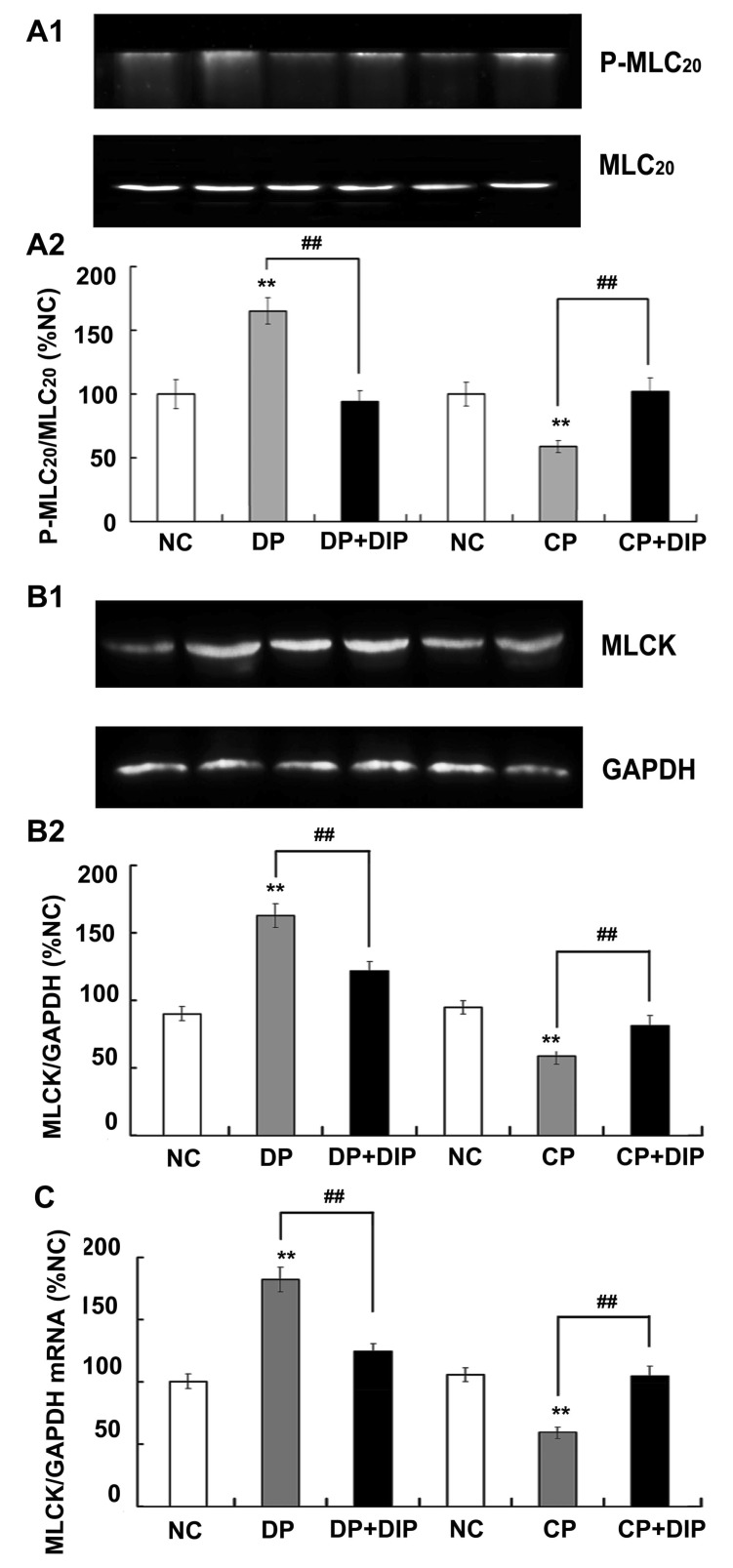
Myosin light chain phosphorylation is positively related to the smooth muscle contractility. The effects of diprophylline on myosin phosphorylation were observed directly from the determination of the extent of P-MLC20 and measurement of protein contents and mRNA expression of MLCK. As shown in Fig. 5A, P-MLC20 in CP rats and DP rats was significantly decreased and increased respectively compared with that in normal rats. Gavage of diprophylline (20 mg/kg) once daily for 7 days, P-MLC20 was significantly increased in diprophylline-treated CP rats compared with that of untreated CP rats and decreased in diprophylline-treated DP rats compared with that of untreated DP rats. The effects of diprophylline on MLCK protein contents were in accordance with the effects of diprophylline on the extent of P-MLC20 (Fig. 5B). As indicated in Fig. 5C, MLCK mRNA expression in CP rats was significantly decreased compared with that in normal rats; MLCK mRNA expression in diprophylline-treated CP rats was significantly increased. Results obtained from DP rats indicated that MLCK mRNA expression was significantly increased compared with that in normal rats; the expression of MLCK mRNA in diprophylline-treated DP rats was significantly decreased. The effects of diprophylline on MLCK protein contents were in accordance with the effects of diprophylline on MLCK mRNA expression.
Fig. 5.
Effects of diprophylline on myosin phosphorylation. (A1) Representative traces and (A2) statistical analysis of the phosphorylation of 20-kDa regulatory light chain of myosin (p-MLC20). (B1) Representative traces and (B2) statistical analysis of the protein content of myosin light chain kinase (MLCK). (C) Statistical analysis of the expression of MLCK mRNA. Data obtained from normal control (NC) is set to 100%. Data are expressed as the mean±SEM (% NC, n=6). **p<0.01 compared with the NC. ##p<0.01 compared with the jejunal contractility before diprophylline treatment. CP, Constipation-prominent; DP, diarrhea-prominent; DIP, diprophylline.
DISCUSSION
In the present study, we investigated diprophylline-induced BM on rat jejunal contractility. Diprophylline exerted two types of BM with different characteristics. One was that diprophylline increased and decreased rat jejunal contractility in a concentration range of 5~40 µM and 80~160 µM respectively. Such BM did not trigger our interest because diprophylline in high concentration might induce potential toxicity. The other type of BM was that diprophylline at the fixed concentration of 20 µM exerted stimulatory on jejunal segments in 6 low contractile states and inhibitory on jejunal segments in 6 high contractile states. Different from quantitative structure-activity relationships which seeks mathematical equations that correlate structural descriptors with activities of drugs [19], and different from BM induced by a mixture which contains different chemical compounds, diprophylline-induced BM depends on the contractile states of jejunal segment featured by modulating the abnormal low or high contractility to normal. To characterize and reveal diprophylline-induced BM, the present study investigated the potential role of enteric nervous system, Ca2+, stimulation/inhibition-correlated receptor and enzyme, and myosin phosphoralation related mechanisms.
In the presence of TTX which is a blocker of neuronal conduction [20,21], diprophylline only exerted stimulatory effects on jejunal contractility in normal contractile state, RLCS, and RHCS, suggesting that diprophylline-induced BM requires the presence of enteric nervous system.
In a Ca2+-free assay condition or in the presence of verapamil, which is a Ca2+ channel blocker [22], diprophylline did not exert further stimulatory or inhibitory effects, suggesting that diprophylline-induced BM is Ca2+ dependent.
ICCs, the pacemarker cells, physiologically generate electrical slow waves in the gastrointestinal tract. ICCs express the c-Kit tyrosine kinase that is essential for the ICC phenotype development and is possibly required for spontaneous electrical and mechanical activities [23,24]. The diprophylline-induced BM on jejunal contractility was abolished by imatinib an inhibitor of c-Kit tyrosine kinase indicating that diprophylline-induced BM is related to the cycles of slow waves originated from ICCs.
Activation of muscarinic receptor and histamine H1 receptor increases intestinal motility and activation of alpha and beta-adrenoceptors inhibits intestinal motility [25-27]. Nitric oxide is a nonadrenergic, noncholinergic inhibitory neurotransmitter to mediate smooth muscle relaxation in the gastrointestinal tract [28]. The results demonstrated that atropine rather than histamine H1 receptor antagonist diphenhydramine blocked diprophylline-exerted stimulatory effects on jejunal contractility in RLCS, implicating that the stimulatory effects of atropine is correlated to muscarinic receptor linked stimulation, In contrast, phentolamine, propranolol and L-NNA blocked diprophylline- exerted inhibitory effects on jejunal contractility in RHCS, respectively, suggesting that the inhibitory effects of diprophylline are correlated to the activation of adrenergic alpha, beta receptor and the nitric oxide synthase-linked mechanisms.
The activated MLCK phosphorylates the myosin light chains, which allows cross-bridge interaction with actin and smooth-muscle contraction [29]. Diprophylline exerted stimulatory and inhibitory effects on P-MLC20, MLCK protein contents and MLCK mRNA expression in jejunal segments isolated from CP and DP rats respectively, suggesting that the diprophylline-exerted BM on myosin light chain phosphorylation results from the change in contents and activity of MLCK.
In summary, the present study found for the first time that low and high contractile states of rat jejunal segment decided whether diprophylline-induced effects were stimulatory or inhibitory respectively, showing the characteristics of contractile state dependant BM rather than structure-activity relationship. Diprophylline-induced stimulatory effects in a low contractile state were found to be correlated with cholinergic activation and inhibitory effects in a high contractile state were correlated with the adrenergic activation and nitric oxide synthase-linked mechanisms. Diprophylline-induced BM required the presence of enteric nervous system, Ca2+, and ICCs. In accordance with the effects on jejunal contractility, diprophylline also exerted BM on MLCK contents, regulating myosin phosphorylation. However, the assays should still be considered preliminary, since enteric nervous system is highly interconnected and responsible for secreting at least 50 different modulators and the relationships between these modulators in regulating intestinal motility remain unclear [30]. The results suggest that diprophylline-induced BM can be considered as a potential candidate in relieving alternating symptoms of constipation (hypo-motility) and diarrhea (hyer-motility).
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (Grant No. 30772601). The authors wish to thank Zhi Lin and Fan Yuan for their comments.
ABBREVIATIONS
- BM
bidirectional modulation
- MLCK
myosin light chain kinase
- DP
diarrhea-prominent
- CP
constipation-prominent
- PMLC20
phosphorylation of 20-kDa regulatory light chain subunit of myosin
- TTX
Tetrodotoxin
- RLCS
representative low contractile state
- RHCS
representative high contractile state
- ACh
acetylcholine
- SNP
sodium nitroprusside
- L-NNA
L-N-Nitro-Arginine
- ICCs
interstitial cells of Cajal
- NC
normal control
References
- 1.Xia Z, Ni Y, Kokot S. Simultaneous determination of caffeine, theophylline and theobromine in food samples by a kinetic spectrophotometric method. Food Chem. 2013;141:4087–4093. doi: 10.1016/j.foodchem.2013.06.121. [DOI] [PubMed] [Google Scholar]
- 2.Nadai M, Apichartpichean R, Hasegawa T, Nabeshima T. Pharmacokinetics and the effect of probenecid on the renal excretion mechanism of diprophylline. J Pharm Sci. 1992;81:1024–1027. doi: 10.1002/jps.2600811014. [DOI] [PubMed] [Google Scholar]
- 3.Huang WS, Lin SJ, Wu HL, Chen SH. Simultaneous determination of theophylline and dyphylline by micellar electrokinetic chromatography and application in drug formulations. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;795:329–335. doi: 10.1016/s1570-0232(03)00601-9. [DOI] [PubMed] [Google Scholar]
- 4.Paterson N. High-performance liquid chromatographic method for the determination of diprophylline in human serum. J Chromatogr. 1982;232:450–455. doi: 10.1016/s0378-4347(00)84189-4. [DOI] [PubMed] [Google Scholar]
- 5.Gordjani N, Burghard R, Leititis JU, Brandis M, Püschel CH, Gundert-Remy U. Acute intoxication with theophylline, proxyphylline and diprophylline in a 3-month-old infant after rectal application: pharmacokinetic data under hemoperfusion. Acta Paediatr Scand. 1990;79:112–114. doi: 10.1111/j.1651-2227.1990.tb11342.x. [DOI] [PubMed] [Google Scholar]
- 6.Agbaba D, Zivanov-Stakić D, Vukićević N. Dissolution assay of theophylline, diprophylline and proxyphylline from a sustained release dosage form by high performance thin layer chromatography. Biomed Chromatogr. 1992;6:141–142. doi: 10.1002/bmc.1130060309. [DOI] [PubMed] [Google Scholar]
- 7.Kang J. Effects of diprophylline on pulmonary hypertension in patients with chronic pulmonary disease. Zhonghua Jie He He Hu Xi Za Zhi. 1992;15:153–154. 190. [PubMed] [Google Scholar]
- 8.Zuidema J, Merkus FWHM. Pharmacokinetics and pharmacodynamics of diprophylline. Pharm Weekbl. 1981;3:1320–1325. [Google Scholar]
- 9.McEvoy GK American Society of Health-System Pharmacists. AHFS Drug information 2001. Bethesda: American Society of Health-System Pharmacists; c2001. p. 2296.p. 3486. [Google Scholar]
- 10.Schemann M. Control of gastrointestinal motility by the "gut brain"--the enteric nervous system. J Pediatr Gastroenterol Nutr. 2005;41(Suppl 1):S4–S6. doi: 10.1097/01.scs.0000180285.51365.55. [DOI] [PubMed] [Google Scholar]
- 11.La JH, Kim TW, Sung TS, Kang JW, Kim HJ, Yang IS. Visceral hypersensitivity and altered colonic motility after subsidence of inflammation in a rat model of colitis. World J Gastroenterol. 2003;9:2791–2795. doi: 10.3748/wjg.v9.i12.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou BC, Dong L, Wang Y, Wang SH, Cao MB. Expression and role of 5-HT7 receptor in brain and intestine in rats with irritable bowel syndrome. Chin Med J (Engl) 2007;120:2069–2074. [PubMed] [Google Scholar]
- 13.Tanović A, Fernández E, Jiménez M. Alterations in intestinal contractility during inflammation are caused by both smooth muscle damage and specific receptor-mediated mechanisms. Croat Med J. 2006;47:318–326. [PMC free article] [PubMed] [Google Scholar]
- 14.Frings M, Haschke G, Heinke B, Schäfer KH, Diener M. Spontaneous contractions of intestinal smooth muscle re-aggregates from the new-born rat triggered by thromboxane A2. J Vet Med A Physiol Pathol Clin Med. 2000;47:469–475. doi: 10.1046/j.1439-0442.2000.00306.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen D, Xiong Y, Wang L, Lv B, Lin Y. Characteristics of emodin on modulating the contractility of jejunal smooth muscle. Can J Physiol Pharmacol. 2012;90:455–462. doi: 10.1139/y2012-004. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi K, Murata T, Hori M, Ozaki H. Prostaglandin E2-prostanoid EP3 signal induces vascular contraction via nPKC and ROCK activation in rat mesenteric artery. Eur J Pharmacol. 2011;660:375–380. doi: 10.1016/j.ejphar.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 17.Iwabu A, Smith K, Allen FD, Lauffenburger DA, Wells A. Epidermal growth factor induces fibroblast contractility and motility via a protein kinase C delta-dependent pathway. J Biol Chem. 2004;279:14551–14560. doi: 10.1074/jbc.M311981200. [DOI] [PubMed] [Google Scholar]
- 18.Chitano P, Voynow JA, Pozzato V, Cantillana V, Burch LH, Wang L, Murphy TM. Ontogenesis of myosin light chain kinase mRNA and protein content in guinea pig tracheal smooth muscle. Pediatr Pulmonol. 2004;38:456–464. doi: 10.1002/ppul.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruiz IL, Urbano-Cuadrado M, Gómez-Nieto MÁ. Improving the development of QSAR prediction models with the use of approximate similarity approach. Engineering Letters. 2008;16:36–43. [Google Scholar]
- 20.Neunlist M, Toumi F, Oreschkova T, Denis M, Leborgne J, Laboisse CL, Galmiche JP, Jarry A. Human ENS regulates the intestinal epithelial barrier permeability and a tight junction-associated protein ZO-1 via VIPergic pathways. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1028–G1036. doi: 10.1152/ajpgi.00066.2003. [DOI] [PubMed] [Google Scholar]
- 21.Seiler R, Rickenbacher A, Shaw S, Balsiger BM. alpha- and beta-adrenergic receptor mechanisms in spontaneous contractile activity of rat ileal longitudinal smooth muscle. J Gastrointest Surg. 2005;9:227–235. doi: 10.1016/j.gassur.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Han JH, Chang IH, Myung SC, Lee MY, Kim WY, Lee SY, Lee SY, Lee SW, Kim KD. A novel pathway underlying the inhibitory effects of melatonin on isolated rat urinary bladder contraction. Korean J Physiol Pharmacol. 2012;16:37–42. doi: 10.4196/kjpp.2012.16.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanders KM, Ordög T, Koh SD, Torihashi S, Ward SM. Development and plasticity of interstitial cells of Cajal. Neurogastroenterol Motil. 1999;11:311–338. doi: 10.1046/j.1365-2982.1999.00164.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim BJ, Kwon YK, Kim E, So I. Effects of histamine on cultured interstitial cells of cajal in murine small intestine. Korean J Physiol Pharmacol. 2013;17:149–156. doi: 10.4196/kjpp.2013.17.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li M, Johnson CP, Adams MB, Sarna SK. Cholinergic and nitrergic regulation of in vivo giant migrating contractions in rat colon. Am J Physiol Gastrointest Liver Physiol. 2002;283:G544–G552. doi: 10.1152/ajpgi.00114.2001. [DOI] [PubMed] [Google Scholar]
- 26.Konturek SJ, Siebers R. Role of histamine H1- and H2-receptors in myoelectric activity of small bowel in the dog. Am J Physiol. 1980;238:G50–G56. doi: 10.1152/ajpgi.1980.238.1.G50. [DOI] [PubMed] [Google Scholar]
- 27.Gaddum JH, Holzbauer M. Adrenaline and noradrenaline. Vitam Horm. 1957;15:151–203. doi: 10.1016/s0083-6729(08)60510-5. [DOI] [PubMed] [Google Scholar]
- 28.Kim YC, Choi W, Yun HY, Sung R, Yoo RY, Park SM, Yun SJ, Kim MJ, Song YJ, Xu WX, Lee SJ. Nitric Oxide-mediated Relaxation by High K in Human Gastric Longitudinal Smooth Muscle. Korean J Physiol Pharmacol. 2011;15:405–413. doi: 10.4196/kjpp.2011.15.6.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winquist RJ. Modulators of intracellular calcium. Drug Develop Res. 1984;4:241–256. [Google Scholar]
- 30.Goldstein AM, Nagy N. A bird's eye view of enteric nervous system development: lessons from the avian embryo. Pediatr Res. 2008;64:326–333. doi: 10.1203/PDR.0b013e31818535e8. [DOI] [PMC free article] [PubMed] [Google Scholar]