MicroRNAs (miRNAs) are short, single-stranded, non-coding, highly conserved posttranscriptional regulators found in a variety of tissues including circulating blood.1 MicroRNAs regulate gene expression posttranscriptionally through inhibiting translation from and/or inducing degradation of specific RNAs.2 Certain miRNAs are expressed and function cell-type specifically or in association with particular physiological processes. MicroRNAs contribute to cardiac development and remodeling, and miRNAs found in cardiac tissue show dynamic changes in the setting of heart disease, suggesting their involvement in the regulation of cardiovascular disease (CVD). Mouse and human studies have emphasized the importance of individual miRNAs in different forms of CVD, including myocardial infarction (mirR-1, miR-133a, miR-208a, and miR-499), atrial and ventricular arrhythmias (miR-1, miR-133, and miR-328), fibrosis (miR-21 and miR-29), and ventricular hypertrophy (miR-208 and miR-133).3–7
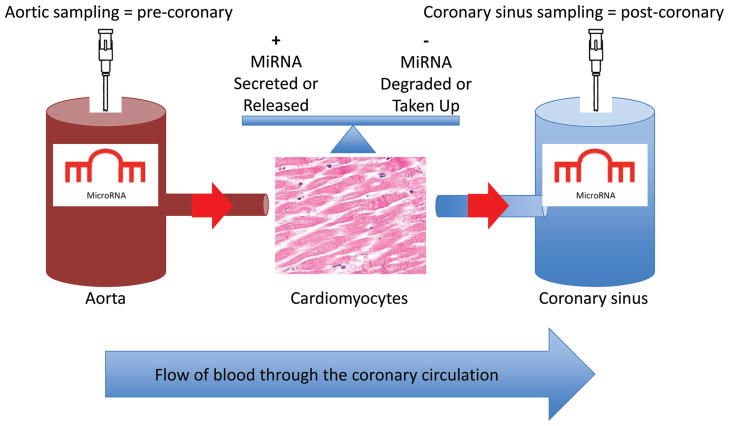
Remarkably, miRNAs can be detected circulating in blood plasma or serum as a result of cellular damage or secretion.8 In contrast to intracellular miRNAs and other extracellular disease mediators, circulating miRNAs have ideal characteristics as risk markers because they are stable and easily detectable (miRNA are comprised of nucleic acids, and their sequences can be amplified).9 Circulating miRNAs have been associated with CVD risk factors (eg, hypertension and diabetes mellitus), and there is an emerging literature associating specific miRNAs with coronary artery disease, myocardial infarction, and heart failure.8,10–12 Although the cellular secretion of specific miRNA suggests process specificity, the source of circulating miRNAs associated with CVD remains unclear in many cases. In the current issue of Circulation,13 De Rosa et al fill a major gap in our knowledge by investigating whether or not circulating miRNAs associated with acute coronary syndrome (ACS) are produced by the human heart. In 57 patients undergoing cardiac catheterization for suspected ACS, they measured the transcoronary concentration gradients of vascular, leukocyte, and platelet-enriched miRNAs (Figure). They report that circulating levels of cardiomyocyte-enriched miR-499, miR-133a, and miR-208a are elevated and correlate with troponin levels in patients with troponin-positive ACS compared with patients with coronary artery disease.
Figure.
Experimental scheme: transcoronary concentration gradients of circulating microRNA. miRNA indicates microRNA.
De Rosa et al also report that levels of cardiomyocyte-enriched miRs-499 and 133a increase across the coronary circulation in patients with troponin-positive ACS and that this is not seen in patients without elevated troponin levels. In demonstrating that miRNA levels differ in plasma obtained directly from the coronary venous sinus and proximal aorta (Figure) of patients with a troponin-positive ACS, De Rosa et al confirm and extend the findings of a study by Widera et al involving 444 patients with ACS, in which patients with myocardial infarction were shown to have higher circulating levels of miR-1, miR-133a, and miR-208b compared with patients who had unstable angina.14 Although the present study does not determine whether or not increased expression or release are responsible for the observed transcoronary miRNA gradients, another study by Kuwabara et al suggests that cardiomyocyte injury promotes increased secretion of exosomes containing miRNAs 133a and 499.15
Intracellular levels of miRNAs provide information about regulatory pathways involved in mediating the cardiomyocyte injury response. MicroRNA 133a, for example, has been shown to target the ion-channel–encoding genes HCN2 and HCN4.6,16 mir-208a is encoded in the intron of the α-myosin heavy chain gene and indirectly influences β-myosin heavy chain and connexin-40 expression,17 and mir-499 is intronic to the myosin gene Myh7b.17 Intronic miRNA are coded within a host gene, often regulating pathways similar to those of the protein encoded by that gene and frequently acting to regulate a single biological process.18 Whether circulating miRNAs such as miR-133a and miR-499 function as signaling molecules or are simply released after cardiomyocyte injury remains unknown, but the functional redundancy of miRNAs would suggest that miRNAs help to mitigate biological perturbations such as occur during an ACS.19 Although De Rosa’s findings firmly establish that miR-133a and miR-499 are produced by the heart and are specific to an ACS, important questions remain unanswered, such as whether or not secreted miRNAs serve to influence gene regulation in other parts of the heart (eg, in peri-infarct or noninfarcted zones) or in other organs.
As evidenced by the authors’ surprising finding that miR-126 levels decrease across the transcoronary circulation (Figure), much remains unknown about the acute processes regulating circulating miRNA levels, especially in the setting of an ACS. The authors propose that the negative miR-126 transcoronary gradient may be explained by the secretion of proteases and/or RNases by injured or ischemic myocytes. Although nonspecific degradation of circulating proteins conjugated to miRNAs or miRNAs themselves may explain the negative miR-126 transcoronary gradient, an alternative hypothesis is that miRNAs such as miR-126 function as signaling molecules and are taken up by injured cardiomyocytes in a specific fashion. More information is needed about the biochemical composition of important circulating miRNAs and how this relates to miRNA stability and uptake.20
Modulation of miRNAs has opened exciting opportunities for miRNA-based therapies in the treatment of CVD.21 Indeed, miRNA mimics and anti-miRs are currently under development and have been advanced to clinical trials.19 Further study is warranted of circulating miRNA secretion, clearance and potential function in patients with CVD. In addition to the potential role of miRNA as biomarkers, miRNAs will provide new insights into CVD pathogenesis and new applications for CVD prevention and treatment in the near future.
Acknowledgments
Sources of Funding
This work was supported by 1U01HL105268-01 from the National Heart Lung and Blood Institute (to D.D.M.) and by R01GM34028 from the National Institute for General Medical Sciences (to V.A.).
Footnotes
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
Disclosures
None.
References
- 1.Lee RC, Ambros V. An extensive class of small RNAs in Caenorhabditis elegans. Science. 2001;294:862–864. doi: 10.1126/science.1065329. [DOI] [PubMed] [Google Scholar]
- 2.Lee R, Feinbaum R, Ambros V. A short history of a short RNA. Cell. 2004;116(Suppl):S89–S92. doi: 10.1016/s0092-8674(04)00035-2. 1 p following S96. [DOI] [PubMed] [Google Scholar]
- 3.Callis TE, Pandya K, Seok HY, Tang RH, Tatsuguchi M, Huang ZP, Chen JF, Deng Z, Gunn B, Shumate J, Willis MS, Selzman CH, Wang DZ. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J Clin Invest. 2009;119:2772–2786. doi: 10.1172/JCI36154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo X, Lin H, Pan Z, Xiao J, Zhang Y, Lu Y, Yang B, Wang Z. Down-regulation of miR-1/miR-133 contributes to re-expression of pacemaker channel genes HCN2 and HCN4 in hypertrophic heart. J Biol Chem. 2008;283:20045–20052. doi: 10.1074/jbc.M801035200. [DOI] [PubMed] [Google Scholar]
- 5.Matkovich SJ, Wang W, Tu Y, Eschenbacher WH, Dorn LE, Condorelli G, Diwan A, Nerbonne JM, Dorn GW., II MicroRNA-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts. Circ Res. 106:166–175. doi: 10.1161/CIRCRESAHA.109.202176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shan H, Zhang Y, Lu Y, Pan Z, Cai B, Wang N, Li X, Feng T, Hong Y, Yang B. Downregulation of miR-133 and miR-590 contributes to nicotine-induced atrial remodelling in canines. Cardiovasc Res. 2009;83:465–472. doi: 10.1093/cvr/cvp130. [DOI] [PubMed] [Google Scholar]
- 7.Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, Zhang Y, Xu C, Bai Y, Wang H, Chen G, Wang Z. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13:486–491. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 8.Laterza OF, Lim L, Garrett-Engele PW, Vlasakova K, Muniappa N, Tanaka WK, Johnson JM, Sina JF, Fare TL, Sistare FD, Glaab WE. Plasma microRNAs as sensitive and specific biomarkers of tissue injury. Clin Chem. 2009;55:1977–1983. doi: 10.1373/clinchem.2009.131797. [DOI] [PubMed] [Google Scholar]
- 9.Wang K, Zhang S, Marzolf B, Troisch P, Brightman A, Hu Z, Hood LE, Galas DJ. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci U S A. 2009;106:4402–4407. doi: 10.1073/pnas.0813371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng Y, Tan N, Yang J, Liu X, Cao X, He P, Dong X, Qin S, Zhang C. A translational study of circulating cell–free microRNA-1 in acute myocardial infarction. Clin Sci (Lond) 119:87–95. doi: 10.1042/CS20090645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corsten MF, Dennert R, Jochems S, Kuznetsova T, Devaux Y, Hofstra L, Wagner DR, Staessen JA, Heymans S, Schroen B. Circulating microRNA-208b and microRNA-499 reflect myocardial damage in cardiovascular disease. Circ Cardiovasc Genet. 3:499–506. doi: 10.1161/CIRCGENETICS.110.957415. [DOI] [PubMed] [Google Scholar]
- 12.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Roxe T, Muller-Ardogan M, Bonauer A, Zeiher AM, Dimmeler S. Circulating microRNAs in patients with coronary artery disease. Circ Res. 107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 13.De Rosa S, Fichtlscherer S, Lehmann R, Assmus B, Dimmeler S, Zeiher AM. Transcoronary concentration gradients of circulating microRNAs. Circulation. 2011;124:1936–1944. doi: 10.1161/CIRCULATIONAHA.111.037572. [DOI] [PubMed] [Google Scholar]
- 14.Widera C, Gupta SK, Lorenzen JM, Bang C, Bauersachs J, Bethmann K, Kempf T, Wollert KC, Thum T. Diagnostic and prognostic impact of six circulating microRNAs in acute coronary syndrome. J Mol Cell Cardiol. 2011;51:872–875. doi: 10.1016/j.yjmcc.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Kuwabara Y, Ono K, Horie T, Nishi H, Nagao K, Kinoshita M, Watanabe S, Baba O, Kojima Y, Shizuta S, Imai M, Tamura T, Kita T, Kimura T. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ Cardiovasc Genet. 2011;4:446–454. doi: 10.1161/CIRCGENETICS.110.958975. [DOI] [PubMed] [Google Scholar]
- 16.Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Rooij E, Olson EN. Searching for miR-acles in cardiac fibrosis. Circ Res. 2009;104:138–140. doi: 10.1161/CIRCRESAHA.108.192492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutter D, Marr C, Krumsiek J, Lang EW, Theis FJ. Intronic microRNAs support their host genes by mediating synergistic and antagonistic regulatory effects. BMC Genomics. 2010;11:224. doi: 10.1186/1471-2164-11-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Rooij E, Olson EN. MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest. 2007;117:2369–2376. doi: 10.1172/JCI33099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39:7223–7233. doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Rooij E, Marshall WS, Olson EN. Toward microRNA-based therapeutics for heart disease: the sense in antisense. Circ Res. 2008;103:919–928. doi: 10.1161/CIRCRESAHA.108.183426. [DOI] [PMC free article] [PubMed] [Google Scholar]