Abstract
High adenine phosphate (HAP) diet serves as an animal model of chronic renal failure (RF). Induction of RF and establishment of end organ damage require long exposure periods to this diet. Previously, we have shown that RF is reversible after diet cessation even after protracted administration. In this study, we explored the underlying renal changes and cellular pathways occurring during administration and after cessation of the diet. Kidneys were obtained from rats fed HAP diet for 7 weeks, and from rats fed HAP diet followed a 10 week recovery period on normal diet. The kidneys of HAP diet group were significantly enlarged due to tubular injury characterized by massive cystic dilatation and crystal deposition. Kidney injury was associated with markers of apoptosis as well as with activation of apoptosis related pathways. Diet cessation was associated with a significant reduction in kidney size, tubules diameter, and crystals deposition. The recovery from renal injury was coupled with regression of apoptotic features. This is the first study showing the potential reversibility of long standing RF model, allowing optimal evaluation of uremia-chronic effects.
Keywords: Renal failure, Animal model, Apoptosis, Reversibility, Diet
Introduction
Renal failure (RF) is associated with significant morbidity and mortality due to severe metabolic and hormonal derangements (Go et al., 2004). Several animal models of RF are used for evaluation of organ damage pathogenesis (Bellomo et al., 2004). Models for chronic RF consist of surgical partial nephrectomy and administration of drugs, whereas models of acute RF are mostly induced by toxins, sepsis or ischemia (Wan et al. 2003; Wichterman et al., 1980; Heyman et al., 2000). In all of these models, RF is irreversible.
Orally administered adenine in rats is metabolized to 2,8-dihydroxyadenine, precipitates and forms tubular crystals leading to kidney injury (Koeda et al., 1998). Consequently, renal dysfunction with elevated serum levels of creatinine, phosphate and parathyroid hormone (PTH) ensues. Late complications of RF such as ectopic calcification and bone metabolic disorders require long duration of RF. Previously, it was suggested that high adenine phosphate (HAP) administration for periods longer than 4 weeks might be associated with irreversible RF organ changes (Katsumata et al., 2003). We recently demonstrated that even long term (7 weeks) HAP diet may result in organ damage which might be reversible after its interruption (Shuvy et al., 2008). Using this model, we induced RF that caused significant aortic valve calcification. RF resolved after diet cessation, and the calcification was markedly reduced. RF resolution was also accompanied by normalization of serum creatinine and phosphate levels. Understanding the cellular and molecular mechanisms of renal injury is highly important for developing acceptable therapeutic interventions. Exposure of renal cells to toxins may induce apoptosis, resulting in extensive cell loss (Padanilam, 2003). In this study, we focused on the pathogenesis of renal injury and evaluated histological changes and cellular apoptotic features in two time points – during administration and after cessation of the diet, demonstrating the reversibility of RF.
Methods
30 Sprague–Dawley female rats, 8 weeks old, weighing about 250 g, were used for the study. The protocol was approved by the Hebrew University Ethics Committee. Kidneys were obtained from 3 different groups (n=10 each). HAP diet group rats were fed exclusively with high-adenine (0.75%), high-phosphate (1.5%) diet (Teklad, Madison, WI, USA) for 7 weeks. The reversibility group rats were fed with the same diet for 7 weeks and then fed with normal rat chow for additional 10 weeks. Controls rats were fed with normal chow. At the end of the study period, all rats were anaesthetized with ketamine/xylazine and sacrificed by exsanguinations after blood sample was collected from abdominal aorta. Kidney tissue was excised and fixed with formalin.
Evaluation of biochemical profile
Plasma was analyzed for potassium, phosphate, calcium and creatinine, using VITRO system 5.1 chemistry (Ortho-Clinical Diagnostics, Johnson & Johnson, Rochester, NY).
Histopathological evaluation
The kidneys were trimmed mid-longitudinally, and 6 μm, paraffin embedded sections were prepared. These sections were stained for hematoxylin and eosin, as well as PAS stainings. A semi-quantitative grading method was applied, details are as follows: The degree of nephropathy changes were scored using 6 grades, based on the method suggested by Shackelford et al. (1992).
Grade 0 – no changes; Grade 1 – up to 10% of the tissue in section is occupied by nephropathy; Grade 2 – between 11% and 20% of the tissue in section is occupied by nephropathy; Grade 3 – between 21% and 40% of the tissue in section is occupied by nephropathy; Grade 4 – between 41% and 60% of the tissue in section is occupied by nephropathy; Grade 5 – between 61% and 100% of the tissue in section is occupied by nephropathy.
Apoptosis assessment using TUNEL
Apoptotic cells were identified with the TUNEL (terminal uridine nick-end labelling) assay carried out using the Promega DeadEndTM Fluorometric TUNEL system, based on a previously described method (Ben-Sasson et al., 1995). Slides were analyzed using a confocal laser scanning microscope (Zeiss 410, Carl Zeiss GmbH, Goettingen Germany).
Apoptosis assessment using western blot analysis
Aortic valves were obtained from all groups; the tissue was hydrolyzed, homogenized under ultrasound and boiled for 5 min. After quantification of the protein concentration, 12 mg of extracts was separated on 5–15% sodium dodecyl sulfate-polyacrylamide gradient gels, and transferred to nitrocellulose membranes. Polyclonal antibodies to caspase 3 and to cleaved caspase 3 were ordered from Sigma (St. Louis, MO, USA). Bands were detected after incubation with western blotting Luminol reagent. The bands on the X-ray film were quantified by scanning densitometry and compared with actin expression; the comparative results were expressed as arbitrary numbers.
Results
Variation of investigated parameters during the HAP diet phase and during the reversibility phase
Effect on electrolytes and kidney function
A biochemical profile was obtained for all groups; diet group rats developed a significant elevation in creatinine levels compared with controls (229 ± 27 vs. 84 ± 16 mmol/L p < 0.05). Phosphate levels were also significantly higher in the HAP diet group compared with controls (6.55 ± 0.13 vs. 2.54 ± 0.26 mmol/L p < 0.05). In the reversibility group renal failure resolved; creatinine and phosphate levels significantly decreased as compared with the diet group (115 ± 13 mmol/L and 1.69 ± 0.16 mmol/L both p < 0.05, respectively). There were no significant differences in potassium or total calcium levels.
Effect on histopathological parameters
On macroscopic evaluation, HAP diet induced significant enlargement of the kidney with granular appearance. Diet cessation resulted in decreased kidney size due to recovery of tubular cystic dilatation and regeneration areas (Fig. 1).
Fig. 1.
Macroscopic morphology of kidneys. Macroscopic morphology of kidneys obtained from 3 groups: 7-weeks adenine-treated, after reversibility and control. The HAP diet induced a significant increase in the size of the kidney due to the cystic dilatation of the tubules. After reversibility the kidney size was decreased and its surface became irregular.
No histopathological lesions were seen in the kidneys of control rats. The changes seen in both the HAP diet rats sacrificed after 7 weeks of treatment and in the rats of the reversibility group were defined as nephropathy. Those changes were characterized by the presence of tubular lumen deposits of 2,8-dihydroxyadenine (i.e., brownish crystals) together with increased basophilic staining of the lining epithelium (regeneration). However, in the HAP diet group’s kidneys, the tubules had more cystic dilation appearance, while in the reversibility group’s kidneys more prominent interstitial inflammation and fibrosis were noted, associated with reduced diameter of the tubules.
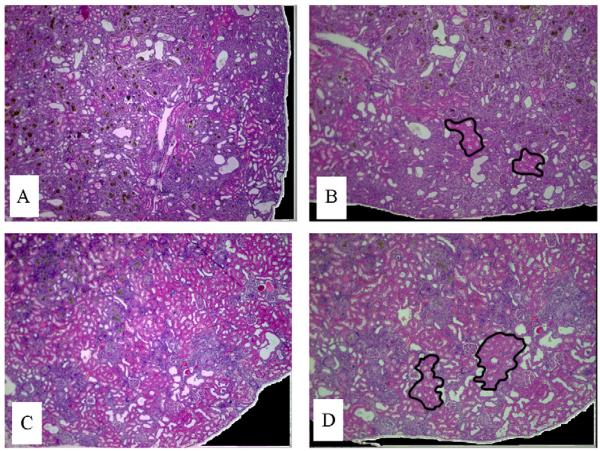
The most striking difference between the kidney histology of the two tested groups was the extent of the damaged area. In the HAP diet group, the damage area was very extensive, consistently involving 70–80% of the tissue (i.e., grade 5). In contrast, a significantly reduced damage area involving not more than 40% of the tissue (i.e., grade 3) was observed in the reversibility group (Fig. 2). The brownish crystals were still present in the tubular lumen only in the areas affected by the nephropathy. However, in the undamaged areas, the tubules seem to have normal basement membrane without interstitial inflammation, suggesting that these characteristics contributed to the resolution seen at this stage.
Fig. 2.
Histological evaluation of kidneys. Sections A and B were obtained from kidney of HAP diet treated rat; A – mid-longitudinal section of the kidney with extensive nephropathy area (i.e., 70–80% of tissue area – grade 5). B – two typical areas not involved by nephropathy are delineated. These areas are characterized by tubules lined by cells having normal eosinophilic-stained cytoplasm. Sections C and D were obtained from kidney of rat treated by HAP diet and then undergoing 10-week reversibility period; C – mid-longitudinal section demonstrating relatively reduced nephropathy area compared with A (i.e., up to 40% of the tissue area – grade 3). D – two typical areas not involved by nephropathy are delineated. These areas are characterized by tubule lined by cells having normal – eosinophilic-stained cytoplasm. In the areas of nephropathy, the brownish crystals were still present in the tubular lumen, as seen in the HAP diet treated rats. Note: for comparative purposes, photos A and B, and C and D, have the same respective magnification (×2).
Apoptosis assessment using TUNEL and caspase 3 protein levels
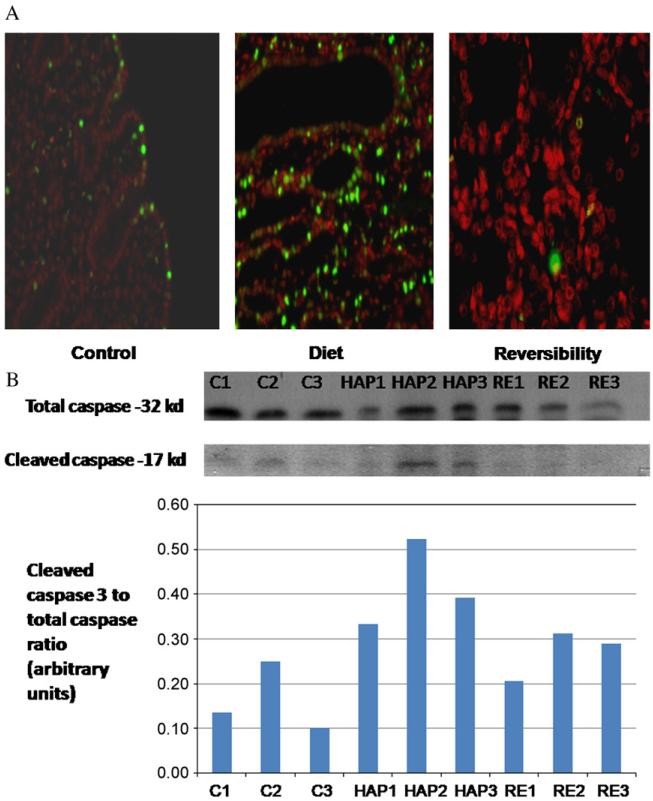
A significant amount of apoptotic cells were found in HAP diet kidneys. (Mean percentage of TUNEL-positive nuclei was 14 ± 4.5.) The apoptotic cells were confined to the tubuli, while the glomeruli were preserved. Only mild apoptosis was found in the controls (3.7 ± of apoptotic cells p < 0.05 compared with HAP diet group). Following diet cessation the number of apoptotic cells decreased, and equaled that of the controls (5.3 ± 2% p=NS as compared with the controls) (Fig. 3A).
Fig. 3.
Apoptosis assessment in kidneys. Apoptosis was assessed using two different methods: 3A – TUNEL staining (original magnification 50) demonstrating a significantly higher level of DNA fragmentation (orange) in HAP diet kidneys. Only scattered apoptotic cells were found in the control and reversibility kidneys. 3B – Western blot analysis and graphic presentation for cleaved caspase 3 and caspase 3 protein levels in control (C), HAP diet (HAP) and reversibility (RE) groups. The analysis demonstrates a significantly higher ratio of cleaved caspase 3 to caspase 3 in HAP diet kidneys. Both methods suggest that apoptosis plays an important part the pathogenesis of this model of RF.
Similarly, apoptosis was confirmed using analysis of caspase 3 and cleaved caspase 3 protein levels, which showed a significant increase in cleaved caspase 3 in the valves of the HAP kidneys compared with the reversibility and control kidneys. There were no significant changes in total caspase level between the groups (Fig. 3B). The elevated levels of cleaved caspase 3 reflect that apoptosis is associated with renal injury and that apoptosis features regress with improvement of renal injury.
Discussion
Our results suggest that HAP diet may serve as an animal model for reversible RF. HAP diet induces severe tubular injury characterized by massive cystic dilatation and deposition of 8-dihydroxyadenine. Renal damage is associated with activation of pro-apoptotic pathway and also with histological features of apoptosis. Western blot analysis of kidneys showed a significant increase in the level of cleaved caspase 3 — a final common pathway and a primary executioner of apoptosis (Kothakota et al., 1997). Diet cessation brought about a significant reduction in damaged area and reduction of the diameter of the tubules, as well as resolution of apoptotic markers.
The role of apoptosis in renal injury emerged from the recognition that apoptosis is triggered by the same cellular insults that precipitate necrosis. It was suggested that apoptosis commonly results when renal cells are exposed to less severe injury that is needed to induce necrosis. The importance of apoptosis in mediating renal injury was shown in both in vivo and in vitro models of renal damage (Padanilam, 2003; Schumer et al., 1992; Lieberthal and Levine, 1996). Furthermore, overexpression of the anti-apoptotic protein Bcl-2 has been shown to prevent apoptosis in renal proximal tubules (Lin et al., 1999).
Resolution of RF depends on the ability of the tubular cells to regenerate and reline the damaged areas along the nephron (Miller et al., 1992). Decline in apoptosis and regeneration of the renal tubule brings about a new equilibrium between cell apoptosis and renewal. The reversibility of the process and the ability to modify apoptosis gives hope for several therapeutic options that might alleviate renal injury
The unique characteristics of this model may open new horizons in the study of RF and its sequels. The relatively long duration of RF allows evaluation of the pathophysiological processes underlying the long term effects, such as vascular calcification, inflammation and bone disease. The reversibility of this model may give an insight into the pathological processes involved in RF. As far as we know this is the first study showing the potential reversibility of a model of chronic RF. Further studies are required in order to more accurately define the degree and the mechanism of the reversibility process.
Acknowledgments
Funding
The Chief Scientist Office of the Ministry of Health, Israel; The Berman Foundation for Cardiovascular Research of Hadassah Medical Center; The Joint Research Fund of the Hebrew University and Hadassah Medical Center.
References
- Bellomo R, Ronco C, Kellum J, Mehta R, Palevsky P, The ADQI workgroup Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Critical Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson S, Sherman Y, Gavrieli Y. Identification of dying cells – in situ staining. Methods Cell Biol. 1995;46:29–39. [PubMed] [Google Scholar]
- Go A, Chertow GM, Fan D, McCulloch CE, Hsu C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- Heyman SN, Rosen S, Darmon D, Goldfarb M, Bitz H, Shina A, et al. Endotoxin-induced renal failure: II. A role for tubular hypoxic damage. Exp Nephrol. 2000;8:275–82. doi: 10.1159/000020679. [DOI] [PubMed] [Google Scholar]
- Katsumata K, Kenichiro K, Michinori H, Kunihiko T, Nobuo N, Steven KB, et al. Sevelamer hydrochloride prevents ectopic calcification and renal osteodystrophy in chronic renal failure rats. Kidney Int. 2003;64:441–50. doi: 10.1046/j.1523-1755.2003.00126.x. [DOI] [PubMed] [Google Scholar]
- Koeda T, Wakaki K, Koizumi F, Yokozawa T, Oura H. Early changes of proximal tubules in the kidney of adenine-ingesting rats, with special reference to biochemical and electron microscopic studies. Nippon Jinzo Gakkai Shi. 1998;30:239–46. [PubMed] [Google Scholar]
- Kothakota S, Azuma C, Reinhard A, Klippel J, Tang K, Chu TJ, et al. Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science. 1997;278:294–8. doi: 10.1126/science.278.5336.294. [DOI] [PubMed] [Google Scholar]
- Lieberthal W, Levine JS. Mechanisms of apoptosis and its potential role in renal tubular epithelial cell injury. Am J Physiol Renal Physiol. 1996;271:F477–88. doi: 10.1152/ajprenal.1996.271.3.F477. [DOI] [PubMed] [Google Scholar]
- Lin HH, Yang TP, Jiang ST, Yang HY, Tang MJ. Bcl-2 overexpression prevents apoptosis-induced Madin–Darby canine kidney simple epithelial cyst formation1. Kidney Int. 1999;55:168–78. doi: 10.1046/j.1523-1755.1999.00249.x. [DOI] [PubMed] [Google Scholar]
- Miller SB, Martin DR, Kissane J, Hammerman MR. Insulin-like growth factor I accelerates recovery from ischemic acute tubular necrosis in the rat. Proc Nat Acad Sci USA. 1992;89:11876–80. doi: 10.1073/pnas.89.24.11876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padanilam BJ. Cell death induced by acute renal injury: a perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol. 2003;284:F608–27. doi: 10.1152/ajprenal.00284.2002. [DOI] [PubMed] [Google Scholar]
- Schumer MM, Colombel C, Sawczuk IS, Gobe G, Connor J, O’Toole KM, et al. Morphologic biochemical, and molecular evidence of apoptosis during the reperfusion phase after brief periods of renal ischemia. Am J Pathol. 1992;140:831–8. [PMC free article] [PubMed] [Google Scholar]
- Cynthia Shackelford, Long G, Wolf J, Okerberg C, Herbert R. Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol Pathol. 1992;30:93–6. doi: 10.1080/01926230252824761. [DOI] [PubMed] [Google Scholar]
- Shuvy M, Abedat S, Beeri R, Danenberg HD, Planer D, Ben-Dov IZ, et al. Uraemic hyperparathyroidism causes a reversible inflammatory process of aortic valve calcification in rats. Cardiovasc Res. 2008;79:492–9. doi: 10.1093/cvr/cvn088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L, Bellomo R, Giantomasso DD, Ronco C. The pathogenesis of septic acute renal failure. Curr Opin Crit Care. 2003;9:496–502. doi: 10.1097/00075198-200312000-00006. [DOI] [PubMed] [Google Scholar]
- Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock: a review of laboratory models and a proposal. J Surg Res. 1980;29:189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]