Abstract
Background
Calcific aortic valve disease is the most common indication for surgical valve replacement in the United States. The cellular mechanisms of valve calcification are not well understood. We have previously shown that cellular proliferation and osteoblastogenesis are important in the development of valvular heart disease. Lrp5, a known low-density receptor-related protein, plays an essential role in cellular proliferation and osteoblastogenesis via the β-catenin signaling pathway. We hypothesize that Lrp5 also plays a role in aortic valve (AV) calcification in experimental hypercholesterolemia.
Methods and Results
We examined the effects of cholesterol and atorvastatin in Watanabe rabbits (n=54). Group I (n=18) received a normal diet, group II (n=18) a 0.25% cholesterol diet, and group III (n=18) a 0.25% (w/w) cholesterol diet with atorvastatin for the development of calcification. The AVs were examined for cellular proliferation, Lrp5/β-catenin, and bone matrix markers. Bone formation was assessed by micro-computed tomography, calcein injection, and osteopontin expression. Low-density lipoprotein with and without atorvastatin was also tested in AV myofibroblasts for cellular proliferation and regulation of the Lrp5/β-catenin pathway. Our results demonstrate that the cholesterol diet induced complex bone formations in the calcified AVs with an increase in the Lrp5 receptors, osteopontin, and p42/44 expression. Atorvastatin reduced bone formation, cellular proliferation, and Lrp5/β-catenin protein levels in the AVs. In vitro analysis confirmed the Lrp5/β-catenin expression in myofibroblast cell proliferation.
Conclusion
Hypercholesterolemic AV calcification is attenuated by atorvastatin and is mediated in part by the Lrp5/β-catenin pathway. This developmental pathway may be important in the signaling pathway of this disease.
Keywords: hypercholesterolemia, pharmacology, valves
Calcific aortic stenosis is the most common indication for surgical valve replacement in the United States.1 The number of valve replacements is increasing in the United States because of the aging population.2 Until recently, the etiology of valve calcification has been thought to be due to a degenerative process related to the passive accumulation of calcium binding to the surface of the valve leaflet. Descriptive studies have demonstrated the critical features of aortic valve (AV) calcification, which are osteoblast expression, cell proliferation, and atherosclerosis.3–6 The cellular mechanisms for AV calcification, however, are still not known.
Recent epidemiological analysis of the risk factors leading to aortic valve disease have identified causative factors similar to those of vascular atherosclerosis, such as smoking, male gender, hypertension, and elevated cholesterol levels.7 Low-density lipoprotein receptors (LDLR) are critical in the uptake, processing, and cellular metabolism of cholesterol. Recently, the LDL receptor-related protein 5 (Lrp5), a coreceptor of LDL receptor family, has been discovered as an important receptor in the activation of skeletal bone formation and also implicated in cholesterol metabolism.8,9 In this article, we studied the Watanabe rabbit with the naturally occurring genetic LDLR mutation to determine whether chronic experimental hypercholesterolemia with and without atorvastatin induces calcification in the aortic valves as compared with our previous short-term cholesterol model demonstrating atherosclerosis.6 We also measured the Lrp5/β-catenin protein expression to determine whether this pathway is upregulated in this disease process.
Methods
Experimental Animal Model
Male Watanabe rabbits weighing 2.5 to 3.0 kg (Covance Kalamazoo, MI) were assigned to a control (n=18), a 0.25% cholesterol-fed group (n=18), or a cholesterol-fed and atorvastatin-treated group (n=18). All animals were fed ad libitum for 24 weeks. Control rabbits were fed a standard diet. Cholesterol-fed animals received a diet supplemented with 0.25% (w/w) cholesterol (Purina Mills), and the cholesterol-fed and atorvastatin group were given atorvastatin 2.5 mg/kg daily orally. One week before euthanasia, the rabbits received an intravenous injection of calcein10 to identify areas of new bone formation. After this 24-week period, the rabbits were anesthetized using intramuscular ketamine/xylazine (40/5 mg/kg) and then euthanized with intracardiac administration of 1 mL of Beuthanasia. All experiments were performed in an animal facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, Inc (ACUC-A3283-01, 1-08-382). Immediately after dissection from the heart, 1 leaflet from each aortic valve and the aortic attachment was fixed in 4% buffered formalin for 24 hours and then embedded in paraffin. Paraffin embedded sections (6 µm) were cut and stained with hematoxylin and eosin, Masson trichrome, and elastin Van Geison (EVG) stains for histopathologial examination.
Lipid Levels Measurements
Blood samples were centrifuged at 2000 rpm for 10 minutes at 4°C, and the serum was stored at −70°C. Total serum cholesterol levels were measured by standard enzymatic techniques as previously shown.6
Immunohistochemistry
Immunostaining of the aortic valves was performed to identify proliferating cell nuclear antigen (PCNA; Dako), Lrp5 receptor expression (Biovision), and osteopontin bone matrix protein expression (University of Iowa Hybridoma Bank). Alpha actin immunostain identified the aortic valve myofibroblasts.11 β-catenin expression identified the mechanism by which the Wnt pathway is activated (Spring Bioscience). Mitogen-activated protein kinase (p42/44) was tested to confirm the activation of cellular proliferation (Cell Signaling). Immunohistochemistry and quantification of PCNA expression was determined using digital image analysis as previously described.6
Micro-Computed Tomography
After fixing in formalin, the rabbit aortic valves were examined with a Scanco micro-computed tomography-40 system operated at 45 kV. Sampling was with ≈8 µm voxels (volume elements) and maximum sensitivity (1000 projections, 2048 samples and 0.3 sec/projection integration). The total volume of valve tissue and the volume of mineral within the valve were determined using the Scanco software; because the available tissue volumes differed somewhat from sample to sample, comparisons were made by computing the volume fraction of mineral for each.
Reverse Transcriptase Polymerase Chain Reaction
Immediately after dissection from the heart, the leaflets from each aortic valve were frozen for RNA extraction. Total RNA was obtained and semiquantitative reverse transcriptase polymerase chain reaction analysis was performed for the expression of Lrp5 and GAPDH as previously described.6
Western Blot
Equivalent amounts of protein fractions were subjected to SDS-PAGE on 12.5% acrylamide gels. After electrophoresis, the proteins were transferred to nitrocellulose membranes. The membranes were then incubated with blocking buffer containing 2% bovine serum albumin and 2% evaporated milk in Tris-buffered saline plus 0.05% Tween for 1 hour. Anti-Lrp5, β-catenin, OPN, p42/44, and α-actin were used at 1:1000 dilution in blocking buffer and added to the membranes overnight. After 2 washes with Tris-buffered saline plus Tween, the membranes were incubated in an anti-mouse immunoglobulin G-horseradish peroxidase secondary antibody at 1:2000 dilution for 30 minutes. Immune complexes were visualized using enhanced chemiluminescence. We have performed replicates (n=3) on each Western blot experiment and show a representative experiment for each blot in the data.
In Vitro Model of Valve Proliferation
Valvular myofibroblast cells were obtained from mature Yorkshire pigs (Oakhill Genetics, Erving, Il) and isolated from the cardiac aortic valves by collagenase digestion.11 Cells were cultured in medium 199 with 10% (v/v) heat-inactivated fetal bovine serum at 37°C in a humidified atmosphere of 5% CO2 in air. Cells were used between the third and tenth passage. Cardiac valve fibroblast cells were grown to confluence in 24-well plates and then growth-arrested by incubation in serum-free medium for 24 hours. Low-density lipoprotein (LDL; 10 ng/mL) (Intracell), LDL=atorvastatin (10−6 M), and atorvastatin (10−6 M) alone were tested and then incubated with cells for 18 hours in quadruplicate wells and pulsed with 1 µCi/well of tritiated thymidine for the final 4 hours of the incubation. Dose response experiments for LDL and atorvastatin were performed (data not shown), and the data shown are the peak doses for these effectors. Newly synthesized DNA was identified by incorporation of radioactivity into acid-precipitated cellular material.11 Platelet-derived growth factor served as a positive control for proliferation, and incubation in serum-free medium alone as a negative control (12 replicates were performed). Western blot analysis was also performed on the different treatment groups as outlined in the Western blot methods (n=3 replicates).
Statistics
Comparison was made among the 3 groups using ANOVA. All statistical tests were 2-tailed, and P<0.05 was considered significant.
Results
Light Microscopy of the Aortic Valves
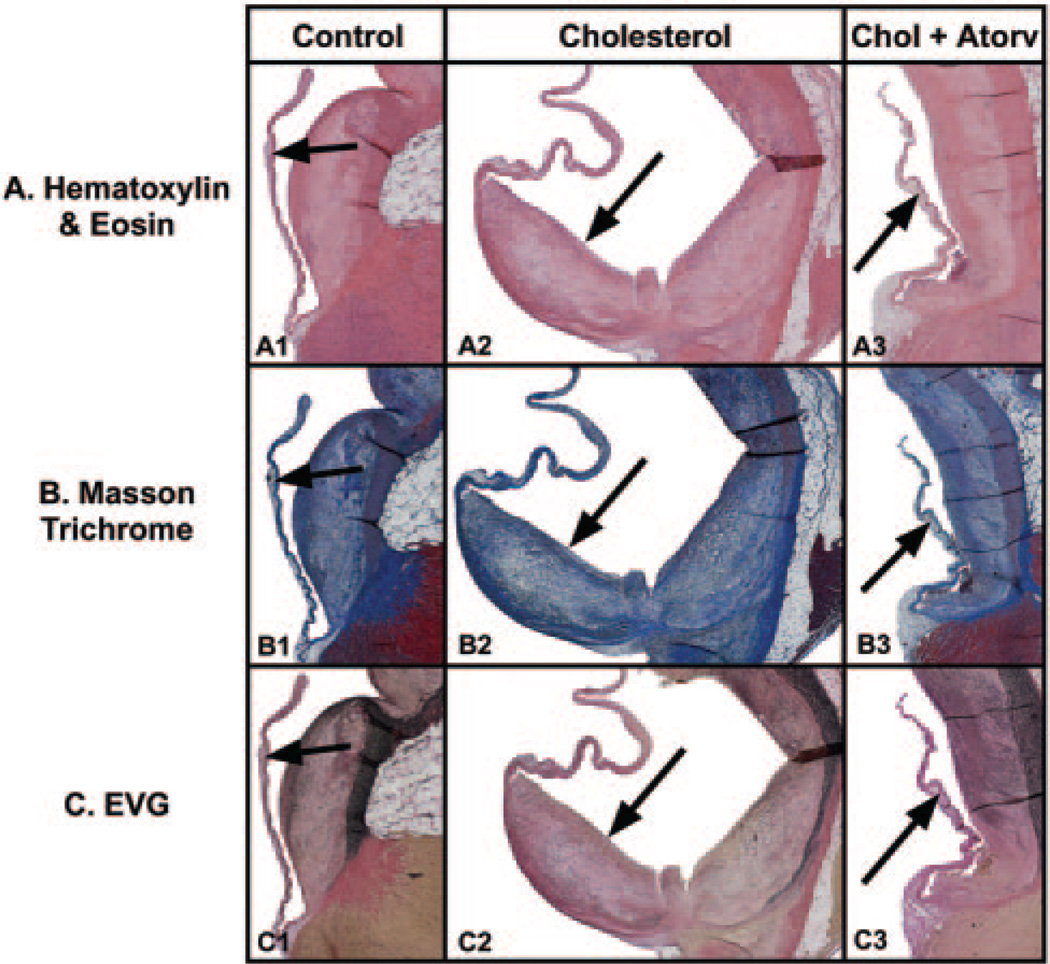
The hematoxylin and eosin, Masson trichrome, and EVG stained hypercholesterolemic aortic valves as shown in Figure 1A2, 1B2, and 1C2 demonstrate an increase in leaflet thickness (0.06±0.03 mm) that begins at the base of the aortic valve leaflet and extends along the valve leaflet from the attachment to the aorta. There is a marked increase in cellularity and collagen staining in the blue Masson trichrome and black staining elastin fibers in the EVG stain throughout the leaflet lesion. The aortic valve surface from control animals appeared normal, thin, and intact, with a smooth endothelial cell layer covering the entire surface and a thin collagen layer in the spongiosa of the valve (0.65±0.10 mm, P<0.05) (Figure 1A1, 1B1, 1C1). Abnormal leaflet thicknesses did not develop when cholesterol-fed rabbits received atorvastatin (0.23±0.10 mm, P<0.05) (Figure 1A3, 1B3, and 1C3). Table 1 compares the aortic valve thickness and cholesterol levels from the rabbits in the different treatment groups. The total serum cholesterol level is significantly higher in the cholesterol-fed animals compared with control animals (1329±141.32 mg/dL versus 284.67±28.8 mg/dL, P<0.001). Atorvastatin-treated rabbits had lower cholesterol levels than the rabbits receiving the cholesterol diet alone (1057±103.86 mg/dL, P<0.001).
Figure 1.
Light microscopy of rabbit aortic valves and aorta. Left column, control diet; middle column, cholesterol diet; right column, cholesterol diet plus atorvastatin. In each panel, the aortic valve leaflet is positioned on the left, with the aorta on the right. Arrow points to aortic valve in each figure. All frames 12.5× magnification. A, Hematoxylin and eosin stain. B, Masson trichrome stain. C, elastin Van Geison stain.
TABLE 1.
Quantification of the In Vivo Aortic Valve Results
| Control | Cholesterol | Chol+Atorv | |
|---|---|---|---|
| AV leaflet thickness, mm | 0.06±0.03 | 0.65±0.1** | 0.23±0.1* |
| Cholesterol | 284.67±28.8 | 1329±141.32** | 1057±103.56 |
| Osteopontin protein, % of control | 111.7% | 174.6% | 125.3% |
| P42/44 protein, % of control | 54.8% | 105% | 83.3% |
| PCNA | 4.4±3.88 | 11.87±7.6** | 3.22±1.07* |
| LRP5/GAPDH ratio | 0.97±1.34 | 3.5±6.66** | 0.837±0.8* |
| MicroCT | 1.07±0.72 | 11.34±7.27** | 1.02±0.55* |
AV indicates Aortic Valve; Chol, cholesterol; Atorv, atorvastatin.
P<0.05 for cholesterol vs Atorvastatin;
P<0.05 for control vs cholesterol.
Proliferation Marker and Lrp5 Expression
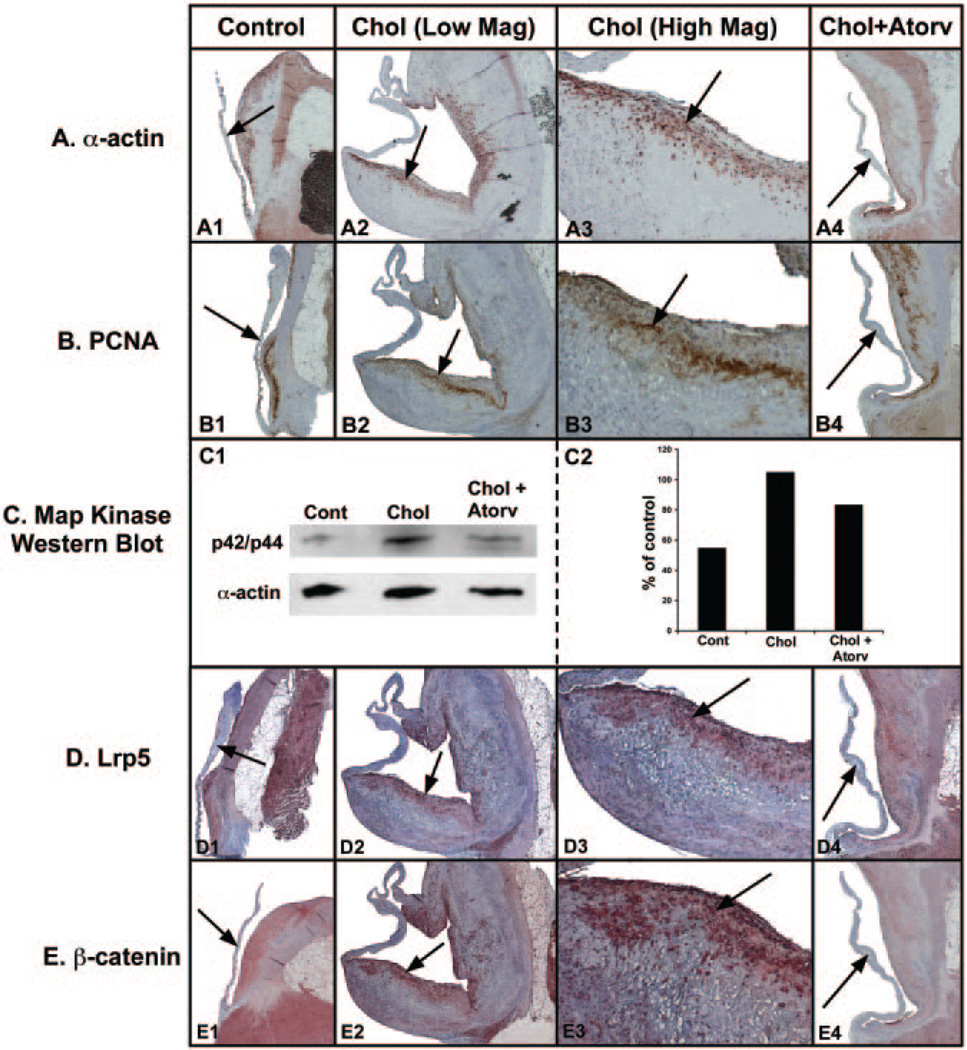
Figure 2A demonstrates the α-actin staining cells within the valve leaflet. The control aortic valve in Figure 2A1 shows that there are few actin-positive cells within the control-reated leaflets. Figure 2A2 and 2A3 of cholesterol treated leaflets show many actin-positive staining cells along the growing surface of the valve leaflet. Figure 2A4 demonstrates few positive staining cells along the surface of the atorvastatin-treated valve leaflet. Figure 2B shows the results of the PCNA stain, which is a DNA polymerase upregulated during cell division. The control valve leaflet demonstrates few PCNA-positive staining cells (Figure 2B1). Figure 2B2 and 2B3 demonstrates positive staining PCNA staining in the same area as the actin-positive staining cells of the leaflets from the cholesterol-fed animals. Figure 2B4 indicates that there were few PCNA staining-positive cells in the atorvastatin-treated valves. Figure 2C demonstrates the Western blot for the p42/44 expression in the valve leaflets from the different treatment arms, again indicating that protein expression increased in the cholesterol leaflets and decreased in the atorvastatin-treated leaflets. Figure 2D demonstrates the immunostain for the Lrp5 receptor. The Lrp5 receptor is present along the normal valve leaflet (Figure 2D1). There was a marked increased in the hypercholesterolemic valve leaflet (Figure 2D2 and 2D3). Again, the Lrp5 receptors colocalize with the actin-positive cells along the proliferating edge of the valve leaflet. Figure 2D4 demonstrates a decrease in the Lrp5 receptors in the valve leaflet. Figure 2E demonstrates the immunohistochemistry for β-catenin in the valve leaflet. β-catenin is a critical regulatory protein that, when activated, translocates to the nucleus to activate osteoblastogenesis. β-catenin is expressed in low levels in the control aortic valve, as shown in Figure 2E1. The hypercholesterolemic aortic valve leaflet has increased β-catenin in the proliferating edge of the valve leaflet, as shown in Figure 2E2 and 2E3. Atorvastatin decreased the β-catenin expression in the leaflet (Figure 2E4). Table 1 demonstrates the quantification of Lrp5 gene expression, PCNA staining in the valve leaflets, and p42/44 protein levels in the aortic valves.
Figure 2.
Proliferation marker and Lrp5/β-catenin protein expression. Left column, control diet; middle column, cholesterol diet; right column, cholesterol diet plus atorvastatin. Arrow points to aortic valve in each figure. A, α-actin immunostain. B, PCNA immunostain. C, p42/44 Western blot (1) and quantification (2). D, Lrp5 receptor immunostain. E, β-catenin immunostain.
Establishment of the Bone-Like Phenotype in the Calcified Aortic Valves
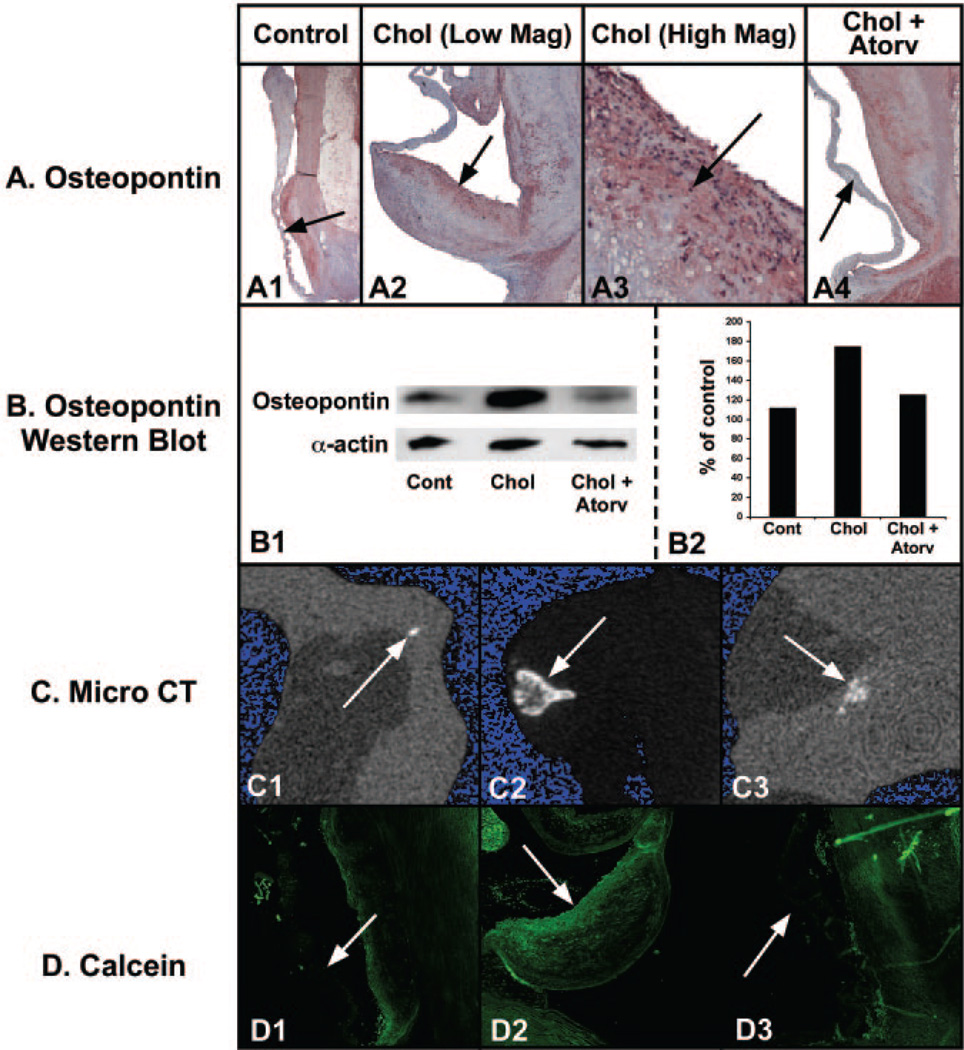
Light microscopy evaluating the osteopontin immunostain in the control aortic valves revealed a low level of osteopontin protein expression (Figure 3A1). Osteopontin is a glycosylated phosphoprotein that is important in mineralization. In the hypercholesterolemic valves, the osteopontin expression increased along the aortic valve leaflet edge and also in the center of valve, as shown in Figure 3A2 and 3A3. The osteopontin protein expression was significantly decreased with the atorvastatin treatment, as shown in Figure 3A4. Osteopontin protein levels were measured in the valve leaflets as indicated in the Western blot data (Figure 3B1). The Western blot confirms that osteopontin protein concentration increased in the cholesterol-treated aortic valves and decreased in the atorvastatin-treated aortic valves.
Figure 3.
Establishment of the bone-like phenotype in the calcified aortic valves. Left column, control diet; middle column, cholesterol diet; right column, cholesterol diet plus atorvastatin. A, Osteopontin immunostain light microscopy; aortic valve on the left and aorta on the right. Arrow points to aortic valve in each figure. All frames 12.5× magnification. B, Osteopontin Western blot and quantification. C, micro-computed tomography 2-dimensional reconstructed slices of each valve (3.4 mm horizontal field of view). D, Confocal microscopy of the calcein labeling in the mineralizing valve.
Micro-computed tomography of the aortic valves revealed the depth and extent of calcification in each nodule on the valve, as shown in Figure 3C. Figure 3C1 shows a typical 2-dimensional reconstructed slice of a control aortic valve, and Figure 3C2 shows a slice of a hypercholesterolemic aortic valve. The images indicate that mineral level is greatest at the outer edge of each nodule and decreases toward the center of the mineralized lesion, similar to what is seen in skeletal bone formation. Figure 3C3 demonstrates a marked reduction in the amount of calcification present by micro-computed tomography. Table 1 lists the amount of mineral in AVs from the different treatment groups.
Finally, we injected intravenous calcein to determine localization of in vivo new bone formation. Figure 3D shows the in vivo calcein label incorporation into the proliferating valve myofibroblast cells. In the control group, there was little calcein label in the valve leaflet (Figure 3D1). In the hypercholesterolemic valves, calcein colocalized to the proliferating valve leaflet edge, shown in Figure 3D2. The localization of calcein in the hypercholesterolemic aortic valves is parallel to the localization of the proliferating, actin-positive myofibroblast cells. There is a marked increase in the calcein label in the valve leaflets. The aortic valves in the atorvastatin-treated animals demonstrated no incorporation of calcein (Figure 3D3).
In Vitro Aortic Valve Myofibroblast Model of Cell Proliferation
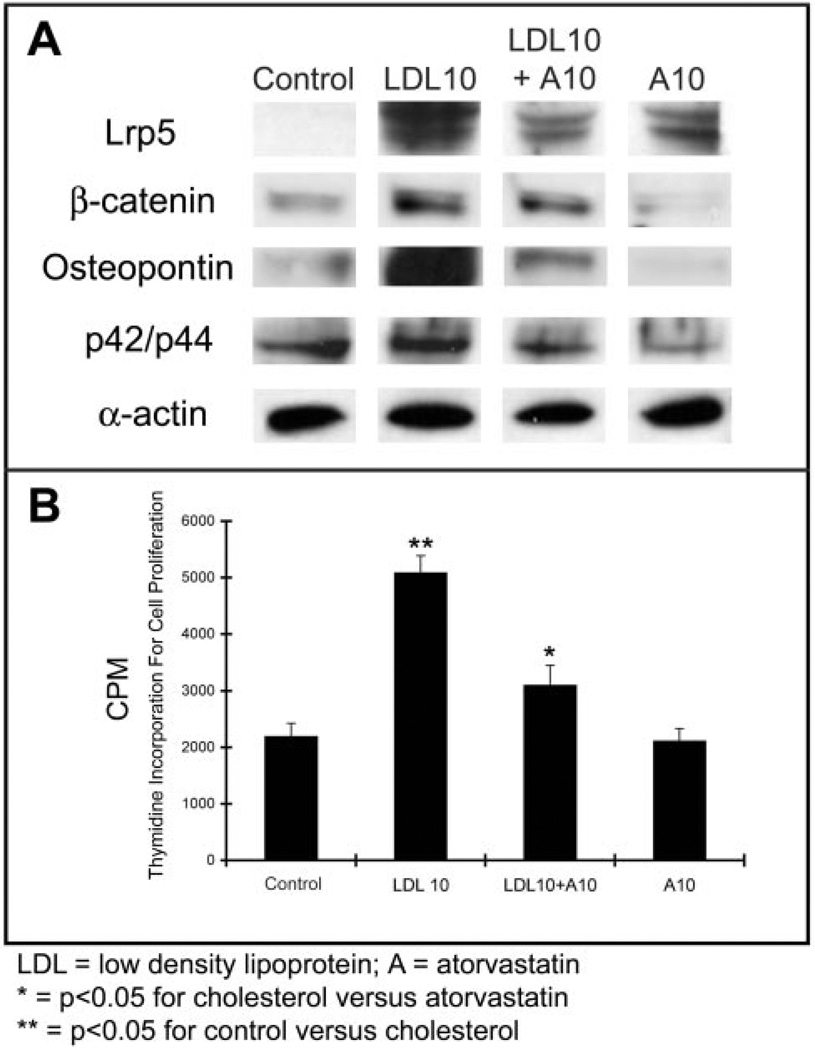
We tested in vitro aortic valve myofibroblast cells for cell proliferation and measured osteopontin/Lrp5/β-catenin and p42/44 protein expression in response to LDL and atorvastatin treatments. Figure 4 demonstrates that myofibroblast cells isolated from the aortic valve treated with LDL, with and without atorvastatin, develop an increase in thymidine incorporation with an upregulation of Lrp5 receptor, β-catenin, osteopontin, and p42/44 protein expression in the myofibroblast cells by Western blot analysis. Atorvastatin attenuates these markers. Table 2 shows the measurements of the protein levels indicated by Western blot and thymidine incorporation levels in the different treatment groups.
Figure 4.
In vitro myofibroblast cell model of proliferation and osteopontin expression in the presence of LDL with and without atorvastatin. A, Western blot for Lrp5, βcatenin, osteopontin, and p42/44. B, Thymidine incorporation in the LDL treated cells with and without atorvastatin. *P<0.001.
TABLE 2.
Quantification of the In Vitro Aortic Valve Results
| Control | LDL10 | LDL10+A10 | A10 | |
|---|---|---|---|---|
| Lrp5 | 10.44% | 105.06% | 66.99% | 67.99% |
| β-catenin | 39.54% | 68.68% | 65.51% | 24.04% |
| Osteopontin | 48.94% | 115.15% | 71.72% | 24.30% |
| P42/p44 | 93.61% | 93.85% | 87.73% | 52.07% |
| Thymidine incorporation | 2190±241 | 5087±305** | 3094±358* | 2111±227 |
LDL indicates low density lipoprotein; A, atorvastatin.
P<0.05 for cholesterol vs atorvastatin;
P<0.05 for control vs cholesterol.
Discussion
Many surgical pathological studies have demonstrated the presence of LDL cholesterol in calcified valves, indicating similarities between the genesis of valvular and vascular diseases.12 In this study, we demonstrate that calcification of the aortic valve leaflets develops within the valve similar to skeletal bone formation and that this mineralization occurs with the upregulation of Lrp5 receptors and bone formation in the cholesterol-treated rabbit valve and a decrease in the receptor and bone formation with the atorvastatin treatment. The gene for Lrp5 is not known for extensive regulation and it is expressed at low levels in nearly all tissues.13 Lrp5 is a critical LDL coreceptor important in the differentiation of osteoblast cells in skeletal bone formation.8,9 The naturally occurring LDLR defect in Watanabe rabbits has made this species a parallel model to familial hypercholesterolemia patients who have mutations in the LDLR.14 Electron beam computed tomography studies demonstrate that familial hypercholesterolemia patients have accelerated vascular and valvular atherosclerosis and calcification.15 This study extends our original study demonstrating atherosclerosis and cell proliferation in a short-term cholesterol feeding in the rabbit model6 to demonstrate the findings in a long-term lower concentration of cholesterol feeding.
Bone is a mineralized connective tissue, comprising an exquisite assembly of functionally distinct cell populations that are required to support the structural integrity of the skeleton. Although ectopic calcification in aortic valves has been described for more than 100 years, little is known regarding the synthesis of bone matrix proteins in the valve or how the genes for these proteins become active. We have demonstrated the development of an osteoblast phenotype in human aortic valves removed at the time of surgical valve replacement.5 The new data that we report in this study confirm the appearance of calcification and cellular proliferation in the hypercholesterolemic aortic valve associated with the upregulation of Lrp5 receptors. The data also confirm the aortic valve myofibroblast as the critical cell important in cell proliferation and extracellular matrix synthesis that is activated by Lrp5 receptors regulated within the myofibroblast cell. Lrp5 is a well-established coreceptor to signal osteoblast differentiation by activating the β-catenin developmental signaling pathway.16 When activated, β-catenin translocates to the nucleus to start osteoblastogenesis and new bone formation. Kim et al17 have previously demonstrated the increase in Lrp5 receptor protein expression in the arteries of the hypercholesterolemic Watanabe rabbit. In the in vivo model, atorvastatin decreased the amount of calcification in the aortic valve, with an associated decrease in Lrp5 receptor protein and gene expression. The only difference between the 2 models is the level of Lrp5 protein expression in the in vitro model versus the in vivo model. The in vitro model demonstrated no difference in the receptor protein expression with atorvastatin versus the LDL treatment, which may be because of the different time points tested in the in vivo model (6 months) versus the in vitro model (24 hours). Together, these new observations in our in vivo and in vitro models support the hypothesis that degenerative valvular aortic stenosis is the result of active bone formation in the aortic valve, which may be mediated through a process of osteoblast differentiation, and that statins inhibit this calcification process.
Acknowledgments
This work was completed with the support of an American Heart Association Grant-in-Aid (0350564Z) and a grant from the US National Institutes of Health (1K08HL073927-01). The authors would like to thank Jason Gocek and Konstantin Ignatiev for their technical support.
References
- 1.Bonow RO, Carabello B, de Leon AC, Edmunds LH, Jr, Fedderly BJ, Freed MD, Gaasch WH, McKay CR, Nishimura RA, O’Gara PT, O’Rourke RA, Rahimtoola SH, Ritchie JL, Cheitlin MD, Eagle KA, Gardner TJ, Garson A, Jr, Gibbons RJ, Russell RO, Ryan TJ, Smith SC., Jr ACC/AHA Guidelines for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on Practice Guidelines (Committee on Management of Patients With Valvular Heart Disease) J Heart Valve Dis. 1998;7:672–707. [PubMed] [Google Scholar]
- 2.STS TECOM web site. [Accessed March 3, 2005]; Available at: http://www.sts.com.
- 3.O’Brien KD, Kuusisto J, Reichenbach DD, Ferguson M, Giachelli C, Alpers CE, Otto CM. Osteopontin is expressed in human aortic valvular lesions. Circulation. 1995;92:2163–2168. doi: 10.1161/01.cir.92.8.2163. [DOI] [PubMed] [Google Scholar]
- 4.Mohler ER, 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 5.Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg T. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–2184. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajamannan NM, Subramaniam M, Springett M, Sebo TC, Niekrasz M, McConnell JP, Singh RJ, Stone NJ, Bonow RO, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced cellular proliferation and bone matrix production in the rabbit aortic valve. Circulation. 2002;105:2660–2665. doi: 10.1161/01.cir.0000017435.87463.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 8.Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, Lappe MM, Spitzer L, Zweier S, Braunschweiger K, Benchekroun Y, Hu X, Adair R, Chee L, FitzGerald MG, Tulig C, Caruso A, Tzellas N, Bawa A, Franklin B, McGuire S, Nogues X, Gong G, Allen KM, Anisowicz A, Morales AJ, Lomedico PT, Recker SM, Van Eerdewegh P, Recker RR, Johnson ML. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Human Genet. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML. Osteoporosis-Pseudoglioma Syndrome Collaborative Group. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 10.Turner RT. Cancellous bone turnover in growing rats: time-dependent changes in association between calcein label and osteoblasts. J Bone Mineral Res. 1994;9:1419–1424. doi: 10.1002/jbmr.5650090913. [DOI] [PubMed] [Google Scholar]
- 11.Rajamannan NM, Caplice N, Anthikad F, Sebo TJ, Orszulak TA, Edwards WD, Tajik J, Schwartz RS. Cell proliferation in carcinoid valve disease: a mechanism for serotonin effects. J Heart Valve Dise. 2001;10:827–831. [PubMed] [Google Scholar]
- 12.O’Brien KD, Reichenbach DD, Marcovina SM, Kuusisto J, Alpers CE, Otto CM. Apolipoproteins, B, (a), and E accumulate in the morphologically early lesion of “degenerative” valvular aortic stenosis. Arterioscler Thromb Vasc Biol. 1996;16:523–532. doi: 10.1161/01.atv.16.4.523. [DOI] [PubMed] [Google Scholar]
- 13.The Wnt home page. [Accessed March 3, 2005]; Available at: http://www.stanford.edu/~rnusse/wntwindow.html.
- 14.Aliev G, Burnstock G. Watanabe rabbits with heritable hypercholesterolaemia: a model of atherosclerosis. Histol Histopathol. 1998;13:797–817. doi: 10.14670/HH-13.797. [DOI] [PubMed] [Google Scholar]
- 15.Hoeg JM, Feuerstein IM, Tucker EE. Detection and quantitation of calcific atherosclerosis by ultrafast computed tomography in children and young adults with homozygous familial hypercholesterolemia. Arterioscler Thromb. 1994;14:1066–1074. doi: 10.1161/01.atv.14.7.1066. [DOI] [PubMed] [Google Scholar]
- 16.Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA, 2nd, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157:303–314. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim DH, Inagaki Y, Suzuki T, Ioka RX, Yoshioka SZ, Magoori K, Kang MJ, Cho Y, Nakano AZ, Liu Q, Fujino T, Suzuki H, Sasano H, Yamamoto TT. A new low density lipoprotein receptor related protein, LRP5, is expressed in hepatocytes and adrenal cortex, and recognizes apolipoprotein E. J Biochem. 1998;124:1072–1076. doi: 10.1093/oxfordjournals.jbchem.a022223. [DOI] [PubMed] [Google Scholar]